Abstract
Background
Repeatedly hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) are often exposed to more antibiotics, but the distribution of pathogenic bacteria in these patients is poorly understood. The objectives of this study were to analyze the distribution of pathogenic bacteria and the risk factors associated with multidrug-resistant (MDR) bacteria infection in early re-admission patients with AECOPD.
Methods
We retrospectively reviewed charts for patients with AECOPD admitted to our hospital between January 2011 and November 2012. The early re-admission group and non-early readmission group were determined by whether patients were readmitted within 31 days after discharge. Detection of potentially pathogenic microorganisms (PPMs) and MDR bacteria were analyzed. Logistic regression analysis was performed to identify independent risk factors for MDR bacteria infection.
Results
PPMs were isolated from 230 (32.0%) cases of respiratory tract specimens; MDR bacteria accounted for 24.7% (57/230). Pseudomonas aeruginosa (43.7%), Klebsiella pneumoniae (15.6%), and Acinetobacter baumannii (12.5%) were the top three PPMs in the early readmission group, while the top three PPMs in the non-early readmission group were K. pneumoniae (23.7%), P. aeruginosa (21.2%), and Streptococcus pneumoniae (17.1%). Multivariate analysis showed that use of antibiotics within 2 weeks (odds ratio [OR] 8.259, 95% confidence interval [CI] 3.056–22.322, p = 0.000) was the independent risk factor for MDR bacteria infection.
Conclusion
Non-fermentative Gram-negative bacilli (NFGNB) and enterobacteria were the predominant bacteria in early readmission patients with AECOPD. The detection rate of MDR bacteria was high which was related to the use of antibiotics within 2 weeks before admission in these patients.
Keywords: AECOPD, re-admission, bacteria, multidrug-resistant (MDR), risk factors
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic airway disease, and is characterized by persistent airflow limitation that is usually progressive. COPD is a leading cause of morbidity, mortality, and utilization of health care resources worldwide1,2. In China, the incidence of COPD is 8.2% in the population over the age of 403, so the number of COPD patients in China is estimated to be close to 43 million. Acute exacerbation is an important clinical course in patients with COPD. On average, acute exacerbation of COPD (AECOPD) rates are approximately one to two per patient-year; with hospitalization averaging approximately 0.1 to 0.2 per patient-year4. Acute exacerbation is the predominant cause of hospitalization and death, and is also the main expenditure component of medical expenses in patients with COPD. For example, in-hospital mortality of AECOPD in the United States was 4.3% in 2006, with mean costs of 9,545 USD per patient for hospitalization5. According to an epidemiological study, the cost of AECOPD for an inpatient in the People's Republic of China has been 1692 USD a year6.
AECOPD is mostly caused by bacterial infection of the trachea and bronchi. The bacterial load was increased in patients with AECOPD and at least 50% of the patients had a high concentration of bacteria in the lower respiratory tract7. Up to 80% of AECOPD is caused by microbial pathogens, including bacteria, viruses, atypical bacteria, and fungi8,9, but the usage of antibiotics in AECOPD is more extensive than expected in the clinical practice10.
Although large-scale, multi-center studies about the bacterial etiology of AECOPD are lacking, the existing research revealed that the distribution of pathogenic bacteria in AECOPD was related to the severity of airflow limitation. The common pathogenic bacteria of community acquired respiratory tract infection, such as Haemophilus influenzae and Streptococcus pneumoniae, were mainly found in mild to moderate AECOPD patients, but Pseudomonas aeruginosa was more common in patients with severe COPD7,9,11,12. Due to the heterogeneity of patients with COPD, the patients with similar forced expiratory volume in one second (FEV1) percentage of the predicted value may have different clinical status. Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) 2011 recommended a new comprehensive assessment method, covering the degree of airflow limitation, symptoms, acute exacerbations, and comorbidities. Patients were divided into A, B, C, and D groups by the former three, but research about the distribution of pathogenic bacteria in each group was lacking.
The unplanned readmission rate within 31 days for the same or related disease is one of the medical quality index evaluation criteria in the China Healthcare Quality Indicators System (CHQIS). The early readmission rate of COPD in five general hospitals in Beijing was 2.67% to 6.3%13 - lower than the 30 days readmission rate of 13.6% to 24.2% in other countries or regions14–16. In a previous study, the 31 days readmission rate for AECOPD was 6.8%17.
The risk of readmission within 31 days is associated with the severity of the disease, and these patients are often exposed to more antibiotics, but the distribution of pathogenic bacteria in these patients is poorly understood so far. This study aimed to analyze the distribution characteristics, drug resistance of pathogenic bacteria, and risk factors associated with multidrug-resistant (MDR) bacteria infection in early readmission patients with AECOPD, which may provide reference for the clinical practice.
Materials and methods
Patients
Charts for patients with AECOPD who were discharged from Taizhou Hospital of Wenzhou Medical University (Zhejiang, P. R. China) over a 23-month period between January 1, 2011 to November 30, 2012 were retrospectively reviewed. The diagnosis of COPD was based on patients with long-term smoking and other risk factors, history of chronic cough, expectoration, and dyspnea. Physical examination showed signs of emphysema, chest X-ray or computer tomography (CT) showed the signs of chronic bronchitis and emphysema, and blood gas analysis was associated with varying degrees of hypoxemia and/or increased carbon dioxide pressure. Some patients completed spirometric tests before admission or during hospitalization which met the GOLD criteria18 for COPD. Exclusion criteria were (1) other lung disorders such as asthma, lung cancer, bronchiectasis, active tuberculosis, pneumothorax, or pleural effusion, and (2) Chest X-ray or CT showed shadow of pulmonary exudation.
Data collection
We selected patients with a primary diagnosis of COPD using international classification of diseases (ICD) coding (ICD-9: 490–492, 494–496) from the information and electronic medical record system of Taizhou Hospital. We collected the patient's demographics, comorbidities, and bacteriological test results (17). Arterial partial pressure of oxygen (PaO2) and arterial partial pressure of carbon dioxide (PaCO2) were recorded according to the last blood test results during hospitalization. Patients had undergone intubation and mechanical ventilation, which was defined as within 6 months of this hospitalization. The interval between discharge and readmission was recorded for the readmitted patients, and we confirmed the same patient by the same hospital medical record number and name. The early readmission group and non-early readmission group were determined by whether patients were readmitted within 31 days after discharge. Study design has been approved by the ethics committee of the Taizhou Hospital of Zhejiang Province, China. Written informed consent was not required because of the retrospective nature of the investigation.
Bacteriological study
Qualified sputum samples (10 squamous cells and > 25 leukocytes per low-power field) were collected on the day of admission or the next morning. All samples were plated onto blood agar, chocolate agar, and MacConkey agar within 4 hours of being collected, and the growth of bacteria was observed within 24–48 h. VITEK® 2 COMPACT 60 automatic microorganism analyzer (bioMérieux, France) and its corresponding identification card were used for strain identification. Drug susceptibility testing and the results were interpreted using the 2008 criteria of the Clinical and Laboratory Standards Institute (CLSI)19. Classification of potentially pathogenic microorganisms (PPMs) or non-PPMs depended on the description in the literature20. Viridans group streptococci, Neisseria species, and Candida species were considered non-PPMs. MDR bacteria were judged by the international experts' recommendation relating to the provisional standard definition of drug resistant bacteria21.
Statistical analysis
All data were analyzed by SPSS 21.0. Numerical variables were analyzed by non-parametric test, and chi-squared test was used for categorical variables. A P of <0.05 was accepted as indicating a statistically significant difference. The MDR bacteria isolated from sputum samples were regarded as dependent variables, and the indicators with statistically significant difference in univariate analysis were considered as independent variables. Finally, multivariate logistic regression analysis was performed to determine the independent risk factors associated with MDR bacteria infection in AECOPD patients.
Results
Patient characteristics
Between January 1, 2011 to November 30, 2012, 515 patients who were admitted to our hospital with AECOPD met the inclusion criteria for the study. The total number of AECOPD admissions during this time period was 718 cases. Baseline characteristics of patients are shown in Table 1. The age of the patients ranged from 47 to 90 years, with an average of 73.31 ± 7.657 years. Four hundred and eleven patients were male (79.8%) and 104 patients were female (20.2%). Sixty one cases were readmitted within 31 days after discharge which met our criteria for early readmission. The interval between discharge and early readmission was from 2 to 31 days, with an average of 15.16 ± 7.27 days, and 10 cases were readmitted within 7 days after discharge (10/61, 16.4%). One hundred cases had a history of antibiotic treatment within 2 weeks before admission, and 41 cases had received intubation and mechanical ventilation within 6 months of this hospitalization (Table 1).
Table 1.
Baseline characteristics of patients in two groups admitted with AECOPD
| Characteristics | Early readmissions (n = 61) |
Non-early readmissions (n = 657) |
p value |
| Gender (male/female) | 55/6 | 529/128 | 0.064 |
| Age (years) | 72.52 ± 8.555 | 73.73 ± 7.551 | 0.239 |
| Use of antibiotics within 2 weeks before admission (%) |
23 (37.7%) | 77 (11.7%) | 0.000 |
| Have received intubation and mechanical ventilation |
10 | 31 | 0.001 |
| PaO2 (mmHg) | 63.38 ± 8.091 | 64.39 ± 8.981 | 0.394 |
| PaCO2 (mmHg) | 62.41 ± 9.155 | 58.05 ± 10.589 | 0.001 |
| PPMs (%) | 32 (52.4%) | 198 (30.1%) | 0.000 |
| MDR bacteria (%) | 14 (22.9%) | 43 (6.5%) | 0.000 |
Data are presented as mean ± standard deviation.
PPMs, potentially pathogenic microorganisms; MDR, multidrug-resistant.
Distribution of PPMs and MDR bacteria
PPMs were isolated from 230 cases of respiratory tract specimens, with 32.0% (230/718) of detection rate. The top five bacteria were P. aeruginosa (56 cases, 24.3%), Klebsiella pneumoniae (52 cases, 22.6%), S. pneumoniae (36 cases, 15.6%), Acinetobacter baumannii (28 cases, 12.1%), and Escherichia coli (12 cases, 5.2%), respectively (Table 2). MDR bacteria were isolated from 57 cases of respiratory tract specimens, accounting for 24.7% of total PPMs (57/230). Non-fermentative Gram-negative bacilli (NFGNB) such as A. baumannii (15 cases, 26.3%) and P. aeruginosa (12 cases, 21.1%) were the predominant MDR bacteria, followed by K. pneumoniae (8 cases, 14.0%) and E. coli (4 cases, 7.02%) which belonged to Extended-Spectrum Beta-Lactamase (ESBL)-producing Enterobacteriaceae. Among Gram-positive cocci, methicillin-resistant Staphylococcus aureus (MRSA) (5 cases, 8.77%) and Enterococcus faecium (2 cases, 3.51%) were the common MDR bacteria (Table 2).
Table 2.
Distribution of PPMs and MDR bacteria in 230 cases of sputum samples with AECOPD
| Bacterial species | Cases of PPMs (%) | Cases of MDR (%) |
| Pseudomonas aeruginosa | 56 (24.3) | 12 (21.1) |
| Klebsiella pneumoniae | 52 (22.6) | 8 (14.0) |
| Streptococcus pneumoniae | 36 (15.6) | 0 (0.00) |
| Acinetobacter baumannii | 28 (12.1) | 15 (26.3) |
| Escherichia coli | 12 (5.2) | 4 (7.02) |
| Staphylococcus aureus | 10 (4.3) | 5 (8.77) |
| Haemophilus influenzae | 10 (4.3) | 0 (0.00) |
| Moraxella catarrhalis | 6 (2.6) | 0 (0.00) |
| Enterobacter cloacae | 5 (2.1) | 1 (1.75) |
|
Stenotrophomonas maltophilia |
3 (1.3) | 3 (5.26) |
| Enterococcus faecium | 2 (0.8) | 2 (3.51) |
| Burkholderia cepacia | 2 (0.8) | 2 (3.51) |
| Staphylococcus haemolyticus | 1 (0.4) | 0 (0.00) |
| Staphylococcus epidermis | 1 (0.4) | 0 (0.00) |
| Flavobacterium | 1 (0.4) | 1 (1.75) |
| P. aeruginosa + A. baumannii | 3 (1.3) | 3 (5.26) |
| P. aeruginosa + S. aureus | 1 (0.4) | 1 (1.75) |
| K. pneumoniae + A. baumannii | 1 (0.4) | 1 (1.75) |
| Total | 230 (100) | 57 (100) |
PPMs, potentially pathogenic microorganisms; MDR, multidrug-resistant.
Distribution of pathogenic bacteria in two groups of AECOPD
The detection rate of PPMs in the early readmission group was higher than that in the non-early readmission group (P = 0.000), with 52.4% and 30.1%, respectively (Table I). The distribution of PPMs was different in the two groups. In the early readmission group, the top three bacteria were P. aeruginosa (14 cases, 43.7%), K. pneumoniae (5 cases, 15.6%), and A. baumannii (4 cases, 12.5%), but K. pneumoniae (47 cases, 23.7%), P. aeruginosa (42 cases, 21.2%) and S. pneumoniae (34 cases, 17.1%) were the top three PPMs in the non-early readmission group (Table 3). Compared with the non-early readmission group, isolation of NFGNB in early readmission group, especially P. aeruginosa (P = 0.006), were much higher, while Gram-positive cocci were relatively low (P = 0.002) (Table 3). The detection rate of MDR bacteria in the early readmission group (14/61, 22.9%) was higher than non-early readmission group (43/657, 6.5%) (P = 0.000) (Table 1).
Table 3.
Distribution of pathogenic bacteria in the early readmission and nonearly readmission group with AECOPD
| PPMs | Early readmissions (n = 32) |
Non-early readmissions (n = 198) |
p value |
| Traditional pathogenic bacteria of community |
3 (9.3%) | 49 (24.7%) | 0.054 |
| S. pneumoniae | 2 (6.2%) | 34 (17.1%) | 0.115 |
| NFGNB | 19 (59.3%) | 71 (35.8%) | 0.011 |
| P. Aeruginosa | 14 (43.7%) | 42 (21.2%) | 0.006 |
| A. baumannii | 4 (12.5%) | 24 (12.1%) | 1.000 |
| Enterobacteriaceae | 7 (21.8%) | 62 (31.3%) | 0.280 |
| K. pneumoniae | 5 (15.6%) | 47 (23.7%) | 0.309 |
| Gram-positive cocci (include S. pneumoniae) |
4 (12.5%) | 46 (23.2%) | 0.002 |
| Mixed infection | 1 (3.1%) | 4 (2.0%) | 1.000 |
Traditional pathogenic bacteria of community, including S. pneumoniae, H. influenzae and M catarrhalis; NFGNB, non-fermentative Gram-negative bacilli; Gram-positive cocci, including S. Aureus, S. haemolyticus, S. epidermis and S. pneumoniae.
Risk factors associated with MDR bacteria infection
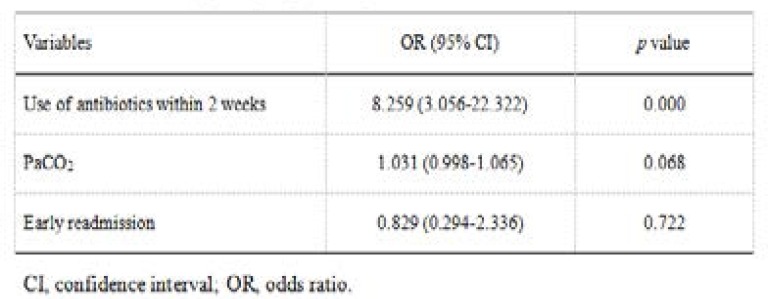
Two hundred and thirty cases of PPMs isolated from respiratory tract specimens were divided into MDR bacteria infection group (57 cases) and non-MDR bacteria infection group (173 cases). Compared with non-MDR bacteria infection group, MDR bacteria infection group had higher levels of PaCO2, more use of antibiotics within 2 weeks, and more frequent unplanned early re-admisson cases (Table 4). Based on the results of univariate analysis, PaCO2, use of antibiotics within 2 weeks, and early re-admission were included in the multivariate analysis. The logistic regression analysis showed that use of antibiotics within 2 weeks (odds ratio [OR] 8.259, 95% confidence interval [CI] 3.056–22.322, P = 0.000) was the independent risk factor associated with MDR bacteria infection (Table 5).
Table 4.
Comparison of patients with AECOPD according to MDR bacteria infection status
| Characteristics | Non-MDR infection (n = 173) |
MDR infection (n = 57) | p value |
| Gender (years) | 73.09 ± 8.799 | 73.65 ± 7.873 | 0.668 |
| PaO2 (mmHg) | 63.08 ± 8.213 | 65.35 ± 9.129 | 0.079 |
| PaCO2 (mmHg) | 58.77 ± 10.853 | 62.46 ± 8.065 | 0.007 |
| Intubation and mechanical ventilation within 6 months |
20 | 7 | 0.884 |
| Use of antibiotics within 2 weeks |
10 | 19 | 0.000 |
| Early readmission | 18 | 14 | 0.007 |
Data are presented as mean ± standard deviation.
Table 5.
Odds ratio by binary logistic regression of MDR bacteria infection
Discussion
AECOPD is defined as an acute event characterized by a worsening of COPD patient's respiratory symptoms (typically presented with dyspnea, cough, increased sputum production, and/or purulent sputum) that is beyond normal day-to-day variations and leads to a change in medicaton18. Based on the definition and process of disease, infection of AECOPD generally refers to the infection of the trachea-bronchus, which belongs to the field of lower respiratory tract infections, but is different from pneumonia. Strictly, COPD patients with pneumonia belong to COPD combined with community acquired pneumonia (CAP), rather than AECOPD, which have quite a difference in etiology and prognosis. Therefore, the patients with exudative shadow in X-ray or CT on admission were excluded in the present study.
Compared with CAP and hospital acquired pneumonia (HAP), research about the microbial etiology of AE-COPD were less in the world. Recommendations for the use of antimicrobial agents in GOLD tended to be programmatic from 2007 edition to 2015 edition, which may be related to the complexity and diversity of individual clinical manifestations in COPD. Species of bacteria were associated with the status of disease, course of disease, lung function, and previous treatment. H. influenzae (including Haemophilus parainfluenzae), S. pneumoniae, and M. catarrhalis were mostly found in mild COPD patients with exacerbation, while P. aeruginosa was more common in patients with severe airflow limitation and A. baumannii was related to the prolonged hospital stay7,11,22.
Many problems still exist in the detection of microbial etiology in AECOPD, except for patients with artificial airway. The reliability of bacteria detected from sputum via spitting through the mouth was under suspicion because it may be affected by oral bacterial colonization, but sputum bacterial culture was still the most commonly used method for diagnosis and treatment in the clinical practice. Bacteria can be isolated from the sputum in 40% to 60% of patients with AECOPD, with the three most common pathogens being H. influenzae, M. catarrhalis, and S. pneumoniae, followed by P. aeruginosa, Enterobacteriaceae, S. aureus, and H. parainfluenzae7,8,23. Recently, a large multicenter study in China showed that 331 pathogenic bacterial strains (37.4%) were isolated from 844 patients with AECOPD. Gram-negative bacilli accounted for the 78.8% of the total strains, and the predominant bacteria were P. aeruginosa, K. pneumoniae, and H. influenzae; 15% of which were Gram-positive cocci, including S. pneumoniae, and S. Aureus24.
In our study, P. aeruginosa, K. pneumoniae, and S. pneumoniae were the predominant PPMs isolated from sputum culture of patients, while H. influenzae and M. catarrhalis (which belong to conventional pathogenic bacteria of community acquired respiratory tract infection) accounted for a low proportion, which may attribute to that all the patients were hospitalized and the degree of airflow limitation was severe in this group. The distribution of major pathogens in this study, including P. aeruginosa and Enterobacteriaceae, is similar to the main clinically isolated strains in the domestic homogeneous patients of China, in addition to many research results throughout the world8,20,22–25.
Previous studies have found that the risk of readmission for AECOPD is related to factors including the frequency of acute exacerbations in the previous year, FEV1%, low body mass index, PaCO2, chronic cor pulmonale, short duration of hospitalization, hyperglycemia, and hypoproteinemia13–16,26. In our research data, compared with the non-early re-admission group, the early readmission group demonstrated higher levels of PaCO2, percentage of antibiotic treatment within 2 weeks, and percentage of intubation and mechanical ventilation within 6 months. The detection rate of PPMs and the percentage of MDR bacteria in the early re-admission group were higher than the non-early re-admission group. Simultaneously, the distribution of PPMs was different in the two groups. In the early readmission group, the top three bacteria were P. aeruginosa, K. pneumoniae1, and A. baumannii, but K. pneumoniae, P. aeruginosa, and S. pneumoniae were the top three PPMs in the non-early readmission group. Compared with the non-early re-admission group, isolation of NFGNB in the early re-admission group, especially P. aeruginosa, was much higher, while Gram-positive cocci isolation was relatively low. The MDR bacteria were more common in the early re-admission group than the non-early readmission group. The differences in the distribution of PPMs and the detection rate of MDR bacteria may be related to the application of antibiotics and the severity of the disease itself.
Previous studies have indicated that the use of antibiotics and endotracheal intubation were the independent risk factors for the infection of MDR bacteria27. This study showed that the detection rate of MDR bacteria was high in the early readmission group, and multivariate regression analysis revealed that infection of MDR bacteria was associated with use of antibiotics within 2 weeks. Antibiotics have been considered as an independent risk factor for MDR infection in AECOPD; the occurrence and development of MDR bacteria are a serious consequence of the wide use, especially the unreasonable application of antibiotics27,28.
There are limitations of this study. Reliance on clinical diagnosis rather than international guideline diagnosis for COPD (GOLD criteria) is a weakness. The patients with AECOPD who did not meet the criteria of admission were not included in our study because all of the patients were hospitalized. Few women were identified in the review, which may reflect under-diagnosis or a lower prevalence of COPD in women in China. This was also a retrospective study, and confirmation of the findings should be sought in prospective studies.
Conclusion
NFGNB and entero bacteria accounted for a higher proportion in unplanned early readmission patients with AECOPD, while the detection rate of MDR bacteria was also high. For such patients, more attention should be paid in detecting bacterial etiology and the results of drug susceptibility testing, strictly mastering the indications for application of antibiotics, and reducing the possibility of MDR bacteria infection in patients with AECOPD.
Acknowledgments
We are grateful to Abigail Howard (school of medicine, the University of Chicago) for revision of this manuscript. This research was supported by Taizhou Science and Technology Planning Project (1702KY26).
Conflicts of interest
None.
References
- 1.Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 3.Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176:753–760. doi: 10.1164/rccm.200612-1749OC. [DOI] [PubMed] [Google Scholar]
- 4.Chenna PR, Mannino DM. Outcomes of severe COPD exacerbations requiring hospitalization. Semin Respir Crit Care Med. 2010;31:286–294. doi: 10.1055/s-0030-1254069. [DOI] [PubMed] [Google Scholar]
- 5.Perera PN, Armstrong EP, Sherrill DL, Skrepnek GH. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD. 2012;9:131–141. doi: 10.3109/15412555.2011.650239. [DOI] [PubMed] [Google Scholar]
- 6.Chen YH, Yao WZ, Cai BQ, Wang H, Deng XM, Gao HL, et al. Economic analysis in admitted patients with acute exacerbation of chronic obstructive pulmonary disease. Chin Med J (Engl) 2008;121:587–591. [PubMed] [Google Scholar]
- 7.Hill AT, Campbell EJ, Hill SL, Bayley DL, Stockley RA. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med. 2000;109:288–295. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]
- 8.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 9.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris S, Anderson P, Irwin DE. Acute exacerbations of chronic bronchitis: a pharmacoeconomic review of antibacterial use. Pharmacoeconomics. 2002;20:153–168. doi: 10.2165/00019053-200220030-00002. [DOI] [PubMed] [Google Scholar]
- 11.Eller J, Ede A, Schaberg T, Niederman MS, Mauch H, Lode H. Infective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung function. Chest. 1998;113:1542–1548. doi: 10.1378/chest.113.6.1542. [DOI] [PubMed] [Google Scholar]
- 12.Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD Study Group of Bacterial Infection in COPD. Chest. 1999;116:40–46. doi: 10.1378/chest.116.1.40. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Jiao YH, Zhao MG, Liang MH, Ma XM, Wang XN, et al. A comparative analysis of “re-entry category” indicators in five major general hospitals in Beijing. Chinese Health Quality Management. 2010;17:4–7. [Google Scholar]
- 14.Nantsupawat T, Limsuwat C, Nugent K. Factors affecting chronic obstructive pulmonary disease early rehospitalization. Chronic Respiratory Disease. 2012;9:93–98. doi: 10.1177/1479972312438703. [DOI] [PubMed] [Google Scholar]
- 15.Chan FW, Wong FY, Yam CH, Cheung WL, Wong EL, Leung MC, et al. Risk factors of hospitalization and readmission of patients with COPD in Hong Kong population: analysis of hospital admission records. BMC Health Serv Res. 2011;11:186. doi: 10.1186/1472-6963-11-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Xu Y, Wu X, Chen M, Lin L, Gong L, et al. Risk factors associated with chronic obstructive pulmonary disease early readmission. Curr Med Res Opin. 2014;30:315–320. doi: 10.1185/03007995.2013.858623. [DOI] [PubMed] [Google Scholar]
- 18.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 19.Clinical Laboratory Standards Institute (CLSI), author Performance standards for antimicrobial susceptibility testing; Eighteenth Informational Supplement. M100-S18. Wayne, PA, USA: 2008. [Google Scholar]
- 20.Lin SH, Kuo PH, Hsueh PR, Yang PC, Kuo SH. Sputum bacteriology in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease in Taiwan with an emphasis on Klebsiella pneumoniae and Pseudomonas aeruginosa. Respirology. 2007;12:81–87. doi: 10.1111/j.1440-1843.2006.00999.x. [DOI] [PubMed] [Google Scholar]
- 21.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakou A, Papaparaskevas J, Diamantea F, Skarmoutsou N, Polychronopoulos V, Tsakris A. A prospective study on bacterial and atypical etiology of acute exacerbation in chronic obstructive pulmonary disease. Future Microbiol. 2014;9:1251–1260. doi: 10.2217/fmb.14.90. [DOI] [PubMed] [Google Scholar]
- 23.Cai BQ, Cai SX, Chen RC, Cui LY, Feng YL, Gu YT, et al. Expert consensus on acute exacerbation of chronic obstructive pulmonary disease in the People's Republic of China. Int J Chron Obstruct Pulmon Dis. 2014;9:381–395. doi: 10.2147/COPD.S58454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye F, He LX, Cai BQ, Wen FQ, Chen BY, Hadiarto M, et al. Spectrum and antimicrobial resistance of common pathogenic bacteria isolated from patients with acute exacerbation of chronic obstructive pulmonary disease in mainland of China. Chin Med J (Engl) 2013;126:2207–2214. [PubMed] [Google Scholar]
- 25.Domenech A, Puig C, Marti S, Santos S, Fernandez A, Calatayud L, et al. Infectious etiology of acute exacerbations in severe COPD patients. J Infect. 2013;67:516–523. doi: 10.1016/j.jinf.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 26.McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132:1748–1755. doi: 10.1378/chest.06-3018. [DOI] [PubMed] [Google Scholar]
- 27.Nseir S, Di Pompeo C, Cavestri B, Jozefowicz E, Nyunga M, Soubrier S, et al. Multiple-drug-resistant bacteria in patients with severe acute exacerbation of chronic obstructive pulmonary disease: Prevalence, risk factors, and outcome. Crit Care Med. 2006;34:2959–2966. doi: 10.1097/01.CCM.0000245666.28867.C6. [DOI] [PubMed] [Google Scholar]
- 28.Falagas ME, Bliziotis IA, Kasiakou SK, Samonis G, Athanassopoulou P, Michalopoulos A. Outcome of infections due to pandrug-resistant (PDR) Gram-negative bacteria. BMC Infect Dis. 2005;5:24. doi: 10.1186/1471-2334-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]