Abstract
Background
Quorum sensing inhibitionis an advanced strategy that aims to interfere with bacterial cell-to-cell communication systems (quorum sensing), which regulate virulence factors production in Pseudomonas aeruginosa, in order to overcome the globalcrisis of antimicrobial resistance.
Objectives
Study the potential quorum sensing inhibitory effect of Zinc oxide (ZnO)nanoparticlesin Pseudomonas aeruginosa and the impact on production of virulence factors.
Methods
Quorum sensing inhibitory effect of ZnO was evaluated by assessing its ability to reducePseudomonas aeruginosa virulence factors production; rhamnolipids, pyocyanin, pyoverdin, hemolysins, elastase and proteases. Furthermore, qRT-PCR was performed to determine ZnO inhibitory effect onQS-regulatory geneslasI, lasR, rhlI, rhlR, pqsA and pqsR that control virulence factors secretion. Moreover, mice survival test was conducted to investigate the influence of ZnO on Pseudomonas aeruginosa-induced mortality in vivo.
Results
ZnO revealed a statistically significant reduction in the production of QS-controlled virulence factors rhamnolipids, pyocyanin, pyoverdin, hemolysins, elastase and proteases. Furthermore, ZnO exhibited a significant decrease in the relative expression of QS-regulatory geneslasI, lasR, rhlI, rhlR, pqsA and pqsR. Additionally, ZnO significantly reduced the pathogenesis of Pseudomonas aeruginosa in vivo
Conclusion
ZnO nanoparticles can be used as a quorum sensing inhibitor in Pseudomonas aeruginosa infections either as an adjuvant or alternative to conventional antimicrobials.
Keywords: Pseudomonas aeruginosa, ZnO, quorum sensing, virulence inhibition
Introduction
Pseudomonas aeruginosa (Ps. aeruginosa) is considered one of the most frequent opportunistic pathogens worldwide that can infect patients with severe medical conditions, particularly immunosuppressed patients. It is responsible for many nosocomial diseases including respiratory tract infections, urinary tract infections, burn and surgical wound infections1. The fast expansion of bacterial resistance to antibioticsmakes it urgent to discover new therapeutic agents in order to combat this issue2.
In Ps. aeruginosa, bacterial cells communicatewith each other through a process known as quorum sensing (QS) which plays a major role in bacterial pathogenesis3. In Ps. aeruginosa, there are three major QS systems, lasI/R system, rhlI/R system and pqsA/R system which are interconnected together through signaling chemical molecules (autoinducers); oxododecanoyl-homoserine lactone (C12-HSL), butyryl-homoserine lactone (C4-HSL) and Pseudomonas quinolone-based intracellular signal (PQS) produced by bacterial cells. When chemical autoinducers reach a certain threshold, the quorum, they trigger the genes that regulate the production of virulence factorssuch as pyocyanin, pyoverdin, hemolysins, elastase and proteases4–6.
Quorum sensing inhibitors are agents that disrupt QS systems in bacterial cells leading to a reduction of virulence factors production and suppression of virulence without interrupting the bacterial growth and so no or low resistance is anticipated to arise against these agents7. In the recent years, the advances accomplished in the field of nanotechnology resulted in an increase in the applications of nanoparticles in the medical sector andas a therapy for infectious diseases. Superior effectiveness on resistant strains of metal oxide nanoparticles such as Zinc oxide (ZnO) and silver has been reported. ZnO nanoparticles were found to exert a potent antimicrobial activity and significantly reduced skin infections and inflammation in mice8–9.
The current study aimed to investigate the possible quorum sensing inhibiting activity of ZnO nanoparticles and their potential role in reducing QS-controlled virulence factors production and pathogenesis in Ps. aeruginosa.
Materials and methods
Bacterial isolates and their identification
Ps.aeruginosa PAO1 wild-type standard strain and five clinical isolates(Ps1, Ps2, Ps3, Ps4 and Ps5) were used in this study. Ps.aeruginosa PAO1 was provided from the stock culture collection of Microbiology and Immunology Department, Faculty of Pharmacy, Zagazig University. Clinical isolates were isolated from patients with burn and surgical wound infections admitted to Port Said General Hospital, Egypt. Clinical isolates were identified by Gram-stain, production of green pigmentson nutrient agar, growth on MacConkey agar, oxidase test, motility, growth on selective mediumcetrimide agar and the ability to grow at 42°C as stated by Koneman et al10.
Media and chemicals
Mueller-Hinton broth, nutrient agar, MacConkey agar, cetrimide agar, tryptone and yeast extract were purchased from (Oxoid, UK), ZnO nanoparticles, Tris-base andElastin Congo Red (ECR) from (Sigma, St. Louis, USA). Other chemicals were of pharmaceutical grade.
Antibiotic susceptibility testing of the clinical isolates
The antibiotic susceptibility testing for the clinical isolates was carried out using the disc diffusion technique as described by the Clinical Laboratory and Standards Institute (CLSI)11 against 10 anti-pseudomonal antibiotics including, aztreonam 30 µg (ATM), piperacillin 100 µg (PRL), ceftazidime 30 µg (CAZ), cefepime 30 µg (FEP), ciprofloxacin 5 µg (CIP), levofloxacin 5 µg (LEV), amikacin 30 µg (AK), gentamicin 10 µg (CN), colistin sulfate 10 µg (CT) and imipenem 10 µg (IPM). The anti-pseudomonal discs were purchased from (Oxoid, UK).
Determination of minimum inhibitory concentration and investigating the effect of sub-inhibitory concentration of ZnO nanoparticles on bacterial growth
The minimum inhibitory concentration (MIC) of ZnO nanoparticles was determinedby using the agar dilution method according to(CLSI)11. Briefly, overnight bacterial cultures of the tested isolateswere diluted, each with Mueller-Hinton broth to reach a turbidity matching that of 0.5 MacFarland Standard and then with sterile saline to achieve a final concentration of 107 CFU/ml. Nutrient agar plates with different concentrations of ZnO (1, 2,4, 8, 16, 32 and 64mg/ml) were prepared in addition to control plates without ZnO. The plates' surfaces were inoculated with 1µl of the suspensions of the tested isolates and incubated overnight at 37°C. The MIC was calculated as the least concentration of ZnO that prevented the visible growth of bacteria.
To ensure that ZnO sub-MIC that would be used in further experiments had no influence on bacterial viability, the effect of ¼ MIC of ZnO on bacterial growth was assessed following Nalca et al12. The tested isolates were incubated in Luria-Bertani (LB) broth (tryptone 10 g, yeast extract 5g and 10 g sodium chloride in 1000 ml distilled H2O) with and without ¼ MIC of ZnO under the same conditions. After 24h of incubation at 37°C, the optical densities of ZnO-treated and untreated cultures were measured at OD600 using spectrofluorometer (Biotek, USA).
The phenotypic effect of ZnO nanoparticles on QS-controlled virulence factors production
Effect on rhamnolipids
Rhamnolipids production, in the presence and absence of ZnO, was assessed by oil spreading method according to Morikawa et al13. A thin oily layer was formed on the surface of water by addition of 20µl of crude oil to 15 ml of distilled H2O in a Petri dish. Ten µl of cell-free supernatants of the tested isolates with and without ¼ MIC of ZnO was added to the center of the oily layer. The diameters of the clear zones formed that are related to the biosurfactant activity and the amounts of rhamnolipids produced by the tested isolates were measured and compared.
Effect on pyocyanin
Pyocyanin determination was performed in King A medium (peptone 20 g, MgCl2 1.4 g and 10 g K2SO4 in 1000 ml distilled H2O)according to Essar et al14. The tested isolates were grown in King A mediumwith and without¼ MIC of ZnO for 48 h at 37° C. Pyocyanin was extracted by addition of aliquots of 2.5 ml of bacterial supernatants to 3 ml chloroform followed by mixing with 1 ml of 0.2 N HCl. The pigment in chloroform layer was measured at OD520 using spectrofluorometer (Biotek, USA).
Effect on pyoverdin
In order to estimate pyoverdin, the method of CoxandAdams15 was used. Overnight cultures of the tested isolatesin LB broth were prepared in the presence and absence of ¼ MIC of ZnO and then centrifuged at 10000 rpm for 10 min. The cell-free supernatants were diluted to 1/10 with 50 mM Tris-HCl and pH adjusted to 7.4. The pyoverdin fluorescence in supernatants was measured at 460 nm, where the samples were excited at 400 nm using spectrofluorometer (Biotek, USA).
Effect on hemolysins
Production of hemolysin was determined following the modified method ofDacheux et al16. Aliquots of 0.5 ml of cell-free supernatants of tested isolates in LB broth with and without ¼ MIC of ZnO were mixed with 0.7 ml of 2% sheep RBCs in saline followed by incubation at 37°C for 2 h. After centrifugation of assay mixtures at 2500 rpm for 5 min to remove any cells, the released hemoglobin was measured at OD540 nm. Percentage lysis was calculated from the formula: [X-B/T-B]x 100, where B is the negative control corresponding to RBCs in LB broth, T is the positive control corresponding to completely lysed RBCs with 0.1%SDS and X is the ZnO-treated or untreated isolates. The hemolytic activity percentage produced byZnO-treated isolates was compared to that produced by untreated isolates.
Effect on elastase
Elastase assay was evaluated according to Ohman et al17 using ECR. Briefly, an aliquot of 0.5 ml of ECR solution (10 mg/ml) in Tris buffer (pH 7.0) was inoculated with 0.25ml of each cell-free supernatant of the tested isolates prepared in the presence and absence of ¼ MIC of ZnO. The mixtures were left at 37°C for 6 h and then centrifuged at 10000 rpm for 10 min to remove insoluble ECR pellets. The color of released ECR in supernatantswas measured at OD495 nm using spectrofluorometer( Biotek, USA).
Effect on proteases
The total proteases were estimated by the modified skim milk assayas described by El-Mowafy et al18. An aliquot of 0.5 ml cell-free supernatant of each of the testedisolates prepared with and without ¼ MIC of ZnOwasmixed with 1 ml of skim milk solution (1.25% in distilled H2O) and incubated at 37°C for 30 minutes. The turbidities of assay mixtures were measured at OD600using spectrofluorometer as a measure of the proteolytic activity.
Estimation of relative gene expression of QS-regulatory genes using qRT-PCR
For molecular determination of QS-regulatory genes, total bacterial RNA was extracted at the middle of the log phase, corresponding to OD600of 0.5–0.6, from the tested strains cultivated overnight in LB broth at 37°C in presence and absence of ¼ MIC of ZnO using Gene-JET RNA Purification Kit following the manufacturer instructions. Reverse transcription followed by qRT-PCR of QS-regulatory genes lasI, lasR, rhlI, rhlR, pqsA and pqsR was carried out using SensiFAST™ SYBR® Hi-ROX One-Step Kit.StepOne Real-Time PCR thermal cycler utilizing primers illustrated in Table 1 was used to setup the qRT-PCR analysis. The relative expression values of QS-regulatory genes were normalized to the housekeeping gene rpoD and agarose gel electrophoresis was used to confirm the specific PCR amplification. The relative gene expression in ZnO-treatedcultures was compared to their expression levels in untreated ones following the 2-ΔΔCt method19.
Table 1.
Primers used in qRT-PCR (El-Mowafy et al18)
| Gene name | Primer sequence | Annealing temp. | Amplicon size (bp) |
| ropD (Fa) | 5′-CGAACTGCTTGCCGACTT-3′ | 56°C | 131 |
| ropD (Rb) | 5′-GCGAGAGCCTCAAGGATAC-3′ | ||
| lasI (F) | 5′-CGCACATCTGGGAACTCA-3′ | 56°C | 176 |
| lasI (R) | 5′-CGGCACGGATCATCATCT-3′ | ||
| lasR (F) | 5′-CTGTGGATGCTCAAGGACTAC-3′ | 55°C | 133 |
| lasR (R) | 5′-AACTGGTCTTGCCGATGG-3′ | ||
| rhlI (F) | 5′-GTAGCGGGTTTGCGGATG-3′ | 58°C | 101 |
| rhlI (R) | 5′-CGGCATCAGGTCTTCATCG-3′ | ||
| rhlR (F) | 5′-GCCAGCGTCTTGTTCGG-3′ | 58°C | 160 |
| rhlR (R) | 5′-CGGTCTGCCTGAGCCATC-3′ | ||
| pqsA (F) | 5′-GACCGGCTGTATTCGATTC-3′ | 58°C | 74 |
| pqsA (R) | 5′-GCTGAACCAGGGAAAGAAC-3′ | ||
| pqsR (F) | 5′-CTGATCTGCCGGTAATTGG-3′ | 58°C | 142 |
| pqsR (R) | 5′-ATCGACGAGGAACTGAAGA-3′ |
Forward
Reverse
Mice survival test
The influence of ZnO on Ps. aeruginosa pathogenesis was assessed by the mice survival in vivo model following the method of Kim et al20. The ethical standards of Medical Research Center, Ain Shams University, Cairo, Egypt, where the experiment was conducted and the mice were provided, were followed in the animal study. An approximate cell density of 2.5 x 107 CFU/ml in phosphate-buffered saline (PBS) of Ps. aeruginosa PAO1 was prepared from overnight bacterial cultures in LB broth with and without ¼ MIC of ZnO. Four random groups of three-weeks-old healthy female albino mice (Mus musculus) with the same weight were used, each comprising 10 mice. In Group 1, mice were injected intraperitoneallywith 100 µl of ZnO-treated bacteria in sterile PBS, while group 2 was injected with 100 µl of untreated bacteria. Two negative control groups are included also;group 3 miceare injected with 100 µl of sterile PBS and group 4 mice were left uninoculated. All groups were kept with normal feeding and aeration at room temperature. The survival of mice in each group was recorded every day for 3 successive days.The results were calculated using Log-rank test, GraphPad Prism 5 and plotted using Kaplan-Meier method.
Statistical analysis
The influence of ZnO on Ps. aeruginosa QS-controlled virulence factors production was analyzed using Graph-Pad Prism 5 software package with One Way ANOVA according to Dunnet's or Tukey's Multiple Comparison Tests < 0.05 or P < 0.001 for significance. Results were calculated as the means ± standard errors of three biological experiments with three technical replicates each.
Results
Identification of clinical isolates
Ps. aeruginosa clinical isolates were identified from the following characters: they were Gram-negative rods and grew as non-lactose fermenters on MacConkey agar, they grew on cetrimide agar at 42°C and showed green pigmentation on nutrient agar, they were motile and oxidase positive.
Antibiotic susceptibility and resistance pattern of clinical isolates
The tested clinical isolates of P s. aeruginos ashowed high resistance against the various antibiotics used in this study. They all were found to be multi-drug resistant (MDR). The full results of antibiotic susceptibility test are illustrated in Table 2.
Table 2.
Antibiotic susceptibility pattern of the five clinical isolates
| Antibiotics Isolate No. |
ATM | PRL | CAZ | FEP | CIP | LEV | AK | CN | CT | IPM |
| Ps1 | S | I | R | R | R | R | R | R | R | R |
| Ps2 | I | R | S | R | R | R | R | R | R | R |
| Ps3 | R | R | R | R | R | R | R | R | S | R |
| Ps4 | R | R | I | R | R | R | R | R | S | R |
| Ps5 | R | R | R | R | S | S | R | R | R | R |
Antibacterial activity of ZnO and growth inhibition assay
ZnO prevented the growth of tested Ps. aeruginosa isolates at a concentration of 8 mg/ml and ¼ MIC (2mg/ml) was selected to test the effect of ZnO against QS-controlled virulence factors.
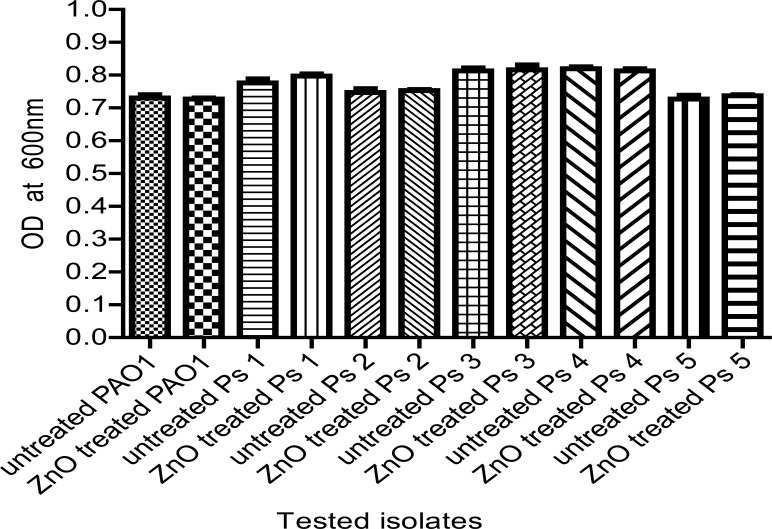
The efficacy of ZnO on QS-controlled virulence factors could be due to its effect on the growth of Pseudomonas isolates. To exclude this possibility, the effect of ¼ MIC of ZnO on bacterial growth was assessed by measuring the optical density of overnight culturesin LB broth at 600 nm and no statistically significant difference in the growth rate was found in the presence or absence of ZnO (Fig. 1).
Figure 1.
The OD600 of Ps.aeruginosa isolates was measured after overnight incubation in LB broth with and without ¼ MIC of ZnO. No significant difference was observed in the growth rates of ZnO-treated and untreated isolates.
ZnO inhibits rhamnolipids and pyocyanin production
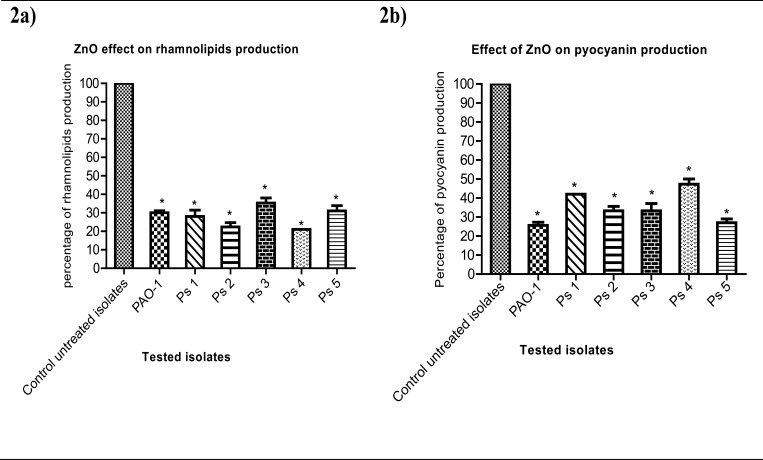
ZnO could significantly reduce rhamnolipids production from 100% in untreated isolates to 30%, 28%, 22%, 35%, 21% and 31% in PAO-1, Ps1, Ps2, Ps3, Ps4 and Ps5 ZnO-treated isolates respectively (Fig. 2a); while pyocyanin production was significantly decreased to 26%, 42%, 33%, 33%, 47% and 27% in PAO-1, Ps1, Ps2, Ps3, Ps4 and Ps5 ZnO-treated isolates respectively (Fig. 2b).
Figure 2.
(a) Rhamnolipids production reduction in ZnO-treated bacteria. The diameters of clear zones produced by addition of the supernatants obtained after culturing in LB broth in the presence and absence of¼ MIC of ZnO were measured. (b) Pyocyanin production reduction in ZnO-treated bacteria. The absorbance of pyocyanin was measured at 520 nm in the supernatants obtained from the cultures in King A broth in the presence and absence of¼ MIC of ZnO. The data shown represent the means ± standard errors of three biological experiments with three technical replicates each. *, significant P< 0.05.
ZnO inhibits pyoverdin and hemolysins production
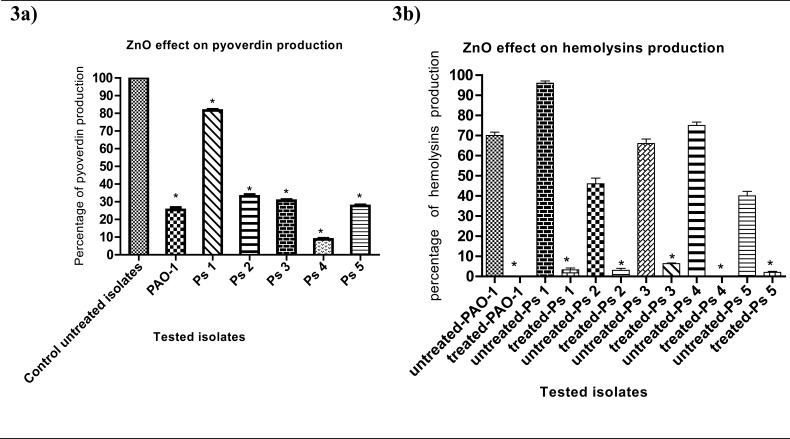
ZnO-treated cultures showed a remarkable decrease in pyoverdin production to 25%, 82%, 33%, 31%, 9% and 28% in PAO-1, Ps1, Ps2, Ps3, Ps4 and Ps5 ZnO-treated isolates respectively (Fig. 3a). Furthermore, hemolysins production was completely blocked in PAO-1 and Ps4 (0%) and was decreased tomerely 3%, 3%, 6% and 2% in Ps1, Ps2, Ps3 and Ps5 ZnO-treated isolates respectively (Fig. 3b).
Figure 3.
(a) Pyoverdin production reduction in ZnO-treated isolates. Pyoverdinfluorescence was measured at 460 nm while the samples were excited at 400 nmafter overnight culture in LB broth in the presence and absence of¼ MIC of ZnO.(b) Hemolysin reduction in ZnO-treated isolates. Absorbance of hemoglobin red color released by hemolysisofRBCs was measured at 540 nm.The data shown are the means ± standard errors of three biological experiments with three technical replicates each. *, significant P< 0.05.
ZnO inhibits elastase and proteases production
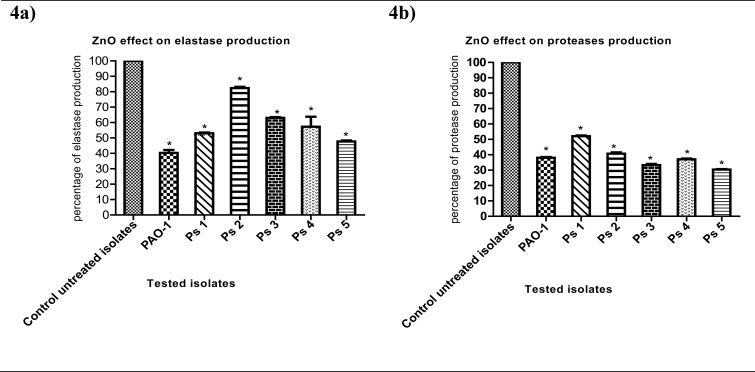
Elastase production in the presence of ZnO was significantly diminished to 40%, 53%, 82%, 63%, 57% and 48% in PAO-1, Ps1, Ps2, Ps3, Ps4 and Ps5 ZnO-treated isolates respectively (Fig. 4a). Moreover, proteases production was significantly decreased to 38%, 52%, 41%, 33%, 37% and 30% in PAO-1, Ps1, Ps2, Ps3, Ps4 and Ps5 ZnO-treated isolates respectively (Fig. 4b).
Figure 4.
(a) Elastase production reduction in ZnO-treated isolates. Absorbance at 495 nm was measured to show the effect of elastase on ECR after culture of bacteria in LB broth with and without¼ MIC of ZnO for 6 h.(b) Proteases production reduction in ZnO-treated bacteria. OD600 was measured following overnight culture of bacteria in LB broth with and without¼ MIC of ZnO and incubation of supernatants with skim milk for ½ hr at 37°C. The data shown are the means ± standard errors of three biological experiments with three technical replicates each. *, significant P<0.05.
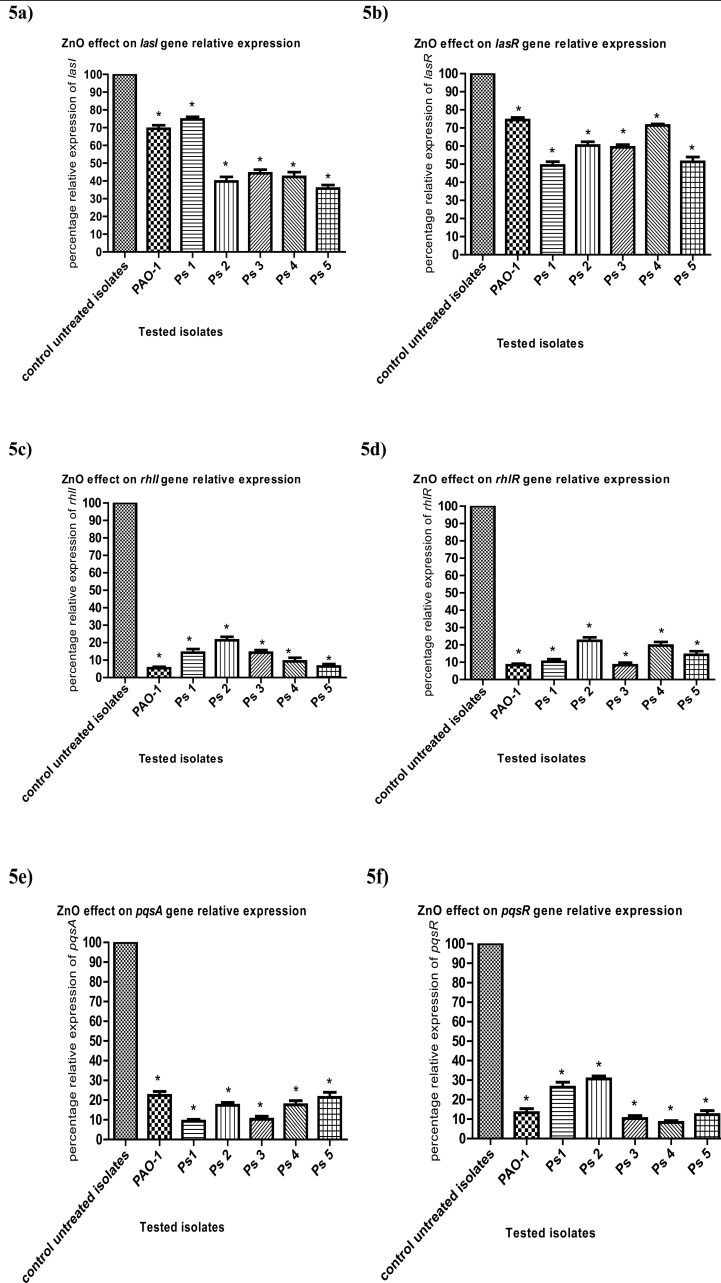
Estimation of relative gene expression of QS-regulatory genes using qRT-PCR
The relative expression of the genes regulating virulence factors production was assessed in ZnO-treated and untreated strains and analyzed using the 2-ΔΔCt method. The relative expression levels of lasI, lasR, rhlI, rhlR, pqsA and pqsR were significantly reduced under ZnOsub-MIC treatment (Figs. 5a, b, c, d, e and f), respectively. The relative expression of lasI gene was significantly decreased from 100% in untreated isolates to 69%, 75%, 40%, 44%, 42% and 36% in PAO-1, Ps1, Ps2, Ps3, Ps4 and Ps5ZnO-treated isolates respectively, and was also reduced to 74%, 49%,60%, 59%, 71% and 51% in PAO-1, Ps1, Ps2, Ps3, Ps4 and Ps5 ZnO-treated isolates respectivelyfor lasRgene as compared to untreated strains. Moreover, the relative expression of rhlI gene wassignificantly reduced to 5%, 14%, 21%, 14%, 9% and 6% in PAO-1, Ps1, Ps2, Ps3, Ps4 and Ps5 ZnO-treated isolates respectively, while rhlR gene relative expression was dropped to 8%, 10%, 22%, 8%, 20% and 14% in PAO-1, Ps1, Ps2, Ps3, Ps4 and Ps5 ZnO-treated isolates respectively. Furthermore, the relative expression of pqsA wassignificantly diminished to 22%, 9%, 17%, 10%, 18% and 21% in PAO-1, Ps1, Ps2, Ps3, Ps4 and Ps5 ZnO-treated isolates respectively and the relative expression of pqsR gene was also reduced to 13%, 26%, 31%, 10%, 8% and 12% in PAO-1, Ps1, Ps2, Ps3, Ps4 and Ps5 ZnO-treated isolates respectively.
Figure 5.
RT-qPCR showedreduced expressionof (a) lasI, (b) lasR, (c) rhlI, (d) rhlR, (e) pqsA and (f) pqsR in ZnO-treated bacteria as compared to untreated ones.The data shown are the means ± standard errors of three biological experiments with three technical replicates each. *, significant P< 0.05.
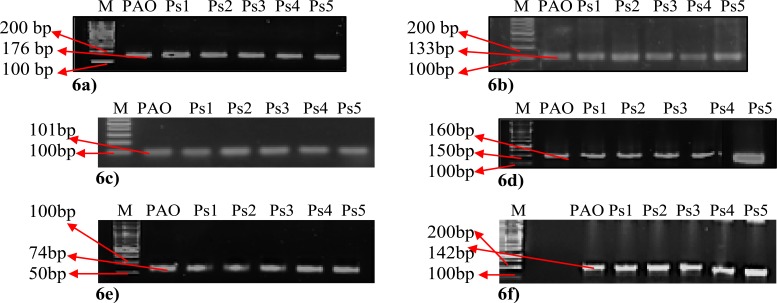
Specific PCR amplification products of the tested genes lasI, lasR, rhlI, rhlR, pqsA and pqsR were confirmed by agarose gel electrophoresis (Figs. 6a, b, c, d, e and f) respectively.
Figure 6.
Agarose gel electrophoresis of (a) lasI amplicon (176bp), (b) lasR amplicon (133bp), (c) rhlI amplicon (101bp), (d) rhlR amplicon (160bp), (e) pqsA amplicon (74bp) and (f) pqsR amplicon (142bp). All the tested PCR products (amplified genes) were detected using 1.5% agarose gel stained with ethidium bromide. Lane 1 (M): represent DNA ladder.
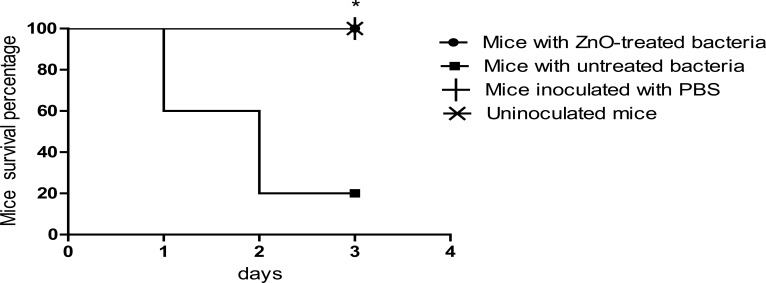
ZnO decreased pathogenesis of Ps. aeruginosa in vivo
In mice survival test shown in (Fig. 7), mice injected with positive control (untreated) Ps. aeruginosa began to die after 24 h and only 20% of mice in this group were still alive at the end of the experiment. Importantly, at an infectious dose of approximately 2.5 x 107 CFU, mice injected with bacteria treated with ZnO showed a significantly higher survival rate as compared to positive control bacteria as all mice in this group remained alive at the end of the experiment; a result similar to that of the negative control groups in which no mice in these groups died at the end of the experiment. Our resultssuggest a protective role of ZnO nanoparticles against Ps.aeruginosa pathogenesis and virulence in mice.
Figure 7.
Survival rate was significantly reduced in mice injected with untreated bacteria in comparison to ZnO-treated bacteria and control groups. Mice survival was monitored every day for 3 days and plotted using Kaplan-Meier method. ZnO-treated Ps. aeruginosa killed significantly fewer mice as compared to untreated bacteria and (n= 10 mice in each group). *, significant P< 0.001.
Discussion
Ps. aeruginosa has become a principal etiologic agent of nosocomial infections which necessitates the urgent and efficient application of proper infection control policies to combat its spread. It shows both natural resistance and acquired multi-drug resistance to antimicrobial agents bydifferentmechanisms21. As an alternative strategy to antimicrobial therapy, targeting of QS has become an attractive option due to its involvement in regulating virulence factors production and pathogenesis in Ps. aeruginosa22. Anti-virulence therapy (as QS inhibitors) would be valuable in the management of microbial diseases due to the lack of pressures affecting bacterial growth upon using anti-virulence agents and so microbial resistance against them is not expected18.
In the present study, ZnO inhibited the growth of all the tested Pseudomonas isolates at 8mg/ml. Testing the effect of ¼ MIC of ZnO nanoparticles(2mg/ml) on microbial growth revealed the absence of statistically significant difference between both ZnO-treated and untreated cultures. As a result, any possible effect on QS is not due to adverse impact on bacterial viability and growth. Instead, it may be attributed to disrupting essential bacterial functions.
Ps. aeruginosa produces an arsenal of virulence factors including pyocyanin, pyoverdin, proteases, elastase and rhamnolipids which take partinestablishing infections and making the dissemination and invasion of hosttissues easier23. Recently, nanotechnology has been used to develop new nanoparticles that can target QS and virulence factors24–25.
In the current study, ZnO nanoparticles at ¼ MIC showed a potent inhibitory effect on the production ofrhamnolipids, pyocyanin, pyoverdin, hemolysins, elastase and proteases. In accordance with our data, Singh et al26 reported that another type of metal nanoparticles, silver nanoparticles, at sub-MIC exhibited a remarkable reduction in production of proteases, elastase, pyocyanin and rhamnolipids.
LasI/R and rhlI/R are two principle QS systems that regulate virulence genes in Ps. aeruginosa. LasI andrhlI synthases are responsible for the production of C12-AHL and C4-AHL autoinducers, respectively. At a threshold concentration of autoinducers, C12-AHL binds with lasR and induces the expression of genes control production of elastase, exotoxin and proteases and also activates the rhlI/R system. In addition, C4-AHL binds with rhlR andplays role in controlling the expression of genes encoding production of elastase, and pyocyanin. IflasI/R and rhlI/R are interrupted, virulence factors will be inhibited27–28.
To further explore the potential quorum quenching effect of ZnO nanoparticles, relative expression of QS-regulatory genes that controlvirulence factors in Ps. aeruginosa was examined using qRT-PCR.Importantly, ZnO nanoparticles significantly down-regulate the relative expression of QS regulatory genes, lasI, lasR, rhlI, rhlR, pqsA and pqsRwhich confirm the phenotypic results. Similarly, it was proved by Singh et al26 that silver nanoparticles down-regulated the expression of lasI, lasR, rhlI and rhlR at the molecular level by inhibiting lasR and rhlR. ZnO nanoparticles effect might be similar to that of silver nanoparticles by inhibiting both lasR and rhlR resulting in disruption of QS circuits with subsequent inhibition of virulence factors production. The molecular basis and full mechanism of nanoparticles impact as quorum sensing inhibitor need more investigations in the future. For more confirmation, the effect of ZnO nanoparticles on Ps. aeruginosa pathogenesis was determined in vivo. Interestingly, a significant increase in the survival rate of mice injected with ZnO-treated isolates was found in comparison to those injected with untreated ones. Previous reports also showed the protective effect of QSinhibitors against bacterial pathogenesisin mice injected with Ps. aeruginosa. This was reported for gingerol19 and polyphenolic compounds of honey29. Our collective phenotypic, genotypic and in vivo results strongly potentiate the potential use of ZnO nanoparticles as a powerful QS inhibitor in Ps. aeruginosa.
Conclusion
The spread of MDR strains of Ps. aeruginosa is of a particular concern causing healthcare-associated infections and increasing the challenge both in the clinical treatment of patients and in the prevention of the cross-transmission of this problematic pathogen. QS controls the production of many of virulence factors in Ps. aeruginosa and plays an essential role in antimicrobial resistance. ZnO nanoparticles is a promising QS inhibitor and anti-virulence compound that can be used as an adjunct for the treatment of Ps. aeruginosa infections such as burns and surgical wound infectionsmainly those caused by MDR isolates.
Acknowledgment
We would like to thank members of Medical Research Center, Ain Shams University, Cairo, Egypt, for providing us with mice and for their help in mice infection model experiment.
Conflict of interest
The authors declare that they have no conflict of interest.
List of abbreviations
AK= amikacin, ATM= aztreonam, CAZ= ceftazidime, CIP= ciprofloxacin, CT= colistin sulfate, CN= gentamicin, C4-HSL= butyryl-homoserine lactone, C12-HSL=oxododecanoyl-homoserine lactone, CLSI= Clinical Laboratory and Standards Institute, ECR= Elastin congo red, FEP= cefepime, IPM= imipenem, LB= Luria-Bertani, LEV= levofloxacin, MDR= multi-drug resistant, MIC= minimum inhibitory concentration, OD= optical density, PBS= phosphate-buffered saline, PRL= piperacillin, Ps= Pseudomonas, PQS=Pseudomonas quinolone-based intracellular signal, QS= quorum sensing, SDS= Sodium dodecyl sulfate, and ZnO= Zinc oxide.
References
- 1.Sangani MH, Moghaddam MN, Forghanifard MM. Inhibitory effect of zinc oxide nanoparticles on Pseudomonas aeruginosa biofilm formation. Nanomedical Journal. 2015;2(2):121–128. [Google Scholar]
- 2.Fernebro J. Fighting bacterial infections: future treatment options. Drug Resistance Updates. 2011;14:125–139. doi: 10.1016/j.drup.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Antunes LC, Ferreira RB, Buckner MM, Finlay BB. Quorum sensing in bacterial virulence. Microbiology. 2010;156:2271–2282. doi: 10.1099/mic.0.038794-0. [DOI] [PubMed] [Google Scholar]
- 4.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 5.Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annual Reviews of Genetics. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 6.Häussler S. Multicellular signalling and growth of Ps. aeruginosa. International Journal of Medical Microbiology. 2010;300:544–548. doi: 10.1016/j.ijmm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Lara B, Saucedo-Mora MA, Roldan-Sanchez JA, Perez-Eretza B, Ramasamy M, Lee J, et al. Inhibition of quorum-sensing-dependent virulence factorsand biofilm formation of clinical and environmental Pseudomonas aeruginosa strains by ZnO nanoparticles. Letters in Applied Microbiology. 2015;61:299–305. doi: 10.1111/lam.12456. [DOI] [PubMed] [Google Scholar]
- 8.Aysa NH, Salman HD. Antibacterial activity of modified zinc oxide nanoparticles against Ps. aeruginosa isolates of burn infections. World Scientific News. 2016;33:1–14. [Google Scholar]
- 9.Pati R, Mehta RK, Mohanty S, Padhi A, Sengupta M, Vaseeharan B, et al. Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomedicine. 2014;10(6):1195–208. doi: 10.1016/j.nano.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Koneman EW, Allen SD, Janda WM, Scheckenberger PC, Winn WC. Color atlas and textbook of diagnostic microbiology. 6th ed. Philadelphia, USA: Lippincott; 2006. [Google Scholar]
- 11.Clinical and Laboratory Standards Institute, author. Approved standard, CLSI document M100-S-25. 3. Vol. 35. Wayne, PA, USA: 2015. [Google Scholar]
- 12.Nalca Y, Jansch L, Bredenbruch F, Geffers R, Buer J, Häussler S. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1, a global approach. Antimicrobial Agents and Chemotherapy. 2006;50:1680–1688. doi: 10.1128/AAC.50.5.1680-1688.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morikawa M, Hirataa y, Imanaka T. A study on the structure-function relationship of lipopeptide biosurfactants. Biochimica et Biophysica Acta. 2000;1488(3):211–218. doi: 10.1016/s1388-1981(00)00124-4. [DOI] [PubMed] [Google Scholar]
- 14.Essar DW, Eberly L, Hadero A, Crawford IP. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. Journal of Bacteriology. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox CD, Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infection and Immunity. 1985;48:130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dacheux D, Goure J, Chabert J, Usson Y, Attree I. Pore-forming activity of type III system-secreted proteins leads to oncosis of Pseudomonas aeruginosa-infected macrophages. Molecular Microbiology. 2001;40:76–85. doi: 10.1046/j.1365-2958.2001.02368.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohman DE, Cryz SJ, Iglewski BH. Isolation and characterization of a Pseudomonas aeruginosa PAO1 mutant that produces altered elastase. Journal of Bacteriology. 1980;142:836–884. doi: 10.1128/jb.142.3.836-842.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Mowafy SA, Shaaban M, Abd El-Galil KH. Sodium ascorbate as a quorum sensing inhibitor of Pseudomonas aeruginosa. Journal of Applied Microbiology. 2014;117:1388–1399. doi: 10.1111/jam.12631. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Lee S, Byun Y, Park H. 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Scientific Reports. 2015;5:8656. doi: 10.1038/srep08656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma U, Kulshreshtha S, Khatri PK. MDR Pseudomonas aeruginosa in Nosocomial Infection: Burden in ICU and Burn Units of a Tertiary Care Hospital. International Journal of Current Microbiology and Applied Science. 2018;7(1):1267–1274. [Google Scholar]
- 22.Zhao T, Liu Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiology. 2010;10:140. doi: 10.1186/1471-2180-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gellatly L, Hancock W. Pseudomonas aeruginosa, new insights into pathogenesis and host defenses. Pathogens and Disease. 2013;67:159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 24.Kalishwaralal K, BarathManiKanth S, Pandian SR, Deepak V, Gurunathan S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surfaces B Biointerfaces. 2010;79:340–344. doi: 10.1016/j.colsurfb.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Kumar CG, Mamidyala SK. Extracellular synthesis of silver nanoparticles using culture supernatant of Pseudomonas aeruginosa. Colloids Surfaces B Biointerfaces. 2011;84:462–466. doi: 10.1016/j.colsurfb.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 26.Singh BR, Singh BN, Singh A, Khan W, Naqvi AH, Singh HB. Mycofabricated biosilver nanoparticles interrupt Pseudomonas aeruginosa quorum sensing systems. Scientific Reports. 2015;5:13719. doi: 10.1038/srep13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsek MR, Val DL, Hanzelka BL, Val DL, Hanzelka BL, CronanJr JE, et al. Acyl-homoserine lactone quorum sensing signal generation. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4360–4365. doi: 10.1073/pnas.96.8.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsador L, Cook JM, Gambello MJ, Rust L, Iglewski BH. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 29.Prateeksha, Singh BR, Shoeb M, Sharma S, Naqvi AH, Gupta VK, et al. Scaffold of selenium nanovectors and honey phytochemicals for inhibition of Pseudomonas aeruginosa quorum sensing and biofilm formation. Frontiers in Cellular and Infection Microbiology. 2017;7:1–14. doi: 10.3389/fcimb.2017.00093. (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]