Abstract
Background
Annona stenophylla is a folk medicine popularly used in Zimbabwe for the treatment of many ailments. This study was carried out to determine some of the possible anti diabetic mechanisms of its action using in vitro cell culturing methods.
Methods
A. stenophylla's effects on glucose uptake were tested using muscle cells (C2Cl2). Expression of glucose 4 transporters was determined by treating cell lines with plant extract. Total RNA was isolated and using RT-PCR, GLUT 4 expression levels were quantified. Translocation of GLUT 4 was assessed using FITC fluorescence measured by flow cytometry.
Results
Treatment of cells with plant extract significantly increased glucose uptake in a concentration dependent manner, with the highest concentration (250 µg/ml) giving 28% increased uptake compared to the negative control. The increase in glucose uptake (2.5 times more than control) was coupled to increase in GLUT 4 mRNA and subsequently GLUT 4 translocation. Wortmannin expunged the A. stenophylla induced increase in GLUT 4 mRNA and glucose uptake.
Conclusion
The results suggest that A. stenophylla aqueous extract increases glucose uptake partly through increasing the GLUT 4 mRNA and translocation potentially acting via the PI-3-K pathway. This study confirms the ethnopharmacological uses of A. stenophylla indicating potential for anti-diabetic products formulation.
Keywords: Annona stenophylla, glucose uptake, GLUT 4, diabetes, wortmannin
Introduction
The rapidly increasing incidence of diabetes mellitus is becoming a serious threat to human health in all parts of the world. The control and treatment of diabetes and its complications mainly depend on chemical or biochemical agents1. There is need for tight regulation of glucose which is the main source of energy for the body. After a meal insulin is released by the pancreatic islets inducing glucose uptake by tissues that are sensitive to it. Glucose transporter type 4 (GLUT4) is the major transporter in the GLUT family, that mediates glucose uptake by the muscle and adipose tissues2. Under insulin resistance, translocation of insulin-sensitive GLUT4 is impaired, resulting in the consequent defect in the insulin-stimulated glucose uptake, a rate-limiting step for glucose disposal3,4. In skeletal muscle, insulin stimulates glucose uptake primarily by increasing translocation and redistribution of the GLUT4 from internal membrane to the plasma membrane5,6. The increased expression of GLUT4 has previously been shown to lower blood glucose levels and enhance glucose transport and utilisation in skeletal muscles7.
While conventional treatments such as sulfonylureas, metformin and thiazolidinediones are effective, they have several limitations, including adverse side effects, secondary failure or the inability to halt further loss of insulin secretory capacity8 and in many cases the precise mechanism of action remains to be completely clarified9.
The insulin stimulation followed by cascade signalling enhances glucose uptake, utilisation and storage in various tissues10. Therefore, muscle glucose-uptake and suppression of gluconeogenesis could be considered as an excellent target for treatment of T2DM. Therapeutic approaches with natural products provide a fruitful source for searching safe, effective and relatively inexpensive new remedies for diabetes mellitus and associated metabolic disorders3,11.
Annona stenophylla (family: Annonaceae), commonly known as the dwarf custard apple, is a small rhizomatous shrub found in some regions of Zimbabwe12. This plant has been used in traditional medicine and scientific studies have validated some of the folkloric claims of different activities such as antiparasitic, anti-infective, antiviral and antidiabetic activities12,13,14. Earlier in our laboratory we have demonstrated that A. stenophylla plant extract possess antioxidant activity, antimicrobial activity and has potential hypoglycaemic effects. The hypoglycaemic activity has partly been attributed to its ability to inhibit carbohydrate metabolising enzymes and a polyherbal formulation encoperating A. stenophylla has been formulated15,16,17,18,19. The extract has been found to be relatively safe to use through brineshrimp lethality tests and subacute toxicity tests using a rat model20,21.
New insights into the mechanisms of action in glucose lowering and insulin resistance may provide new treatment strategies for diabetes. In a bid to explore the observed effects of A. stenophylla plant extract, mechanisms of antidiabetic activity were investigated using established muscle cell lines.
Materials and methods
This was an experimental study performed at the University of Zimbabwe, Physiology laboratory.
Reagents
Dulbecco's Minimum Essential Medium DMEM, fetal bovine serum (FBS), trypsin-EDTA, antibiotic (strep/penicillin) and Phosphate buffered saline (PBS) reagents were from Gibco, USA. Whatman No. 1 filter paper Merk (South Africa) Antibodies to GLUT4 were from Indaba Biotechnology (South Africa)
Plant material and extraction
The plant roots of Annona stenophylla were collected from Mazowe District in Harare, Zimbabwe GPS coordinates were latitude 17 ° 24 14.25 Southings and longitude 30 ° 41 36.13 Eastings, using guidelines for sustainable harvesting of traditional medicinal plants in Zimbabwe23,24. Identification and authentication was done with the aid of a botanist from the National Herbarium and Botanic Gardens of Zimbabwe, and a voucher specimen tagged (2540) was kept for reference. The roots were air dried for 4 weeks in the laboratory at ambient temperature, ground, and extracted overnight with distilled water at 37 °C. The filtered extract was lyophilized in a freeze dryer and stored at −20 °C until use.
Cell culture
C2Cl2 myocytes were maintained in Dulbecco's Modified Eagles Medium (DMEM) supplemented with 10% Foetal Bovine Serum (FBS) and 1% antibiotic solution (10,000 U/ml penicillin G, 10 mg/ml streptomycin) in a humidified atmosphere of air and 5% CO2 at 37°C1. The myocytes were left without change of medium for 6–7 days after seeding into 48 well plates for differentiation to occur and experiments were performed in the differentiated myotubes which express GLUT 4 insulin responsive transporters.
Glucose uptake
The determination of glucose uptake in C2Cl2 myotubes was performed using a modified method described previously25. Briefly, media was removed from cell wells and replaced with 1ml of fresh medium containing increasing concentrations of A. stenophylla aqueous extract, PBS as negative control and positive control insulin, followed by an overnight incubation period. Glucose concentration in the medium was determined after the incubation period by the glucose oxidase method using a commercial kit (KAT medicals) following the manufactures instructions. Test samples were tested in quadruplicates and repeated at a later occasion. Absorbances were read at 540nm in a microplate reader named (Anthos 2010). The amount of glucose taken up by the cells was regarded as being proportional to the absorbance readings. A standard curve using known glucose concentrations was constructed and used to extrapolate the glucose levels.
Extraction of RNA and analysis of gene expression
After exposure to plant or control treatments, total RNA was extracted from C2Cl2 myotubes (GeneJET RNA kit, Thermo Scientific). RNA was measured using a Qubit machine (Invitrogen) and normalised for cDNA synthesis (Revert Aid 1st strand cDNA KIT, Thermo Scientific). RT-PCR was done using Thermo Scientific Dream Taq Green following manufacturer's instructions. The PCR products were run on 1% agarose gels stained with ethidium bromide and quantified using ChemDoc Imager software. GAPDH was used as an internal control. The primers used were:
GAPDH: 5′-AACTTTGGCATTGTGGAAGG-3′ (forward) and 5′-ACACATTGGGGGTAGGAACA-3’ (reverse)
Glut4: 5′-ACATACCTGACAGGGCAAGG-3′ (forward) and 5′-CGCCCTTAGTTGGTCAGAAG-3′ (reverse)
GLUT4 translocation
The levels of GLUT 4 transporters in C2Cl2 myotubes (non permeabilised) were measured by flow cytometry. After cells were treated with plant extract or the controls, they were harvested and washed twice with 2% FBS in PBS. Cells were blocked using CD16/32 for 15 min and then incubated with a conjugated anti-GLUT4 antibody solution (1.0 µg/ml in PBS) for 1 h at 4 °C. Excess antibodies after labelling were removed by washing twice in ice-cold PBS as described by Wang et al26. The cells on the surface membrane were measured in duplicate and assay repeated at a later occasion. The fraction of GLUT 4 was expressed as increase in FITC fluorescence with respect to untreated stained cells.
Statistical analysis
Values are given as mean ± SE. Analysis of statistical significance of differences in measurements between samples was done by one-way ANOVA followed by Dunnet's post hoc test (GraphPad Prism version 5). P < 0.05 was considered statistically significant.
Results
Effect of A. stenophylla plant extract on glucose uptake in C2Cl2 cells
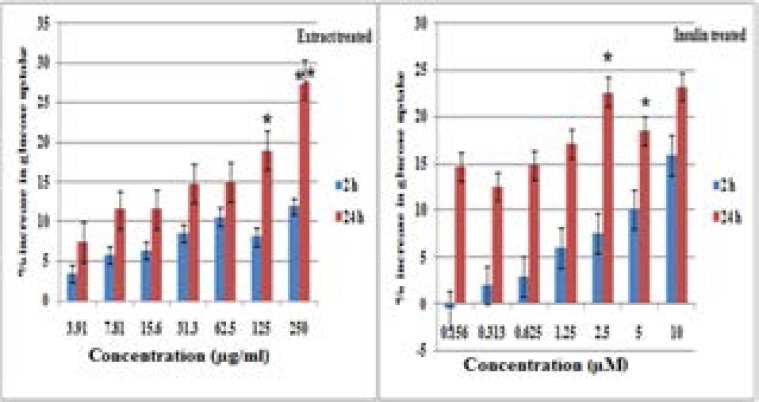
There was a general dose dependent increase in glucose uptake after 2 and 24 h of both plant extract and insulin positive control administration (Figure 1). Significant differences in the 2h compared to 24h glucose uptakes were recorded for concentrations 125 µg/ml and 250 µg/ml (p = 0.011 and 0.014 respectively). A. stenophylla plant extract showed percentage increase in glucose uptake that was comparable to insulin (positive control).
Figure 1.
Effect of different treatments on glucose uptake in C2Cl2 muscle cells.
Changes in glucose uptake were measured after 2h and 24hours for the plant extract compared to the positive control insulin. % increase in glucose uptake was calculated as a fraction of the untreated control. Each assay was done in triplicate (N=6) and repeated on a separate occasion and results are the average (± SD). * (P< 0.05) shows significant differences upon comparing 2 h percentage glucose uptakes to 24 h ones at a particular concentration.
Effect of A. stenophylla extract on GLUT4 mRNA levels in C2Cl2 cells
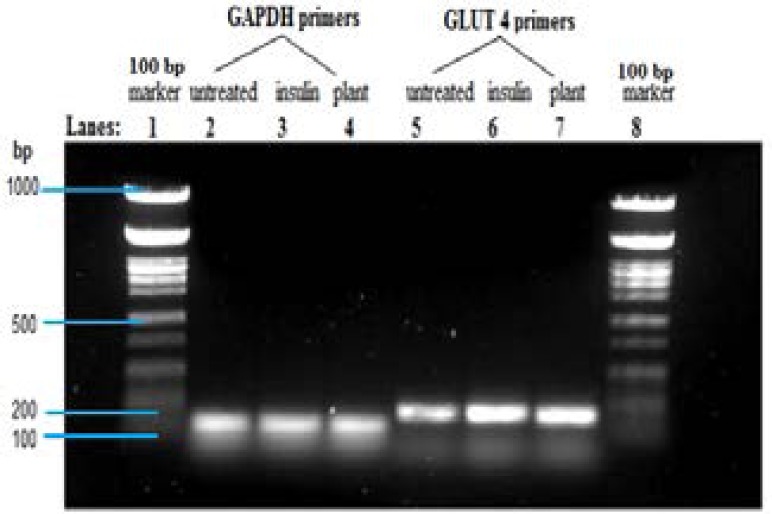
Incubation of C2Cl2 cells with A. stenophylla and insulin resulted in increased mRNA levels of GLUT 4 when compared to the untreated control (Figure 2). The RT-PCR products were approximately 150 bp.
Figure 2.
Effect of A. stenophylla extract on GLUT 4 mRNA levels in C2Cl2 cells using RT-PCR.
cDNA prepared from total RNA was amplified using GLUT 4 primer after treatment with plant extract (250 µg/mL) and insulin positive control (10 µM). Amplicon band sizes were equated to amounts of mRNA levels of the GLUT 4. cDNA loading was normalised by use of the housekeeping gene (GAPDH). Results are the average (± SD) of two assays (Each assay done in duplicate), N=4.
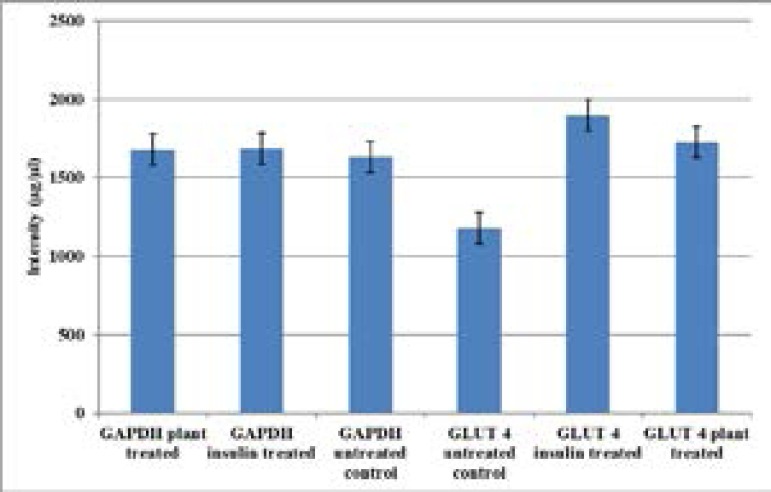
Equal amounts of cDNA material loaded were shown by analysis of the band sizes using Chemidoc Imager and this was confirmed by the almost equal amounts of band intensities in GAPDH (Figure 3). The untreated control showed the least amount of amplicon band intensities when compared to those from plant extract treated and insulin treated cells. Differences in band intensities were not significant when treated samples were compared to the untreated control (p>0.05)
Figure 3.
GLUT 4 mRNA amplicon intensities in C2Cl2 myotubes.
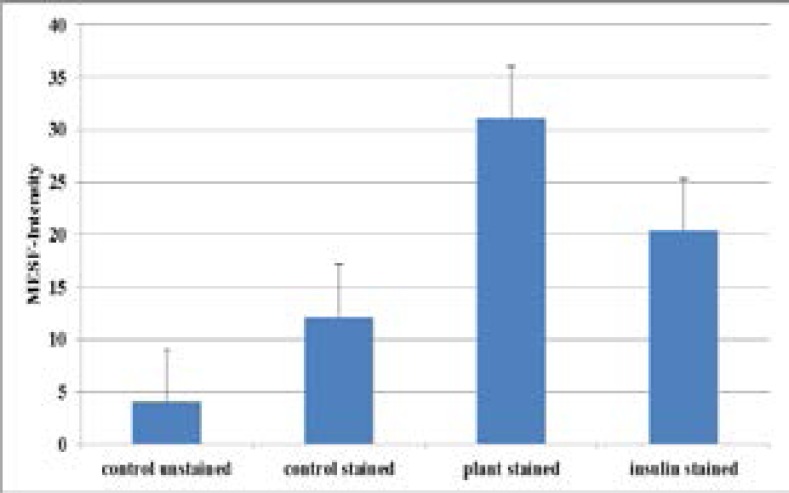
GLUT 4 mRNA amplicons resulting from cells treated with plant extract compared to the positive control insulin. The amplicons' differences in size were equated to differences in the mRNA levels of GLUT 4 expressed. The housekeeping gene (GAPDH) primers were used to normalize the cDNA loaded. Results are the average (± SD) of two assays (Each assay done in duplicate) N=4. GLUT4 translocation measurement using flow cytometry Thenumbers of GLUT 4 on C2Cl2 myotubes plasma membrane surface were measured using flow cytometry. The plant extract showed an increase in GLUT 4 cell surface transporters measured as 31.1± 11 MESF -FITC arbitrary units of molecules of equivalent soluble FITC fluorescence (figure 4). The untreated stained control had 4.0 ± 0.46 MESF -FITC. As a percentage of the untreated stained control the plant extract caused more increase in FITC levels compared to the positive control insulin. All the groups tested showed no significant differences of levels of surface GLUT 4 translocated to the surface membrane when compared amongst each other (p>0.14) in all cases.
Figure 4.
The effect of A. stenophylla aqueous extract on GLUT4 translocation in C2Cl2 myotubes.
The numbers of translocated GLUT 4 after treatment with plant and insulin standard control were quantified. Baseline fluorescence is shown in untreated stained cells (negative control). The intensities of MESF-FITC were equated to amounts of GLUT 4 translocated. Results are the average (± SD) of three assays (Each assay done in duplicate), (N=6).
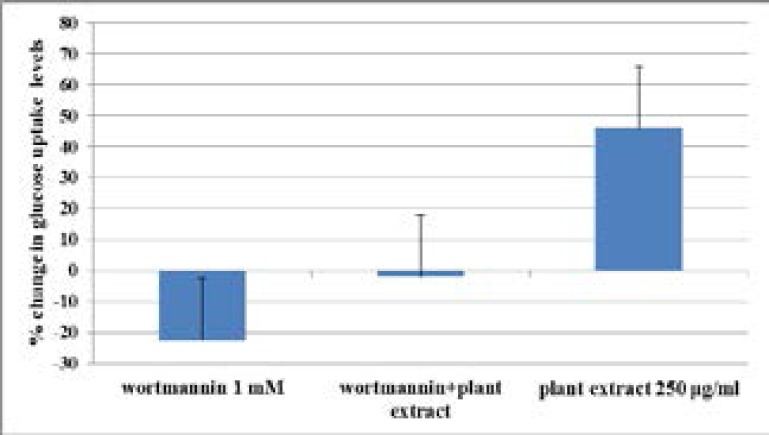
Effect of wortmannin inhibitor on glucose uptake in C2Cl2 myotubes
Wortmannin inhibitor showed a decrease in the glucose uptake when administered to C2Cl2 cells over 24 h (Figure 5). The 1.3 fold decrease in the glucose uptake was slightly reversed to a 1.0 fold decrease when wortmannin was incubated together with 250 µg/ml of plant extract. The difference between the change in glucose uptake when treatment with inhibitor was compared to plant extract + inhibitor was, however, not significant (p = 0.264).
Figure 5.
Effects of wortmannin on glucose uptake in C2Cl2 cells.
Effects of treatment with wortmannin alone, plant extract + wortmannin and plant extract alone was measured after 24 hours. Results are the average (± SD) of two assays done in duplicate (N=4).
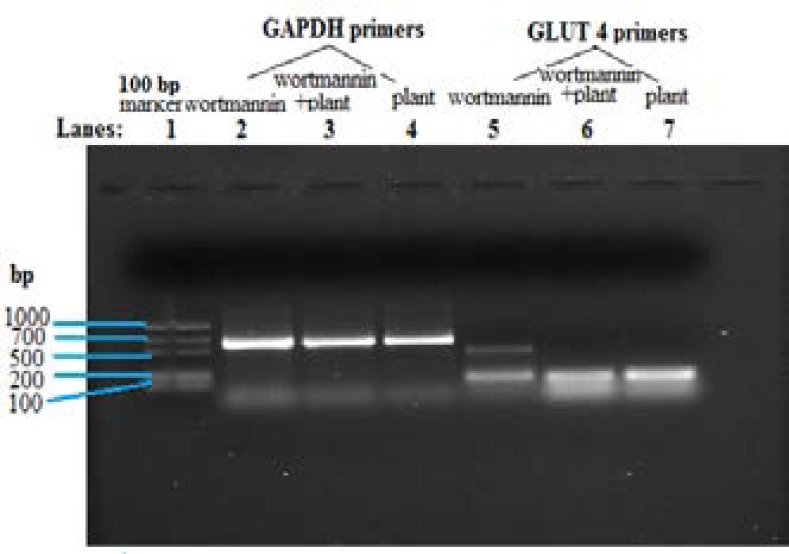
Effect of wortmannin on GLUT 4 mRNA levels in C2Cl2 muscle cells
Wortmannin treatment on C2Cl2 muscle cells decreased the levels of GLUT 4 mRNA expressed (Figure 6) when compared to plant extract treatment alone and combined wortmannin and plant extract treatment.
Figure 6.
RT-PCR showing effect of wortmannin on GLUT 4 mRNA levels in C2Cl2 cells.
GLUT 4 primers were used to amplify the cDNA from cells treated with wortmannin compared to wortmannin + plant extract and plant extract alone. Differences in amplicon band sizes were equated to differences in the expressed mRNA levels of GLUT 4. Concentration of plant extract used was 250 µg/ml and wortmannin 1mM. Results are the average (± SD) of two assays (Each assay done in duplicate), N=4.
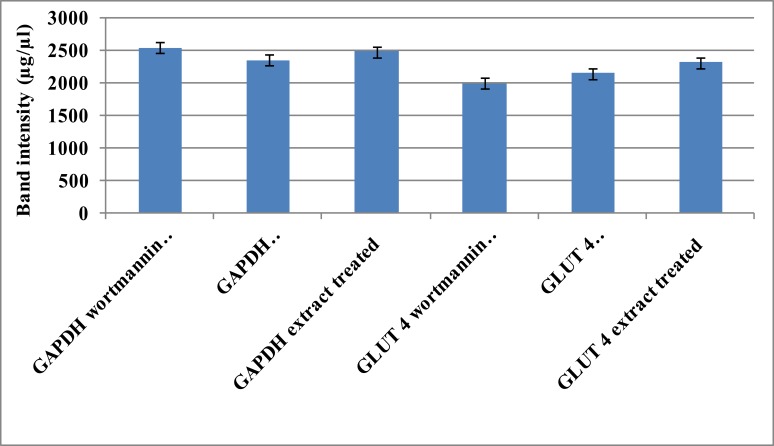
The differences in amplicon band sizes were equated to differences in the expressed mRNA levels of GLUT 4. Band densitometry analysis confirmed the decrease in GLUT 4 mRNA levels, using GAPDH as an internal control (Figure 7).
Figure 7.
Band intensities of GLUT 4 mRNA levels in C2Cl2 myotubes treated with wortmannin.
Band intensities of the GLUT 4 amplicons resulting from cells treated with wortmannin compared to wortmannin + plant extract and extract alone were measured. Differences in amplicon band sizes were equated to differences in themRNA levels of GLUT 4 expressed. The housekeeping gene (GAPDH) primers were used to normalize the cDNA loaded. Visual appreciation of the differences in band densities is shown. Results are the average (± SD) of two assays (Each assay done in duplicate) N=4.
Discussion
In previous studies the aqueous extract of Annona stenophylla demonstrated hypoglycaemic and insulinotropic effects in alloxan-induced diabetic rats18. Western diabetic drugs correct hypoglycemia by supplementing insulin, improving insulin sensitivity, increasing insulin secretion from the pancreas and/or glucose uptake by tissue cells27. Certain herbs may lower blood glucose28, however, their test results are subject to several factors and hence the present study was conducted to determine the potential mechanism of action of glucose-lowering effect shown by aqueous extract of Annona senophylla.
Skeletal muscle is the principal site for postprandial glucose utilization and a major element in the maintenance of glucose homeostasis9,29. A wide array of plant-derived molecules has been reported to be associated with beneficial effects on glucose transport and metabolism in skeletal muscle cells1. In this study, plant extract treatment increased glucose uptake in a concentration-dependent manner which shows that the more concentrated the drug the more the effect. The effect of extract treatment on GLUT 4 mRNA transcription was determined and proposed as a potential mechanism through which glucose uptake was increased. Gel electrophoresis showed a visual appreciation of how the different treatments affected glucose uptake in the C2Cl2 myotubes. The least uptake was shown in the untreated cells and amplification shown would possibly be due to the basal mRNA available. Extract and insulin treatment did show an increase in the transcription of GLUT 4 mRNA which would subsequently influence the amount of glucose taken up by the cells (Figures 1 & 2). Translocation of GLUT4 to the cell surface could be a part of the underlying molecular mechanism responsible for the insulin-mediated increased glucose transport30. In cell cultures the delivery of glucose to the cell surface is not rate limiting like it sometimes is in animals. Increased glucose entry depends on amount of GLUT 4 translocation and other facilitative transporters. The differences in expression of GLUT 4 in cell line experiments can, therefore, be attributed to different treatment responses.
GLUT4 gene expression is also subject to up or down regulation depending on the physiologic state of the organism. Changes in GLUT4 gene expression are observed in physiologic states of altered glucose homeostasis30. Further to glucose uptake and mRNA assays, effect of A. stenophylla extract on translocation of GLUT 4 from the cytoplasm to the plasma membrane was assessed. GLUT 4 is only transiently expressed at the plasma membrane of cells and is endocytosed. The bound antibodies remain attached to the membrane, even during endocytosis, facilitating measurement of the total fluorescence signal of exposed GLUT 4 molecules31. The marginal increase in glucose uptake after treatment could be due to plants ability to enhance GLUT 4 translocation followed by increased gene transcription and expression4. Although the difference in the effects of untreated control compared to treatments were not significant, the influence of the extract can be considered for use in combination with other allopathic drugs or herbal formulations like proposed by Mohammad32.
Glucose uptake and GLUT 4 translocation has been shown to be mediated through two major pathways, insulin-mediated PI3K pathway and insulin-independent AMPK pathway. Wortmannin, a drug which inhibits insulin release and blocks many short term metabolic effects induced by insulin receptor activation, decreased glucose uptake in muscle cells. Decrease in glucose uptake was accompanied by decreased levels of GLUT 4 mRNA levels. Wortmannin did not completely abolish glucoseuptake when incubated together with plant extract and this could be because A. stenophylla plant extract partly reversed the inhibitory effect of wortmannin. The role of PI3K in A. stenophylla plant extract induced glucose uptake and increased mRNA levels affected by coincubation with wortamannin suggest that uptake was partly PI3K dependent33.
Conclusion
The major goal in treating diabetes is to minimize elevation of blood glucose without causing abnormally low levels of blood glucose. The action mechanisms for hypoglycemic herbs are multiple and A. stenophylla extract stimulates glucose transport in C2Cl2 myotubes by enhancing GLUT4 translocation from the internal membrane to plasma membrane accompanied with change in the total amount of GLUT4 or its gene expression.
Our findings show that A. stenophylla appear to have the ability to modulate glucose metabolism and provide the molecular basis of antihyperglycemic activity of the extract, which can be a promising candidate for the management of diabetes and associated metabolic disorders.
Acknowledgements
Many thanks go out to the Biomedical Department of Tshwane University of Technology through Dr L.J Shai and Professor D Katerere for the collaboration and all the assistance that made the work possible. The research work for the Doctoral research project was funded by the Southern African Consortium for Research Excellence (SACORE) grant number 087537/F/08/A. This work is part of a PhD project presented to theUniversity of Zimbabwe in 2016. Supplementary research material was received from the University of Zimbabwe.
Conflict of interest
There is no conflict of interest from all the authors and the funders. I also affirm that all the authors have seen and agreed to the submission of the paper and their inclusion of name(s) as co-author(s).
References
- 1.Anandharajan R, Jaiganesh S, Shankernarayanan NP, Viswakarma RA, Balakrishnan In vitro glucose uptake activity of Aegles marmelos and Syzygium cumini by activation of Glut-4, PI3 kinase and PPARgamma in L6 myotubes. Phytomedicine. 2006;13(6):434–441. doi: 10.1016/j.phymed.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Campbell RK. Type 2 diabetes: where we are today: an overview of disease burden, current treatments, and treatment strategies. Journal of American Pharmaceutical Association. 2009;49(Suppl):3–9. doi: 10.1331/JAPhA.2009.09077. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes care. 2009;32(Suppl 2):157–163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efrat S, Surana M, Fleischer N. Glucose induces insulin gene transcription in a murine pancreatic beta-cell line. Journal of Biological Chemistry. 1991;266:11141–11143. [PubMed] [Google Scholar]
- 5.Gelfand M, Drummond RB, Mavi S, Ndemera B. The traditional medical practitioner in Zimbabwe: his principles of practice and pharmacopoeia. Gweru: Mambo Press; 1985. [Google Scholar]
- 6.Gustavsson J, Parpal S, Strålfors P. Translocation of insulin-regulated glucose transporter is stimulated by longchain 1, 2-diacylglycerol in rat adipocytes. Experimental Cell Research. 1995;221(2):438–442. [PubMed] [Google Scholar]
- 7.Hou JC, Min L, Pessin JE. Insulin granule biogenesis, trafficking and exocytosis. Vitamins and Hormones. 2009;80:473–506. doi: 10.1016/S0083-6729(08)00616-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishi K, Muromoto N, Nakaya Y, Miyata I, Hagi A, Hayashi H, Ebina Y. Bradykinin directly triggers GLUT4 translocation via an insulin-independent pathway. Diabetes. 1998;47:550–558. doi: 10.2337/diabetes.47.4.550. [DOI] [PubMed] [Google Scholar]
- 9.Koistinen HA, Galuska D, Chibalin AV, Yang J, Zierath JR, Holman GD, Wallberg-Henriksson H. 5-Amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes. 2003;52:1066–1072. doi: 10.2337/diabetes.52.5.1066. [DOI] [PubMed] [Google Scholar]
- 10.Lee MS, Sohn CB, Hoang DM, Kim BY, Sohn CB, Kim MR, Ahn JS. Anti-diabetic properties of Chrysophanol and Its Glucoside from Rhubarb Rhizome Biological and Pharmaceutical Bulletin. 2008. 31(11):2154–2157. doi: 10.1248/bpb.31.2154. [DOI] [PubMed] [Google Scholar]
- 11.Liu IM, Liou SS, Cheng JT. Mediation of β -endorphin by myricetin to lower plasma glucose in Streptozotocin induced diabetic rats. Journal of Ethnopharmacology. 2006;104(1–2):199–206. doi: 10.1016/j.jep.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Malviya N, Jain S, Malviya S. Anti-diabetic potential of medicinal plants. Acta Poloniae Pharmaceutica. 2010;67(2):113–118. [PubMed] [Google Scholar]
- 13.Olaokun OO, McGaw LJ, Awouafack MD, Eloff JN, Naidoo V. Potential role of GLUT4 transporters and insulin receptors in the hypoglycaemic activity of Ficus lutea acetone leaf extract. BMC Complementary and Alternative Medicine. 2014;14:269. doi: 10.1186/1472-6882-14-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen KF, Shulman GI. New insights into the pathogenesis of insulin resistance in humans using magnetic resonance spectroscopy. Obesity. 2006;14(Suppl 2) doi: 10.1038/oby.2006.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munodawafa T, Chagonda LS, Viol ID, Muchuweti M, Moyo RS. Total phenolic content and antioxidant activity of some Zimbabwean traditional medicinal plants. Recent Progress in Medicinal Plants, Drug Plants III. 2010;29:364–373. [Google Scholar]
- 16.Munodawafa T, Chagonda L.S, Moyo SR. Antimicrobial and Phytochemical Screening of some Zimbabwean Medicinal Plants. Journal of Biologically Active Products from Nature. 2013;3(5–6):323–330. [Google Scholar]
- 17.Phiri J, Chagonda LS. Hypoglycemic effects of Annona stenophylla and Morus alba plant extracts in alloxan-induced diabetic mice. Journal of Biologically Active Products from Nature. 2012;2(6):377–381. [Google Scholar]
- 18.Taderera T, Gomo E, Chagonda LS. The Antidiabetic Activity of an Aqueous Root Extract of Annona stenophylla Engl. and Diels in Non-diabetic Control and Alloxan-induced Diabetic rats. Journal of Biologically Active Plants from Nature. TBAP. 2016;6(4):315–322. 2016. [Google Scholar]
- 19.Taderera T, Chagonda LS, Gomo E, Shai LJ. Inhibitory activity of α-glucosidase and α-amylase by Annona stenophylla root extract as mechanism for hypoglycaemic control of DM. International Journal of Pharmacy, Photon. 2015;106:436–444. [Google Scholar]
- 20.Verengai W, Chagonda L, Chitindingu K, Marume A, Taderera T. An anti-diabetic poly-herbal medicine prepared from extracts of Annona stenophylla, Citrus limon and Zingiber officinal. International Journal of Pharmaceutical Sciences and Research. 2017;8(3):1049–1055. [Google Scholar]
- 21.Munodawafa T, Moyo S, Chipurira B, Chagonda LS. Brine shrimp lethality bioassay of some selected Zimbabwean traditional medicinal plants. International Journal of Phytopharmacology. 2016;7(4):229–232. [Google Scholar]
- 22.Munodawafa T, Tagwireyi D, Gadaga L, Gomo E, Bierman F, Chagonda LS. Acute and subacute oral toxicity of hydroethanolic root extract of Annona stenophylla Engl. andDiels in Sprague dawley rats. Journal of Biologically Active Products from Nature. 2015;5(5):349–356. [Google Scholar]
- 23.Bukuluki P, Luwangula R, Walakira EJ. Harvesting of Medicinal Plants in Uganda: Practices, Conservation and Implications for Sustainability of Supplies. 2014. pp. 7–13. [Google Scholar]
- 24.Khumalo S, Fröde A, Sola P. Guidelines for the Sustainable Harvesting of Traditional Medicinal Plants in Zimbabwe: For Ministry of Environment and Tourism. Research and Development Section Southern Alliance for Indigenous Resources (Safire) Harare; 1998. pp. 7–18. [Google Scholar]
- 25.Tamrakar AK, Schertzer JD, Chiu TT, Foley KP, Bilan PJ, Philpott DJ, Klip A. NOD2 activation induces muscle cell-autonomous innate immune responses and insulin resistance. Endocrinology. 2010;151(12):5624–5637. doi: 10.1210/en.2010-0437. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Khayat Z, Kishi K, Ebina Y, Klip A. GLUT4 translocation by insulin in intact muscle cells: detection by a fast and quantitative assay. FEBS Letters. 1998;427:193–197. doi: 10.1016/s0014-5793(98)00423-2. [DOI] [PubMed] [Google Scholar]
- 27.Hui H, Tang G, Go VL. Hypoglycemic herbs and their action mechanisms. Chinese Medicine. Biomedical Central. 2009;4:11. doi: 10.1186/1749-8546-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin J, Zhang H, Ye J. Traditional Chinese medicine in treatment of metabolic syndrome. Endocrine, Metabolic and Immune Disorders-Drug Targets. 2008;8(2):99–111. doi: 10.2174/187153008784534330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zierath JR, He L, Guma A, Odegoard Wahlstrőm E, Klip A, Wallberg-Henriksson H. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia. 1996;39:1180–1189. doi: 10.1007/BF02658504. [DOI] [PubMed] [Google Scholar]
- 30.Stuart CA, Howell ME, Zhang Y, Yin D. Insulin-stimulated translocation of glucose transporter (GLUT) 12 parallels that of GLUT4 in normal muscle. Journal Clinical and Endocrinology Metabolism. 2009;94:3535–3542. doi: 10.1210/jc.2009-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koshy S, Alizadeh P, Timchenko LT, Beeton C. Quantitative Measurement of GLUT4 Translocation to the Plasma Membrane by Flow Cytometry. Journal of visualised experiments. 2010;45 doi: 10.3791/2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammad S, Taha A, Bamezai RNK, Baquer NZ. Modulation of glucose transporter (GLUT4) by vanadate and Trigonella in alloxan-diabetic rats. Life Science. 2006;78:820–824. doi: 10.1016/j.lfs.2005.05.105. [DOI] [PubMed] [Google Scholar]
- 33.Tamrakar AK, Jaiswal N, Yadav PP, Maurya R, Srivastava AK. Pongamol from Pongamia pinnata stimulates glucoseuptake by increasing surface GLUT4 level in skeletal muscle cells. Molecular and Cellular Endocrinology. 2011;339:98–104. doi: 10.1016/j.mce.2011.03.023. [DOI] [PubMed] [Google Scholar]