Abstract
Synthesis of a highly responsive fluorescent ribonucleoside analogue based on a 5-methoxybenzofuran uracil core, enzymatic incorporation of its triphosphate substrate into RNA transcripts, and its utility in the specific detection and estimation of Hg2+-ion-mediated metallo-base pair formation in DNA–RNA and RNA–RNA duplexes are described.
Selective binding of metal ions to natural and modified nucleobases has rendered such noncanonical metallo-base pairs as useful motifs to devise tools to sense and trap heavy-metal ions, detect single-nucleotide polymorphism, and fabricate electronic wires and nanomachines.1,2 In particular, pyrimidine-metal-pyrimidine base pairs formed by Hg2+ and Ag+ ions have received significant attention, because of their diagnostic and material applications3 and recent findings linking the metal toxicity and mutagenicity to metallo-base pair formation.3a,4 It is now clear that T-Hg2+-T can serve as a stable analogue of T-A pair5 and could potentially introduce mutations by facilitating enzymatic misincorporation of dTTP opposite to T.6 A recent study demonstrates that the addition of Hg2+ ions to duplexes containing T-T mismatch significantly reduces the efficiency of DNA polymerase activity, compared to equivalent duplexes containing T-A pair, suggesting that the metallo-base pair can hamper the DNA metabolism.4 Similarly, in the presence of Ag+ ions, the DNA polymerases do incorporate dATP/dTTP/dCTP opposite to dC, which is largely dependent on the sequence and substrate/enzyme concentration.7
Several biophysical methods, including ultraviolet (UV), nuclear magnetic resonance (NMR), electron paramagnetic resonance (EPR), X-ray, and isothermal titration calorimetry (ITC) methods are commonly used to study metallo-base pair structure, kinetics, and thermodynamics.8 Alternatively, fluorescence resonance energy transfer (FRET)-pair-labeled T-rich oligonucleotides (ONs), hairpin-forming motifs, and a thrombin binding aptamer have been developed to monitor the formation and stability of metallo-base pairs.9 Similarly, label-free Hg2+ and Ag+ sensors have been developed by using intercalating organic dyes and metal complexes, which show changes in fluorescence upon metal ion-induced base pairing.10 Usually, these methods use higher concentrations of ON duplexes and or stretches of T-T mismatches to obtain reliable response upon base-pair formation.1b,3 However, detecting and estimating site-specific nucleobase-metal interactions in duplexes at submicromolar concentrations would provide a better understanding of their actual biological impact (e.g., point mutations and conformation changes induced by metal ion binding).4 In this context, environment-sensitive fluorescent nucleoside analogues based on a dimethylaminobenzene core and pyrrolo-dC were successfully used in detecting and determining the local kinetics and thermodynamic parameters of metallo-base pairs formed by Hg2+ and Ag+ ions in DNA ON duplexes.11 However, to the best of our knowledge, there is no report that describes the fluorescence analysis of site-specific metallo-base pair interaction in DNA-RNA heteroduplexes and RNA–RNA duplexes. Such an effort, apart from aiding material design, could also be useful in understanding the impact of metallo-base pairs in RNA folding, function, and recognition.
Here, we report the development of a new microenvironment-sensitive ribonucleoside analogue, 5-methoxybenzofuran uridine (1), which after triphosphorylation (2) serves as a good substrate for RNA polymerase in in vitro transcription reaction and selectively reports dT-1 and U-1 mismatches in model DNA–RNA and RNA–RNA ON duplexes, respectively, with significant enhancement in fluorescence. This property of the nucleoside analogue was aptly utilized in establishing a method to selectively detect single Hg2+-mediated base pair in different duplexes. Furthermore, by monitoring the changes in fluorescence intensity as a function of Hg2+ ion concentration, it was found that the metal ion binds to dT−U better than the U–U mismatch.
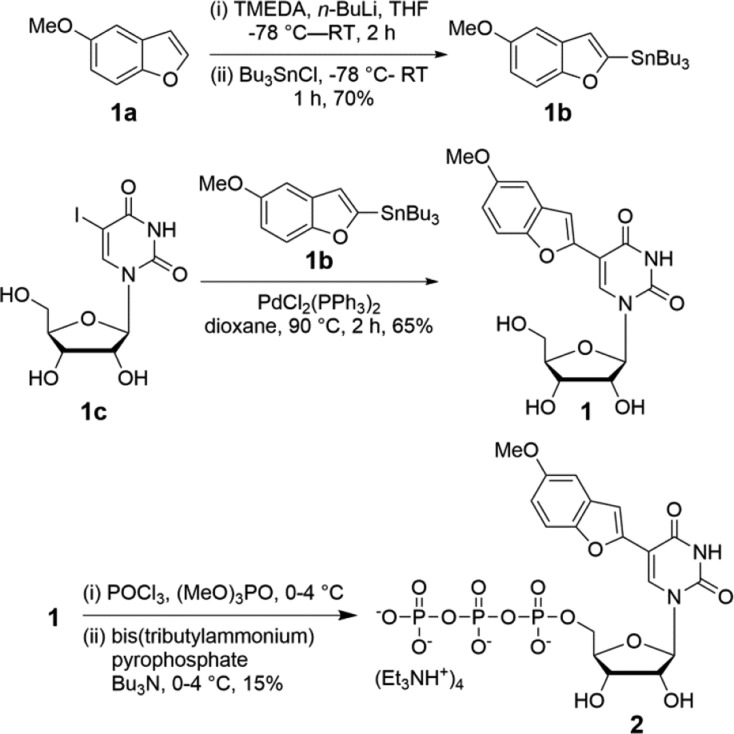
It has been shown that conjugating or fusing heterocyclic rings onto purine and pyrimidine bases can produce responsive nucleoside analogues.12 Their implementation in nucleic acid analysis has been largely empirical, based on the fluorescence outcome in model systems. Following a similar probe design approach, 5-methoxybenzofuran uridine 1 was synthesized according to the steps given in Scheme 1. 5-Methoxybenzofuran (1a) was first stannylated and then cross-coupled with 5-iodouridine (1c) in the presence a palladium-catalyst under Stille reaction conditions. The corresponding triphosphate substrate 2 required for in vitro transcription was synthesized by reacting nucleoside 1 with POCl3, followed by a reaction with pyrophosphate (Scheme 1).
Scheme 1. Synthesis of Nucleoside 1 and Its Triphosphate 2.
Photophysical analysis in solvents of different polarity and viscosity confirmed the environment-sensitive of the nucleoside analogue. The longest wavelength absorption maximum of 1 was found to be slightly red-shifted and hyperchromic as the polarity was reduced from water to methanol to dioxane (see Figure 1 and Table 1). Notably, the nucleoside exhibited excellent fluorescence solvatochromism (Figure 1, Table 1). In water, it was practically nonemissive, and, with a change in solvent polarity, the quantum yield increased dramatically by 250-fold in the least polar solvent tested (dioxane). The emission maximum was also found to be significantly blue-shifted. Interestingly, a comparison of relative quantum yields (Φx/Φwater) of 1 and few other heterocycle-conjugated nucleoside probes13 in different solvents, as a function of ET(30) (Reichardt’s microscopic solvent polarity parameter) revealed that nucleoside 1 is highly sensitive to even small changes in micropolarity (Figure S1 in the Supporting Information). Other probes showed very small differences in the quantum yield. As the solvent viscosity was increased from water to ethylene glycol to glycerol, the quantum yield also increased significantly, because of rigidification of the fluorophore, which was further confirmed by the higher anisotropy values exhibited by the probe in a more-viscous medium (see Figure 1 and Table 1).14 While the viscosity of methanol and dioxane is considerably lower, compared to that of ethylene glycol and glycerol, higher or comparable fluorescence efficiency suggests that the fluorescence outcome in different solvents is largely due to a polarity effect.
Figure 1.
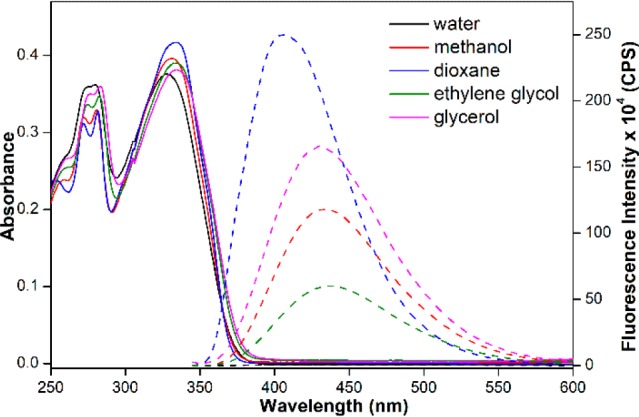
Absorption (25 μM, solid lines) and emission (5.0 μM, dashed lines) spectra of nucleoside 1 in different solvents. See the Supporting Information (SI) for details.
Table 1. Fluorescence Properties of Nucleoside Analogue 1.
| solvent | λmaxa (nm) | λem (nm) | ET(30) | Φb | Φx/Φwaterc | rd |
|---|---|---|---|---|---|---|
| water | 329 | – | 63.1 | 0.0008 | 1 | nd |
| methanol | 331 | 433 | 55.5 | 0.11 | 137 | nd |
| dioxane | 335 | 406 | 36.0 | 0.20 | 250 | 0.03 |
| ethylene glycol | 332 | 435 | 56.3 | 0.07 | 88 | 0.26 |
| glycerol | 335 | 430 | 57.2 | 0.21 | 263 | 0.33 |
The lowest energy absorption maximum is given.
The standard deviation of Φ in water is 0.00006, and in other solvents, it is ≤0.004.
Relative quantum yield (Φx/Φwater), where x is the solvent).
The standard deviation of r (anisotropy) in different solvents is ≤0.004; nd = not determined.
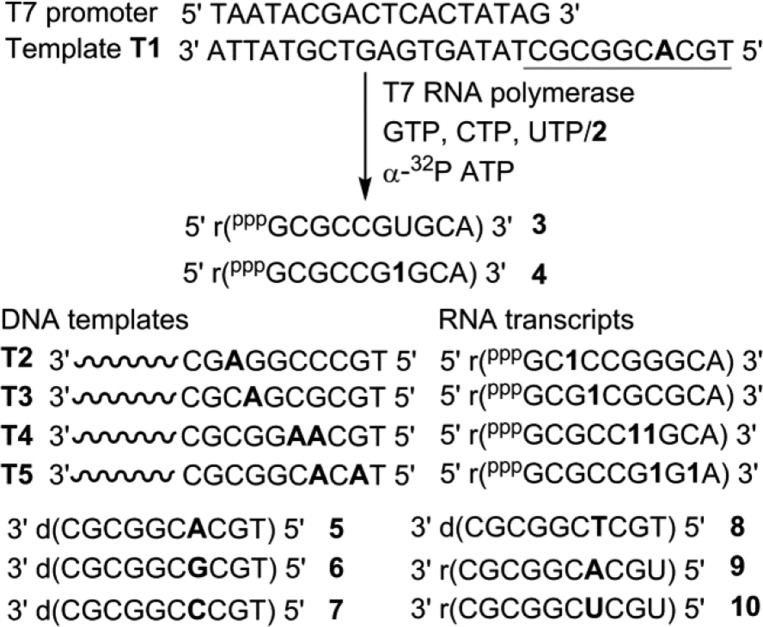
To evaluate the responsiveness in duplexes, we sought to incorporate nucleoside analogue 1 into RNA transcripts by in vitro transcription reaction, using modified nucleoside 5′-triphosphate 2. The templates were custom-made to contain one or two dA residues in the coding region to guide the incorporation of modified nucleoside 1 into the transcripts (Figure 2). To facilitate easy visualization of full-length transcription products by phosphor imaging, all templates contained a dT at the 5′ end to guide the incorporation of an α-32P adenosine label. Transcription was initiated by adding T7 RNA polymerase to a reaction mixture containing T7 promoter-template duplex, GTP, CTP, UTP/2, and α-32P ATP, and was resolved by PAGE under denaturing conditions (see Figure 3, as well as Figure S2 in the SI). Using template T1, singly modified full-length transcript 4 was formed in very good yields (81%, Figure 3, lane 2). Incorporation of nucleoside 1 was evident from the slower mobility exhibited by transcript 4, compared to native transcript 3. Along with the full-length product, trace amounts of nontemplated incorporation products were also formed, which is common in in vitro transcription reactions. Importantly, when UTP or 2 was not added, full-length product was not formed, which indicated that there was no random misincorporation of the nucleotide analogue (Figure 3, lane 3). Notably, when the reaction was performed in the presence of an equimolar concentration of 2 and UTP, both of the triphosphates were incorporated with comparable efficiency (Figure 3, lane 4). Reactions with other templates indicated that modifications near the promoter region using T2 and T3 were less tolerated and adjacently placed modification using T4 was produced in good yields, compared to nucleoside analogue placed in an alternating position using T5 (Figure 3, lanes 5–12). Furthermore, modified transcript 4 obtained from a large-scale reaction with T1 was purified and its purity and integrity were confirmed by HPLC, mass analysis, and enzymatic digestion (see Figures S3 and S4 and Table S1 in the SI).
Figure 2.
Enzymatic incorporation of triphosphate 2 into RNA ON transcripts in the presence of DNA templates T1–T5. Complementary and mismatched ONs (3–10) used in this study. Prefixes “d” and “r” represent DNA and RNA ONs, respectively.
Figure 3.
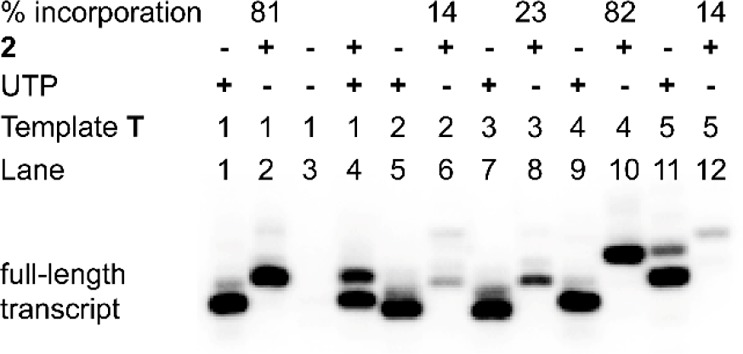
Phosphor image of PAGE resolved transcription products obtained using DNA templates T1–T5 in the presence of UTP and or 2. See Figure S2 in the SI for a complete gel picture and conditions.
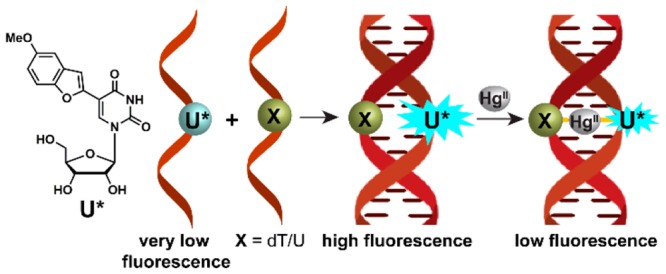
To understand the behavior of nucleoside 1 in different neighboring base environment, a model RNA transcript 4 labeled with 1 was hybridized to complementary DNA and RNA ONs (Figure 2). The duplexes were designed such that the emissive base was placed opposite to complementary and mismatched bases. The emissive analogue placed opposite to a complementary base dA/A (4·5 and 4·9) and mismatched bases dG and dC (4·6 and 4·7) showed comparable fluorescence intensity (Figure 4). Notably, when placed opposite to dT (4·8) or U (4·10), nucleoside 1 showed a significant enhancement in fluorescence intensity, compared to other duplexes (see Figure 4, as well as Figure S5 in the SI). Thus, the emissive analogue 1 was able to selectively detect dT and U mismatches in a DNA-RNA and RNA-RNA hetero and homo duplexes, respectively. CD analysis suggests that hybrid duplexes and RNA duplexes adopt a similar secondary structure, which resembles the A-form (Figure S6 in the SI). In the absence of further structural information, it is proposed that small differences in stacking interaction and electron transfer between the fluorophore and adjacent bases, the solvation–desolvation effect, and rigidification–derigidification of the fluorophore could have influenced the fluorescence outcome.14,15
Figure 4.
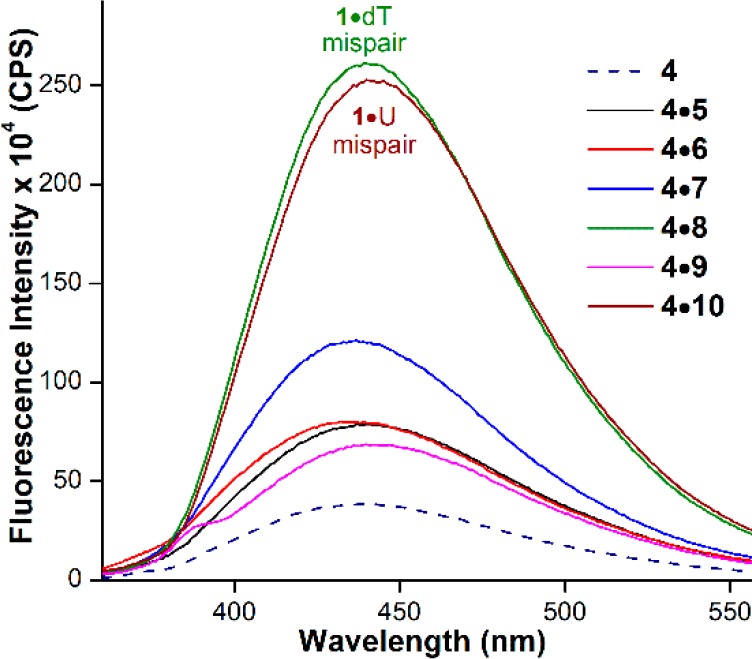
Emission spectra of duplexes (1 μM) formed by hybridizing transcript 4 with complementary and mismatched DNA and RNA ONs (5–10).
The ability of the probe to signal dT and U mismatches was put to use in devising a simple fluorescence method to detect and estimate metallo base-pairs as we envisioned that Hg2+ ion binding to these mismatches could alter the electronics, as well as the environment of the probe, thus, influencing its fluorescence.11,16 Rewardingly, the addition of 1 equiv of Hg2+ ions significantly reduced the fluorescence intensity of duplexes 4·8 and 4·10 containing dT and U mispairs, respectively (see Figure 5, as well as Figure S7 in the SI). However, free nucleoside 1, single-stranded RNA ON 4, and perfect and other mismatched duplexes displayed only minor differences in fluorescence in the presence and absence of Hg2+ ions (see Figure S7). Furthermore, as compared to Hg2+ ions, the addition of 1 equiv of different metal ions (Ag+, Ca2+, Cu2+, Cd2+, Pb2+, Mn2+, Mg2+, Co2+, Zn2+, Fe2+, and Ni2+) had only a minor influence on the fluorescence of duplexes containing the probe placed opposite to dT and U mismatches (see Figure S8 in the SI). These findings confirm that our probe reliably and selectively reports Hg2+ ion-mediated metallo-base pair formation in DNA–RNA and RNA–RNA duplexes. The reduction in fluorescence intensity in the presence of Hg2+ is more likely due to the base-paired state of the nucleoside analogue, which experiences more stacking interaction and quenching effect from adjacent guanosine bases.11b,15,16 Furthermore, the fluorescence could also be quenched by the coordinated heavy atom.4
Figure 5.
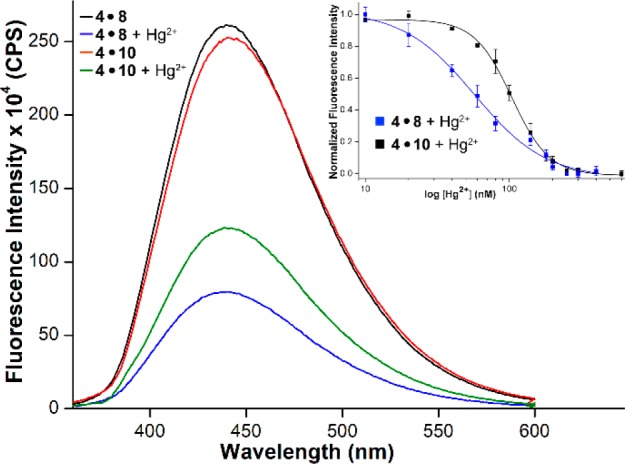
Fluorescence spectra of duplexes 4·8 and 4·10 (1 μM) in the presence and absence of Hg2+ ions (1 μM). Inset shows the curve fit for the titration of duplexes with Hg2+ ions. See the SI for details.
The responsiveness of the probe also enabled determination of the binding affinity of the Hg2+ ion to duplexes. Titrating with the metal ion resulted in a dose-dependent quenching in fluorescence intensity, which gave an apparent dissociation constant Kd of 57 ± 7 nM for 4·8 and 104 ± 2 nM for 4·10 (see Figure 5, as well as Figure S9 in the SI). While Kd values are in the range observed for the dT–Hg2+-dT pair, our results suggest that the metal ion has a comparatively higher binding affinity for dT–U mismatch in a DNA–RNA duplex than U–U mismatch in an RNA–RNA duplex.
The formation of dT–Hg-1 and U–Hg-1 base pairs was ascertained by performing the following experiments. In the presence of Hg2+ ions, both control (3·8, 3·10) and modified duplexes (4·8, 4·10) showed significant increase in thermal melting (ΔTm = 17–21 °C) due to stabilization of the mispairs by metal ion coordination (see Figure S10 and Table S2 in the SI). As expected, perfect duplexes (3·5, 4·5, 3·9, and 4·9) showed only minor differences in Tm upon the addition of Hg2+ ions (see Table S2). Furthermore, the addition of increasing concentrations of the metal ion to duplexes 4·8 and 4·10 resulted in the complete disappearance of respective imino proton signals (10.6 and 10.4 ppm, corresponding to mispair) at 1 equiv of the Hg2+ ion (see Figure S11 in the SI). Consistent with the literature reports,11a these results confirm that the label is minimally perturbing and the fluorescence response is a true reflection of the formation of metallo base-pairs.
In summary, we have introduced a new fluorescent ribonucleoside analogue that is minimally perturbing, highly sensitive to its local environment, and a good substrate for T7 RNA polymerase. The analogue incorporated into an RNA transcript and hybridized to complementary and mismatched ONs served as a fluorescence turn-on reporter for dT and U mismatches. This behavior was used in the specific detection and estimation of Hg2+-mediated metallo-base pair formation. It was found that the Hg2+ ion binds relatively better to a dT mispair in a hybrid duplex, compared to a U mispair in a RNA homo duplex. Taken together, the utility of our probe and these findings are important, because it could further elucidate the implications of metallo-base pairs in RNA folding and recognition.
Acknowledgments
S.M. thanks UGC, India for a graduate research fellowship. This work was supported by Wellcome Trust-DBT India Alliance (No. IA/S/16/1/502360) grant to S.G.S.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.9b01544.
Experimental details, characterization data, supporting figures and tables (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Clever G. H.; Kaul C.; Carell T. DNA–Metal base pairs. Angew. Chem., Int. Ed. 2007, 46, 6226–6236. 10.1002/anie.200701185. [DOI] [PubMed] [Google Scholar]; b Tanaka Y.; Kondo J.; Sychrovský V.; Šebera J.; Dairaku T.; Saneyoshi H.; Urata H.; Torigoe H.; Ono A. Structures, physicochemical properties, and applications of T–HgII–T, C–AgI–C, and other metallo-base-pairs. Chem. Commun. 2015, 51, 17343–17360. 10.1039/C5CC02693H. [DOI] [PubMed] [Google Scholar]; c Zhou W.; Saran R.; Liu J. Metal sensing by DNA. Chem. Rev. 2017, 117, 8272–8325. 10.1021/acs.chemrev.7b00063. [DOI] [PubMed] [Google Scholar]
- a Ono A.; Togashi H. Highly selective oligonucleotide-based sensor for mercury(II) in aqueous solutions. Angew. Chem., Int. Ed. 2004, 43, 4300–4302. 10.1002/anie.200454172. [DOI] [PubMed] [Google Scholar]; b Hollenstein M.; Hipolito C.; Lam C.; Dietrich D.; Perrin D. M. A highly selective DNAzyme sensor for mercuric ions. Angew. Chem., Int. Ed. 2008, 47, 4346–4350. 10.1002/anie.200800960. [DOI] [PubMed] [Google Scholar]; c Dave N.; Chan M. Y.; Huang P.-J. J.; Smith B. D.; Liu J. Regenerable DNA-functionalized hydrogels for ultrasensitive, instrument-free mercury(II) detection and removal in water. J. Am. Chem. Soc. 2010, 132, 12668–12673. 10.1021/ja106098j. [DOI] [PubMed] [Google Scholar]; d Lin Y.-W.; Ho H.-T.; Huang C.-C.; Chang H.-T. Fluorescence detection of single nucleotide polymorphisms using a universal molecular beacon. Nucleic Acids Res. 2008, 36, e123 10.1093/nar/gkn537. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Wang Z.-G.; Elbaz J.; Remacle F.; Levine R. D.; Willner I. All-DNA finite-state automata with finite memory. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 21996–22001. 10.1073/pnas.1015858107. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Liu S.; Clever G. H.; Takezawa Y.; Kaneko M.; Tanaka K.; Guo X.; Shionoya M. Direct conductance measurement of individual metallo-DNA duplexes within single-molecule break junctions. Angew. Chem. 2011, 123, 9048–9052. 10.1002/ange.201102980. [DOI] [PubMed] [Google Scholar]; g Tanaka K.; Clever G. H.; Takezawa Y.; Yamada Y.; Kaul C.; Shionoya M.; Carell T. Programmable self-assembly of metal ions inside artificial DNA duplexes. Nat. Nanotechnol. 2006, 1, 190–194. 10.1038/nnano.2006.141. [DOI] [PubMed] [Google Scholar]; h Kim E.-K.; Switzer C. Bis(6-carboxypurine)-Cu2+: a possibly primitive metal-mediated nucleobase pair. Org. Lett. 2014, 16, 4059–4061. 10.1021/ol5018728. [DOI] [PubMed] [Google Scholar]
- a Ono A.; Torigoe H.; Tanaka Y.; Okamoto I. Binding of metal ions by pyrimidine base pairs in DNA duplexes. Chem. Soc. Rev. 2011, 40, 5855–5866. 10.1039/c1cs15149e. [DOI] [PubMed] [Google Scholar]; b Scharf P.; Müller J. Nucleic acids with metal-mediated base pairs and their applications. ChemPlusChem 2013, 78, 20–34. 10.1002/cplu.201200256. [DOI] [Google Scholar]
- Schmidt O. P.; Mata G.; Luedtke N. W. Fluorescent base analogue reveals T-HgII-T base pairs have high kinetic stabilities that perturb DNA metabolism. J. Am. Chem. Soc. 2016, 138, 14733–14739. 10.1021/jacs.6b09044. [DOI] [PubMed] [Google Scholar]
- Miyake Y.; Togashi H.; Tashiro M.; Yamaguchi H.; Oda S.; Kudo M.; Tanaka Y.; Kondo Y.; Sawa R.; Fujimoto T.; Machinami T.; Ono A. MercuryII-mediated formation of thymine-HgII-thymine base pairs in DNA duplexes. J. Am. Chem. Soc. 2006, 128, 2172–2173. 10.1021/ja056354d. [DOI] [PubMed] [Google Scholar]
- a Urata H.; Yamaguchi E.; Funai T.; Matsumura Y.; Wada S.-I. Incorporation of thymine nucleotides by DNA polymerases through T–HgII–T base pairing. Angew. Chem., Int. Ed. 2010, 49, 6516–6519. 10.1002/anie.201002142. [DOI] [PubMed] [Google Scholar]; b Funai T.; Nakamura J.; Miyazaki Y.; Kiriu R.; Nakagawa O.; Wada S.-i.; Ono A.; Urata H. Regulated incorporation of two different metal ions into programmed sites in a duplex by DNA polymerase catalyzed primer extension. Angew. Chem., Int. Ed. 2014, 53, 6624–6627. 10.1002/anie.201311235. [DOI] [PubMed] [Google Scholar]; c Park K. S.; Jung C.; Park H. G. ″Illusionary″ polymerase activity triggered by metal ions: use for molecular logic-gate operations. Angew. Chem., Int. Ed. 2010, 49, 9757–9760. 10.1002/anie.201004406. [DOI] [PubMed] [Google Scholar]
- Funai T.; Miyazaki Y.; Aotani M.; Yamaguchi E.; Nakagawa O.; Wada S.; Torigoe H.; Ono A.; Urata H. AgI ion mediated formation of a C–A mispair by DNA polymerase. Angew. Chem., Int. Ed. 2012, 51, 6464–6466. 10.1002/anie.201109191. [DOI] [PubMed] [Google Scholar]
- a Tanaka Y.; Oda S.; Yamaguchi H.; Kondo Y.; Kojima C.; Ono A. 15N–15N J-Coupling Across HgII: Direct observation of HgII-Mediated T–T Base Pairs in a DNA duplex. J. Am. Chem. Soc. 2007, 129, 244–245. 10.1021/ja065552h. [DOI] [PubMed] [Google Scholar]; b Yamaguchi H.; Šebera J.; Kondo J.; Oda S.; Komuro T.; Kawamura T.; Dairaku T.; Kondo Y.; Okamoto I.; Ono A.; Burda J. V.; Kojima C.; Sychrovský V.; Tanaka Y. The structure of metallo-DNA with consecutive thymine–HgII–thymine base pairs explains positive entropy for the metallo base pair formation. Nucleic Acids Res. 2014, 42, 4094–4099. 10.1093/nar/gkt1344. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Torigoe H.; Miyakawa Y.; Ono A.; Kozasa T. Positive cooperativity of the specific binding between Hg2+ ion and T:T mismatched base pairs in duplex DNA. Thermochim. Acta 2012, 532, 28–35. 10.1016/j.tca.2011.03.018. [DOI] [Google Scholar]; d Jakobsen U.; Shelke S. A.; Vogel S.; Sigurdsson S. T. Site-directed spin-labeling of nucleic acids by click chemistry: detection of abasic sites in duplex DNA by EPR spectroscopy. J. Am. Chem. Soc. 2010, 132, 10424–10428. 10.1021/ja102797k. [DOI] [PubMed] [Google Scholar]; e Liu H.; Cai C.; Haruehanroengra P.; Yao Q.; Chen Y.; Yang C.; Luo Q.; Wu B.; Li J.; Ma J.; Sheng J.; Gan J. Flexibility and stabilization of HgII-mediated C:T and T:T base pairs in DNA duplex. Nucleic Acids Res. 2016, 45, 2910–2918. 10.1093/nar/gkw1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Wang Z.; Lee J. H.; Lu Y. Highly sensitive “turn-on” fluorescent sensor for Hg2+ in aqueous solution based on structure-switching DNA. Chem. Commun. 2008, 6005–6007. 10.1039/b812755g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Teh H. B.; Wu H.; Zuo X.; Li S. F. Y. Sensors and Actuators B. Detection of Hg2+ using molecular beacon-based fluorescent sensor with high sensitivity and tunable dynamic range. Sens. Actuators, B 2014, 195, 623–629. 10.1016/j.snb.2014.01.089. [DOI] [Google Scholar]; c Liu C.-W.; Huang C.-C.; Chang H.-T. Chang, H.-T. Highly selective DNA-based sensor for lead(II) and mercury(II) ions. Anal. Chem. 2009, 81, 2383–2387. 10.1021/ac8022185. [DOI] [PubMed] [Google Scholar]
- a Chan D. S.-H.; Lee H.-M.; Che C.-M.; Leung C.-H.; Ma D.-L. A selective oligonucleotide-based luminescent switch-on probe for the detection of nanomolar mercury(II) ion in aqueous solution. Chem. Commun. 2009, 7479–7481. 10.1039/b913995h. [DOI] [PubMed] [Google Scholar]; b Zhang X.; Li Y.; Su H.; Zhang S. Highly sensitive and selective detection of Hg2+ using mismatched DNA and a molecular light switch complex in aqueous solution. Biosens. Bioelectron. 2010, 25, 1338–1343. 10.1016/j.bios.2009.10.023. [DOI] [PubMed] [Google Scholar]; c Wang Y.; Geng F.; Xu H.; Qu P.; Zhou X.; Xu M. A label-free oligonucleotide based thioflavin-T fluorescent switch for Ag+ detection with low background emission. J. Fluoresc. 2012, 22, 925–929. 10.1007/s10895-011-1031-z. [DOI] [PubMed] [Google Scholar]; d Ma D.-L.; He H.-Z.; Leung K.-H.; Zhong H.-J.; Chan D. S. -H.; Leung C.-H. Label-free luminescent oligonucleotide-based probes. Chem. Soc. Rev. 2013, 42, 3427–3440. 10.1039/c2cs35472a. [DOI] [PubMed] [Google Scholar]
- a Schmidt O. P.; Benz A. S.; Mata G.; Luedtke N. W. HgII binds to C–T mismatches with high affinity. Nucleic Acids Res. 2018, 46, 6470–6479. 10.1093/nar/gky499. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Park K. S.; Lee J. Y.; Park H. G. Mismatched pyrrolo-dC-modified duplex DNA as a novel probe for sensitive detection of silver ions. Chem. Commun. 2012, 48, 4549–4551. 10.1039/c2cc17148a. [DOI] [PubMed] [Google Scholar]
- a Sinkeldam R. W.; Greco N. J.; Tor Y. Fluorescent analogs of biomolecular building blocks: design, properties, and applications. Chem. Rev. 2010, 110, 2579–2619. 10.1021/cr900301e. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tanpure A. A.; Pawar M. G.; Srivatsan S. G. Fluorescent nucleoside analogs: probes for investigating nucleic acid structure and function. Isr. J. Chem. 2013, 53, 366–378. 10.1002/ijch.201300010. [DOI] [Google Scholar]
- a Pawar M. G.; Nuthanakanti A.; Srivatsan S. G. Heavy atom containing fluorescent ribonucleoside analog probe for the fluorescence detection of RNA-ligand binding. Bioconjugate Chem. 2013, 24, 1367–1377. 10.1021/bc400194g. [DOI] [PubMed] [Google Scholar]; b Pawar M. G.; Srivatsan S. G. Synthesis, photophysical characterization, and enzymatic incorporation of a microenvironment-sensitive fluorescent uridine analog. Org. Lett. 2011, 13, 1114–1117. 10.1021/ol103147t. [DOI] [PubMed] [Google Scholar]; c Tanpure A. A.; Srivatsan S. G. A Microenvironment-sensitive fluorescent pyrimidine ribonucleoside analogue: synthesis, enzymatic incorporation, and fluorescence detection of a DNA abasic site. Chem. - Eur. J. 2011, 17, 12820–12827. 10.1002/chem.201101194. [DOI] [PubMed] [Google Scholar]; d Milisavljevič N.; Perlíková P.; Pohl R.; Hocek M. Enzymatic synthesis of base-modified RNA by T7 RNA polymerase. A systematic study and comparison of 5-substituted pyrimidine and 7-substituted 7-deazapurine nucleoside triphosphates as substrates. Org. Biomol. Chem. 2018, 16, 5800–5807. 10.1039/C8OB01498A. [DOI] [PubMed] [Google Scholar]
- a Sinkeldam R. W.; Wheat A. J.; Boyaci H.; Tor Y. Emissive nucleosides as molecular rotors. ChemPhysChem 2011, 12, 567–570. 10.1002/cphc.201001002. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dziuba D.; Jurkiewicz P.; Cebecauer M.; Hof M.; Hocek M. A Rotational BODIPY nucleotide: an environment-sensitive fluorescence-lifetime probe for DNA interactions and applications in live-cell microscopy. Angew. Chem., Int. Ed. 2016, 55, 174–178. 10.1002/anie.201507922. [DOI] [PubMed] [Google Scholar]; c Manna S.; Sarkar D.; Srivatsan S. G. A dual-app nucleoside probe provides structural insights into the human telomeric overhang in live cells. J. Am. Chem. Soc. 2018, 140, 12622–12633. 10.1021/jacs.8b08436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Rachofsky E. L.; Osman R.; Ross J. B. A. Probing structure and dynamics of DNA with 2-aminopurine: effects of local environment on fluorescence. Biochemistry 2001, 40, 946–956. 10.1021/bi001664o. [DOI] [PubMed] [Google Scholar]; b Doose S.; Neuweiler H.; Sauer M. Fluorescence quenching by photoinduced electron transfer: a reporter for conformational dynamics of macromolecules. ChemPhysChem 2009, 10, 1389–1398. 10.1002/cphc.200900238. [DOI] [PubMed] [Google Scholar]; c Teppang K. L.; Lee R. W.; Burns D. D.; Turner M. B.; Lokensgard M. E.; Cooksy A. L.; Purse B. W. Electronic Modifications of fluorescent cytidine analogues control photophysics and fluorescent responses to base stacking and pairing. Chem. - Eur. J. 2019, 25, 1249–1259. 10.1002/chem.201803653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kim S. J.; Kool E. T. Sensing metal ions with DNA building blocks: fluorescent pyridobenzimidazole nucleosides. J. Am. Chem. Soc. 2006, 128, 6164–6171. 10.1021/ja0581806. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Blanchard D. J. M.; Manderville R. A. An internal charge transfer-DNA platform for fluorescence sensing of divalent metal ions. Chem. Commun. 2016, 52, 9586–9588. 10.1039/C6CC04613D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.