Abstract
Background:
The progression from acute kidney injury (AKI) to chronic kidney disease (CKD) is not well understood in children.
Objectives:
We aimed to develop a pediatric CKD definition using administrative data and use it to evaluate the association between AKI in critically ill children and CKD 5 years after hospital discharge.
Design:
Retrospective cohort study using chart collection and administrative data.
Setting:
Two-center study in Montreal, Canada.
Patients:
Children (≤18 years old) admitted to two pediatric intensive care units (ICUs) between 2003 and 2005. We a priori excluded patients with end-stage renal disease or no health care number. Only the first admission during the study period was included. We excluded patients who could not be linked to administrative data, did not survive hospitalization, or had preexisting renal disease.
Measurements:
Acute kidney injury was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. Patients were defined as having CKD 5 years post-discharge if they had ≥1 CKD diagnostic code or ≥1 CKD-specific medication prescription.
Methods:
Chart data used to define the exposure (AKI) were merged with provincial administrative data used to define the outcome (CKD). Cox regression was used to evaluate the AKI-CKD association.
Results:
A total of 2235 (56% male) patients were included, and the median admission age was 3.7 years. A total of 464 (21%) patients developed AKI during pediatric ICU admission. At 5 years post-discharge, 43 (2%) patients had a CKD diagnosis. Patients with both stage 1 and stage 2-3 AKI had increased risk of a CKD diagnosis, with the adjusted hazard ratios (95% confidence intervals) of 2.2 (1.1-4.5) and 2.5 (1.1-5.7), respectively (P < .001).
Limitations:
Results may not be generalizable to non-ICU patients. We were not able to control for post-discharge variables; future research should try to explore these additional potential risk factors further.
Conclusions:
Acute kidney injury is associated with 5-year post-discharge CKD diagnosis defined by administrative health care data.
Keywords: acute kidney injury, pediatrics, chronic kidney disease, children, administrative data
Abrégé
Contexte:
Chez l’enfant, la progression de l’insuffisance rénale aigüe (IRA) vers l’insuffisance rénale chronique (IRC) est encore mal connue.
Objectifs:
Nous souhaitions élaborer une définition de l’IRC pédiatrique à partir des données administratives, et l’employer pour évaluer l’association entre l’IRA chez les enfants gravement malades et un diagnostic d’IRC cinq ans après leur sortie de l’hôpital.
Type d’étude:
Étude de cohorte rétrospective réalisée à partir des dossiers médicaux et des données administratives.
Cadre:
Deux centres hospitaliers de Montréal (Canada).
Sujets:
L’étude porte sur des enfants (≤18 ans) admis à deux unités de soins intensifs (USI) pédiatriques entre 2003 et 2005. Les patients atteints d’insuffisance rénale terminale ou sans numéro d’assurance-maladie ont été exclus d’emblée. Seule la première admission survenue au cours de l’étude a été retenue. Les patients n’ayant pu être reliés aux données administratives, n’ayant pas survécu à l’hospitalisation ou souffrant d’une néphropathie préexistante ont été exclus.
Mesures:
L’IRA a été définie selon les critères KDIGO (Kidney Disease: Improving Global Outcomes) et l’IRC cinq ans après la sortie de l’hôpital par la présence d’au moins un code diagnostique pour l’IRC ou la prise d’au moins un médicament spécifique au traitement de l’IRC.
Méthodologie:
Les données des dossiers médicaux, utilisées pour définir l’exposition (IRA), ont été couplées aux données administratives provinciales, utilisées pour définir le résultat (IRC). Un modèle de régression de Cox a servi à établir l’association entre IRA et IRC.
Résultats:
Ont été inclus 2 235 patients (56 % de garçons), dont l’âge médian à l’admission était de 3,7 ans. De ce nombre, 464 (21 %) ont développé une IRA en cours d’hospitalisation à l’USI pédiatrique. Cinq ans après leur sortie de l’hôpital, 43 patients (2 %) avaient reçu un diagnostic d’IRC. Les patients atteints d’une IRA de stade 1 et de stade 2-3 ont présenté un plus grand risque de progresser vers l’IRC (rapport de risque ajusté [IC à 95 %] 2,2 [1,1 – 4,5] et 2,5 [1,1 – 5,7] respectivement, P < 0,001).
Limites:
Les résultats pourraient ne pas s’appliquer aux patients non admis aux USI pédiatriques. Nous n’avons pu ajuster les résultats avec les variables après la sortie de l’hôpital. Des études futures devraient examiner plus attentivement ces potentiels facteurs de risque supplémentaires.
Conclusion:
L’IRA chez l’enfant a été associée à une progression vers l’IRC cinq ans après la sortie de l’hôpital, telle que définie par les données administratives de santé.
What was known before
Acute kidney injury is common in the pediatric intensive care unit. Acute kidney injury in adults is associated with increased risk of long-term chronic kidney disease, but little is known about this association in children.
What this adds
We designed an algorithm for defining pediatric chronic kidney disease using administrative health data. We then used this to evaluate the association between acute kidney injury and 5-year chronic kidney disease in a large pediatric cohort.
Introduction
Acute kidney injury (AKI) is defined as an abrupt onset of kidney dysfunction and is common in the pediatric intensive care unit (ICU). Children who develop AKI in the pediatric ICU are at increased risk of mortality and morbidity.1-4 In adults, AKI increases risk for long-term incident chronic kidney disease (CKD).5-7 In both adults and children, CKD is defined as the presence of markers of kidney injury (eg, albuminuria; known renal malformation) or decreased kidney function for more than 3 months.8 In the general population, the prevalence of CKD is extremely low in children; however, pediatric studies have reported higher than expected long-term CKD prevalence after AKI. Most studies have lacked non-AKI comparison groups9 or have not evaluated pediatric ICU patients specifically.10,11 Thus, the extent to which children with AKI should be followed for long-term CKD development remains unclear.
The objectives of this study were to develop a pediatric CKD definition using administrative data and assess it for face validity. We also used this administrative data definition to evaluate if AKI is associated with a diagnosis of CKD 5 years after hospital discharge. We hypothesized that children with AKI in the ICU are at increased risk of CKD compared with patients with no AKI.
Methods
Design, Setting, and Patients
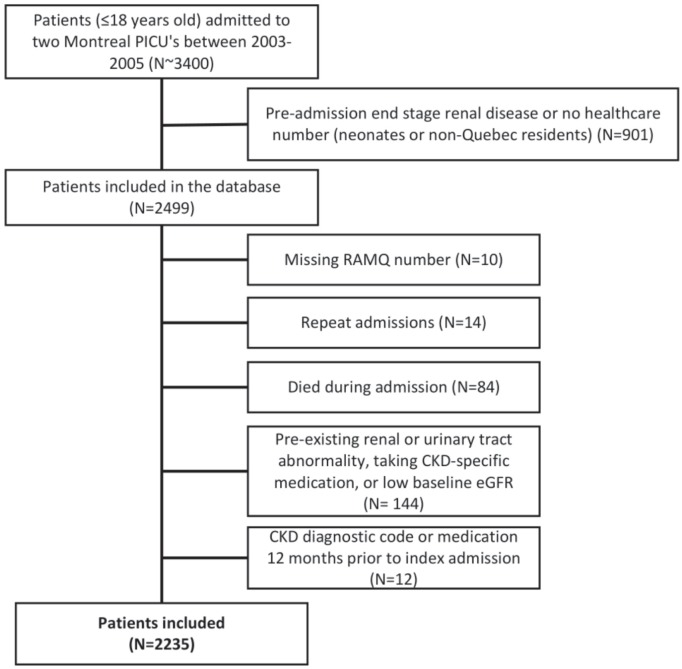
We performed a retrospective cohort study of children (≤18 years old) admitted to two pediatric ICUs in Montreal, Canada between January 1, 2003 and March 31, 2005. We a priori excluded patients with preadmission end-stage renal disease or if this diagnosis was made during index admission, and patients with no health care number. We included the first hospitalization per patient during the study period. We excluded patients who could not be linked to provincial data, did not survive hospitalization, or had preexisting renal or urinary tract abnormalities (by chart review), or a diagnostic code for CKD or a prescription of CKD-specific medication 12 months before admission (per administrative data). We also excluded patients with low baseline estimated glomerular filtration rate (eGFR) (≥1 month old: eGFR < 35 mL/min/1.73m2 using the CKD in Children [CKiD] formula; <1 month old: eGFR <2 standard deviations from mean normative value, because glomerular filtration rate [GFR] = 35 mL/min/1.73m2 is within 2 standard divisions of the mean normative value; see Figure 1).12,13 Approvals from institutional research ethics boards and the Commission d’accès à l’information du Québec (CAI; provincial ethics body) were obtained. Requirement for patient consent was waived.
Figure 1.
Study flow and patient selection criteria leading to the analysis population.
Note. PICU = pediatric intensive care unit; RAMQ = Régie de l’assurance maladie du Québec; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate.
Data Collection and Sources
Index hospitalization data were collected by retrospective chart review (described previously).4,14 Variables collected included primary ICU admission diagnosis, treatment details, and illness severity measures (Supplemental Table 1). Chart data were merged with the Quebec Vital Statistics Registry and administrative health databases (Régie de l’assurance maladie du Québec [RAMQ] and MED-ECHO) from which data from January 1, 2002 to March 31, 2010 (12 months before the first hospitalization and 5 years after the last hospital admission) were available. The RAMQ database contains (1) a demographic database; (2) a medical services database (including outpatient services date and International Classification of Diseases, Ninth Revision [ICD-9] and International Classification of Diseases, Tenth Revision [ICD-10] diagnostic codes); and (3) a outpatient prescription database for patients with provincial medication insurance. The MED-ECHO database contains acute care hospitalization data including admission and discharge dates, primary diagnosis, procedures, and up to 15 secondary diagnoses (ICD-9/10 codes). The codes from the index admission were used to calculate the Pediatric Medical Complexity Algorithm, which classifies children with chronic disease according to medical complexity.15 Emigration could not be determined with certainty; however, <0.5% of the Quebec population emigrated per year during this study period.16
Primary Exposure: AKI
Acute kidney injury was defined using serum creatinine (SCr) and urine output criteria, based on the Kidney Disease: Improving Global Outcomes (KDIGO) definition.17,18 AKI was classified as stage 1 (SCr rise ≥ 1.5-1.9 times baseline in 7 days or ≥26.5 µmol/L within 48 hours or urine output < 0.5 mL/kg/h for 8 hours), stage 2 (SCr rise ≥ 2.0-2.9 times baseline or urine output < 0.5 mL/kg/h for 16 hours), and stage 3 (SCr rise ≥ 3.0 times baseline, SCr ≥ 353.6 µmol/L, dialysis treatment for AKI, or eGFR < 35 mL/min/1.73m2 [if >3 months old] or urine output < 0.3 mL/kg/h for 24 hours, or anuric for 12 hours). Baseline SCr was the lowest measurement 3 months before admission. If this was unavailable, baseline was estimated by back-calculation using a previously validated method.19 The maximum AKI stage defined by SCr or urine output criteria was used to classify severity. To maximize sample size and reduce bias, patients without SCr or urine output measurements were classified as non-AKI, as previously performed.20
Outcome: CKD
A detailed literature review focused on administrative data–defined CKD was performed. A total of 11 original articles and 1 review article containing CKD algorithms using ICD-9/10 codes were evaluated.21-33 A list of codes used to define CKD was created, excluding codes that were not applicable to children or too general. Remaining codes were ranked from 1 to 3, where 1 indicated the most specific codes based on validation studies and coauthor opinions (adult/pediatric nephrologists, pharmaco-epidemiologist, statistician).21,25,26,30,31,33 We designed two algorithms for initial evaluation, including one stricter definition and one broader definition. The stricter algorithm only includes codes consistent with the KDIGO CKD criteria (CKD, proteinuria, outpatient dialysis); the broader algorithm includes CKD codes as well as other codes associated with CKD from the literature review (Supplemental Table 2). These diagnostic codes were combined with CKD-specific medications, primarily selected by expert opinion (see Table 1).27
Table 1.
CKD Definition: One or More Diagnostic Codes, OR CKD Outpatient Medication Prescription.
| CKD algorithm | |||
|---|---|---|---|
| Inpatient or outpatient diagnostic (ICD-9 or ICD-10)/procedural codes (CCP or CCI)a | Medications | ||
| Dialysis related procedural codes | |||
| CKD | 585 | N18.x | Erythropoietin Activated vitamin D Potassium-binding agents Phosphate-binding agents |
| Unspecified renal failure | 586 | N19 | |
| Proteinuria | 791.0 | R80 | |
| Kidney transplant | V42.0, 996.8, 590.8 | Z94.0, T86.100, T86.101, N16.5 | |
| Transplant related procedural codes | |||
| Dialysis related procedural codes | 5127, 5142, 5129, 660 | 1KY76LA, 1KY80LA, 1KG76MZXXA, 1KG76MZXXN, 1SY55LAFT | |
| Transplant related procedural codes | 6751, 6759, 6483 | 1PC83LA, 1PC85LAXXJ, 1PC85LAXXK, 1OK85TNXXK, 1OK85XTXXK, 1OK85XUXXK, 1OK85XVXXK | |
| Outpatient diagnostic (ICD-9 or ICD-10)/procedural codes (CCP or CCI) only | |||
| Dialysis related procedural codes | |||
| Dialysis related diagnostic codes | V45.1, V53.9, V56.0, V56.8, 996.1, E87.02, E87.12, E87.22, E87.91 | Z99.2, Z49.0, Z49.1, Z49.2, T82.4, Y60.2, Y61.2, Y62.2, Y84.1 | |
| Transplant related procedural codes | |||
| Dialysis related procedural codes | 6698, 5195, 0789, 6693 | 1PZ21HPD4, 1PZ21HQBR, 7SC59QD, 1OT53DATS, 1OT53HATS, 1OT53LATS | |
Note. CKD = chronic kidney disease; ICD-9/10 = International Classification of Diseases, Ninth Revision; ICD-10 = International Classification of Diseases, Tenth Revision; CCP = Canadian Classification of Diagnostic, Therapeutic, and Surgical Procedure; CCI = Canadian Classification of Health Interventions.
In the province of Quebec, administrative health care data used ICD-9 until the fiscal year of 2006; thereafter, ICD-10 coding was used.
Three different combinations of codes were evaluated (diagnostic codes alone [1], diagnosis AND [2]/OR medication [3]). These combinations were made with the stricter and broader definition diagnostic codes separately. A preliminary univariable analysis comparing CKD between AKI and non-AKI patients using all algorithms was performed: All algorithms showed a statistically significant difference (not shown).
Table 1 shows the final CKD algorithm (expanded; Supplemental Table 3). We selected the stricter definition codes to be confident of AKI-outcome associations. The diagnostic and procedural codes were divided into two categories: (1) inpatient or outpatient codes and (2) outpatient codes only. A patient was defined as having CKD if he or she had ≥1 CKD diagnostic codes and/or ≥1 prescription for CKD-specific medication 5 years post-hospital discharge. This allowed the inclusion of patients uninsured by provincial medication coverage (~70%).
Analysis
Associations between baseline patient and treatment characteristics with AKI and CKD were evaluated using appropriate univariable analyses. Associations with AKI and CKD individually, as well as CKD stratified by AKI (yes/no), were performed. All analyses were planned a priori and conducted using SAS statistical software, release 9.2 (SAS Institute Inc, Cary, NC, USA). Reporting was prepared in accordance to guidelines.34
Cumulative incidence curves were plotted by no vs stage 1 vs stage 2-3 AKI. Multivariable Cox regression was performed to evaluate the association between AKI and 5-year CKD. Variables were included in the multivariable analysis if they were associated with the exposure (AKI) and the outcome (CKD) at the P < .01 level.
The characteristics between the patients without an SCr or urine output measurement during ICU admission and those with measurements but with no AKI during ICU admission were compared. Sensitivity analysis was performed, excluding patients without SCr or urine output measurements. To ensure that we were not including patients with a CKD diagnosis prior to admission (but not recorded in the data), or those with temporary post-AKI dialysis, in the outcomes, cumulative incidence curves excluding CKD diagnoses made 90 days post-hospital discharge were made.
We also compared nephrology specialist visit follow-up after hospital discharge between patients with vs without AKI and patients with vs without CKD diagnosis. After detailed group discussion, we used the presence of ≥2 nephrology visits within 16 months to indicate the presence of regular nephrology follow-up.
Results
Patient Characteristics
A total of 2235 patients were included (56% male), with the median (interquartile range [IQR]) age of 3.7 (10.4) years and the median (IQR) follow-up of 5.0 (0) years; of these, 464 (21%) developed AKI during ICU admission. A total of 43 (2%) patients had algorithm-defined CKD diagnosis 5 years post-hospital discharge (see Table 1; 39 diagnostic codes only, 2 medications only, 2 both diagnostic codes and medications). Few children fulfilled the medication criteria; however, only ~30% had medication coverage through provincial insurance plans. Chronic kidney disease diagnosis was ascertained a median (IQR) of 1.1 (2.4) years post-discharge. During follow-up, 95 (4%) patients died.
Table 2 shows univariable associations between baseline characteristics with AKI and 5-year CKD. Variables associated with AKI and 5-year CKD with P < .01 included chronic illness by the pediatric medical complexity algorithm15 and nephrotoxic antibiotics use in the ICU. Patients with AKI and CKD had longer ICU and hospital stays (see Table 2). Among the patients with AKI in the pediatric ICU, those who had a diagnosis of CKD compared with those without a CKD diagnosis had a higher prevalence of admission diagnosis under the category of gastrointestinal or oncologic, nephrotoxic antibiotics, and had longer hospital stay (Supplemental Table 4).
Table 2.
Univariable Analysis of Patient and Pediatric ICU Characteristics by AKI and by 5-Year CKD Diagnosis.
| Variables | No AKI (N = 1771) | AKI (N = 464) | No CKD (N = 2192) | CKD (N = 43) |
|---|---|---|---|---|
| Patient characteristics | ||||
| ICU admission age, years | 3.7 (10.3) | 3.6 (10.6) | 3.6 (10.4) | 4.7 (11.4) |
| Female sex | 787 (44%) | 212 (46%) | 966 (44%) | 28 (65%)* |
| Center 2 | 988 (56%) | 310 (67%)** | 1271 (58%) | 27 (63%) |
| ICU diagnosis | ||||
| Cardiac surgery | 163 (9%) | 167 (36%)** | 323 (15%) | 7 (16%) |
| Cardiac (nonsurgical) | 100 (6%) | 27 (6%) | 123 (6%) | 4 (9%) |
| Trauma | 180 (10%) | 34 (7%) | 213 (10%) | 1 (2%) |
| Infection (nonbronchiolitis) | 298 (17%) | 72 (16%) | 362 (17%) | 8 (19%) |
| Neurologic/neurosurgical | 243 (14%) | 38 (8%)* | 277 (13%) | 4 (9%) |
| Gastrointestinala | 46 (3%) | 22 (5%)* | 61 (3%) | 7 (16%)** |
| Oncologic | 43 (2%) | 7 (2%) | 47 (2%) | 3 (7%) |
| Respiratory | 186 (11%) | 28 (6%)* | 212 (10%) | 2 (5%) |
| Diabetes | 39 (2%) | 16 (3%) | 54 (2%) | 1 (2%) |
| Otherb | 473 (27%) | 53 (11%)** | 520 (24%) | 6 (14%) |
| Postoperative (noncardiac) | 580 (33%) | 83 (18%)** | 654 (30%) | 9 (21%) |
| PRISM death rate | 1.9 (3.7) | 4.9 (13.9)** | 2.2 (4.7) | 2.0 (7.8) |
| Pediatric Medical Complexity Algorithm | ** | ** | ||
| No chronic disease | 333 (19%) | 52 (11%) | 383 (17%) | 2 (5%) |
| Noncomplex chronic disease | 436 (25%) | 75 (16%) | 509 (23%) | 2 (5%) |
| Complex chronic disease | 1002 (57%) | 337 (73%) | 1300 (60%) | 39 (91%) |
| ICU characteristics and outcomes | ||||
| Nephrotoxic antibiotics in the ICUc | 304 (17%) | 156 (34%)** | 439 (20%) | 21 (49%)** |
| NSAIDs | 178 (10%) | 90 (19%)** | 263 (12%) | 5 (12%) |
| Vasopressors used (yes/no) | 178 (10%) | 215 (46%)** | 382 (17%) | 11 (26%) |
| Steroids used (yes/no) | 444 (25%) | 142 (31%)* | 570 (26%) | 16 (37%) |
| Mechanically ventilated (yes/no) | 697 (39%) | 327 (70%)** | 1007 (46%) | 17 (40%) |
| Length of mechanical ventilation, days | 0 (2) | 2 (6)** | 0 (2) | 0 (3) |
| ICU length of stay, days | 1.1 (1.9) | 3.7 (7.1)** | 1.5 (2.7) | 2.5 (5.6)* |
| Hospital length of stay | 7 (9) | 13 (18)** | 8 (11) | 19 (40)** |
| Kidney related | ||||
| Baseline eGFR | 120 (36) | 120 (49) | 120 (36) | 120 (49) |
| Renal replacement therapy in ICU | 0 | 4 (0.9%)** | 3 (0.1%) | 1 (2%) |
Note. Continuous variables are presented as median (IQR) and categorical variables are presented as number (percentage). Associations between continuous variables were determined using Student t test or Kruskal-Wallis test depending on the distribution. Categorical variables were evaluated using chi-square test or Fisher exact test. ICU = intensive care unit; AKI = acute kidney injury defined using serum creatinine and urine output; CKD = chronic kidney disease; PRISM = Pediatric Risk of Mortality; NSAIDs = nonsteroidal anti-inflammatory drugs; eGFR = estimated glomerular filtration rate; IQR = interquartile range.
Includes the liver, stomach, pancreas, and intestine.
Includes hematologic (nononcologic), inborn error of metabolism and metabolic (noninborn error of metabolism), immunologic, intoxication, burn, orthopedic, otolaryngologic, endocrinologic (nondiabetes), and bronchiolitis.
Includes aminoglycosides, acyclovir/ganciclovir, amphotericin, and vancomycin.
P < .05. **P < .001.
AKI-CKD Association
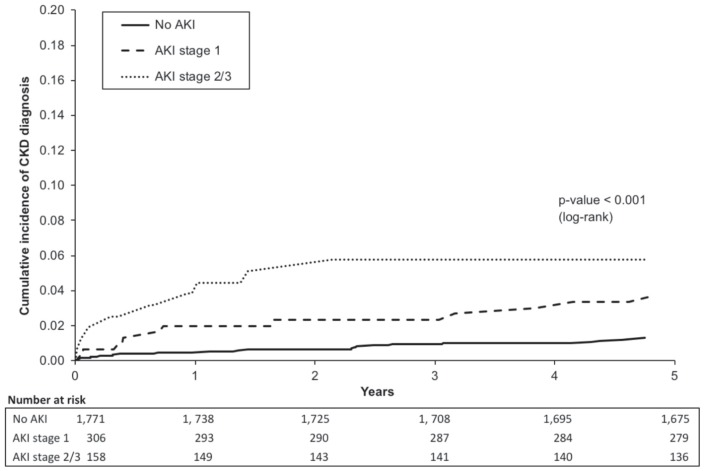
Figure 2 shows the cumulative incidence of CKD by AKI strata. Patients with stage 2-3 AKI had higher incidence of CKD diagnosis, especially 1 year after discharge. In univariable and multivariable analyses, AKI was associated with CKD 5 years post-discharge (see Table 3). Patients with either stage 1 or stage 2-3 AKI had more than 2 times higher adjusted risk of CKD diagnosis 5 years post-discharge relative to non-AKI patients (see Table 3).
Figure 2.
Cumulative incidence curves for CKD stratified by AKI severity.
Note. The figure compares the incidence of a CKD diagnosis in patients with no AKI (solid line), stage 1 AKI (dashed line), and stage 2-3 AKI (dotted line). Time 0 is the date of hospital discharge. Log-rank test was used to compare groups. Incidence of a CKD diagnosis increases with the increasing severity of AKI. CKD = chronic kidney disease; AKI = acute kidney injury.
Table 3.
Proportion of Patients With CKD and Univariable and Multivariable Cox Regression for Association Between AKI and 5-Year CKD.
| Classification | CKD | P value | Univariable HR (95% CI) | Multivariable HR (95% CI)a |
|---|---|---|---|---|
| No AKI (N = 1771) AKI (N = 464) |
23 (1%) 20 (4%) |
<.001 | 1.00 3.4 (1.9-6.2)** |
1.00 2.3 (1.3-4.3)* |
| No AKI/stage 1 (N = 2077) Stage 2/3 (N = 158) |
34 (2%) 9 (6%) |
<.001 | 1.00 3.6 (1.7-7.6)** |
1.00 2.1 (1.0-4.4) |
| No AKI (N = 1771) Stage 1 (N = 306) Stage 2/3 (N = 158) |
23 (1%) 11 (4%) 9 (6%) |
<.001 | 1.00 2.8 (1.4-5.8)* 4.6 (2.1-9.9)** |
1.00 2.2 (1.1-4.5)* 2.5 (1.1-5.7)* |
Note. CKD = chronic kidney disease; AKI = acute kidney injury; HR = hazard ratio; CI = confidence interval; ICU = intensive care unit.
Adjusted for Pediatric Medical Complexity Algorithm and nephrotoxic antibiotic use in the pediatric ICU.
P < .05. **P < .001.
Sensitivity Analyses
Of all 2235 patients, 1833 had measured SCr and/or urine output in the ICU (852 had both; 45 only urine output; and 936 only SCr). The 402 patients without measurements had lower illness severity scores, were less likely to receive vasopressors and mechanical ventilation, had shorter ICU stays, higher baseline GFR, and slightly lower 5-year mortality (3% vs 5%, P = .05) vs known non-AKI patients (Supplemental Table 5). When excluding patients without SCr or urine output measurements, AKI remained associated with CKD in adjusted analysis, with adjusted hazard ratio (95% confidence interval [CI]): 2.6 (1.3-5.0), P = .005 (Supplemental Table 6).
After excluding patients diagnosed with CKD 90 days after discharge, the association of increasing risk of CKD diagnosis with increasing AKI severity remained significant (Supplemental Figure 1).
In our cohort, out of the 2235 patients, 110 (5%) saw a nephrologist ≥2 times within 16 months during the 5 years of follow-up. Of patients with AKI, 36/464 (8%) saw a nephrologist ≥2 times within 16 months compared to 74/1771 (4%) non-AKI patients (P = .002). A higher proportion of patients with a CKD diagnosis had ≥2 nephrology visits within 16 months during the 5 years of follow-up (CKD: 19/43 [44%] vs non-CKD: 91/2192 [4%], P < .001).
Discussion
Using a proposed algorithm to define CKD in children, we found that pediatric ICU AKI is independently associated with increased risk of a CKD diagnosis 5 years post-hospital discharge.
Although the association between AKI and CKD is well characterized in adults, it is less well understood in children. Potential mechanisms for AKI to CKD progression include loss of renal mass causing hyperfiltration damage and cellular pathophysiological events leading to fibrosis.35 Pediatric studies evaluating the AKI-CKD association have been limited by sample size, lack of non-AKI comparison groups, and inconsistent definitions, making it difficult to understand if these children are at risk.9,36,37 A recent Danish population–based registry study found that pediatric cardiac surgery patients with AKI are at greater risk of CKD (based on eGFR measurements) 5 years post-discharge.10 We found a lower incidence (2%); however, this is somewhat expected as the adult literature has demonstrated that using administrative data tends to underestimate CKD incidence, generally capturing more late–stage disease. Considering this and that the general pediatric population CKD prevalence is extremely low, our findings suggest that the pediatric ICU population is globally at higher risk for long-term CKD.38,39 This patient population should be targeted for research to identify risk factors for CKD development and follow-up strategies to reduce CKD incidence, followed by an evaluation of the effects of such interventions.
Compared with the past pediatric AKI literature, our study is unique because it combines chart data to define our exposure with administrative data to define outcomes, allowing for a large sample size and multivariable analysis controlling for potential confounders. Our finding of an AKI-CKD association justifies the need for future research to validate this and other CKD algorithms. Adult studies evaluating the validity of administrative data–based CKD definitions have shown that these algorithms often have low sensitivity but high specificity (>95%).21,26,30,40 Our pediatric definition likely follows similar patterns. This lack in sensitivity is a limitation for all studies using administrative data.41
Our CKD definition and methodology were designed to evaluate the face validity of using administrative data to evaluate long-term kidney outcomes in children. We did not validate the definition. Rather, we determined if CKD using administrative data was detectable in a pediatric population (as childhood CKD prevalence is low) and whether, in a pediatric ICU population, AKI showed evidence of association with long-term CKD, as observed in adults.5-7 Despite the lack of validity evaluation (which would require population-level laboratory review), as described above, studies in adults show that CKD diagnosis using administrative data has high specificity.21,26,30,40 If specificity of administrative data–defined CKD is lower than expected in children, the effect would most likely have been to reduce the magnitude of the AKI-CKD association. Moving forward, our study supports the large task involved in performing a validation study of this and other CKD algorithms to better interpret associations with different exposures, including AKI.
In secondary analyses, we evaluated if AKI or CKD were associated with nephrology follow-up. We did this for 3 reasons. First, we wanted to explore other measures in administrative data that may increase sensitivity for CKD diagnosis. Second, we sought to begin understanding the extent to which these patients are or are not being followed by nephrologists. Third, we wanted to evaluate for potential ascertainment bias; if patients with AKI diagnosis were more likely to be seen by a nephrologist after discharge, it follows that CKD diagnosis may be more common due to increased diagnostic testing (ie, ascertainment). Only 8% of patients with AKI saw a nephrologist ≥2 times within 16 months; this occurred twice more often than in non-AKI patients, suggesting that there may have been some ascertainment bias in our findings on AKI-CKD associations. Prospective studies with unbiased laboratory measurements should be conducted to validate our findings. The nephrology follow-up prevalence in our study also highlights that few AKI patients were followed by a nephrologist. There are no pediatric-specific international guidelines on how children with AKI should be followed. Future research should attempt to better understand AKI follow-up patterns and reasons for follow-up/non-follow-up and evaluate the impact of systematic AKI follow-up.
Our study had limitations. We only had access to administrative data 12 months before admission. It is possible that some patients had a CKD diagnosis before index admission which was not detected. We attempted to limit this by excluding patients with any indication of renal or urinary tract issues from chart review. Moreover, sensitivity analysis excluding patients diagnosed with CKD 90 days post-discharge showed a persistent association with severe AKI and CKD diagnosis. Given that a significant proportion of patients excluded were neonates at pediatric ICU admission for whom we did not have access to a health care number and could not match to the administrative health care data, our study may be biased toward nonneonates. However, given the unique risk factors for acute and chronic kidney injury in neonates, it is worthwhile in future research to evaluate long-term outcomes of AKI in this population. Only ~30% of our population had medications covered by the provincial drug plan, and therefore this aspect of the algorithm contributed little to our results. However, we included medications in our definition so future researchers with access to medication information can evaluate this algorithm. Although we controlled for the effects of multiple index admission variables, there are post-discharge variables (new treatments, diagnoses) that we could not account for. Future research should try to explore these additional potential risk factors further. A challenge present in all studies evaluating the association of AKI with later outcomes is that many of the risk factors or potential confounders that are traditionally adjusted for in analyses may be causes of AKI; thus, AKI would be on the causal pathway to CKD (eg, nephrotoxic medication or illness severity leads to AKI, followed by CKD). Future research should attempt to elucidate the extent to which different causes of AKI may independently contribute to CKD development. As noted above, we were not able to validate this algorithm with the data we had available; however, the goal of this study was to design and assess the face validity of a pediatric-specific CKD definition and to determine whether it is feasible to study pediatric CKD using administrative data. This population is from 2003 to 2005, with follow-up until 2010; this may limit the generalizability of this study, and it is possible that trends in follow-up or CKD detection could have changed. However, no guidelines on how children with AKI should be followed have been established. This research may not be generalizable to non-critically ill populations. We were unable to determine the impact of emigration on our associations. However, during the study period, Quebec population’s yearly emigration was <0.5% and there is little reason to suspect a difference between AKI and non-AKI patients.16
Conclusions
In conclusion, patients admitted to the pediatric ICU are at overall high risk for long-term CKD development. Patients with AKI are at increased risk of CKD diagnosis 5 years post-discharge compared with patients without AKI. Future research should validate the pediatric-specific CKD definition and focus on developing follow-up guidelines for children that are at higher risk of developing CKD after ICU admission. We propose that administrative health care data may provide a rich source from which to evaluate AKI’s association with long-term renal outcomes and understand follow-up patterns and costs associated with pediatric AKI. We also believe that this study contributes to justification of the need to devote resources to perform large, prospective studies of long-term kidney outcomes in critically ill children with AKI.
Supplemental Material
Supplemental material, CKD_Supplemental_Material_Revisions_1_07_Aug_2019 for Acute Kidney Injury in Critically Ill Children and Subsequent Chronic Kidney Disease by Erin Hessey, Sylvie Perreault, Marc Dorais, Louise Roy and Michael Zappitelli in Canadian Journal of Kidney Health and Disease
Acknowledgments
The authors acknowledge the individuals who performed the data collection and helped with data management at Hospital Sainte Justine and the Montreal Children’s Hospital. They also acknowledge Nellie Kamkar, MSc, PhD (c), for helping with manuscript preparation and submission. M.Z. received research support from Fonds de recherche du Québec—Santé, which allowed him to perform this work.
Footnotes
Ethics Approval and Consent to Participate: Ethics approval was provided by the Research Institute of the McGill University Health Centre Pediatric Research Ethics Board (Study code 10-399-PED) and Commission d’accès à l’information du Québec (CAI, provincial ethics body #10-15-04). Requirement for patient consent was waived.
Consent for Publication: All authors reviewed the final manuscript and provided consent for publication.
Availability of Data and Materials: Data and materials may be made available upon written request to the corresponding author.
Author’s Note: Erin Hessey is also affiliated with Faculty of Medicine, University of Alberta, Edmonton, Alberta, Canada.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: All phases of this study were supported by Fonds de recherche du Québec—Santé (FRQS). M.Z. was also supported by a research salary award and E.H. by a graduate studies award from the FRQS.
ORCID iD: Erin Hessey  https://orcid.org/0000-0002-6115-6872
https://orcid.org/0000-0002-6115-6872
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Alkandari O, Eddington KA, Hyder A, et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 2011;15(3):R146. doi: 10.1186/cc10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; for the AWARE Investigators. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11-20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554-561. doi: 10.2215/CJN.01900214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hessey E, Morissette G, Lacroix J, et al. Long-term mortality after acute kidney injury in the pediatric ICU. Hosp Pediatr. 2018;8(5):260-268. doi: 10.1542/hpeds.2017-0215. [DOI] [PubMed] [Google Scholar]
- 5. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442-448. http://www.nature.com/ki/journal/v81/n5/suppinfo/ki2011379s1.html. Accessed August 19, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heung M, Steffick DE, Zivin K, et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis. 2016;67(5):742-752. doi: 10.1053/j.ajkd.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta RH, Honeycutt E, Patel UD, et al. Impact of recovery of renal function on long-term mortality after coronary artery bypass grafting. Am J Cardiol. 2010;106(12):1728-1734. doi: 10.1016/j.amjcard.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2012;3:1-150. [DOI] [PubMed] [Google Scholar]
- 9. Greenberg JH, Coca S, Parikh CR. Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC Nephrol. 2014;15:184. doi: 10.1186/1471-2369-15-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Madsen NL, Goldstein SL, Froslev T, Christiansen CF, Olsen M. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int. 2017;92(3):751-756. doi: 10.1016/j.kint.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 11. Hollander SA, Montez-Rath ME, Axelrod DM, et al. Recovery from acute kidney injury and CKD following heart transplantation in children, adolescents, and young adults: a retrospective cohort study. Am J Kidney Dis. 2016;68(2):212-218. doi: 10.1053/j.ajkd.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 12. Piepsz A, Tondeur M, Ham H. Revisiting normal (51)Cr-ethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging. 2006;33(12):1477-1482. doi: 10.1007/s00259-006-0179-2. [DOI] [PubMed] [Google Scholar]
- 13. Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629-637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hessey E, Ali R, Dorais M, et al. Renal function follow-up and renal recovery after acute kidney injury in critically ill children. Pediatr Crit Care Med. 2017;18(8):733-740. doi: 10.1097/PCC.0000000000001166. [DOI] [PubMed] [Google Scholar]
- 15. Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. doi: 10.1542/peds.2013-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ministère de l’Immigration, de la Diversité et de l’Inclusion. Recueil de statistiques sur l’immigration et la diversité au Québec. Montreal, Canada: Gouvernement du Québec; 2014. [Google Scholar]
- 17. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Inter, Suppl. 2012;2:1-138. doi: 10.1038/kisup.2012.4. [DOI] [Google Scholar]
- 18. Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028-1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 19. Hessey E, Ali R, Dorais M, et al. Evaluation of height-dependent and height-independent methods of estimating baseline serum creatinine in critically ill children. Pediatr Nephrol. 2017;32(10):1953-1962. doi: 10.1007/s00467-017-3670-z. [DOI] [PubMed] [Google Scholar]
- 20. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20. doi: 10.2215/CJN.02730313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fleet JL, Dixon SN, Shariff SZ, et al. Detecting chronic kidney disease in population-based administrative databases using an algorithm of hospital encounter and physician claim codes. BMC Nephrol. 2013;14:81. doi: 10.1186/1471-2369-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Navaneethan SD, Jolly SE, Schold JD, et al. Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol. 2011;6(1):40-49. doi: 10.2215/CJN.04230510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nelson SE, Shroff GR, Li S, Herzog CA. Impact of chronic kidney disease on risk of incident atrial fibrillation and subsequent survival in Medicare patients. J Am Heart Assoc. 2012;1(4):e002097. doi: 10.1161/JAHA.112.002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parasa S, Navaneethan U, Sridhar AR, Venkatesh PG, Olden K. End-stage renal disease is associated with worse outcomes in hospitalized patients with peptic ulcer bleeding. Gastrointest Endosc. 2013;77(4):609-616. doi: 10.1016/j.gie.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 25. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 26. Ronksley PE, Tonelli M, Quan H, et al. Validating a case definition for chronic kidney disease using administrative data. Nephrol Dial Transplant. 2012;27(5):1826-1831. doi: 10.1093/ndt/gfr598. [DOI] [PubMed] [Google Scholar]
- 27. Roy L, White-Guay B, Dorais M, Dragomir A, Lessard M, Perreault S. Adherence to antihypertensive agents improves risk reduction of end-stage renal disease. Kidney Int. 2013;84(3):570-577. doi: 10.1038/ki.2013.103. [DOI] [PubMed] [Google Scholar]
- 28. Siddiqui NF, Coca SG, Devereaux PJ, et al. Secular trends in acute dialysis after elective major surgery—1995 to 2009. CMAJ. 2012;184(11):1237-1245. doi: 10.1503/cmaj.110895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sood P, Kumar G, Nanchal R, et al. Chronic kidney disease and end-stage renal disease predict higher risk of mortality in patients with primary upper gastrointestinal bleeding. Am J Nephrol. 2012;35(3):216-224. doi: 10.1159/000336107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57(2):131-141. doi: 10.1016/S0895-4356(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 31. Winkelmayer WC, Schneeweiss S, Mogun H, Patrick AR, Avorn J, Solomon DH. Identification of individuals with CKD from Medicare claims data: a validation study. Am J Kidney Dis. 2005;46(2):225-232. doi: 10.1053/j.ajkd.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 32. Wu MY, Hsu YH, Su CL, Lin YF, Lin HW. Risk of herpes zoster in CKD: a matched-cohort study based on administrative data. Am J Kidney Dis. 2012;60(4):548-552. doi: 10.1053/j.ajkd.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 33. Vlasschaert MEO, Bejaimal SAD, Hackam DG, et al. Validity of administrative database coding for kidney disease: a systematic review. Am J Kidney Dis. 2011;57(1):29-43. doi: 10.1053/j.ajkd.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 34. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; for the STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 35. Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11(5):264-276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cooper DS, Claes D, Goldstein SL, et al. Follow-up renal assessment of injury long-term after acute kidney injury (FRAIL-AKI). Clin J Am Soc Nephrol. 2016;11(1):21-29. doi: 10.2215/CJN.04240415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morgan CJ, Zappitelli M, Robertson CM, et al. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr. 2013;162(1):120-127.e1. doi: 10.1016/j.jpeds.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 38. Collins AJ, Foley RN, Chavers B, et al. United States Renal Data System 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59:A7, e1-420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 39. Warady BA, Chadha V. Chronic kidney disease in children: the global perspective. Pediatr Nephrol. 2007;22(12):1999-2009. doi: 10.1007/s00467-006-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, Ghali WA. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424-1441. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schaffzin JK, Dodd CN, Nguyen H, Schondelmeyer A, Campanella S, Goldstein SL. Administrative data misclassifies and fails to identify nephrotoxin-associated acute kidney injury in hospitalized children. Hosp Pediatr. 2014;4(3):159-166. doi: 10.1542/hpeds.2013-0116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CKD_Supplemental_Material_Revisions_1_07_Aug_2019 for Acute Kidney Injury in Critically Ill Children and Subsequent Chronic Kidney Disease by Erin Hessey, Sylvie Perreault, Marc Dorais, Louise Roy and Michael Zappitelli in Canadian Journal of Kidney Health and Disease