Abstract
Microneedle (MN) delivery system has been greatly developed to deliver drugs into the skin painlessly, noninvasively, and safety. In the past several decades, various types of MNs have been developed by the newer producing techniques. Briefly, as for the morphologically, MNs can be classified into solid, coated, dissolved, and hollow MN, based on the transdermal drug delivery methods of “poke and patch,” “coat and poke,” “poke and release,” and “poke and flow,” respectively. Microneedles also have other characteristics based on the materials and structures. In addition, various manufacturing techniques have been well-developed based on the materials. In this review, the materials, structures, morphologies, and fabricating methods of MNs are summarized. A separate part of the review is used to illustrate the application of MNs to deliver vaccine, insulin, lidocaine, aspirin, and other drugs. Finally, the review ends up with a perspective on the challenges in research and development of MNs, envisioning the future development of MNs as the next generation of drug delivery system.
Keywords: microneedle, transdermal drug delivery, drug delivery methods, materials, structures, fabricating methods
Introduction
Skin is an important organ evolved to protect the body against unwanted influences, such as excessive water loss, invasion of harmful chemicals, and to prevent the pathogens. There are 3 layers in human skin, including the epidermis, dermis, and hypodermis.1–3 The stratum corneum is part of the epidermis and the outermost skin layer, which is the primary barrier of skin and consists of 15 to 20 layers of corneocytes.4–7
Transdermal drug delivery is an essential alternative to oral and hypodermic administrations to deliver drugs. Compared with oral and hypodermic administrations, transdermal drug delivery can overcome the problem of absorption and degradation of drugs occurring in the gastrointestinal tract or the liver; it is convenient, inexpensive, noninvasive, painless, and self-administrated; as well as it can provide sustained release of drugs to improve patient compliance.2,8,9 However, the stratum corneum imposes significant restrictions on the successful delivery of drugs, especially the high-molecular-weight drugs.1,10,11 Therefore, numerous technologies have been developed to enhance the permeability of drugs via stratum corneum, including chemical enhancement approaches, such as applying the chemical penetration enhancers, physical and electrical enhancement approaches, such as thermal ablation, electroporation, ultrasound, jet injection, and microneedles (MNs).3,10,12 Generally speaking, these methods enhance the delivery of the drugs through stratum corneum either via pore formation or through improved diffusive interaction, and the principles and mechanisms of these methods have been illustrated well in the studies by Zhang10 and Leite-Silva.12 Hereinto, MN, as the novel technique to increase the permeability of drugs, has been used widely in recent years.
Microneedle can be single or an array, consisting of hundreds or even thousands of microprojections, with a length up to 2 mm and a diameter up to hundreds of microns, which are attached to a base support.13,14 The major advantage of MN delivery system is that the system can be used to penetrate into the skin noninvasively and painlessly to improve the safety and comfort for the patients.2 In addition, MN delivery system can precisely deliver drugs from small molecules to macromolecule (eg, protein, insulin) and vaccine.15
Since the first concept of MN was proposed as a drug delivery device in a US patent in 1971 by Gerstel and Place,16 the MNs have been developed for delivery of drugs over several decades. Now, MN has been explored into various structures (out-of-plane MNs and in-plane MNs), materials (silicon, ceramic, glass, metal, and polymer), geometries (octagonal,15 cylindrical,17 rectangular,18 pyramidal,11,19–21 conical,22,23,24 and quadrangular25), and morphologies, that is, solid MN (first reported in 1971 by Gerstel and Place16), hollow MN (first reported in 1971 by Gerstel and Place16), dissolving MN (first reported in 2005 by Miyano et al26), and coated MN (first reported in a patent in 1975 by Pistor27). Meanwhile, a variety of manufacturing techniques (such as etching, micromolding techniques, and lithography) for any MNs mentioned above, comprehensively considering the factors, including materials, structures, morphologies, geometry, and size, have also been reported in numbers of literatures.
In this article, we pay attention to MN as a transdermal drugs delivery system and make an overview of the MN delivery system. First of all, types of MNs are summarized. After that, MNs fabricating techniques based on materials are summarized. Then, the applications of MNs in drug delivery are reviewed. Finally, a perspective on the challenges in research and development of MNs is illustrated. We hope this review, providing the basic information related to MNs, could be helpful for the further researching of MN delivery system.
Classification of MNs
The main purpose of MNs is to penetrate into skin via the microprojections, without hurting any nerves to improve the patient comfort and ensure the safety. Microneedles can be classified into different types based on the parameters, including drug delivery methods, materials, and structures.
Classified by Drug Delivery Methods
Solid MNs
Solid MNs are developed to deliver drugs into skin based on the mechanism of “poke-and-patch” approach. In this approach, solid MNs are penetrated into the skin to disrupt the stratum corneum and create transient microchannels and then a patch with the drug formulations is applied onto the skin so that the drug can diffuse slowly into the skin through the transient microchannels,1,13,28–30 as shown in Figure 1A.
Figure 1.
Diagrams showing various microneedle drug delivery approaches. (A) Solid microneedles, for skin pretreatment to create microchannels, followed by the application of transdermal patch; (B) coated microneedles, for deposition of drug formulations into the skin, followed by removal of microneedles; (C) dissolving microneedles, incorporated into the substrate of microneedles, remaining in the skin and dissolving over time to release the drugs; and (D) hollow microneedles, for inserted into the skin and continuous infusion of drug through the created microchannels.
The solid MNs are efficient enough to enhance the delivery of drug with wide range of molecules. For examples, Zhang et al31 fabricated the solid silicon MN arrays with the length of 150 µm to deliver peptides. The porcine ear skin was pretreated with solid MNs and then 4 types of peptides with different molecular weight were used to illustrate the drug permeability. The experiment results showed that the solid MN arrays promoted the delivery of peptides, but the peptides permeability decreased with the increase in molecular weight of peptides. Nayak et al32 used the “poke-and-patch” method to enhance the permeation of lidocaine hydrochloride. Following solid MNs treatment about 5 minutes, lidocaine hydrochloride was efficiently delivered into the skin and crossed the minimum therapeutic drug threshold in less than 70 minutes. Recently, Bhatnagar et al33 fabricated the MNs, containing 36 needles in 1 cm2 area, to deliver 2 antibreast cancer drugs, such as tamoxifen and gemcitabine. It was confirmed that the “poke-and-patch” approach greatly enhanced the permeation of gemcitabine.
Coated MNs
Coated MNs are used for transdermal drug delivery based on the “coat-and-poke” manner. Specifically, MNs coated with drug formulation are inserted into the skin, then the drugs dissolve from the MNs and enter into the skin, and finally MNs are pulled out of the skin,29,34,35 as shown in Figure 1B. This approach is simple because it just takes one step to deliver the drug through the skin. However, the MNs can only be coated with a small amount of drugs, resulting in a very low drug delivery efficiency.1 Multiple coating approaches have been developed, including dip-coating,36,37 gas-jet drying,38 spray coating,39 electrohydrodynamic atomization (EHDA)-based process,40 and ink-jet printing,41–44 to overcome the drawback of drug wastage and loss, variable coating thickness, and inaccurate drug dosage. These coating approaches are illustrated well in Figure 2. Briefly, the dip-coating process is a simple process to coat the MNs. As shown in Figure 2A, the MNs are dipped into the formulation and then removed from the formulation to form a liquid film on the MNs. Finally, the liquid layer dries to form a solid film coating on the MNs. Gas-jet drying was proposed to overcome the slow drying process, which always induce the reducing and varying dose in the dip-coating process. The gas-jet can disperse a small amount of coating solution to wet the MNs, remove the excess coating solution, and dry the coating solution simultaneously, as shown in Figure 2B. Spray-coating process is similar to the conventional coating method to obtain the film coating on the MNs. As shown in Figure 2C, there are 3 steps to form the intact film coating. The spray coater generates the formulated microdroplets, such asatomization. After that, the droplets deposit and adhere onto the surface of the MNs and then the drops coalesce to form the film coating on the substrate. The principle of EHDA process is that the atomized droplets are produced by electrical force. The atomized liquids jet out of the capillary nozzle exit by jet and then are collected onto the electrically grounded substrate below the nozzle tip, as shown in Figure 2D. In the ink-jet printing process, as shown in Figure 2E, the microdroplets are ejected by a piezoelectric dispenser onto the surface of MNs to form the uniform, accurate, and reproducible coating films. The droplet size depends on the parameters, such as amplitude, pulse width, and excitation frequency. More illustrations of these approaches are discussed by Haj-Ahmad et al.9
Figure 2.
Illustration of coating approaches used to coat microneedles: (A) dip-coating, (B) gas-jet drying, (C) spray coating, and (D) electrohydrodynamic atomization–based process, and (E) ink-jet printing. (Images reprinted with permission from Haj-Ahmad et al.9)
Besides, numbers of proper surfactants, such as utrol F-68 NF36 and poloxamer 188,38 and thickening agents, such as alginic acid45 and CpG,46 were used to facilitate MN coating, and stabilizer, such as trehalose, was used to reduce the damage of bioactive drugs in the process of coating/drying.47 In addition, optimizing MN structures (eg, grooves,48,49 pockets,50 nanopatterning,51 pores52) was employed to increase coating amounts or facilitate the delivery of drugs into the skin.
Coated MNs can be used to deliver various drugs into the skin. For example, Baek et al18 fabricated the biocompatible coated MNs made of poly (l-lactide) (PLLA), which were coated with optimized coating formation of lidocaine on the tips by the dip-coating device. It was confirmed that the PLLA MN arrays released rapidly the lidocaine into the phosphate-buffered saline within 2 minutes and into skin more efficiently than EMLA cream and provided rapid local anesthesia in a painless way. Gill and Prausnitz50 designed the MNs with holes cut through the shafts to form “pockets,” which were dip coated with fluorescent model drugs and various surfactants and viscosity enhancers. It was concluded that the coated MNs released rapidly the model drugs after inserted into the skin, indicating the capabilities of MNs to control the drug delivery into skin. Additionally, Jung et al51 designed the MNs with nanopatterns on the surface and dip coated with plasmid DNA vaccinations. It was concluded that the improved hydrophilicity of nanopatterned MNs induced to enhance the loading capacity of MNs. The in vivo study revealed that higher immune responses were induced by the nanopattered MNs than by unmodified MNs. In short, the above results proved that the coated MNs have a promising potential to deliver drugs in transdermal drug delivery systems.
Dissolving MNs
Dissolving MNs deliver drugs into skin based on the mechanism of “poke-and-release” method. Quite different from “poke-and-patch” method, drugs are usually encapsulated within MNs, and MNs remain on the skin after being inserted into the skin and then the drug releasing is realized when MNs completely degrade or dissolve in the skin,1,8 as shown in Figure 1C. These MNs have various advantages, such as easily-made, convenient, and high drug loading.53 Furthermore, these MNs can eventually dissolve in the skin, thus leaving no biohazardous sharp waste and ensuring a safe disposal for remaining MNs.54
To date, minimally invasive dissolvable MNs are effective and convenient for transdermal drug delivery. Li et al55 fabricated the dissolving MNs made of polylactic acid (PLA) for the delivery of insulin using thermal micromolding technique. The relationships between MN dimensions, drug concentration, drug viscosity, administration time, and drug penetration into the skin were discussed. It was concluded that the shorter MNs had a better mechanical stability, while the longer MNs were more appropriate for drug permeation. The increasing of drug concentration induced the increasing of drug permeation amount, but not affected the drug permeation rate. However, with the increase in drug viscosity, the drug permeation amount decreased. Prolonging the administration time on the skin at 1 hour, the drug permeation amount achieved to a stable value and essentially unchanged after 1 hour. The in vivo study revealed that dissolving MN system promoted the insulin absorption to reduce the blood glucose levels to 29% of initial level at 5 hours. Bhatnagar et al20 fabricated the dissolvable MNs made of the mixture of polyvinyl alcohol (PVA) and polyvinylpyrrolidone (PVP) for the codelivery of doxorubicin and docetaxel. It was concluded that the pyramidal-shaped MNs fully dissolved within 1 hour after inserted into the excised skin, reduced the tumor volume and tumor weight in 4T1 tumor-bearing athymic nude mice, and indicated greater survival compared with intratumoral injection. Besides, Sullivan et al54 fabricated the PVP MNs to deliver influenza vaccine. In detail, the MNs have a length of 650 µm and a diameter of 20 µm in the tip. The MNs dissolved 89% ± 3% (by mass) after 5 minutes application in ex vivo study and 83% ± 6% (by mass) after 15 minutes application in in vivo study and induced a strong cellular and humoral immune response after a single immunization with a low antigen dose, which can confer protective immunity against fatal viral challenge.
Hollow MNs
Hollow MN deliver drugs via the “poke-and-flow” approach. Similar to the hypodermic injection, the liquid drug can continuously flow into the skin via the holes in the hollow MNs driven by the pressure, as shown in Figure 1D. Hence, the flow rate of drug can be accurately controlled by special equipment, such as micropump.34 Comparing with the solid MNs, hollow MNs are likely to promote force-driven fluid flow, thereby allowing faster drug delivery rates. Furthermore, the painless, long-term, and continuous drug delivery can be achieved by hollow MNs with precise and tunable dosage according to the need of the patients.
Hollow MNs have also been extensively used to deliver various drugs. For examples, Vinayakumar et al56 developed a hollow stainless MN to deliver insulin into a diabetic rat. In detail, the hollow MN array with a height of 300 µm and an outer diameter of 110 µm at tip and 150 µm at the base was fabricated using femtosecond laser micromachining. The insulin was delivered into the dermis of the rat skin through hollow MN by peristaltic pump. The insulin successfully diffused into the blood stream and the blood glucose level significantly decreased to the normal level after 5 hours, indicating that the hollow MNs can efficiently replace the subcutaneous insulin delivery. Pamornpathomkul et al57 used the hollow MNs combined with a nanocarrier delivery system to deliver plasmid DNA vaccine encoding ovalbumin. It was concluded that this complex system was good at enhancing the permeation of plasmid DNA vaccine encoding ovalbumin into skin, without inducing infection or pinpoint bleeding and inducing a strong immunoglobulin G immune response. Besides, hollow MNs are suitable for the blood extraction based on the transportation mechanism of poke and flow. Li et al58 fabricated a hollow MN by drawing lithography technique. In detail, the hollow MN with a bevel angle of 15° has a length, inner diameter, and tip diameter of 1800, 60, and 120 μm, respectively. The hollow MNs are penetrated into the skin without rupture and extracts 20 mL mouse blood in vivo, indicating that the hollow MNs have potential applications in blood analysis systems for diagnosis.
Classified by Structure of MNs
Microneedles can be divided into 2 types based on the structures, including in-plane and out-of-plane MNs. The out-of-plane MNs show that the length of MNs is perpendicular to the substrate,59–61 as shown in Figure 3A. It is easy to enhance the efficiency of drug delivery on large areas of skin by out-of-plane MNs through increasing the density of MN arrays. Out-of-plane MNs can be hollow or solid MNs. Furthermore, it is also easy to manufacture the out-of-plane MNs, and the out-of-plane MNs have widely used to deliver drugs, as shown in Table 1. However, it is a challenging task to fabricate the out-of-plane MNs with high aspect ratio structures.69 In contrast, the in-plane MNs show that the length of MNs is parallel to the substrate,69,70 as shown in Figure 3B. The in-plane MNs can be produced into production with a wide range of lengths and easily combined with the microfluid chip techniques, but the density of in-plane MNs cannot be high enough.1,2,8,113 Until now, only some literatures have been reported related to the in-plane MNs to deliver drugs, as shown in Table 1. For examples, Li et al70 fabricated the in-plane silicon MNs with conical structure, tapering smoothly from the base to the apex by micromachining method. The surface of the MNs was treated with oxygen plasma to make the surface hydrophilic. These in-plane MNs were robust enough to penetrate porcine skin, being intact after the repetitive penetration. Khandan81 developed the in-plane titanium (Ti)-based MNs for passive ocular drug delivery. In detail, the in-plane MNs with length from 500 to 1500 μm, width from 50 to 200 μm, thickness of 50 μm, and needle tip angle of 60° were developed using methods of photolithographic patterning and dry-etching. The results showed that the MNs were stiffness enough for reliable corneal insertion and provided potential for enhanced safety. Additionally, Kolli and Banga25 fabricated the solid maltose in-plane MNs for transdermal delivery. The in-plane MNs were manufactured to be 508.46 ± 9.32 µm in length with a radius of curvature of 3 µm at the tip, and to create microchannels with 55.42 ± 8.66 µm in diameter in the skin to improve the delivery of nicardipine hydrochloride.
Figure 3.
(A) Out-of-plane and (B) in-plane microneedles.
Table 1.
Overview of Microneedle Arrays Manufactured by Various Methods Based on Materials.
| Method/Material | Microneedles Type | Structure | References |
|---|---|---|---|
| Inorganic materials | |||
| Wet-etching/silicon | Solid MN | Out-of-plane | 62 |
| Wet-etching/silicon | Solid MN | Out-of-plane | 63 |
| Wet-etching/silicon | Solid MN | Out-of-plane | 64 |
| Wet-etching/silicon | Hollow MN | Out-of-plane | 61 |
| Dry-etching/silicon | Solid MN | In-plane | 65 |
| Dry-etching/silicon | Hollow MN | Out-of-plane | 66 |
| Dry-etching/silicon | Solid MN | Out-of-plane | 67 |
| Reactive ion etching/silicon | Solid MN | Out-of-plane | 68 |
| Deep reactive ion etching/silicon | Hollow MN | Out-of-plane | 69 |
| Deep reactive ion etching/silicon | Solid MN | In-plane | 70 |
| Deep reactive ion etching/silicon | Solid MN | Out-of-plane | 71 |
| Deep reactive ion etching/silicon | Hollow MN | Out-of-plane | 72 |
| Electrochemical micromachining/silicon | Hollow MN | Out-of-plane | 73 |
| Micromolding/ceramic | Solid MN | Out-of-plane | 74 |
| Micromolding/bioceramic | Solid MN | Out-of-plane | 75 |
| Micromolding/bioceramic | Solid MN | Out-of-plane | 76 |
| Micromolding/bioceramic | Solid MN | Out-of-plane | 77 |
| Two photon polymerization micromolding and pulsed laser deposition/ceramic | Solid MN | Out-of-plane | 78 |
| Metal MNs | |||
| Micromolding/Ti | Solid MN | Out-of-plane | 79 |
| Wire-electrode cutting and wet-etching/Ti | Solid MN | Out-of-plane | 80 |
| Dry-etching/Ti | Solid MN | In-plane | 81 |
| Sputter deposition/Ti | Hollow MN | Out-of-plane | 82 |
| Laser cutting/Ti | Solid MN | In-plane | 83 |
| Laser cutting/SS | Solid MN | Out-of-plane | 84 |
| Laser cutting/SS | Solid MN | Out-of-plane | 85 |
| Laser cutting/SS | Solid MN | In-plane | 86 |
| Laser cutting/SS | Solid MN | In-plane | 87 |
| Laser cutting/SS | Coated MN | In-plane | 47 |
| EDM and femtosecond laser machining/SS | Hollow MN | Out-of-plane | 56 |
| EDM/SS | Solid MN | Out-of-plane | 88 |
| Mechanical dicing and electrochemical corrosion/SS | Solid MN | Out-of-plane | 89 |
| Micromilling/SS | Solid MN | Out-of-plane | 90 |
| Electroplating/Ni | Solid MN | Out-of-plane | 91 |
| Electrodeposition/Ni | Hollow MN | Out-of-plane | 92 |
| Electrodeposition/Ni | Hollow MN | Out-of-plane | 93 |
| Polymeric MNs | |||
| Hot embossing/PLLA | Coated MN | Out-of-plane | 48 |
| Hot embossing/PMMA | Solid MN | Out-of-plane | 92 |
| Hot embossing/PLA | Coated MN/dissolving MN | Out-of-plane | 94 |
| Hot embossing/PLGA | Dissolving MN | Out-of-plane | 95 |
| Hot embossing/PCL | Dissolving MN | Out-of-plane | 96 |
| Injection/PMMA | Solid MN | Out-of-plane | 97 |
| Injection/PGA | Dissolving MN | Out-of-plane | 98 |
| Injection/nylon-6 | Solid MN | Out-of-plane | 98 |
| Injection/liquid crystal polymer | Coated MN | Out-of-plane | 99 |
| Injection/polycarbonate | Hollow MN | Out-of-plane | 100 |
| Micromolding/PLA | Dissolving MN | Out-of-plane | 55 |
| Micromolding/PGA, PLA, PLGA | Dissolving MN | Out-of-plane | 101 |
| Micromolding/PCL | Dissolving MN | Out-of-plane | 102 |
| Micromolding/PVA | Dissolving MN | Out-of-plane | 103 |
| Casting/mixture of PVA and PVP | Dissolving MN | Out-of-plane | 104 |
| Casting/mixture of PVA, dextran, and CMC | Dissolving MN | Out-of-plane | 105 |
| Casting/mixture of CMC and AP | Dissolving MN | Out-of-plane | 106 |
| Casting/PGA | Dissolving MN | Out-of-plane | 107 |
| Photolithography/PEGDA | Solid MN | Out-of-plane | 108 |
| Photolithography/PEGDA | Solid MN | Out-of-plane | 109 |
| Photolithography/PEGDA | Solid MN | Out-of-plane | 110 |
| Heat imprint lithography/PLA | Dissolving MN | Out-of-plane | 111 |
| Laser writing/SU-8 | Hollow MN | Out-of-plane | 5 |
| 3-Dimensional printing/PLA | Dissolving MN | Out-of-plane | 112 |
Abbreviations: AP, amylopectin; CMC, carboxymethyl cellulose; EDM, electric discharge machining; MNs, microneedles; PEGDA, polyethylene glycol diacrylate; PCL, polycaprolactone; PGA, polyglycolic acid; PLA, polylactic acid; PLGA, polylactic-co-glycolic acid; PLLA, poly (l-lactide); PMMA, polymethyl methacrylate; PVA, polyvinyl alcohol; PVP, polyvinylpyrrolidone; SS, stainless steel.
Classified by Materials of MNs
Traditionally, materials used for MNs are inorganic materials, metals, and polymer. We subsequently introduce each type of materials in detail.
Inorganic materials
The inorganic materials used to fabricate MNs are silicon,62,63,70,114 glass,115–117 and ceramic.74,75 Until now, the inorganic materials have been used as structural materials to fabricate solid MNs, coated MNs, and hollow MNs.39,61,63–65,70,75,76,118 Hereinto, silicon is the most common inorganic materials for manufacturing various MNs. The silicon is relative high hardness; therefore, the silicon MNs are easily to be penetrated into skin. However, silicon MNs are easy to break off during the process of insertion into the skin due to the fragile property. Therefore, these MNs may stay underneath the skin after used and induce inflammation because silicon is not well established as biocompatible materials like some polymers and metals (such as Ti, stainless steel [SS]), which limits their wide applications.1,8,66 Glass is also a kind of brittle material, and the glass MNs may meet the same problems after penetrated into skin. Ceramic has generated great interest from researchers over years and also widely been used to produce MNs. Alumina, zirconia, and calcium sulfate hemihydrate are commonly used to fabricate MNs. However, most sintered ceramics (eg, alumina) are brittle and nonresorbable, which may induce unwanted inflammation after breaking in the skin.75,119 Hence, biodegradable bioceramic MNs (BCMNs) have been developed and proved to have improved mechanical strength to be penetrated into the skin, without keeping so much biohazardous sharp waste in the skin. Moreover, the BCMNs can control the drug release well via controlling the factors, such as the porosity, surface, and degradation.75,76
Metal
Various metals, such as SS,51,61,84,88 Ti,81,83,120 and nickel (Ni),92 have been used as structural materials to fabricate solid MNs, coated MNs, and hollow MNs. Metals have strong mechanical strength and toughness for the transdermal drug delivery system.53 However, metals are nonbiodegradable, which may produce unwanted biohazardous tip waste induced by the broken MNs left behind in the skin, even though some metals, such as SS and Ti, have been safely used as biomaterials in medical treatment for decades.121,122
Polymer
Nowadays, polymeric MNs have attracted great attention of researchers because most polymeric MNs are biocompatible and biodegradable to avoid harsh side effects in the skin, providing a safe device for the delivery of drugs.30,104,112,123 Polymeric materials have been efficiently fabricated into solid MNs, coated MNs, dissolving MNs, and hollow MNs, including polymethyl methacrylate,21,97,124 PLA,55,94,112 polyglycolic acid,101,98 polylactic-co-glycolic acid),95,125 PVP,22,54,104,126 polycaprolactone (PCL),102,127 PVA,103,105 and carboxymethyl cellulose (CMC).106,128,129 However, most polymers are too soft to induce the buckling failure during the insertion process.8 Therefore, some complex structural materials such as PCL-polyethylene glycol (PCL/PEG),127 CMC-amylopectin,106 PVP-cyclodextrin,130 and PVA-PLA131 are used to prepare the MNs to increase the mechanical strength during insertion process. Besides, geometry properties of polymeric MNs have been investigated well to improve the mechanical strength. For example, Li et al55 systemically investigated the mechanical stability of PLA MNs, evaluated by the relationship between percentage of successful insertions and the number of MNs on the patch. In detail, the MN array with heights of 0, 600, 700, and 800 µm were prepared and used to treat porcine cadaver skins. The results showed that the MN patches with lower height resulted in better mechanical strength. Chen et al102 fabricated MNs with various types of aspect ratio and the results demonstrated that the MNs with a smaller aspect ratio also exhibited higher mechanical strength. Gittard et al132 also proved that a decrease in the aspect ratio of MN induced to an increase in mechanical strength. Recently, a type of MN array with separable arrowhead was fabricated. This MN array combines the mechanical strength of metal and dissolving property of polymer to eliminate biohazardous sharp waste and improve strength of MNs so as to be penetrated into skin for the enhancing capacity of delivering drugs.133
Other materials such as sugars (eg, maltose, trehalose, sucrose) are also attractive materials to fabricate the dissolving MNs. For example, Loizidou et al134 fabricated the MNs based on sugar materials of CMC/maltose, CMC/trehalose, and CMC/sucrose, respectively, by the vacuum deposition method. Nguyen and Banga135 also prepared the MNs based on maltose by micromolding technique. The maltose MNs can rapidly dissolve and increase the permeability of drugs through skin.
Fabrication of MNs
To accurately produce the microstructures of the MNs, many researchers have attempted to develop fabricating methods of MNs based on the materials. Table 1 presents an overview of various techniques to fabricate the MNs made of polymer, inorganic materials, and metal.
Fabrication of Inorganic Material MNs
Since the first solid MNs made of silicon by reactive ion etching (RIE)136 was reported, the techniques to fabricate the silicon MNs have been developed for years. Not only wet-etching and dry-etching but also electrochemical micromachining have already been used to fabricate silicon MNs, such as solid, hollow, in-plane, and out-of-plane MNs. These methods have also been used together to make silicon MNs. For example, Jurčíček et al61 prepared the hollow silicon MNs with a height of more than 100 µm and a hole diameter of 10 to 25 µm under the condition of 40% KOH solution and a water bath temperature of 87°C using the deep RIE and anisotropic wet-etching technique. These manufacturing techniques in Table 1 offer the potential for high production of MNs, but the processing of some methods for making silicon MNs requires clean room and the manufacturing methods for MNs are relative expensive and complicated.2,8,35
Micromolding is the most common method to make BCMNs or ceramic MNs. Generally, in this fabricating method, the MN master templates were first prepared by microfabrication methods, after that the ceramic slurry was filled into the cavities of molds, and then the ceramic MNs were formed when the ceramic materials were dried.119 Traditionally, poly(dimethyl siloxane) mold74,77 was usually used to fabricate ceramic MNs or BCMNs with nanopores. The porosity, grain size, and morphology of MNs can be controlled well through slurry process.137 Cai et al76 have prepared the BCMNs with flexible and self-swelling substrate (BCMN-Gs) by the micromolding technique. The process of micromolding technique used to produce this BCMNs is illustrated in Figure 4. Briefly, the homogenous paste, which is the mixture of α calcium sulfate hemihydrate and water, was filled in the cavities of the negative replica and then the needles formed after cured in the ambient conditions. Finally, the warmed gelatin solution was poured on the top of the mold and cross-linked in the desiccators overnight under ambient conditions. In addition, 2 photon polymerization micromolding was also developed to fabricate the ceramic MNs.7,8,138
Figure 4.
Micromolding process to fabricate BCMN-G. BCMN-G indicates bioceramic microneedle with gelatin substrate. (Images reprinted with permission from Cai et al.76)
Glass MNs are usually manufactured by pulling of glass rods using pipette puller.115–117,139 For example, Mahadevan et al116 used a pipette puller to fabricate the glass MNs based on borosilicate glass capillary tubes. Peter et al139 fabricated the MNs with fine gradually tapered shape (angles between 0.02° and 0.66°) by pulling of borosilicate glass rods using a micropipette puller. The MNs were cut into different finial length by platinum wire. After that, the tips were fire polished until smooth and then the MNs were bent into a 90° cantilever by the microforge.
Fabrication of Metal MNs
Ti and SS are the 2 types of metals to fabricate MNs due to their biocompatibility and good fracture toughness. Various fabricating methods, including dry-etching, wet-etching, laser cutting, electroplating (or electrodeposition), micromilling, electric discharge machining (EDM), and micromolding techniques, have been used either alone or in combination to make Ti or SS MNs, as shown in Table 1. Ni, as a common metal, has also been used to make hollow and solid MNs by electrodeposition or electroplating. Other methods, such as twisted-light laser ablation140 and electrochemical corrosion, have been developed to make metal MNs. Hereinto, electrodeposition and laser machining are often used to produce the hollow metal MNs.
Fabrication of Polymeric MNs
Polymer materials are now widely used to fabricate the MNs because polymers are inexpensive and easy to mass production due to their uncomplicated fabrication and low cost.1,3,141–143 The most common manufacturing technique for polymeric MNs is micromolding method, including hot embossing, injection, and casting.144–148 After the master mold for MNs are fabricated, MNs can be produced efficiently and stably via micromolding techniques until the mold breaks. These maser molds with desired microstructures were usually produced via techniques such as LIGA (the abbreviation of lithographie, galvanoformung, and abformung), EDM, micromilling and microgrinding techniques, and laser percussion drilling, which have been described in detail in the reference.149 Other methods, such as photolithography, heat imprint lithography, and laser writing, were also used to fabricate polymeric MNs. Dardano et al108 have used the photolithographic approach to fabricate MNs made of polyethylene glycol diacrylate (PEGDA) with different shape, length, and tip. In the group of Kang,109,110 photolithographic approach was widely used to prepare the polymeric MNs. Recently, the 3-dimensional printing has been developed for polymeric MNs. All these methods have been achieved based on the advantage of versatility of polymer, including viscosity, dissolution properties, and postmodification.13,150–156
Application of MNs
Microneedles are convenient, safe, and painless enough to achieve the comfort of patient and now widely used in transdermal, ocular, and intracellular delivery.10,12,34,131 Hereinto, transdermal drug delivery is the main area for the application of MNs.
Microneedle Delivery of Vaccine
Traditionally, most vaccines are administered intramuscularly or subcutaneously, and the route of administration is relatively painful. Today, MNs array system has been widely studied for delivery of vaccine and is comparable to the conventional routes of administration.
DNA vaccines are considered to be the effective candidates of conventional vaccines because they can generate strong cellular and humoral immune responses, are inexpensively, and can be manufactured easily.51,157,158 However, DNA vaccines often exhibit an immune response weaker than expected when administered intramuscularly to patients because of the low efficient delivery of plasmid DNA into host cell, which induces the low expression of encoded antigen.159 Thus, a suitable DNA vaccines delivery system is considered a key method to improve the immunization results. Microneedles can deliver DNA vaccine into the skin to improve the immune responses induced by the enhancing expression of encoded antigen and become an effective delivering method for DNA vaccines.
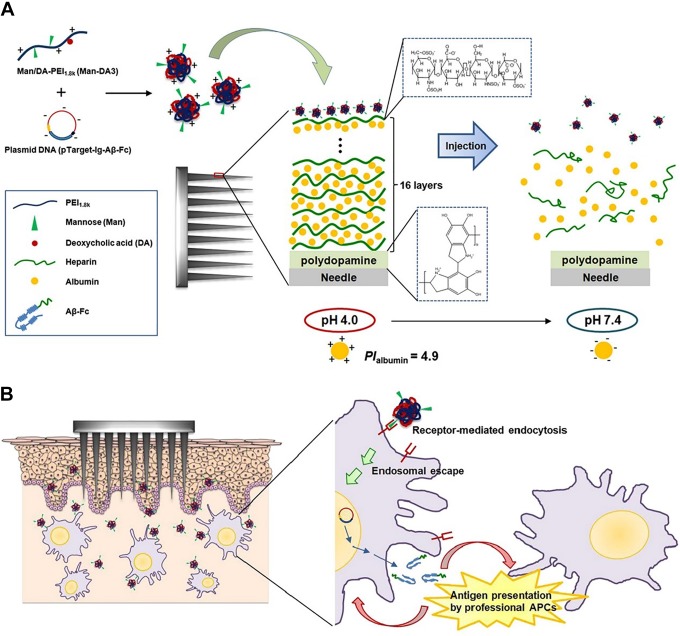
Kim et al158 used the MNs coated with pH-responsive polyelectrolyte multilayer assembly to deliver functional polyplexes containing DNA vaccines. It was concluded that in in vivo experiment on mice, compared with the traditional subcutaneous injection method, MNs were able to release polyplexes rapidly into skin and induced strong humoral immune response. The mechanism of polyplexes containing DNA vaccine releasing from MNs is shown in Figure 5A. In brief, after the 16 bilayers of heparin and albumin were coated alternatively on the MNs, cationic polyplexes containing plasmid DNA were deposited on the outermost of heparin film layers via electrostatic interaction. Finally, multilayer films rapidly disintegrated to release polyplexes from MN arrays after inserted into skin at pH 7.4. In addition, Man-DA3 is utilized to form the polyplexes for target gene delivery to the resident antigen-presenting cells (APCs) into dermis, and dermal APCs can express high levels of mannose receptors and mannose receptor-related receptors. The mechanism of delivery of polyplexes with surface mannose moieties into intradermal resident APCs after release from the MNs was illustrated in Figure 5B.
Figure 5.
(A) Description of functional polyplexes released from MNs coated with pH-responsive PMA after applied on the skin. (B) Illustration of delivery of polyplexes with surface mannose moieties to intradermal resident APCs after releasing from MNs. APC indicates antigen-presenting cells; MNs microneedles; PMA, polyelectrolyte multilayer assembly. (Images reprinted with permission from Kim et al.158)
Zhang et al159 conducted the experiment of delivery of DNA vaccine by MNs with pyramid shape on the shaved mouse ear pinna or dorsal skin. The results showed that the MNs can be superior to conventional syringe injection in respect of both protein expression resulting from intradermal delivery of DNA vaccine and immunogenicity. Microneedles also prolonged the protein expression compared to the syringe injection. For example, MN delivery gave a 18.6-fold higher level of green fluorescent protein expression in the ear and more than 4-fold higher in dorsal skin than syringe injection.
Every year, there is about 3 to 5 million serious diseases and 250 000 to 500 000 deaths induced by influenza.160,161 Vaccination is the main method to control seasonal influenza via improving protection against the illness and reducing the risk of death and hospitalization caused by influenza-related complications.162 The MNs array system has been investigated for influenza immunization due to the efficient and precise delivery of vaccine. Kim et al47 used the fabricated SS-coated MNs to deliver influenza vaccine into skin. After coating the SS MNs with the optimized vaccine formulation, vaccine delivered into the skin via MNs induced strong systemic and functional antibodies, and provided complete protection against fatal challenge infection, similar to conventional intramuscular injection. Later on, Kim et al45 investigated the SS-coated MNs to deliver influenza virus-like particle vaccine into skin. It was also concluded that the vaccine generated strong antibody response and provided full protection against high-dose fatal challenge infection. Numbers of human trials for assessing the safety, tolerability, and immune response generation with various MN devices, such as MicronJet (Nanopass Technologies Ltd., Ness Ziona, Israel)160 and BD Soluvia (New Jersey, USA),163 have been conducted. Bhatnagar et al2 have already reviewed in detail the successful application of these MN devices on the delivery of influenza vaccine and the results reveal that the influenza vaccine is well against influenza. In summary, MN array system provides an important advance in the delivery of vaccine to enhance the strong cellular immunity, indicating that MNs will have a great influence on drugs used for vaccination in the future.
Microneedle Delivery of Insulin
Diabetes mellitus is a complex metabolic diseases caused by abnormal insulin level in the whole world. Its main manifestations are increased glucose production in the liver and decreased clearance of glucose into muscle and fat, resulting in obvious hyperglycemia in the blood.10,164–165 There is approximately 425 million adults suffering from diabetes, and the number of globally diabetic patients is estimated to be 439 million by 2030.10,53,166 Insulin administration is required to control blood glucose levels for patients suffering from various types of diabetes. Traditionally, the delivery of insulin is conducted by methods ranging from smaller gauge needles to insulin pen to insulin jet injector and to insulin pump.167 However, the exogenous insulin delivered by these methods does not closely match the physiological release of insulin, which often causes inadequate glycemic control and subsequent negative consequences. For example, too low dose of insulin will induce the blindness and kidney failure for patients, while too high dose of insulin will induce hypoglycemia, which will cause seizures, loss of consciousness, and even death.53 Microneedle delivery system is the expected technique to deliver the insulin, closely matching the need of patient. Numbers of published literatures have grown steadily these years, showing that the MN delivery system is attractive carrier for insulin delivery.
Ling and Chen168 fabricated a dissolving MN patch composed of starch and gelatin for the transdermal delivery of insulin to diabetic rats. The dissolving MNs were administrated by a homemade applicator to the diabetic rat, as shown in Figure 6. It was concluded that the dissolving MNs are strength enough to penetrate into skin painlessly and completely dissolve after being inserted into the skin for 5 minutes. Insulin was rapidly released into skin, resulting in similar hypoglycemic effect in rats like a subcutaneous injection.
Figure 6.
The schematic illustration of the microneedles used for delivery of insulin. (Images reprinted with permission from Ling and Chen.168)
Resnik et al118 used the hollow silicon MNs with length of 220 μm, out diameter of 130 μm, and inner diameter of 50 µm to deliver insulin. The in vivo results implied that the hollow MNs improved transdermal delivery of fast-acting insulin without causing any skin irritation or inflammation at the delivery sites. At almost the same injection dose, compared with the subcutaneous injection, hollow MNs showed no significant decrease in glucose level, but significant increase in serum insulin. Zhang et al169 used the calcium ion cross-linked alginate/maltose composite MNs with pyramidal shape to deliver insulin into rats. These prepared MNs exhibited strong mechanical properties with the maximum failure force of about 0.41 N/needle to be penetrated into the skin. It was concluded that the MNs successfully triggered the releasing of insulin and had obvious and effective hypoglycemic effect compared with subcutaneous injection. Additionally, Yu et al77 used the fabricated BCMNs for insulin transdermal delivery. The made MNs were strength enough to be penetrated into skin. Insulin released from the MNs also exhibited an effective and obvious hypoglycemic effect. There are still many other literatures related to insulin delivered by MNs, which have not been illustrated in this review. Jin et al170 have comprehensively introduced the MNs for insulin delivery in clinical trials. It was indicated that the MNs for insulin delivery are expected to pave the way for noninvasive regulation of glucose level for diabetic patients.
Microneedle Delivery of Other Drugs
Lidocaine is a class drug for local anesthesia and is usually delivered for pre- and postoperative anesthesia either alone or in combination with other drugs.99,171 Transcutaneous injection are often used to deliver lidocaine. However, the traditional method can cause local and systemic effect to the patients, including unpleasant feeling (eg, fear, pain, anxiety), erythema, and edema occurred in topical application, increasing risk of unexpected diffusion and inadequate placement of lidocaine.172,173 Hence, MN delivery system has been investigated to be an alternative traditional delivery method of lidocaine.
Kathuria et al171 used the MNs made of PEGDA by photolithography to deliver lidocaine into skin. The in vitro and in vivo experiment results showed that MN can deliver lidocaine into the skin perpendicularly and release the active ingredient to alleviate acute and chronic pain. Zhang et al99 fabricated the coated MNs via injection. The lidocaine was coated to the MNs by dip-coating process. The MN was used to deliver lidocaine into the skin of swine. The in vivo results revealed that the lidocaine dissolved rapidly off the MNs into skin within seconds and induced local analgesia about 1 minute, facilitating routine or emergency procedures. Additionally, Baek et al18 also prepared the coated MNs made of PLLA using micromolding technique for the delivery of lidocaine into porcine ears. Dip-coating device was used to coat lidocaine onto the MNs. The results revealed that the lidocaine on the MNs was released into phosphate-buffered saline within 2 minutes and its storages stability could last for 3 weeks at different temperatures. These MNs also showed more efficient delivery of lidocaine than the commercial EMLA cream.
Acetyl salicylic acid (ASA, aspirin) is commonly used for pain relief and anti-inflammatory and cardiovascular treatment. Due to the gastrointestinal side effects and low bioavailability of oral administration, MNs delivery system is becoming an alternative method for the delivery of ASA.174,175 Olatunji et al175 conducted the experiment on the delivery of ASA on the porcine skin. The result revealed that the release of ASA from fish scale biopolymer transdermal patches via the “poke-and-patch” method was greatly enhanced after pretreating the porcine skin with the solid metal MNs.
In addition, MNs are widely used to deliver other large-molecular-weight drugs, such as protein,28,176 DNA,177,178 and peptides,31,179 and small-molecular-weight drugs, such as dyclonine developed for topical anesthesia,180 zolmitriptan developed for acute treatment of migraine,181 naltrexone used to treat opiate and alcohol dependence,182 and so on. Besides, MNs can be also one of the methods used to detect the tumors183,184 and to continuously real time monitor alcohol,185 glucose,186 and so on for patients, since they can be penetrated into skin with negligible damage or pain.
Conclusions and Outlook
After the first MN reported for the drug delivery in 1971, MNs have been developed over 4 decades. Compared to the traditional drug delivery system, MNs have been demonstrated to be safe and successful enough to deliver various drugs. So far, MNs have been made of a variety of materials, including polymers, metals, silicon, ceramics, glass, and sugar. Researchers have already made great achievements in the fabrication techniques of MNs based on these materials. Solid, coated, dissolved, and hollow MNs have been developed to deliver drugs with wide range of molecules. Furthermore, the in-plane and out-of-plane MNs are also fabricated for the special requirement in the delivery of drugs.
Despite great successes of the MNs in the transdermal drug and vaccine delivery, there still exist some challenges for the long-term use of MNs. One of the challenges of MNs is that although doses and delivering rate of drug can be controlled well by MNs through some devices, and some MNs can be used to monitor situation of patients, most current MNs are unable to change the delivery parameters in time upon the changing condition of patients. Hence, it is urgent to develop the super-MNs in the future, which are consisted of MNs, biosensor, bioelectronics, automation, and so on, and are able to monitor patient conditions, rapidly change the delivery parameters (such as pH, temperature, and dose) in response to the information of patient, realize the diagnostic and treatment purpose simultaneously, and increase the compliance of patient with the minimal side effects.
In addition, there are already some MN devices on the market, which have brought good clinical outcomes for the delivery of insulin, influenza vaccine, and lidocaine. However, most MNs are still in experimental phases. To move more MNs into market further, narrowing the gap between laboratory research and clinical applications is a must in the future. To achieve this goal, manufacturing process should be optimized and validated to the current manufacturing standards, and more comprehensive in vivo study and clinical trials are important to achieve the requirements of regulatory system. Moreover, MNs should be designed well to balance the pain, mechanical strength, quantity of drugs, and stable drug formulation; be fabricated with a relatively low cost to obtain sufficient and reproducible penetration; and be easily handled by all patients.
In spite of these problems, due to the unique properties of MNs, more efficient and advanced MN system will be developed for the market in the near future. Definitely, MNs system for transdermal drug and vaccine delivery will have a great impact on the future medicine.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (Nos. 51673020 and 51173015), the Fundamental Research Funds for Central Universities (No. JD1910), and the Talents Introduction Project in Beijing University of Chemical Technology (No. buctrc201909).
ORCID iD: Jingyao Sun  https://orcid.org/0000-0002-0140-0212
https://orcid.org/0000-0002-0140-0212
References
- 1. Van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161(2):645–655. [DOI] [PubMed] [Google Scholar]
- 2. Bhatnagar S, Dave K, Venuganti VVK. Microneedles in the clinic. J Control Release. 2017;260:164–182. [DOI] [PubMed] [Google Scholar]
- 3. Kim BH, Seo YH. Transdermal drug delivery devices based on microneedles: a review. J Biomed Nanotechnol. 2015;1(1):5–14. [Google Scholar]
- 4. Lee JW, Prausnitz MR. Drug delivery using microneedle patches: not just for skin. Expert Opin Drug Deliv. 2018;15(6):541–543. [DOI] [PubMed] [Google Scholar]
- 5. Mishra R, Maiti TK, Bhattacharyya TK. Design and scalable fabrication of hollow SU-8 microneedles for transdermal drug delivery. IEEE Sens J. 2018;18(14):5635–5644. [Google Scholar]
- 6. Ventrelli L, Marsilio Strambini L, Barillaro G. Microneedles for transdermal biosensing: current picture and future direction. Adv Healthc Mater. 2015;4(17):2606–2640. [DOI] [PubMed] [Google Scholar]
- 7. Ito Y, Hirono M, Fukushima K, Sugioka N, Takada K. Two-layered dissolving microneedles formulated with intermediate-acting insulin. Int J Pharm. 2012;436(1-2):387–393. [DOI] [PubMed] [Google Scholar]
- 8. Ma G, Wu C. Microneedle, bio-microneedle and bio-inspired microneedle: a review. J Control Release. 2017;251:11–23. [DOI] [PubMed] [Google Scholar]
- 9. Haj-Ahmad R, Khan H, Arshad MS, et al. Microneedle coating techniques for transdermal drug delivery. Pharmaceutics. 2015;7(4):486–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Yu J, Kahkoska AR, Wang J, Buse JB, Gu Z. Advances in transdermal insulin delivery. Adv Drug Deliv Rev. 2019;139:51–70. doi:10.1016/j.addr.2018.1012.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bekir B, Emrullah K, Rakesh K, et al. Dissolvable microneedle arrays for intradermal delivery of biologics: fabrication and application. Pharm Res. 2013;31(1):117–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leite-Silva VR, Mariana Mandelli DA, Aurélie F, Jeffrey EG, Michael SR. Delivery of drugs applied topically to the skin. Exp Rev Dermatol. 2014;7(4):383–397. [Google Scholar]
- 13. Fonseca DFS, Vilela C, Silvestre AJD, Freire CSR. A compendium of current developments on polysaccharide and protein-based microneedles. Int J Biol Macromol. 2019;136:704–728. [DOI] [PubMed] [Google Scholar]
- 14. Donnelly RF, Singh TRR, Woolfson AD, Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv. 2010;17(4):187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gerstel MS, Place VA. Drug delivery device. 1976, Google Patents. U.S. Patent No. 3,964,482.
- 16. Martin CJ, Allender CJ, Brain KR, Morrissey A, Birchall JC. Low temperature fabrication of biodegradable sugar glass microneedles for transdermal drug delivery applications. J Control Release. 2012;158(1):93–101. [DOI] [PubMed] [Google Scholar]
- 17. Xie Y, Xu B, Gao Y. Controlled transdermal delivery of model drug compounds by MEMS microneedle array. Nanomedicine. 2005;1(2):184–190. 2005;1(2): 184–190. [DOI] [PubMed] [Google Scholar]
- 18. Baek SH, Shin JH, Kim YC. Drug-coated microneedles for rapid and painless local anesthesia. Biomed Microdevices. 2017;19(1):2. [DOI] [PubMed] [Google Scholar]
- 19. González-Vázquez P, Larrañeta E, McCrudden MTC, et al. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J Control Release. 2017;265:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhatnagar S, Bankar NG, Kulkarni MV, Venuganti VVK. Dissolvable microneedle patch containing doxorubicin and docetaxel is effective in 4T1 xenografted breast cancer mouse model. Int J Pharm. 2019;556:263–275. [DOI] [PubMed] [Google Scholar]
- 21. Moon SJ, Lee SS. A novel fabrication method of a microneedle array using inclined deep X-ray exposure. J Micromech Microeng. 2005;15(5):903–911. [Google Scholar]
- 22. Deng YL, Juang YJ. Polydimethyl siloxane wet etching for three dimensional fabrication of microneedle array and high-aspect-ratio micropillars. Biomicrofluidics. 2014;8(2):026502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun J, Li H, Huang Y, et al. Simple and affordable way to achieve polymeric superhydrophobic surfaces with biomimetic hierarchical roughness. ACS Omega. 2019;4(2):2750–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun J, Xiaobing W, Jinghua Wu, et al. Biomimetic moth-eye nanofabrication: enhanced antireflection with superior self-cleaning characteristic. Sci Rep. 2018;8(1):5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kolli CS, Banga AK. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharmaceut Res. 2007;25(1):104–113. [DOI] [PubMed] [Google Scholar]
- 26. Miyano T, Tobinaga Y, Kanno T, et al. Sugar micro needles as transdermic drug delivery system. Biomed Microdevic. 2005;7(3):185–188. [DOI] [PubMed] [Google Scholar]
- 27. Pistor MLP. Device for cutaneous therapeutic treatment. 1975;Google Patents. U.S. Patent No. 3,918,449.
- 28. Witting M, Obst K, Pietzsch M, Friess W, Hedtrich S. Feasibility study for intraepidermal delivery of proteins using a solid microneedle array. Int J Pharm. 2015;486(1-2):52–58. [DOI] [PubMed] [Google Scholar]
- 29. Giri Nandagopal MS, Rahul A, Rangabhashiyam S, Nidhin S, Selvaraju N. Overview of microneedle system: a third generation transdermal drug delivery approach. Microsyst Technol. 2014;20(7):1249–1272. [Google Scholar]
- 30. Sun J, Shen J, Chen S, et al. Nanofiller reinforced biodegradable PLA/PHA composites: current status and future trends. Polymers. 2018;10(5):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang S, Qiu Y, Gao Y. Enhanced delivery of hydrophilic peptides in vitro by transdermal microneedle pretreatment. Acta Pharm Sin B. 2014;4(1):100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nayak A, Das DB, Vladisavljević GT. Microneedle-assisted permeation of lidocaine carboxymethylcellulose with gelatine co-polymer hydrogel. Pharmaceut Res. 2013;31(5):1170–1184. [DOI] [PubMed] [Google Scholar]
- 33. Bhatnagar S, Kumari P, Pattarabhiran SP, Venuganti VVK. Zein microneedles for localized delivery of chemotherapeutic agents to treat breast cancer: drug loading, release behavior, and skin permeation studies. AAPS Pharm Sci Technol. 2018;19(4):1818–1826. [DOI] [PubMed] [Google Scholar]
- 34. Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tuan-Mahmood TM, McCrudden MT, Torrisi BM, et al. Microneedles for intradermal and transdermal drug delivery. Eur J Pharm Sci. 2013;50(5):623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117(2):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caudill CL, Perry JL, Tian S, Luft JC, DeSimone JM. Spatially controlled coating of continuous liquid interface production microneedles for transdermal protein delivery. J Control Release. 2018;284:122–132. [DOI] [PubMed] [Google Scholar]
- 38. Chen X, Prow TW, Crichton ML, et al. Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J Control Release. 2009;139(3):212–220. [DOI] [PubMed] [Google Scholar]
- 39. McGrath MG, Vrdoljak A, O’Mahony C, Oliveira JC, Moore AC, Crean AM. Determination of parameters for successful spray coating of silicon microneedle arrays. Int J Pharm. 2011;415(1-2):140–149. [DOI] [PubMed] [Google Scholar]
- 40. Mehta P, Haj-Ahmad R, Rasekh M, et al. Pharmaceutical and biomaterial engineering via electrohydrodynamic atomization technologies. Drug Discov Today. 2017;22(1):157–165. [DOI] [PubMed] [Google Scholar]
- 41. Uddin MJ, Scoutaris N, Klepetsanis P, Chowdhry B, Prausnitz MR, Douroumis D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int J Pharm. 2015;494(2):593–602. [DOI] [PubMed] [Google Scholar]
- 42. Ross S. Inkjet printing of insulin microneedles for transdermal delivery. Drug Deliv Translation Res. 2015;5(4):451–461. [DOI] [PubMed] [Google Scholar]
- 43. Economidou SN, Lamprou DA, Douroumis D. . 3D printing applications for transdermal drug delivery. Int J Pharm. 2018;544(2):415–424. [DOI] [PubMed] [Google Scholar]
- 44. O’mahony C, Leonie H, Tobias K, et al. Accuracy and feasibility of piezoelectric inkjet coating technology for applications in microneedle-based transdermal delivery. Microelectronic Eng. 2017;172:19–25. doi:org/10.1016/j.mee.2017.02.018. [Google Scholar]
- 45. Kim YC, Fu-Shi Q, Richard W, et al. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS Pharm Sci Technol. 2010;11(3):1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeng Q, Gammon JM, Tostanoski LH, Chiu YC, Jewell CM. In vivo expansion of melanoma-specific T cells using microneedle arrays coated with immune-polyelectrolyte multilayers. ACS Biomater Sci Eng. 2017;3(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142(2):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Han M, Dae KK, Seong HK, et al. Improvement in antigen-delivery using fabrication of a grooves-embedded microneedle array. Sens Actuat Chem. 2009;137(1):274–280. [Google Scholar]
- 49. Shen QI, Ying C, Xiang C, Xiaolin Z. The fabrication and property of a novel coated out-of-plane microneedle arrays. Microsys Technol. 2015;22(1):143–149. [Google Scholar]
- 50. Gill HS, Prausnitz MR. Pocketed microneedles for drug delivery to the skin. J Phys Chem Solids. 2008;69(5-6):1537–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jung D, Rejinold NS, Kwak JE, Park SH, Kim YC. Nano-patterning of a stainless steel microneedle surface to improve the dip-coating efficiency of a DNA vaccine and its immune response. Colloids Surf B Biointerf. 2017;159:54–61. [DOI] [PubMed] [Google Scholar]
- 52. Ullah A, Kim C M, Kim GM. Porous polymer coatings on metal microneedles for enhanced drug delivery. R Soc Open Sci. 2018;5(4):171609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hao Y, Li W, Zhou X, Yang F, Qian Z. Microneedles-based transdermal drug delivery systems: a review. J Biomed Nanotechnol. 2017;13(12):1581–1597. [DOI] [PubMed] [Google Scholar]
- 54. Sullivan SP, Koutsonanos DG, Del Pilar Martin M, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li QY, Jia Nan Z, Bo Zhi C, Qi Lei W, Xin Dong G. A solid polymer microneedle patch pretreatment enhances the permeation of drug molecules into the skin. RSC Adv. 2017;7(25):15408–15415. [Google Scholar]
- 56. Vinayakumar KB, Prachit GK, Nayak MM, et al. A hollow stainless steel microneedle array to deliver insulin to a diabetic rat. J Micromech Microeng. 2016;26(6):065013. [Google Scholar]
- 57. Pamornpathomkul B, Wongkajornsilp A, Laiwattanapaisal W, Rojanarata T, Opanasopit P, Ngawhirunpat T. A combined approach of hollow microneedles and nanocarriers for skin immunization with plasmid DNA encoding ovalbumin. Int J Nanomed. 2017;12:885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li CG, Lee CY, Lee K, Jung H. An optimized hollow microneedle for minimally invasive blood extraction. Biomed Microdevices. 2012;15(1):17–25. [DOI] [PubMed] [Google Scholar]
- 59. Rodgers AM, Courtenay AJ, Donnelly RF. Dissolving microneedles for intradermal vaccination: manufacture, formulation, and stakeholder considerations. Expert Opin Drug Deliv. 2018;15(11):1039–1043. [DOI] [PubMed] [Google Scholar]
- 60. Zhu J, Shen QI, Ying C, et al. Characterization of out-of-plane cone metal microneedles and the function of transdermal delivery. Microsyst Technol. 2012;19(4):617–621. [Google Scholar]
- 61. Jurčíček P, Helin Z, Shuiping Z, Chong L. Design and fabrication of hollow out-of-plane silicon microneedles. Micro Nano Lett. 2013;8(2):78–81. [Google Scholar]
- 62. Mulcahy A, Ye SR, Morrissey A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron J. 2005;36(7):650–656. [Google Scholar]
- 63. Pradeep Narayanan S, Raghavan S. Solid silicon microneedles for drug delivery applications. Int J Adv Manuf Technol. 2016;93(1-4):407–422. [Google Scholar]
- 64. Yan XX, Jing-Quan L, Shui-Dong J, et al. Fabrication and testing analysis of tapered silicon microneedles for drug delivery applications. Microelectronic Eng. 2013;111:33–38. [Google Scholar]
- 65. Izumi H, Aoyagi S. Novel fabrication method for long silicon microneedles with three-dimensional sharp tips and complicated shank shapes by isotropic dry etching. IEEJ Trans Elect Electronic Eng. 2007;2(3):328–334. [Google Scholar]
- 66. Vinayakumar KB, Hegde GM, Nayak MM, et al. Fabrication and characterization of gold coated hollow silicon microneedle array for drug delivery. Microelectron Eng. 2014;128:12–18. [Google Scholar]
- 67. Held J, Gaspar J, Ruther P, et al. Design of experiment characterization of microneedle fabrication processes based on dry silicon etching. J Micromech Microeng. 201020(2):025024. [Google Scholar]
- 68. Chen B, Wei J, Tay FEH, et al. Silicon microneedle array with biodegradable tips for transdermal drug delivery. Microsyst Technol. 2008;14(7):1015–1019. [Google Scholar]
- 69. Ashraf MW, Tayyaba S, Nisar A, et al. Design, fabrication and analysis of silicon hollow microneedles for transdermal drug delivery system for treatment of hemodynamic dysfunctions. Cardiovasc Eng. 2010;10(3):91–108. [DOI] [PubMed] [Google Scholar]
- 70. Li Y, Hang Z, Ruifeng Y, et al. In-plane silicon microneedles with open capillary microfluidic networks by deep reactive ion etching and sacrificial layer based sharpening. Sens Actuat A Phy. 2019;292:149–157. doi:org/10.1016/j.sna.2019.04.008. [Google Scholar]
- 71. Rouhi N, Jung-Kubiak C, White V, Wilson D, Anderson J, Marrese-Readin C. Fabrication of 3-D silicon microneedles using a single-step DRIE process. J Microelectromech Sys. 201524(5):1409–1414. [Google Scholar]
- 72. Khanna P, Luongo K, Strom JA, Bhansali S. Sharpening of hollow silicon microneedles to reduce skin penetration force. J Micromech Microeng. 201020(4):045011. [Google Scholar]
- 73. Longo A, Strambini LM, Ventrelli L, Barillaro G. Silicon microneedles for transdermal applications by electrochemical micromachining technology. IEEE Sens. 2014;691–693. doi:10.1109/ICSENS.2014.6985093. [Google Scholar]
- 74. Bystrova S, Luttge R. Micromolding for ceramic microneedle arrays. Microelectronic Eng. 2011;88(8):1681–1684. [Google Scholar]
- 75. Vallhov H, Wei X, Håkan E, et al. Bioceramic microneedle arrays are able to deliver OVA to dendritic cells in human skin. J Mater Chemist B. 2018;6(42):6808–6816. [DOI] [PubMed] [Google Scholar]
- 76. Cai B, Xia W, Bredenberg S, Li H, Engqvist H. Bioceramic microneedles with flexible and self-swelling substrate. Eur J Pharm Biopharm. 2015;94:404–410. [DOI] [PubMed] [Google Scholar]
- 77. Yu W, Jiang G, Liu D, et al. Transdermal delivery of insulin with bioceramic composite microneedles fabricated by gelatin and hydroxyapatite. Mater Sci Eng C. 2017;73:425–428. [DOI] [PubMed] [Google Scholar]
- 78. Gittard SD, Narayan RJ, Jin C, et al. Pulsed laser deposition of antimicrobial silver coating on Ormocer® microneedles. Biofabrication. 2009;1(4):041001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li J, Liu B, Zhou Y, et al. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS One. 2017;12(2):e0172043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yan XX, Liu J, Jiang SD, et al. Fabrication and testing of porous Ti microneedles for drug delivery. Micro Nano Lett. 2013;8(12):906–908. [Google Scholar]
- 81. Khandan O. Titanium-Based, Fenestrated, In-Plane Microneedles for Passive Ocular Drug Delivery. Paper presented at: 34th Annual International Conference of the IEEE EMBS San Diego, California, USA, 28 August–1 September 2012: 6572–6575. [DOI] [PubMed] [Google Scholar]
- 82. Tsuchiya K, Nakanishi N, Uetsuji Y, Nakamachi E. Development of blood extraction system for health monitoring system. Biomed Microdevices. 2005;7(4):347–353. [DOI] [PubMed] [Google Scholar]
- 83. Li W, Zhang YM, Chen J. Design, fabrication and characterization of in-plane titanium microneedles for transdermal drug delivery. Key Eng Mater. 2011;483:532–536. [Google Scholar]
- 84. Rajabi M, Roxhed N, Shafagh RZ, et al. Flexible and stretchable microneedle patches with integrated rigid stainless steel microneedles for transdermal biointerfacing. PLoS One. 2016;11(12):e0166330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011;154(2):148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kim SJ, Shin JH, Noh JY, Song CS, Kim YC. Development of the novel coating formulations for skin vaccination using stainless steel microneedle. Drug Deliv Transl Res. 2016;6(5):486–497. [DOI] [PubMed] [Google Scholar]
- 87. Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS One. 2009;4(3):e4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vinayakumar KB. Out-of-plane cup shaped stainless steel microneedle array for drug delivery. Proceedings of the 11th IEEE Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Japan, Matsushima Bay and Sendai MEMS City, 17–20 April 2016: 172–175. [Google Scholar]
- 89. Yan XX, Liu JQ, Iang SD, Yang B, Yang CS. Tapered metal microneedles fabricated by the hybrid process of mechanical dicing and electrochemical corrosion for drug delivery. Micro Nano Lett. 2012;7(12):1313–1315. [Google Scholar]
- 90. López EG., Siller HR, Rodríguez CA, Study of the fabrication of AISI 316L microneedle arrays. Proc Manuf. 2018;26:117–124. [Google Scholar]
- 91. Bai WQ, Li YG, Yang CS, et al. Fabrication of metal micro needle array by LIGA process. Adv Mater Res. 2011;418:1911–1914. [Google Scholar]
- 92. Zhu MW, Li HW, Chen XL, et al. Silica needle template fabrication of metal hollow microneedle arrays. J Micromech Microeng. 2009;19(11):115010. [Google Scholar]
- 93. Mansoor I, Liu Y, Häfeli UO, Stoeber B. Arrays of hollow out-of-plane microneedles made by metal electrodeposition onto solvent cast conductive polymer structures. J Micromech Microeng. 2013;23(8):85011–85020. [Google Scholar]
- 94. Chen Y, Chen BZ, Wang QL, Jin X, Guo XD. Fabrication of coated polymer microneedles for transdermal drug delivery. J Control Release. 2017;265:14–21. [DOI] [PubMed] [Google Scholar]
- 95. Li J, Zhou Y, Yang J, et al. Fabrication of gradient porous microneedle array by modified hot embossing for transdermal drug delivery. Mater Sci Eng C Mater Biol Appl. 2019;96:576–582. [DOI] [PubMed] [Google Scholar]
- 96. Andersen TE, Andersen JA, Peterse RS, et al. Drug loaded biodegradable polymer microneedles fabricated by hot embossing. Microelectronic Eng. 2018;195:57–61. [Google Scholar]
- 97. Janphuang P, Mongkhol L, Chanwut S, et al. Polymer based microneedle patch fabricated using microinjection moulding. MATEC Web Conf. 2018;192:01039. [Google Scholar]
- 98. Ono A, Azukizawa H, Ito S, et al. Development of novel double-decker microneedle patches for transcutaneous vaccine delivery. Int J Pharm. 2017;532(1):374–383. [DOI] [PubMed] [Google Scholar]
- 99. Zhang Y, Brown K, Siebenaler K, Determan A, Dohmeier D, Hansen K. Development of lidocaine-coated microneedle product for rapid, safe, and prolonged local analgesic action. Pharm Res. 2012;29(1):170–177. [DOI] [PubMed] [Google Scholar]
- 100. Lhernould MS, Deleers M, Delchambre A. Hollow polymer microneedles array resistance and insertion tests. Int J Pharm. 2015. 480(1-2):152–157. [DOI] [PubMed] [Google Scholar]
- 101. Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66. [DOI] [PubMed] [Google Scholar]
- 102. Chen MC, Wang KW, Chen DH, Ling MH, Liu CY. Remotely triggered release of small molecules from LaB6@SiO2-loaded polycaprolactone microneedles. Acta Biomater. 2015;13:344–353. [DOI] [PubMed] [Google Scholar]
- 103. Nguyen HX, Bozorg BD, Kim Y, et al. Poly (vinyl alcohol) microneedles: fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur J Pharm Biopharm. 2018;129:88–103. [DOI] [PubMed] [Google Scholar]
- 104. Chu LY, Choi SO, Prausnitz MR. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs. J Pharm Sci. 2010;99(10):4228–4238. [DOI] [PubMed] [Google Scholar]
- 105. Yang S, Feng Y, Zhang L, Chen N, Yuan W, Jin T. A scalable fabrication process of polymer microneedles. Int J Nanomed. 2012;7:1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Park YH, Keun HS, Choi IW, et al. Fabrication of degradable carboxymethyl cellulose (CMC) microneedle with laser writing and replica molding process for enhancement of transdermal drug delivery. Biotechnol Bioprocess Eng. 2016;21(1):110–118. [Google Scholar]
- 107. Chen MC, Ling MH, Kusuma SJ. Poly-γ-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin. Acta Biomater. 2015;24:106–116. [DOI] [PubMed] [Google Scholar]
- 108. Dardano P, Caliò A, Palma VD, Bevilacqua MF, Matteo AD, Stefano LDA. . Photolithographic approach to polymeric microneedles array fabrication. Materials (Basel). 2015;8(12):8661–8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kochhar JS, Zou S, Chan SY, Kang L. Protein encapsulation in polymeric microneedles by photolithography. Int J Nanomed. 2012;7:3143–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kochhar JS, Quek TC, Soon WJ, Choi J, Zou S, Kang L. Effect of microneedle geometry and supporting substrate on microneedle array penetration into skin. J Pharm Sci. 2013;102(11):4100–4108. [DOI] [PubMed] [Google Scholar]
- 111. Tomono T. Puncture performance of sharpen microneedles by using inclined contact UV lithography. Microsyst Technol. 2018;24(9):3589–3599. [Google Scholar]
- 112. Luzuriaga MA, Berry DR, Reagan JC, Smaldone RA, Gassensmith JJ. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip. 2018;18(8):1223–1230. [DOI] [PubMed] [Google Scholar]
- 113. Paik SJ, Sangwon B, Jung ML, et al. In-plane single-crystal-silicon microneedles for minimally invasive microfluid systems. Sens Actuat A Phys. 2004;114(2-3):276–284. [Google Scholar]
- 114. Li WZ, Huo MR, Zhou JP, et al. Super-short solid silicon microneedles for transdermal drug delivery applications. Int J Pharm. 2010;389(1-2):122–129. [DOI] [PubMed] [Google Scholar]
- 115. Lee S, Jeong W, Beebe DJ. Microfluidic valve with cored glass microneedle for microinjection. Lab Chip. 2003;3(3):164–167. [DOI] [PubMed] [Google Scholar]
- 116. Mahadevan GH, Sheardown P, Selvaganapathy P. PDMS embedded microneedles as a controlled release system for the eye. J Biomater Appl. 2012;28(1):20–27. [DOI] [PubMed] [Google Scholar]
- 117. Hu Q, Yukun R, Xu Z, et al. A micro-needle induced strategy for preparation of monodisperse liquid metal droplets in glass capillary microfluidics. Microfluid Nanofluid. 2019;23:13. [Google Scholar]
- 118. Resnik D, Možek M, Pečar B, et al. In vivo experimental study of noninvasive insulin microinjection through hollow Si microneedle array. Micromachines (Basel). 2018;9(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ita K. Ceramic microneedles and hollow microneedles for transdermal drug delivery: two decades of research. J Drug Deliv Sci Technol. 2018;44:314–322. [Google Scholar]
- 120. Ita K, Hatsakorzian N, Tolstikov V. Microneedle-mediated delivery of atenolol and bisoprolol hemifumarate. J Nanopharma Drug Deliv. 2013. 1(1):38–44. [Google Scholar]
- 121. Gyaneshwar T, Nitesh R, Sagar T, Pranav K, Rustagi N. Treatment of pediatric femoral shaft fractures by stainless steel and titanium elastic nail system: a randomized comparative trial. Chin J Traumatol. 2016;19(4):213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chaudhary S, Gharti A, Adhikari B. An in vivo comparison of accuracy of two electronic apex locators in determining working length using stainless steel and nickel titanium files. Clin Cosmet Investig Dent. 2018;10:75–82. doi:10.2147/CCIDE.S158882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res. 2006;23(5):1008–1019. [DOI] [PubMed] [Google Scholar]
- 124. Moronkeji K, Todd S, Dawidowska I, Barrett SD, Akhtar R. The role of subcutaneous tissue stiffness on microneedle performance in a representative in vitro model of skin. J Control Release. 2017;265:102–112. [DOI] [PubMed] [Google Scholar]
- 125. Nguyen HX, Banga AK. Delivery of methotrexate and characterization of skin treated by fabricated PLGA microneedles and fractional ablative laser. Pharm Res. 2018;35(3):68. [DOI] [PubMed] [Google Scholar]
- 126. Dangol M, Yang H, Li CG, et al. Innovative polymeric system (IPS) for solvent-free lipophilic drug transdermal delivery via dissolving microneedles. J Control Release. 2016;223:118–125. [DOI] [PubMed] [Google Scholar]
- 127. Pei-Ting K, Chi Leea I, Chin Chenb MI, Wei Tsaia S. Polymer microneedles fabricated from PCL and PCL/PEG blends for transdermal delivery of hydrophilic compounds. J Taiwan Instit Chem Eng. 2015;51:1–8. [Google Scholar]
- 128. Chen CH, Shyu V, Chen CT. Dissolving microneedle patches for transdermal insulin delivery in diabetic mice: potential for clinical applications. Materials (Basel). 2018;11(9):1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kim JD, Kim M, Yang H, Lee K, Jung H. Droplet-born air blowing: novel dissolving microneedle fabrication. J Control Release. 2013;170(3):430–436. [DOI] [PubMed] [Google Scholar]
- 130. Chen W, Chong W, Yan LI, et al. Improved polyvinylpyrrolidone microneedle arrays with non-stoichiometric cyclodextrin. J Mater Chemist B. 2014;2(12):1699–1705. [DOI] [PubMed] [Google Scholar]
- 131. He MC, Chen BZ, Ashfaq M, Guo XD. Assessment of mechanical stability of rapidly separating microneedles for transdermal drug delivery. Drug Deliv Transl Res. 2018;8(5):1034–1042. [DOI] [PubMed] [Google Scholar]
- 132. Gittard SD, Chen B, Xu H, et al. The effects of geometry on skin penetration and failure of polymer microneedles. J Adhes Sci Technol. 2013;27(3):227–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chu LY, Prausnitz MR. Separable arrowhead microneedles. J Control Release. 2011;149(3):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Loizidou EZ, Williams NA, Barrow DA, et al. Structural characterisation and transdermal delivery studies on sugar microneedles: experimental and finite element modelling analyses. Eur J Pharm Biopharm. 2015;89(2):224–231. [DOI] [PubMed] [Google Scholar]
- 135. Nguyen HX, Banga AK. Fabrication, characterization and application of sugar microneedles for transdermal drug delivery. Ther Deliv. 2017;8(5):249–264. [DOI] [PubMed] [Google Scholar]
- 136. Hashmi S, Ling P, Hashmi G, Reed M, Gaugler R, Trimmer W. Genetic transformation of nematodes using arrays of micromechanical piercing structures. Biotech. 1995;19(5):766–770. [PubMed] [Google Scholar]
- 137. Domanski M, Winnubst L, Luttge R, et al. Production and characterization of miro- and nano-features in biomedical alumina and zirconia ceramics using a tape casting route. J Mater Sci Mater Med. 2012;23(7):1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Doraiswamy A, Jin C, Narayan RJ, et al. Two photon induced polymerization of organic–inorganic hybrid biomaterials for microstructured medical devices. Acta Biomater. 2006;2(3):267–275. [DOI] [PubMed] [Google Scholar]
- 139. Ayittey PN, Walker JS, Rice JJ, De Tombe PP. Glass microneedles for force measurements: a finite-element analysis model. Pflugers Arch. 2008;457(6):1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Omatsu T, Chujo K, Miyamoto K, et al. Metal microneedle fabrication using twisted light with spin. Optic Exp. 2010;18(17):17967–17973. [DOI] [PubMed] [Google Scholar]
- 141. Sun J, Zhuang J, Jiang H, et al. Thermal dissipation performance of metal-polymer composite heat exchanger with V-shape microgrooves: a numerical and experimental study. Appl Therm Eng. 2017;121:492–500. [Google Scholar]
- 142. Wu H, Zhu J, Huang Y, Wu D, Sun J. Microfluidic-based single-cell study: current status and future perspective. Molecules. 2018;23(9):2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kormakov S, He XX, Huang YA, et al. Mathematical model for predicting conductivity of polymer composites with a forced assembly network obtained by SCFNA method. Polym Comp. 2018;40(5):1819–1827. [Google Scholar]
- 144. Wu D, Sun J, Liu Y, et al. Rapid fabrication of microstructure on PMMA substrate by the plate to plate transition-spanning isothermal hot embossing method nearby glass transition temperature. Polym Eng Sci. 2017;57(3):268–274. [Google Scholar]
- 145. Sun J, Wu D, Liu Y, Dai Le, Jiang C. Numerical simulation and experimental study of filling process of micro prism by isothermal hot embossing in solid-like state. Adv Polym Technol. 2018;37(6):1581–1591. [Google Scholar]
- 146. Zhuang J, Hu W, Fan Y, et al. Fabrication and testing of metal/polymer microstructure heat exchangers based on micro embossed molding method. Microsyst Technol. 2018;25(2):381–388. [Google Scholar]
- 147. Jingyao S, Wu D, Liu Y, et al. Rapid fabrication of micro structure on polypropylene by plate to plate isothermal hot embossing method. Polym Eng Sci. 2018;58(6):952–960. [Google Scholar]
- 148. Zhuang J, Wu DM, Xu H, Huang Y, Liu Y, Sun JY. Edge effect in hot embossing and its influence on global pattern replication of polymer-based microneedles. Int Polym Process. 2019;34(2):231–238. [Google Scholar]
- 149. Juster H, Der Aar BV, Brouwer HD. A review on microfabrication of thermoplastic polymer-based microneedle arrays. Polym Eng Sci. 2019;59(5):877–890. [Google Scholar]
- 150. Huang Y, Kormakov S, He XX, et al. Conductive polymer composites from renewable resources: an overview of preparation, properties, and applications. Polymers. 2019;11(2):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Liu H, Jian R, Chen H, et al. Application of biodegradable and biocompatible nanocomposites in electronics: current status and future directions. Nanomaterials. 2019;9(7):950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. He X, Yao H, Wan C, et al. Enhancing thermal conductivity of polydimethylsiloxane composites through spatially confined network of hybrid fillers. Comp Sci Technol. 2019;172:163–171. [Google Scholar]
- 153. He X, Yao H, Liu Y, et al. Improved thermal conductivity of polydimethylsiloxane/short carbon fiber composites prepared by spatial confining forced network assembly. J Mater Sci. 2018;53(20):14299–14310. [Google Scholar]
- 154. Sun J, Zhao Y, Yang Z, et al. Highly stretchable and ultrathin nanopaper composites for epidermal strain sensors. Nanotechnology. 2018;29(35):355304. [DOI] [PubMed] [Google Scholar]
- 155. Hao F, Li Y, Zhu J, et al. Polyethylenimine-based formulations for delivery of oligonucleotides. Curr Med Chem. 2019;26(13):2264–2284. [DOI] [PubMed] [Google Scholar]
- 156. Sun J, Jia Z, Shi J, et al. Highly elastic and ultrathin nanopaper-based nanocomposites with superior electric and thermal characteristics. J Mater Sci. 2019;54(11):8436–8449. [Google Scholar]
- 157. Yang HW, Ye L, Guo XD, Yang C, Compans RW, Prausnitz MR. Ebola vaccination using a DNA vaccine coated on PLGA-PLL/γPGA nanoparticles administered using a microneedle patch. Adv Healthc Mater. 2017;6(1):1600750. [DOI] [PubMed] [Google Scholar]
- 158. Kim NW, Lee MS, Kim KR, et al. Polyplex-releasing microneedles for enhanced cutaneous delivery of DNA vaccine. J Control Release. 2014;179:11–17. [DOI] [PubMed] [Google Scholar]
- 159. Zhang S, Zhao S, Jin X, Wang B, Zhao G. Microneedles improve the immunogenicity of DNA vaccines. Hum Gen Ther. 2018;29(9):1004–1010. [DOI] [PubMed] [Google Scholar]
- 160. Levin Y, Kochba E, Shukarev G, Rusch S, Herrera-Taracena G, van Damme P. A phase 1, open-label, randomized study to compare the immunogenicity and safety of different administration routes and doses of virosomal influenza vaccine in elderly. Vaccine. 2016;34(44):5262–5272. [DOI] [PubMed] [Google Scholar]
- 161. Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32(16):1856–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Morelon E, Pouteil Noble C, Daoud S, et al. Immunogenicity and safety of intradermal influenza vaccination in renal transplant patients who were non-responders to conventional influenza vaccination. Vaccine. 2010;28(42):6885–6890. [DOI] [PubMed] [Google Scholar]
- 163. Icardi G, Orsi A, Ceravolo A, Ansaldi F. Current evidence on intradermal influenza vaccines administered by Soluvia™ licensed micro injection system. Hum Vaccin Immunother. 2014;8(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP. Type 2 diabetes mellitus and cardiovascular disease: genetic and epigenetic links. Front Endocrinol (Lausanne). 2018;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Trierweiler H, Kisielewicz G, Hoffmann Jonasson T, et al. Sarcopenia: a chronic complication of type 2 diabetes mellitus. Diabetol Metab Syndr. 2018;10(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Xie S, Li Z, Yu Z. Microneedles for transdermal delivery of insulin. J Drug Deliv Sci Technol. 2015;28:11–17. [Google Scholar]
- 167. Peyrot M, Dreon D, Zraick V, Cross B, Tan MH. Patient perceptions and preferences for a mealtime insulin delivery patch. Diabetes Ther. 2018;9(1):297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Ling MH, Chen MC. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Act Biomater. 2013;9(11):8952–8961. [DOI] [PubMed] [Google Scholar]
- 169. Zhang Y, Jiang G, Yu W, Liu D, Xu B. Microneedles fabricated from alginate and maltose for transdermal delivery of insulin on diabetic rats. Mater Sci Eng C Mater Biol Appl. 2018;85:18–26. [DOI] [PubMed] [Google Scholar]
- 170. Jin X, Zhu DD, Chen BZ, Ashfaq M, Guo XD. Insulin delivery systems combined with microneedle technology. Adv Drug Deliv Rev. 2018;127:119–137. [DOI] [PubMed] [Google Scholar]
- 171. Kathuria H, Li H, Pan J, et al. Large size microneedle patch to deliver lidocaine through skin. Pharm Res. 2016;33(11):2653–2667. [DOI] [PubMed] [Google Scholar]
- 172. Ishikawa K, Fukamizu H, Tetsuyai T, Ohta Y, Yoshiki T. Application of a three-microneedle device for the delivery of local anesthetics. Patient Prefer Adher. 2015;9:585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kochhar JS, Lim WX, Zou S, Foo WY, Pan J, Kang L. Microneedle integrated transdermal patch for fast onset and sustained delivery of lidocaine. Mol Pharm. 2013;10(11):4272–4280. [DOI] [PubMed] [Google Scholar]
- 174. Ammar HO, Ghorab M, El-Nahhas SA, Kamel R. Design of a transdermal delivery system for aspirin as an antithrombotic drug. Int J Pharm. 2006;327(1-2):81–88. [DOI] [PubMed] [Google Scholar]
- 175. Olatunji O, Olubowale M, Okereke C. Microneedle-assisted transdermal delivery of acetylsalicylic acid (aspirin) from biopolymer films extracted from fish scales. Polym Bull. 2017;75(9):4103–4115. [Google Scholar]
- 176. Sullivan SP, Murthy N, Prausnitz MR. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv Mater. 2008;20(5):933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Nam H, kim H, Park Y, et al. Fabrication of DNA-coated microneedles for transdermal DNA delivery. Sci Adv Mater. 2014;6(11):2536–2539. [Google Scholar]
- 178. Wei Z, Zheng S, Wang R, et al. A flexible microneedle array as low-voltage electroporation electrodes for in vivo DNA and siRNA delivery. Lab Chip. 2014;14(20):4093–4102. [DOI] [PubMed] [Google Scholar]
- 179. Zhao X, Coulman SA, Hanna SJ, Wong FS, Dayan CM, Birchall JC. Formulation of hydrophobic peptides for skin delivery via coated microneedles. J Control Release. 2017;265:2–13. [DOI] [PubMed] [Google Scholar]
- 180. Li X, Zhao R, Qin Z, et al. Microneedle pretreatment improves efficacy of cutaneous topical anesthesia. Am J Emerg Med. 2010;28(2):130–134. [DOI] [PubMed] [Google Scholar]
- 181. Uppuluri CT, Devineni J, Han T, et al. Microneedle-assisted transdermal delivery of zolmitriptan: effect of microneedle geometry, in vitro permeation experiments, scaling analyses and numerical simulations. Drug Dev Ind Pharm. 2017;43(8):1292–1303. [DOI] [PubMed] [Google Scholar]
- 182. Wermeling DP, Banks SL, Hudson DA, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci U S A. 2008;105(6):2058–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Chiappini C, Campagnolo P, Almeida CS, et al. Mapping local cytosolic enzymatic activity in human esophageal mucosa with porous silicon nanoneedles. Adv Mater. 2015;27(35):5147–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Sun J, Semen K, Ying L, et al. Recent progress in metal-based nanoparticles mediated photodynamic therapy. Molecules. 2018;23(7):1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Mohan AMV, Windmiller JR, Mishra RK, Wang J. Continuous minimally-invasive alcohol monitoring using microneedle sensor arrays. Biosens Bioelectron. 2017;91:574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ester CS, Brady AJ, Eltayib E, et al. Hydrogel-forming microneedle arrays allow detection of drugs and glucose in vivo: potential for use in diagnosis and therapeutic drug monitoring. PLoS One. 2015;10(12):e0145644. [DOI] [PMC free article] [PubMed] [Google Scholar]