Abstract
Delirium is a serious complication of acute illness. Little is known, however, regarding the neurobiology of delirium, largely due to challenges in studying the complex inpatient population. Neuroimaging is one noninvasive method that can be used to study structural and functional brain abnormalities associated with delirium. The purpose of this integrative literature review was to examine the content and quality of current structural neuroimaging evidence in delirium. After meeting inclusion criteria, 11 articles were included in the review. Commonly noted structural abnormalities were impaired white matter integrity, brain atrophy, ischemic lesions, edema, and inflammation. Findings demonstrated widespread alterations in several brain structures. Limitations of the studies in this review included small sample sizes, inappropriate or questionable delirium measurements, and failure to consider confounding variables. This review provides insight into possible structural changes responsible for the signs and symptoms seen in patients with delirium, but more high-quality studies are needed.
Keywords: delirium, neuroimaging, neuropathology, magnetic resonance imaging, computed tomography
Delirium is a serious complication of acute illness that affects approximately 50% of hospitalized older adults (Hshieh, Inouye, & Oh, 2018) and up to 87% of patients in the intensive care unit (ICU; Cavallazzi, Saad, & Marik, 2012). Defined in the Diagnostic and Statistical Manual of Mental Disorders as a fluctuating change in mental status, delirium is characterized by a disturbed level of consciousness with a reduced ability to focus and either (a) a change in cognition or (b) the development of a perceptual disturbance (American Psychiatric Association, 2013). It is independently associated with increases in length of hospital stay (Ely et al., 2001a) and cost of care (Leslie, Marcantonio, Zhang, Leo-Summers, & Inouye, 2008; Milbrant et al., 2004). Patients who experience delirium are at a significantly higher risk of mortality (Shehabi et al., 2010; Witlox et al., 2010), and those who survive experience long-term cognitive impairments up to a year after discharge (Girard et al., 2010; Saczynski et al., 2012) and are at increased risk of dementia (Witlox et al., 2010). Despite widespread knowledge of the negative clinical outcomes associated with the syndrome, the neurobiological basis for delirium and its long-term cognitive consequences remains poorly understood.
Nurse researchers have been influential in the development and implementation of multicomponent interventions to prevent delirium (Balas et al., 2014; Barnes-Daly, Phillips, & Ely, 2017). Nurse-driven, nonpharmacologic interventions such as sleep promotion, early mobility, correction of visual and hearing impairments, and family presence decrease the incidence and duration of delirium (Flannery, Oyler, & Weinhouse, 2016; Hshieh, Yang, Gartaganis, Yue, & Inouye, 2018; Rosa et al., 2017; Schweickert et al., 2009). Yet published nursing research has given little consideration to the pathologic brain changes associated with delirium. Although neuroimaging has been suggested as a noninvasive method for studying both structural (damage to brain structures) and functional (damage to the communication within and between brain structures) brain abnormalities, the method is underutilized in nursing research (Monroe et al., 2017).
In 2008, authors of a systematic review of the delirium neuroimaging literature determined that existing evidence was limited by small sample size, poor study design, and variation in imaging methods (Soiza et al., 2008). They found evidence of associations between delirium and cortical atrophy and white matter lesions but could not exclude age and cognition as covariates. The authors concluded that further studies were needed. By 2017, the use of neuroimaging to understand delirium pathophysiology had grown in popularity, and two additional systematic reviews were published. The first is a comprehensive review of the literature, providing a broad overview of both structural and functional neuroimaging in delirium (Nitchingham, Kumar, Shenkin, Ferguson, & Caplan, 2017), while the second focused exclusively on functional neuroimaging (Haggstrom, Welschinger, & Caplan, 2017).
Both of those previous reviews differed from the present review in several ways. First, this integrative review focuses solely on structural neuroimaging with an overall goal to understand the specific alterations to structures in the brains of patients with delirium. Second, the prior reviews considered neuroimaging performed prior to the onset of delirium to understand pathologic features that may predispose to delirium. In the current review, we excluded studies that only used neuroimaging prior to delirium, as the focus here is on structural alterations that could be directly associated with the experience of delirium. Lastly, all prior reviews have been written with a medical audience in mind. This review is the first in which the discussion is geared for a nursing audience, with the hope of creating impetus for future use of neuroimaging in nursing research. Specifically, we undertook this integrative review of the neuroimaging literature to determine the current state of knowledge regarding the neurobiological foundations of structural brain damage in delirium. We examined the volume and quality of available evidence to answer the following question: What structural changes are occurring in the adult brain during delirium?
Method
In the conduct of the literature search, data extraction, and analysis of articles, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, Altman, & the PRISMA Group, 2009).
Eligibility Criteria
Articles were eligible if they were published in English after 2008 in a peer-reviewed journal. We considered studies conducted in adult (≥18 years) inpatient populations with a focus on delirium during acute illness for inclusion. We excluded studies of delirium as a result of psychiatric illness or other causes. Further, we only considered quantitative analyses and excluded literature reviews.
Search Strategy
To sample a large variety of evidence, we searched the Cochrane Database of Systematic Reviews, Cumulative Index of Nursing and Allied Health Literature, MEDLINE, PsycINFO, PubMed, and Web of Science databases. We conducted a secondary search by reviewing reference lists of relevant articles and literature reviews. Electronic database searches were performed from September 2016 to March 2018.
The search terms we used were modified from those used by Soiza and colleagues (2008). They included delirium, encephalopathy, and acute confusional state to identify articles studying delirium and associated neurologic dysfunction, such as sepsis-associated encephalopathy (SAE); neuroimaging, neuroanatomy, and neuropathology to restrict articles to those looking at neurobiological changes; and various imaging techniques, including CT scan, CAT scan, computed tomography (CT), tomography, X-ray computed, computed axial tomography, magnetic resonance imaging (MRI), magnetic resonance spectroscopy, and nuclear magnetic resonance spectroscopy. We used the search terms in various groupings as key words or medical subject headings when appropriate.
Study Selection
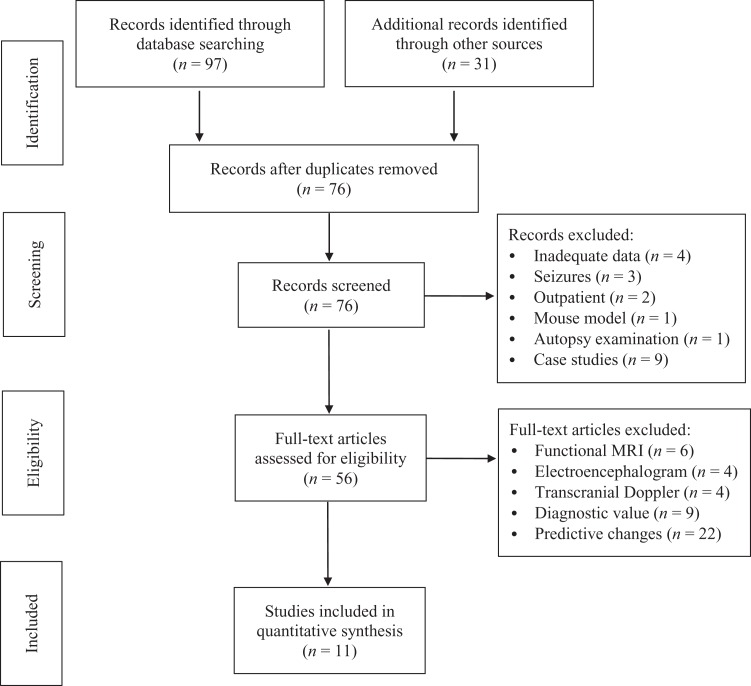
Figure 1 illustrates our process of study selection. After retrieving eligible articles from the databases (n = 97) and through secondary searches of reference lists (n = 31), we removed duplicates, leaving 76 records for screening. After initial review of title and abstract, we removed articles that had incomplete or missing data on the variables of interest (n = 4) or were studies of encephalopathy involving seizures (posterior reversible encephalopathy syndrome and subacute encephalopathy with seizures in alcoholics; n = 3) or of outpatient populations (n = 2), or used a mouse model (n = 1) or data from an autopsy examination (n = 1). We also removed case studies (n = 9) due to the low level of evidence represented by these articles, though we kept case series in the review due to the paucity of higher level evidence.
Figure 1.
Study selection flow diagram. Adapted from Moher et al. (2009). MRI = magnetic resonance imaging.
After initial screening, 56 records remained for full-text review. To narrow articles to those that specifically looked at structural changes, we removed studies using functional magnetic resonance imaging (n = 6), electroencephalogram (n = 4), or transcranial doppler (n = 4). We also excluded studies that focused on the financial utility of imaging patients with altered mental status (n = 9) or looked at baseline neuroimaging to determine structural changes that predisposed patients to postoperative delirium (n = 22), leaving 11 studies for inclusion in the integrative review.
Data Extraction
During review of the 11 articles, the primary author (L.B.K.) complied data into literature review tables with the following column headings: citation, study design, level of evidence, purpose, sample/setting, major variables, measurement, data analysis (if applicable), findings, strengths, limitations, and recommendations. Main variables of interest during data extraction were delirium, imaging technique, and structural abnormalities. After careful review of the articles and abstraction of data, we complied results into a synthesis table (Table 1). The small sample sizes and observational natures of the included studies prevented use of summary statistics.
Table 1.
Synthesis of Findings Featuring Type of Structural Neurobiological Abnormality Observed in Patients With Delirium.
| Study | Design | Sample | Neurologic Process | Imaging Technique | Structural Neurobiological Abnormality | ||||
|---|---|---|---|---|---|---|---|---|---|
| Impaired White Matter Integrity | Brain Atrophy | Ischemic Lesions | Edema | Inflammation | |||||
| Bourgeois, Hategan, Ford, Tisi, and Xiong (2015) | Cross sectional | 45 patients | Delirium | CT | X | ||||
| Brown et al. (2015) | Cohort | 79 CICU patients | Delirium | MRI | X | ||||
| Gunther et al. (2012) | Cohort | 47 ICU patients | Delirium | MRI | X | ||||
| Morandi et al. (2010) | Case series | 8 ICU patients | Delirium | MRI | X | ||||
| Morandi et al. (2012) | Cohort | 47 ICU patients | Delirium | MRI | X | ||||
| Piazza, Cotena, De Robertis, Caranci, and Tufano (2009) | Case series | 4 ICU patients | SAE | MRI | X | X | |||
| Polito et al. (2013) | Cohort | 71 ICU patients | SAE | MRI | X | X | |||
| Rolandi et al. (2018) | Cohort | 16 orthopedic patients | Delirium | MRI | X | X | |||
| Suchyta, Jephson, and Hopkins (2010) | Cohort | 64 ICU patients | Neurologic dysfunction | CT, MRI | X | X | X | X | |
| Sutter et al. (2015) | Cohort | 146 ICU patients | Neurologic dysfunction | MRI | X | X | X | ||
| Yager et al. (2011) | Cohort | 23 BMT patients | Delirium | MRI | X | ||||
Note. BMT = bone marrow transplant; CICU = cardiac intensive care unit; CT = computed tomography; ICU = intensive care unit; MRI = magnetic resonance imaging; SAE = sepsis-associated encephalopathy.
Risk of Bias
To critically appraise the articles and aid in the detection of bias, we used the Joanna Briggs Institute critical appraisal tools (Moola et al., 2017), which include specific criteria that increase the reliability and validity of a research study. Although unique for each study design, common themes among the criteria include clear description of inclusion criteria and sample demographics, valid and reliable measurement of the exposure variable (delirium), appropriate control for confounding factors, valid and reliable measurement of the outcome variable (neuroimaging abnormalities), and the use of appropriate statistical analyses. We marked each criterion as met, not met, or not applicable for each of the 11 studies. After full-text review of the articles, both authors discussed and came to a consensus on the ratings for each study. The degree to which each study met or did not meet the criteria determined its overall value to the synthesis of results.
Results
A total of 503 subjects across 11 studies were included in this integrative review. Of these subjects, 419 (83.3%) were medical, surgical, or cardiac ICU patients. Other inpatient populations included bone marrow transplant (n = 23), orthopedic (n = 16), and general inpatient (n = 45). Study designs were primarily prospective cohort (n = 6) but also included two retrospective cohort studies, a retrospective cross-sectional study, and two case-series reports. Due to the ethical issues related to inducing delirium in human subjects and the logistical difficulties in obtaining neuroimaging prior to acute illness, there were no randomized controlled trials in the literature. Exposure variables of interest included delirium (n = 7), SAE (n = 2), and a cluster of neurologic dysfunction (n = 2) that included delirium, altered level of consciousness, altered mental status, seizures, or focal neurologic deficits. MRI was the most commonly utilized neuroimaging method (n = 10), but one study used CT or MRI and another used CT exclusively. Results are organized in the following sections by the type of abnormality noted on imaging. Structural abnormalities identified in the studies included impaired white matter integrity, atrophy, ischemic lesions, edema, and inflammation.
Impaired White Matter Integrity
The white matter of the brain consists of myelinated nerve fibers that connect the grey matter of the brain, which is composed of neurons (Squire, 1987). Of the studies we reviewed, seven noted abnormalities associated with impaired white matter integrity.
White matter hyperintensities (WMHs)
WMHs are nonspecific changes in white matter that have a variety of possible causes including atherosclerosis, ischemia, and disruption of the blood–brain barrier (Debette & Markus, 2010). In the five studies that identified WMH, investigators diagnosed them through structural neuroimaging on MRI or pulled the data retrospectively from radiology reports. In two studies, researchers used the Schmidt Scale to measure the severity of the hyperintensity, where the lowest grade (0) indicates no hyperintensities and the highest grade (3) indicates widespread, severe hyperintensities (Schmidt et al., 1992).
In a case series of eight ICU patients who underwent MRI for delirium, Morandi et al. (2010) found that six had WMH, with even distribution between the three grades. In another case series of four patients experiencing SAE, researchers found hyperintensities in the frontal lobe and periventricular region of two patients (Piazza, Cotena, De Robertis, Caranci, & Tufano, 2009). In a sample of 71 ICU patients with SAE, Polito et al. (2013) identified WMH in 21% (n = 15); however, delirium was not significantly associated with the presence of WMH on imaging. Suchyta, Jephson, and Hopkins (2010) found WMH in the periventricular regions and subcortical matter in 29.7% (n = 19) of 64 ICU patients with neurological dysfunction who underwent CT or MRI. In an unexpected finding, Sutter et al. (2015) discovered a high burden of WMH in a cohort of 146 ICU patients experiencing neurologic dysfunction, such that 71% (n = 104) of patients had a total of 190 WMHs, with the frontal (n = 83) and parietal (n = 50) lobes most commonly affected.
Authors have debated the clinical relevance of WMH in the literature. WMH are more common among older adults, with estimates of the prevalence among healthy adults aged <75 years ranging from 11% to 21% and among healthy adults aged more than 75 years from 38% to 65% (Ylikoski et al., 1995). However, the patients included in the present review were relatively young, with 8 of the 12 patients in the two case series reports younger than 60 years (Morandi et al., 2010; Piazza et al., 2009) and mean ages of 57.9 ± 16.8 and 54.3 ± 7.3 years in Suchyta et al. (2010) and Sutter et al. (2015), respectively. Additionally, Suchyta et al. found no significant correlation between age and abnormalities on imaging (p = .08).
WMH can also be related to comorbid conditions such as vascular dementia or atherosclerotic disease. While early-onset Alzheimer’s disease does affect approximately half a million Americans under the age of 65 (Alzheimer’s Association, 2006), it remains unlikely that the relatively young patients included in this review had dementia. Furthermore, all five studies that discovered WMH in patients with delirium specifically excluded patients with prior neurologic disease. In Suchyta et al.’s study (2010), 22 (34.4%) of the patients had comorbid atherosclerotic risk factors; however, the presence of multiple comorbidities did not predict abnormalities on imaging (p = .71). In contrast, Sutter et al. (2015) found that subjects with WMH were more likely to be older (p < .001), have a higher severity of illness (p = .012), and generally have more atherosclerotic risk factors (no p value given).
In sum, the relationship between WMH and delirium remains unknown. However, the present evidence suggests that researchers should not ignore the potential contribution of hyperintensities to the neurobiology of delirium, and further investigation is warranted.
Altered diffusivity
Diffusion tensor imaging is an MRI technique that measures the direction of water molecule diffusion along nerve fibers, which becomes altered in areas of impaired white matter integrity (Morandi et al., 2012). This altered diffusion is measured via fractional anisotropy (FA) and quantified on a scale of 0–1, with values close to 1 indicating nerve-fiber integrity. In a sample of 47 ICU survivors who underwent diffusion tensor imaging at discharge and 3 months, Morandi et al (2012) found that patients who experienced a longer duration of delirium had significantly lower FA values at both time points. These lower values indicated impaired white matter integrity in multiple areas of the corpus callosum at discharge (p < .05) and 3 months (p < .05) and in the anterior limb of the internal capsule at discharge (p = .01). The FA values were .01–.02 lower for patients who experienced 3 days of delirium compared to those who experienced no delirium, while a typical decrease associated with aging is .03 (Salat et al., 2005).
In a sample of 16 elderly orthopedic patients who underwent diffusion tensor imaging 3–5 months after surgery, Rolandi et al. (2018) found that those diagnosed with postoperative delirium had areas of altered diffusivity in the corpus callosum, anterior limb of the internal capsule, and several other connective fiber tracts (e.g., anterior and superior corona radiata, left posterior thalamic radiation, superior longitudinal fasciculus of the right hemisphere) within the brain. These areas showed increased diffusivity, indicating an alteration in diffusion that could be related to impaired white matter integrity in these structures.
Brain Atrophy
Of the studies included in this review, five found patients with evidence of brain atrophy. Atrophy of the brain indicates a volume loss of the cerebral tissues and neuronal destruction. A ventricle-to-brain ratio (VBR) can be calculated with MRI images to detect generalized, diffuse brain volume loss, with a higher ratio indicating more atrophy. The VBR is posited to be a more accurate indicator of atrophy than measurement of total brain volume (Bigler et al., 2004). In a cohort of 47 ICU survivors who underwent MRI at discharge and 3 months, a patient who experienced 3 days of delirium had an average VBR .76 greater at discharge (p = .03) and .62 greater at 3 months (p = .05) compared to patients who did not experience delirium (Gunther et al., 2012). The mean VBR of this sample (median age 58 years) at discharge and 3 months was 4.0 ± 1.9 and 3.81.7, respectively, while the normal VBR for adults aged 56–65 years is 2.08 (Blatter et al., 1995).
Researchers in three studies undertook volumetric segmentation of specific brain structures. Using techniques with good test–retest reliability (Han et al., 2006; Jovicich et al., 2006), Gunther et al. (2012) found that an increased duration of delirium was associated with volume loss in the frontal lobes at discharge (p = .03) and 3 months (p = .02) and with smaller hippocampal volumes at discharge (p < .001). Using a previously validated scale to measure cerebral ventricular size (Manolio et al., 1994), Brown et al. (2015) noted a significantly increased ventricular size (p = .003), indicative of atrophy, in cardiac ICU patients with postoperative delirium, although the difference was unadjusted for potential covariates. In a sample of elderly orthopedic patients with postoperative delirium, Rolandi et al. (2018) found reduced grey matter volume in the temporal lobe (p = .014) and amygdala (p = .003) at 3–5 months postsurgery.
In two studies, researchers abstracted the radiological diagnosis of atrophy from medical records. Suchyta et al. (2010) defined atrophy as sulcal widening, ventricular enlargement, or generalized atrophy. Of the 64 ICU patients with neurologic dysfunction who underwent imaging, Suchyta et al. found that 40.6% (n = 26) had atrophy. In a study of the effects of hypovitaminosis D on brain abnormalities, 73.3% (n = 33) of 45 inpatients diagnosed with delirium demonstrated brain atrophy (Bourgeois, Hategan, Ford, Tisi, & Xiong, 2015). The researchers did not find that low levels of vitamin D were associated with deformities on neuroimaging (no p value given).
Brain atrophy is commonly associated with older age and dementia. To control for this, Suchyta et al. (2010) only considered findings of atrophy from radiographic reports that were considered more than was normal for the patient’s age. Additionally, the authors found that age was not significantly correlated with the brain abnormalities noted on imaging reports (p = .08). Gunther et al. (2012), by contrast, did find that older age was associated with increased VBR at discharge (p = .004) and 3 months (p = .004) and smaller hippocampal volumes at discharge (p = 0.04); however, in a linear regression model that considered age as a covariate, they still found that delirium duration was significantly associated with atrophy. Furthermore, Gunther et al. (2012), Rolandi et al. (2018), and Suchyta et al. (2010) all excluded patients with dementia from enrollment. However, the average ages of subjects in Bourgeois, Hategan, Ford, Tisi, and Xiong (2015), Brown et al. (2015), and Rolandi et al. were all 70 years or above, indicating that some findings of atrophy may be attributable to the effects of aging and preexisting cognitive impairment rather than delirium.
Ischemic Lesions
Lesions identified on neuroimaging represent nonspecific areas of ischemic brain damage resulting from multiple possible causes including trauma, hemorrhage, or infection. In the present review, three studies identified ischemic lesions in critically ill patients with acute neurological dysfunction. In a sample of 71 ICU patients with SAE who underwent MRI, ischemic stroke was identified in 29% (n = 21; Polito et al., 2013). Although 35 (49%) of the total sample screened positive for delirium, delirium was not significantly associated with the presence of ischemia on imaging.
Through review of radiographic reports, Suchyta et al. (2010) identified ischemic lesions in 26.6% (n = 17) of 64 ICU patients who experienced acute neurologic dysfunction. These lesions were scattered throughout the brain, with most located in the frontal (n = 7) or parietal (n = 6) lobes. These are the same areas researchers found to be highly damaged by WMH in a separate study (Sutter et al., 2015). Suchyta et al. found no significant associations between brain abnormalities and age, comorbidities, acute respiratory distress syndrome, or duration of hypoxemia or hypotension. Sutter et al. (2015) noted acute cerebral infarcts in 40.4% (n = 59) of 146 ICU patients experiencing acute neurological dysfunction. These lesions were primarily unilateral (50.9%) and located in the anterior circulation (55.9%). The presence of ischemic lesions was not significantly associated with age, severity of illness, or level of consciousness at discharge but was significantly associated with cardiac surgery (p = .001). In both of these studies, subjects exhibited a cluster of neurologic dysfunction. Therefore, the prevalence of delirium in these samples is unknown, which limits the generalizability of these findings.
Cerebral Edema
In three studies, researchers noted findings of cerebral edema, an increase in the fluid content of the brain commonly associated with SAE. One of the explanatory mechanisms for SAE is disruption of the blood–brain barrier due to inflammation (Piazza et al., 2009). This disruption causes vasogenic edema, an interstitial edema that primarily affects white matter. S100 calcium-binding protein B (S100B) is thought to be a diagnostic marker of this blood–brain barrier disruption (Undén, Christensson, Bellner, Alling, & Romner, 2004). In a case-series report of four ICU patients who experienced SAE and underwent MRI, Piazza, Cotena, De Robertis, Caranci, and Tufano (2009) found increased S100B levels in three patients. These increases, however, were not related to abnormalities noted on MRI. Due to the inconclusive nature of the results and small sample size, Piazza et al. were unable to make any definitive conclusions.
Sutter et al. (2015) reported similarly inconclusive findings in a sample of 146 ICU patients experiencing neurologic dysfunction. Using diffusion-weighted MRI, the researchers calculated whole-brain apparent diffusion coefficients. High apparent diffusion coefficients indicate acute vasogenic edema (Sener, 2001). These coefficients were significantly higher in patients with WMH (p = .001); however, they were not in the range expected for vasogenic edema. Suchyta et al. (2010) found that a small portion of the 64 ICU patients they had identified, who underwent neuroimaging for neurologic dysfunction, had edema of an unspecified type (n = 8). These small sample sizes and inconclusive findings make interpretation of the role of cerebral edema in the pathogenesis of delirium difficult.
Inflammation
MRI spectroscopy is a technique used to quantify brain metabolites. Yager et al. (2011) utilized MRI spectroscopy in a study of 14 bone marrow transplant patients and nine healthy controls. They measured three metabolites of interest: choline, creatine, and N-acetyl aspartate. All are sensitive but nonspecific markers of neuronal damage including cellular death and altered cerebral metabolism (Ross & Bluml, 2001; Sibtain, Howe, & Saunders, 2007). Yager et al. found that patients with delirium (n = 5) had an elevated choline/creatine ratio (p = .049) and decreased N-acetyl aspartate/choline ratio (p = .037) compared to nondelirious patients (n = 9). Compared to healthy controls, patients with delirium had a lower N-acetyl aspartate/choline ratio (p = .012). Delirium was significantly associated with increased age (p = .02) and diagnosis of lymphoma (p = .02), while the N-acetyl aspartate/choline ratio was significantly associated with diagnosis of lymphoma (p = .004) and memory scores (p = .002). Although the use of ratios for metabolites allows for more accurate measurement, they do make it difficult to determine which metabolites are increasing or decreasing, prohibiting a more specific understanding of these results.
Risk of Bias
We both evaluated the 11 articles included in this review using The Joanna Briggs Institute critical appraisal tools (Moola et al., 2017). By marking criteria as met, not met, or not applicable for each study, we found several common limitations across studies (Tables 2 and 3).
Table 2.
Critical Appraisal of Nonexperimental Studies.
| Study | Design | Groups Similar With Clear Inclusion Criteria? | Measure of Exposure Valid and Reliable? | Confounding Variables Identified and Dealt With? | Free of Exposure at Start of Study? | Outcomes Measures Valid and Reliable? | Follow-Up Time Sufficient and Complete, Attrition Dealt With? | Appropriate Statistical Analysis? |
|---|---|---|---|---|---|---|---|---|
| Bourgeois et al. (2015) | Cross sectional | Y | N | Y | N | N | N/A | Y |
| Brown et al. (2015) | Cohort | Y | Y | Y | N | Y | N | Y |
| Gunther et al. (2012) | Cohort | Y | Y | Y | Y | Y | N | Y |
| Morandi et al. (2012) | Cohort | Y | Y | Y | Y | Y | N | Y |
| Polito et al. (2013) | Cohort | N/Aa | Y | Y | Y | Y | Y | Y |
| Rolandi et al. (2018) | Cohort | Y | Y | Y | Y | Y | N | Y |
| Suchyta, Jephson, and Hopkins (2010) | Cohort | N/Aa | N | Y | Y | N | N/Ab | Y |
| Sutter et al. (2015) | Cohort | N/Aa | Y | Y | Y | Y | N/Ab | Y |
| Yager et al. (2011) | Cohort | Y | Y | Y | N | Y | N | Y |
Note. Y = met; N = not met; N/A = not applicable.
aUsed a one-group cohort design. bUsed a retrospective cohort design.
Table 3.
Critical Appraisal of Case Series Reports.
| Study | Clear Inclusion Criteria? | Condition Measure Similar, Valid and Reliable? | Consecutive and Complete Inclusion? | Clear Reporting of Patient Demographics and Clinical Information? | Outcomes Clear? | Clear Reporting of Setting Demographics? | Statistical Analysis Appropriate? |
|---|---|---|---|---|---|---|---|
| Morandi et al. (2010) | Y | Y | Y | Y | Y | Y | N/A |
| Piazza et al. (2009) | Y | Y | N | Y | Y | Y | N/A |
Note. Y = met; N = not met; N/A = not applicable.
Researchers used multiple delirium assessment tools across the included studies. The Diagnostic and Statistical Manual of Mental Disorders criteria are the gold standard for delirium assessment (American Psychiatric Association, 2013). Valid and reliable measures of delirium based on these criteria and recommended for use at the bedside (Barr et al., 2013; National Institute for Health and Clinical Excellence, 2010) include the Confusion Assessment Method (Inouye et al., 1990), Confusion Assessment Method for the ICU (Ely et al., 2001b), and the Intensive Care Delirium Screening Checklist (Bergeron, Dubois, Dumont, Dial, & Skrobik, 2001). All of the studies included in this review used one of these recommended delirium screening tools except for four studies. Of these four, one (Yager et al., 2011) used two delirium assessment tools with good reported validity and reliability but less extensive use in the research literature (Breitbart et al., 1997; Trzepacz et al., 2001) and three used chart review to identify delirium (Bourgeois et al., 2015; Brown et al., 2015; Suchyta, Jephson, & Hopkins, 2010). Although Brown et al. (2015) used a validated chart-review method (Inouye et al., 2005), this method has limited sensitivity for use in clinical studies. The remaining two studies extracted delirium diagnoses through chart review without detailing the delirium assessment method used by bedside clinicians. Similarly, the validity and reliability of neuroimaging results were questionable in two of the included studies, which extracted findings from radiographic reports in the medical record without independent examination of the imaging (Bourgeois et al., 2015; Suchyta et al., 2010).
The inability to rule out preexisting cognitive impairment or dementia as the cause of neuroimaging abnormalities was a limitation in three studies that did not exclude patients with dementia (Bourgeois et al., 2015; Brown et al., 2015; Yager et al., 2011). In two of these studies, researchers considered preexisting cognitive impairment as a covariate, and in both they found statistically significant differences in cognitive performance between delirious and nondelirious patients (Brown et al., 2015; Yager et al., 2011). The ability to control for potential covariates such as age and dementia was further limited by small sample sizes. These small samples were due in part to the high rate of attrition in five of the selected studies. Notably, all five of these studies were prospective cohort designs (Brown et al., 2015; Gunther et al., 2012; Morandi et al., 2012; Rolandi et al., 2018; Yager et al., 2011). Subjects were either lost to follow up or unable to complete neuroimaging, which is not uncommon in imaging studies of highly vulnerable populations. In only two of these studies did researchers verify that those unable to complete the study were not significantly different from the remaining sample (Brown et al., 2015; Rolandi et al., 2018).
While each of these studies adds important knowledge to the understanding of delirium, the limited use of valid and reliable measures of delirium coupled with the difficulties in using neuroimaging methods as well as the inability to control potential extraneous variables indicates that study results should be interpreted with caution.
Discussion
Impaired white matter integrity and atrophy were the most common abnormalities we identified in this review. Other abnormalities included ischemic lesions, edema, and inflammation. Abnormalities were frequently located in the frontal lobe and limbic system, as well as the parietal and temporal lobes. The frontal lobe and limbic system are highly connected structures involved in attention, emotional regulation, and the stress response (Squire, 1987). Together with the temporal lobe, these areas are responsible for the formation of memories. The parietal lobe is essential to the integration of sensory information. Abnormalities in these areas paint the clinical picture of a delirious patient: disturbed attention and awareness with a change in cognition (i.e., memory deficit, disorientation) or development of perceptual disturbance (American Psychiatric Association, 2013).
These results are similar to those that Soiza et al. (2008) presented in their systematic review, having also found atrophy and white matter lesions to be highly associated with delirium. That review was limited by small sample sizes and heterogeneity of study design, which has not improved in the present review. The inability to ethically induce delirium in patients after obtaining baseline neuroimaging prevents the design of randomized controlled trials to answer this clinical question. Studies included in the present review were primarily cohort designs. However, we did note improvements in the use of validated methods for neuroimaging and analysis, with 9 of the 11 studies in the present review referencing some standardized analysis method.
Investigators rarely controlled for the confounders of age and cognitive status in studies published prior to 2008. Of the 11 studies included in this review, seven had a patient population with a mean or median age of 60 years or younger, indicating a low probability of large numbers of patients with undiagnosed dementia. To distinguish brain abnormalities due to delirium from those due to chronic neurologic changes, researchers excluded patients with dementia, severe cognitive impairment, or other neurologic conditions from eight studies. Additionally, six of the studies controlled for the effects of age and other confounding variables such as sepsis, acute respiratory distress syndrome, hypotension, and hypoxia in statistical analyses.
The abnormalities and impaired brain structures we have discussed in the present review are similar to those that Nitchingham, Kumar, Shenkin, Ferguson, and Caplan (2017) discussed in their recent systematic review of structural and functional neuroimaging. However, the narrow focus of the present review allowed for an in-depth discussion of those structural abnormalities noted on neuroimaging performed during or after a delirium episode.
Researchers must consider feasibility issues when using neuroimaging in acutely ill populations. The nature of acute illness generally precludes the ability to obtain baseline imaging. Additionally, patients must be stable to travel to obtain imaging, and staff must ensure the safety of patients during this time off the unit. Lastly, many patients are unable to undergo MRI imaging due to feelings of claustrophobia or discomfort, in addition to the strict exclusion of patients with metal implants such as pacemakers, vascular clips, and medication pumps. This inability to undergo MRI was one reason for the large amount of attrition authors noted in five of the included studies. In one study, 42.4% (n = 142) of eligible participants met exclusion criteria for MRI, and 19.4% (n = 12) of the 62 patients enrolled in the study were unable to tolerate scanning (Gunther et al., 2012; Morandi et al., 2012). Of the 34 patients enrolled in Yager et al. (2011), 32.4% (n = 11) were unable to tolerate MRI. Despite these challenges, the studies in the present review demonstrated that neuroimaging techniques can be useful in the search for the underlying neurobiology of delirium.
We should note some limitations of this review. The criteria for selecting articles for inclusion were somewhat strict and may have led to relevant articles being omitted. We included only peer-reviewed articles in English and available in the selected databases. We focused on structural neuroimaging and excluded studies that only performed imaging prior to an episode of delirium. For reviews on functional neuroimaging and imaging prior to delirium, the reader can refer to the existing literature (Haggstrom et al., 2017; Nitchingham et al., 2017). We did include studies of generalized neurologic dysfunction and SAE. While delirium is one clinical presentation of these syndromes, it is not the only possible symptom. Therefore, the abnormalities seen in these studies may be related to a general pattern of neurologic dysfunction rather than delirium. We also included case series reports in this review due to the low number of relevant studies; however, these articles provide little generalizable information.
Only the primary author performed database searching and data abstraction, increasing the chance for human error and bias. However, both authors critically appraised the included articles for risk of bias. Of the 11 included studies, three were conducted by members of the ICU Delirium and Cognitive Impairment Study Group, two of which utilized the same sample of patients (Gunther et al., 2012; Morandi et al., 2010; Morandi et al., 2012). Thus, a portion of the selected literature described studies that operated with similar assumptions, patient populations, and methods. These limitations may have affected the review and analysis of these studies.
Conclusion
Delirium is a common neurologic complication of acute illness that nurses at the bedside frequently encounter. Nursing research has been influential in the development of interventions to prevent and treat delirium, yet little nursing research has been devoted to understanding the pathophysiology of delirium. Neuroimaging is a noninvasive method that is useful for understanding changes occurring in the brain during delirium. Research has shown widespread damage, including atrophy and impaired white matter integrity, in the frontal, temporal, parietal, and limbic systems of patients who experience delirium. These alterations persist for 3–5 months after discharge (Gunther et al., 2012; Morandi et al., 2012; Rolandi et al., 2018), further highlighting the possible link between long-term cognitive impairment and delirium. With the emphasis placed on health promotion and wellness within the nursing profession, nursing researchers should be engaging in interdisciplinary partnerships with the medical and radiology professions to better understand the neurological basis for a syndrome known to cause long-term cognitive impairments in survivors of acute illness. While the research to date in this area has contributed critical knowledge about the neurobiology of delirium, this review, along with others, continues to highlight the need for high-quality experimental studies in this highly vulnerable population.
Footnotes
Authors’ Note: Contents of this article have been presented as a poster presentation (Midwest Nursing Research Society, April 2018, and American Delirium Society, June 2018) and have been published in conference abstract form in Western Journal of Nursing Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: L. B. Kalvas contributed to conception, design, acquisition, analysis, and interpretation drafted manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. T. Monroe contributed to analysis and interpretation, critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Nursing Research of the National Institutes of Health (#T32 NR014225; Pickler & Melnyk, MPI).
ORCID iD: Laura Beth Kalvas  https://orcid.org/0000-0001-9458-514X
https://orcid.org/0000-0001-9458-514X
References
- Alzheimer’s Association. (2006). Early onset dementia: A national challenge, a future crisis. Retrieved from https://www.alz.org/national/documents/report_earlyonset_full.pdf
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed). Washington, DC: Author. [Google Scholar]
- Balas M. C., Vasilevskis E. E., Olsen K. M., Schmid K. K., Shostrom V., Cohen M. Z.…Burke W. J. (2014). Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Critical Care Medicine, 42, 1024–1036. doi:10.1097/CCM.0000000000000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Daly M. A., Phillips G., Ely E. W. (2017). Improving hospital survival and reducing brain dysfunction at seven California community hospitals. Critical Care Medicine, 45, 171–178. doi:10.1097/CCM.0000000000002149 [DOI] [PubMed] [Google Scholar]
- Barr J., Fraser G. L., Puntillo K., Ely E. W., Gélinas C., Dasta J. F.…Jaeschke R. (2013). Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: Executive summary. American Journal of Health-System Pharmacy, 70, 53–58. doi:10.1097/CCM.0b013e3182783b72 [DOI] [PubMed] [Google Scholar]
- Bergeron N., Dubois M. J., Dumont M., Dial S., Skrobik Y. (2001). Intensive care delirium screening checklist: Evaluation of a new screening tool. Intensive Care Medicine, 27, 859–864. [DOI] [PubMed] [Google Scholar]
- Bigler E. D. Neeley E. S. Miller M. J. Tate D. F. Rice S. A. Cleavinger H.…Welsh-Bohmer K. (2004). Cerebral volume loss, cognitive deficit and neuropsychological performance: Comparative measures of brain atrophy: I. Dementia. Journal of the International Neuropsychological Society, 10, 442–452. doi:10.1017/S1355617704103111 [DOI] [PubMed] [Google Scholar]
- Blatter D. D., Bigler E. D., Gale S. D., Johnson S. C., Anderson C. V., Burnett B. M.…Horn S. D. (1995). Quantitative volumetric analysis of brain MR: Normative database spanning 5 decades of life. American Journal of Neuroradiology, 16, 241–251. [PMC free article] [PubMed] [Google Scholar]
- Bourgeois J. A., Hategan A., Ford J., Tisi D. K., Xiong G. L. (2015). Delirium and hypovitaminosis D: Neuroimaging findings. Journal of Neuropsychiatry and Clinical Neurosciences, 27, 69–71. doi:10.1176/appi.neuropsych.13060121 [DOI] [PubMed] [Google Scholar]
- Breitbart W., Rosenfeld B., Roth A., Smith M. J., Cohen K., Passik S. (1997). The memorial delirium assessment scale. Journal of Pain and Symptom Management, 13, 128–137. [DOI] [PubMed] [Google Scholar]
- Brown C. H., Faigle R., Klinker L., Bahouth M., Max L., LaFlam A.…Hogue C. W., Jr (2015). The association of brain MRI characteristics and postoperative delirium in cardiac surgery patients. Clinical Therapeutics, 37, 2686–2699.e9 doi:10.1016/j.clinthera.2015.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallazzi R., Saad M., Marik P. E. (2012). Delirium in the ICU: An overview. Annals of Intensive Care, 2, 49 doi:10.1186/2110-5820-2-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S., Markus H. S. (2010). The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. British Medical Journal, 341, c3666 doi:10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely E., Gautam S., Margolin R., Francis J., May L., Speroff T.…Inouye S. (2001. a). The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Medicine, 27, 1892–1900. doi:10.1007/s00134-001-1132-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely E. W., Inouye S. K., Bernard G. R., Gordon S., Francis J., May L.…Dittus R. (2001. b). Delirium in mechanically ventilated patients: Validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Journal of the American Medical Association, 286, 2703–2710. [DOI] [PubMed] [Google Scholar]
- Flannery A. H., Oyler D. R., Weinhouse G. L. (2016). The impact of interventions to improve sleep on delirium in the ICU: A systematic review and research framework. Critical Care Medicine, 44, 2231–2240. doi:10.1097/CCM.0000000000001952 [DOI] [PubMed] [Google Scholar]
- Girard T. D., Jackson J. C., Pandharipande P. P., Pun B. T., Thompson J. L., Shintani A. K.…Wesley Ely E. (2010). Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Critical Care Medicine, 38, 1513–1520. doi:10.1097/CCM.0b013e3181e47be1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther M. L., Morandi A., Krauskopf E., Pandharipande P., Girard T. D., Jackson J. C.…Ely E. W. (2012). The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: The VISIONS cohort magnetic resonance imaging study. Critical Care Medicine, 40, 2022–2032. doi:10.1097/CCM.0b013e318250acc0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggstrom L., Welschinger R., Caplan G. A. (2017). Functional neuroimaging offers insights into delirium pathophysiology: A systematic review. Australasian Journal on Ageing, 36, 186–192. doi:10.1111/ajag.12417 [DOI] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S.…Fischl B. (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage, 32, 180–194. doi:10.1016/j.neuroimage.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Hshieh T. T., Inouye S. K., Oh E. S. (2018). Delirium in the elderly. Psychiatric Clinics of North America, 41, 1–17. doi:10.1016/j.psc.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Hshieh T. T., Yang T., Gartaganis S. L., Yue J., Inouye S. K. (2018). Hospital elder life program: Systematic review and meta-analysis of effectiveness. American Journal of Geriatric Psychiatry, 26, 1015–1033. doi:10.1016/j.jagp.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S. K., Leo-Summers L., Zhang Y., Bogardus S. T., Leslie D. L., Agostini J. V. (2005). A chart-based method for identification of delirium: Validation compared with interviewer ratings using the confusion assessment method. Journal of the American Geriatrics Society, 53, 312–318. doi:10.1111/j.1532-5415.2005.53120.x [DOI] [PubMed] [Google Scholar]
- Inouye S. K., van Dyck C. H., Alessi C. A., Balkin S., Siegal A. P., Horwitz R. I. (1990). Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Annals of Internal Medicine, 113, 941–948. [DOI] [PubMed] [Google Scholar]
- Jovicich J., Czanner S., Greve D., Haley E., van der Kouwe A., Gollub R.…Dale A. (2006). Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. NeuroImage, 30, 436–443. doi:10.1016/j.neuroimage.2005.09.046 [DOI] [PubMed] [Google Scholar]
- Leslie D. L., Marcantonio E. R., Zhang Y., Leo-Summers L., Inouye S. K. (2008). One-year health care costs associated with delirium in the elderly population. Archives of Internal Medicine, 168, 27–32. doi:10.1001/archinternmed.2007.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio T. A., Kronmal R. A., Burke G. L., Poirier V., O’Leary D. H., Gardin J. M.…Bryan R. N. (1994). Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke, 25, 318–327. [DOI] [PubMed] [Google Scholar]
- Milbrant E. B., Deppen S., Harrison P. L., Shintani A. K., Speroff T., Stiles R. A.…Ely E. W. (2004). Costs associated with delirium in mechanically ventilated patients. Critical Care Medicine, 32, 955–962. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., & the PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6, e1000097 doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe T. B., Beach P. A., Bruehl S. P., Dietrich M. S., Rogers B. P., Gore J. C.…Cowan R. L. (2017). The impact of Alzheimer’s disease on the resting state functional connectivity of brain regions modulating pain: A cross sectional study. Journal of Alzheimer’s Disease, 57, 71–83. doi:10.3233/JAD-161187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R.…Mu P.-F. (2017). Systematic reviews of etiology and risk In Aromataris E., Munn Z. (Eds.), Joanna Briggs Institute reviewer’s manual. Adelaide: The Joanna Briggs Institute; Retrieved from https://reviewersmanual.joannabriggs.org/ [Google Scholar]
- Morandi A., Gunther M. L., Vasilevskis E. E., Girard T. D., Hopkins R. O., Jackson J. C.…Ely E. W. (2010). Neuroimaging in delirious intensive care unit patients: A preliminary case series report. Psychiatry, 7, 28–33. [PMC free article] [PubMed] [Google Scholar]
- Morandi A., Rogers B. P., Gunther M. L., Merkle K., Pandharipande P., Girard T. D.…Hopkins R. O. (2012). The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: The VISIONS prospective cohort magnetic resonance imaging study. Critical Care Medicine, 40, 2182–2189. doi:10.1097/CCM.0b013e318250acdc [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence. (2010). Delirium: Diagnosis, prevention and management (Clinical Guideline 103). Retrieved from www.nice.org.uk/nicemedia/live/13060/49909/49909.pdf
- Nitchingham A., Kumar V., Shenkin S., Ferguson K. J., Caplan G. A. (2017). A systematic review of neuroimaging in delirium: Predictors, correlates and consequences. International Journal of Geriatric Psychiatry, 33, 1458–1478. doi:10.1002/gps.4724 [DOI] [PubMed] [Google Scholar]
- Piazza O., Cotena S., De Robertis E., Caranci F., Tufano R. (2009). Sepsis associated encephalopathy studied by MRI and cerebral spinal fluid S100B measurement. Neurochemical Research, 34, 1289–1292. doi:10.1007/s11064-008-9907-2 [DOI] [PubMed] [Google Scholar]
- Polito A., Eischwald F., Maho A.-L., Polito A., Azabou E., Annane D.…Sharshar T. (2013). Pattern of brain injury in the acute setting of human septic shock. Critical Care, 17, R204 doi:10.1186/cc12899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolandi E., Cavedo E., Pievani M., Galluzzi S., Ribaldi F., Buckley C.…Frisoni G. B. (2018). Association of postoperative delirium with markers of neurodegeneration and brain amyloidosis: A pilot study. Neurobiology of Aging, 61, 93–101. doi:10.1016/j.neurobiolaging.2017.09.020 [DOI] [PubMed] [Google Scholar]
- Rosa R. G., Tonietto T. F., da Silva D. B., Gutierres F. A., Ascoli A. M., Madeira L. C.…Teixera C. for the ICU Visits Study Group Investigators. (2017). Effectiveness and safety of an extended ICU visitation model for delirium prevention. Critical Care Medicine, 45, 1660–1667. doi:10.1097/CCM.0000000000002588 [DOI] [PubMed] [Google Scholar]
- Ross B., Bluml S. (2001). Magnetic resonance spectroscopy of the human brain. Anatomical Record, 265, 54–84. [DOI] [PubMed] [Google Scholar]
- Saczynski J. S., Marcantonio E. R., Quach L., Fong T. G., Gross A., Inouye S. K., Jones R. N. (2012). Cognitive trajectories after postoperative delirium. New England Journal of Medicine, 367, 30–39. doi:10.1056/NEJMoa1112923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat D. H., Tuch D. S., Greve D. N., van der Kouwe A. J. W., Hevelone N. D., Zaleta A. K.…Dale A. M. (2005). Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging, 26, 1215–1227. doi:10.1016/j.neurobiolaging.2004.09.017 [DOI] [PubMed] [Google Scholar]
- Schmidt R., Fazekas F., Kleinert G., Offenbacher H., Gindl K., Payer F.…Lechner H. (1992). Magnetic resonance imaging signal hyperintensities in the deep and subcortical white matter. A comparative study between stroke patients and normal volunteers. Archives of Neurology, 49, 825–827. [DOI] [PubMed] [Google Scholar]
- Schweickert W. D., Pohlman M. C., Pohlman A. S., Nigos C., Pawlik A. J., Esbrook C. L.…Kress J. P. (2009). Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet, 373, 1874–1882. doi:10.1016/S0140-6736(09)60658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener R. N. (2001). Diffusion MRI: Apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Computerized Medical Imaging and Graphics, 25, 299–326. [DOI] [PubMed] [Google Scholar]
- Shehabi Y., Riker R. R., Bokesch P. M., Wisemandle W., Shintani A., Ely E. W. (2010). Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Critical Care Medicine, 38, 2311–2318. doi:10.1097/CCM.0b013e3181f85759 [DOI] [PubMed] [Google Scholar]
- Sibtain N. A., Howe F. A., Saunders D. E. (2007). The clinical value of proton magnetic resonance spectroscopy in adult brain tumours. Clinical Radiology, 62, 109–119. doi:10.1016/j.crad.2006.09.012 [DOI] [PubMed] [Google Scholar]
- Soiza R. L., Sharma V., Ferguson K., Shenkin S. D., Seymour D. G., MacLullich A. M. J. (2008). Neuroimaging studies of delirium: A systematic review. Journal of Psychosomatic Research, 65, 239–248. doi:10.1016/j.jpsychores.2008.05.021 [DOI] [PubMed] [Google Scholar]
- Squire L. R. (1987). Memory and brain. New York, NY: Oxford University Press. [Google Scholar]
- Suchyta M. R., Jephson A., Hopkins R. O. (2010). Neurologic changes during critical illness: Brain imaging findings and neurobehavioral outcomes. Brain Imaging and Behavior, 4, 22–34. doi:10.1007/s11682-009-9082-3 [DOI] [PubMed] [Google Scholar]
- Sutter R., Chalela J. A., Leigh R., Kaplan P. W., Yenokyan G., Sharshar T., Stevens R. D. (2015). Significance of parenchymal brain damage in patients with critical illness. Neurocritical Care, 23, 243–252. doi:10.1007/s12028-015-0110-4 [DOI] [PubMed] [Google Scholar]
- Trzepacz P. T., Mittal D., Torres R., Kanary K., Norton J., Jimerson N. (2001). Validation of the delirium rating scale-revised-98: Comparison with the delirium rating scale and the cognitive test for delirium. Journal of Neuropsychiatry and Clinical Neurosciences, 13, 229–242. doi:10.1176/jnp.13.2.229 [DOI] [PubMed] [Google Scholar]
- Undén J., Christensson B., Bellner J., Alling C., Romner B. (2004). Serum S100B levels in patients with cerebral and extracerebral infectious disease. Scandinavian Journal of Infectious Diseases, 36, 10–13. [DOI] [PubMed] [Google Scholar]
- Witlox J., Eurelings L. S. M., de Jonghe J. F. M., Kalisvaart K. J., Eikelenboom P., van Gool W. A. (2010). Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia. Journal of the American Medical Association, 304, 443–451. doi:10.1001/jama.2010.1013 [DOI] [PubMed] [Google Scholar]
- Yager J. R., Magnotta V. A., Mills J. A., Vik S. M., Weckmann M. T., Capizzano A. A.…Beglinger L. J. (2011). Proton magnetic resonance spectroscopy in adult cancer patients with delirium. Psychiatry Research—Neuroimaging, 191, 128–132. doi:10.1016/j.pscychresns.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski A., Erkinjuntti T., Raininko R., Sarna S., Sulkava R., Tilvis R. (1995). White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke, 26, 1171–1177. [DOI] [PubMed] [Google Scholar]