Abstract
Long noncoding RNAs (lncRNAs) play crucial regulatory roles in fundamental biological processes, and deregulations of lncRNAs have been linked to numerous human diseases, especially cancers. Of particular interest in this regard is lncRNA GAS5, which is mainly identified as a tumor suppressor in several cancers. GAS5 was significantly low expressed in multiple cancers and was associated with clinic-pathological characteristics and patient survival, indicating a novel potential diagnostic and prognostic biomarker, and a therapeutic target for cancer. Functionally, GAS5 is involved in cell proliferation, metastasis, invasion, apoptosis, epithelial–mesenchymal transition (EMT), and drug resistance, among others, via multiple molecular mechanisms, such as binding to DNA sequences, forming RNA–DNA triplex complex, triggering or suppressing the expression of genes, binding proteins to form chromatin-modifying complex, which activates or represses gene expression, and acting as miRNA sponge to suppress miRNA expression, leading to regulation of miRNA target genes. This review provides an overview of the current state of knowledge and role of GAS5 in clinical relevance, biological functions and molecular mechanisms underlying the dysregulation of expression and function of GAS5 in cancer. Finally, the potential prospective role as diagnostic and prognostic biomarker and therapeutic target in cancer is discussed.
Keywords: GAS5, cancer, tumor suppressor, biomarker, therapeutic target
Introduction
There had been a preconception on the primacy of noncoding RNAs (ncRNAs) under the protein-coding RNAs for a long time. The development of techniques, such as genome-wide association studies, RIP-RNA sequencing, computational prediction of lncRNAs from ultra-deep RNA sequencing, and lncRNA chip-based screening, has made it possible for scientists to uncover the widespread expression profile and biological functions of ncRNAs.1 ncRNAs are generally divided into two groups, namely small ncRNAs and long noncoding RNAs (lncRNAs) depending upon their transcript sizes.2 A number of evidence show that lncRNAs function as chromatin modifier, transcriptional, post-transcriptional and translational regulators of gene expression in human physiological and pathological processes.3 Importantly, lncRNAs play key roles in human cancers.4,5 They are dysregulated in multiple cancers and act as tumor suppressors or oncogenes during tumorigenesis and have widespread biological functions, such as regulating cell proliferation, apoptosis, invasion, migration, differentiation, and drug resistance.6–8 Therefore, in-depth understanding of lncRNA biology may provide novel and better strategies for the diagnosis and even therapeutic potential in human cancer.
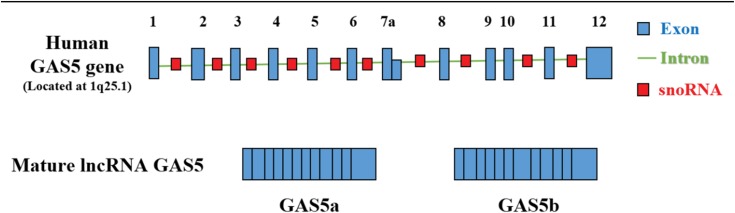
One lncRNA human growth arrest-specific transcript 5 (GAS5), a multi-snoRNA host gene located at chromosome 1q25.1, comprises 12 exons and 11 introns, and encode the same 10 snoRNAs in corresponding introns with a short open reading frame (ORF), and is considered to have no function to encode proteins, rather spliced to yield two mature lncRNAs, termed GAS5a and GAS5b due to the presence of alternative 5′-splice donor sites in exon 7.9,10 As a lncRNA transcript of about 630 nt in length, which was primitively isolated from a subtraction cDNA library of growth-arrested cells, mature GAS5 was mainly identified as a tumor suppressor in human cancer.9
Saturating cell density or nutrient deprivation can increase GAS5 levels and accompany with growth arrest at post-transcriptional level, while GAS5 expression may be regulated in differentiating cells at transcriptional level.11,12 The former mechanism is perhaps best understood and involveed an interplay between the nonsense-mediated decay (NMD) and the mammalian target of rapamycin (mTOR) pathways.13,14 GAS5 is a 5′-terminal oligopyrimidine RNA, whose translation is specifically controlled by the mTOR pathway.10,13,15 NMD is historically identified as a RNA quality control system to eliminate abnormal transcripts and control GAS5 function in mammalian cells.14 When mTOR activity is high, such as in actively growing cells, GAS5 translation is increased due to the presence of the 5ʹ-TOP sequence. However, due to the short ORF and the presence of multiple termination codons in its sequence, the degradation of GAS5 transcripts via NMD happens, which further resulted in decrease in GAS5 levels. On the contrary, when mTOR pathway is inactivated, such as in growth-arrested cells, the translation of GAS5 short ORF is blocked and this in turn prevents NMD pathways, leading to the increased expression of GAS5.12,16
In this review, we will describe the current knowledge about GAS5, from the clinical relevance, biological functions to molecular mechanisms, and the potential role of GAS5 in diagnostic and prognostic biomarker, and therapeutic target in human cancer.
GAS5 In Cancer
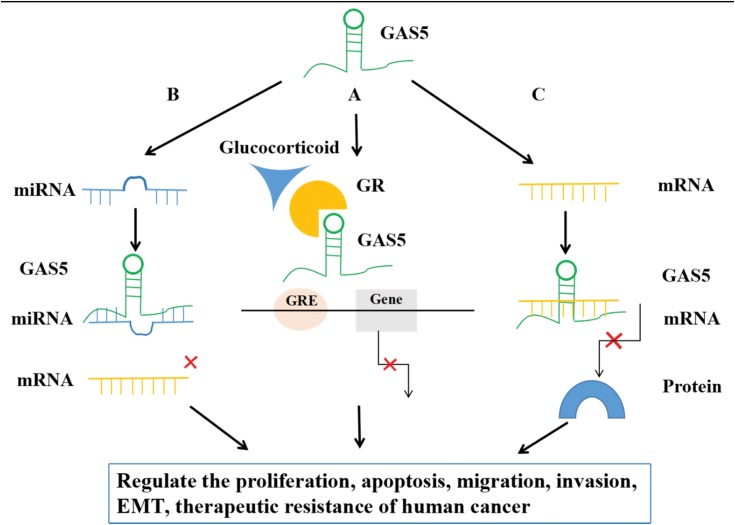
In recent years, GAS5 has raised increased attention due to its downregulation in a wide variety of malignancies, including non-small cell lung cancer (NSCLC),16,17 breast cancer (BC),18,19 hepatocellular carcinoma (HCC),20,21 esophageal carcinoma (EC),22,23 gastric cancer (GC),24,25 colorectal cancer (CRC),26,27 pancreatic cancer (PC),28,29 cervical cancer (CC),30,31 ovarian cancer (OC),32,33 renal cell carcinoma (RCC)34,35 (Figure 1). Clinicopathological characteristics, such as overall survival (OS), disease-free survival (DFS), distant metastasis (DM), lymph node metastasis (LNM), tumor node stage (TNM) and tumor size, are highly correlated with the level of GAS5 expression in cancers,16,19,27,36,37 indicating that GAS5 can be a novel diagnostic and prognostic biomarker, as well as therapeutic target in many human cancers.38,39 Recent studies have demonstrated that GAS5 participates in multiple biological functions, such as cell proliferation, apoptosis, migration, invasion and epithelial–mesenchymal transition (EMT) and DNA repair in cancer.16,40–42 Additionally, GAS5 is also involved in modulating drug- and radio-sensitivities in human cancers.24,43–45 Moreover, GAS5 can exert its biological functions through multiple mechanisms, such as riborepression of steroid hormone, microRNAs (miRNAs) sponging and binding to mRNAs, thereby regulating protein-coding and noncoding gene expressions at transcriptional or post-transcriptional levels (Figure 2).25,38,39 Moreover, recent discovery of the more in-depth mechanisms and roles of GAS5 acting as potential biomarkers and therapeutic targets will open a promising area and hallmark feature for cancer diagnosis and treatment.
Figure 1.
Human GAS5 gene and mature lncRNA GAS5 structure. Blue boxes represent the 12 exons and green lines represent the 11 introns of human GAS5 gene, and red boxes represent the snoRNA sequences present within 10 of its introns. Human GAS5 gene is located at 1q25.1 and comprises 12 exons and 11 introns. GAS5 transcribes to two types of mature lncRNA, namely GAS5a and GAS5b, due to the presence of alternative 5ʹ-splice donor sites in exon 7.
Figure 2.
Mechanism of actions of GAS5 in cancer. There are three different molecular mechanisms of action described for GAS5 to regulate the proliferation, apoptosis, migration, invasion, epithelial–mesenchymal transition (EMT), metastasis and therapeutic resistance of human cancer. GAS5 could affect biological functions through riborepression of steroid hormone, miRNA sponge or binding to mRNAs at transcriptional and translation levels. (A) Transcriptional regulation by GAS5 through riborepression of steroid hormone. The glucocorticoid receptor (GR) regulates cell survival. GAS5 suppressed GR-induced transcriptional activity by acting as a decoy of the glucocorticoid response element (GRE) for binding to the GR. (B) Transcriptional regulation by GAS5 through miRNA sponge. GAS5 may regulate gene expression by binding to miRNAs and consequently preventing specific miRNAs from binding to their target mRNAs, thus regulating the expression of target gene thereby leading to anti-cancer or pro-cancer activities. (C) Translation regulation by GAS5 via binding to mRNAs. GAS5 can regulate gene translation by directly binding to its target mRNAs.
Clinical Relevance
GAS5 is expressed less in a number of malignancies, which was associated with clinio-pathological characteristics and patient prognosis, such as histological differentiation, tumor sizes, tumor node metastasis stage, lymph node metastasis (LNM), infiltration depth, vascular invasion, overall survival (OS), recurrence-free survival (RFS), disease-free survival (DFS), and/or prognostic biomarkers (Table 1). Based on this, GAS5 could be utilized as a diagnostic and prognostic biomarker and therapeutic target in several cancer types. Lung cancer (LC) is one of the most common malignant tumors with the highest morbidity and mortality.46 NSCLC is the most common histological type of LC, which accounts for more than 80% of human LC types.47 In NSCLC, GAS5 levels were reported to be significantly decreased in the cancerous tissues compared to the adjacent normal tissues and healthy controls, and were associated with clinicopathological features.43,44,48–52 One study showed that GAS5 was down-regulated in lung cancerous tissues compared to the adjacent normal tissues and related to tumor sizes and TNM stages.16 NSCLC tissues from patients with progressive disease (PD) had even lower GAS5 expression level.44 Low expression of GAS5 was correlated with advanced clinical stage and larger tumor size in NSCLC51 and was also associated with poorer differentiation and advanced pathological stages.52 In plasma, the levels of GAS5 were significantly lower in patients with NSCLC than those of healthy controls, and GAS5 levels increased after surgery compared to preoperative GAS5 levels.17 The combination of carcinoembryonic antigen (CEA) and GAS5 could produce an area of 0.909 under the receiver-operating characteristic (ROC) curve in distinguishing NSCLC patients from healthy subjects.17 Another group also found that GAS5 expression levels were significantly increased in postoperative groups compared with preoperative ones, and the combination of GAS5, CEA and CA199 showed that the area under the ROC curve was 0.734.49 In addition, single-nucleotide polymorphism (SNP) rs145204276 of GAS5 acted as a protecting factor for LC risk, and patients with del/del genotype showed increased GAS5 expression level and decreased susceptibility of LC.50 Together, these findings indicate that GAS5 is a highly specific and detectable diagnostic biomarker for LC. Breast cancer (BC) is one of the most common types of neoplasms and the second leading cause of death in women worldwide.53 There are nearly 1.3 million newly diagnosed BC cases and approximately 500,000 deaths yearly in the world.54 GAS5 levels were significantly decreased in BC tissues than those in adjacent normal breast epithelial tissues.18,55,56 One study showed low GAS5 expression in triple-negative breast cancer (TNBC) tissues than that of adjacent non-tumor tissues, and was associated with clinical stage, lymph node metastasis (LNM) and OS.19 Decreased expression of GAS5 was found in BC tissues and was negatively associated with later TNM stage and shorter OS time as well.45 Intriguingly, BC patients aged less than 45 years old who had much lower GAS5 levels than those of old cases showed the poorer prognosis.56 Others found that GAS5 levels were decreased frequently in estrogen receptor-negative (ER-) BC tissues and cells, and were related with large tumor size, advanced TNM stage.57 BC patients with low GAS5 levels in plasma and a high Ki67 proliferation index before surgery were positively associated with LNM after surgery, indicating that plasma GAS5 may have the potential to assess the surgical effects and prognosis for patients with BC.58 Together, GAS5 may provide a new perspective for understanding the molecular mechanisms of breast carcinogenesis, and a novel approach for the diagnosis and treatment of BC. Esophageal cancer (EC) is a common malignant tumor with a high mortality rate,53 of which 90% of cases account for esophageal squamous cell carcinoma (ESCC).59 GAS5 was frequently down-regulated in ESCC tissues, and patients with low GAS5 expression were associated with advanced stage of ESCC.22 GAS5 expression levels in serum were significantly lower in tumor tissues of patients with EC than those in adjacent healthy ones.23 Another study also found that GAS5 was downregulated in ESCC tissues and EC cells.60 More studies exploring the contribution of GAS5 in the pathogenesis of ESCC and its complications should be encouraged. Gastric cancer (GC) is one of the most common malignant diseases in the world.61 Although great improvements have been made in the diagnosis and treatment of GC, the outcome of GC patients remains unsatisfactory with the 5-year OS rate less than 30%.62 GAS5 expression was significantly reduced in GC tissues and cells relative to normal counterpart.24,25,37,63–65 Low GAS5 expression was associated with large tumor size and advanced pathologic stage.37 Patients with lower GAS5 expression levels had poor DFS and OS than those with higher GAS5 expression, suggesting that GAS5 could be considered as an independent prognostic indicator for GC.37 Lower expression of GAS5 was also correlated with larger tumor size and advanced clinical stage of GC.64 In addition, the SNP rs145204276 of GAS5 was associated with the development and prognosis of GC, and the allele del of rs145204276 is associated with low GC susceptibility and low incidence rate of cancer progression and metastasis, and patients with del/del genotype had a high survival rate and early tumor stage in GC.66 A similar finding was also reported by others, which showed a significant association with a decreased risk of GC, and patients with allele del were less likely to develop LNM.67,68 Together, the results above could enhance our understanding of the important role of GAS5 played in the molecular etiology and new biomarker for prognosis and a therapeutic target for GC intervention. Colorectal cancer (CRC) was predicted to be the second and third leading cause of cancer-related death in men and women, respectively, in Europe.69 Low expression of GAS5 was observed in CRC tissues and cells compared to the normal ones.26,27,70–73 For example, GAS5 was expressed less in the tissues, plasma and exosomes of 158 cases with CRC patients than that of 173 cases of healthy controls and was associated with the TNM stage, LNM, rates of local recurrence and DM, indicating an independent prognostic factor for CRC.27 Similar findings were reported in others.70–72 Lower expression of GAS5 was also associated with large tumor size, high histological grade and late TNM stage in CRC further confirming that GAS5 was an independent predictor for OS in CRC patients.73 Similarly, reduced GAS5 levels were correlated with advanced clinical stage and LNM in CRC.74 Also, rs145204276 mutation of GAS5 was significantly associated with the susceptibility and progression of CRC.75 The allele del of rs145204276 was associated with 21% decreased susceptibility of CRC, and patients with allele del genotype were less likely to have LNM.75 Together, GAS5 plays a crucial role in the clinicopathological characteristics and prognosis of CRC, which may provide significant clues for potential clinical utilities of CRC in the future. Hepatocellular carcinoma (HCC) ranks as the fifth-leading causes of cancer-related deaths for men in the United State.53 Downregulation of GAS5 was found in HCC tissues and cells than those of adjacent normal tissues and normal liver L02 cells, and predicted poor survival of HCC patients. Moreover, expression of GAS5 was correlated with the clinicopathological characteristics of HCC, and patients with high GAS5 expression had longer OS.20,21 Similarly, the expression level of GAS5 was reduced in HCC cases in comparison to normal ones and was associated with tumor size, LNM and clinical stage of HCC, suggesting a novel independent prognostic biomarker of OS of HCC patients.36 Low expression of GAS5 was positively correlated with differentiation and portal vein tumor thrombosis, tumor size, lymph node metastasis and clinical stage of HCC patients.76 Pancreatic cancer (PC) is one of the most common lethal malignancies, with a 5-year OS rate less than 5% due to the advanced stage of disease at initial diagnosis and the lack of effective therapies.77 GAS5 was found to be decreased in human PC tissues and cells compared with those of normal ones.28,29,78 Lower expression of GAS5 in plasma was found in intraductal papillary mucinous neoplasms (IPMNs) compared to non-diseased controls, suggesting that a GAS5-based blood test may be used as an adjunct diagnostic tool for identifying IPMNs.79 Thus, GAS5 may be used as a potential biomarker for diagnosis in patients with PC. Ovarian cancer (OC) is one of the most common gynecological malignancies in the world with low 5-year OS.53,80 Low expression GAS5 was found in OC tissues and cells, 32,33,81 which was negatively associated with advanced clinical stage.32 Studies found that low GAS5 expression levels were correlated with large tumor size, advanced tumor stage, tumor invasive depth, poor DFS, and OS in patients with OC.81–83 These studies suggested that GAS5 represented a novel and a potential target for diagnosis and treatment of OC. Cervical cancer (CC) is the second most common cancer in women worldwide, and it is the leading cause of cancer death among women in developing countries.61 GAS5 expression was decreased in CC tissues and cells as well30,31,84,85 and was associated with FIGO stage, tumor invasion and metastasis of CC patients.30 Patients with lower expression of GAS5 had shorter OS than those with higher GAS5 expression in CC.85 Thus, the current knowledge about the role of GAS5 played in CC contributed to our better understanding of the pathogenesis of this malignancy and development of lncRNA-mediated diagnostic biomarker and therapeutic target in CC. Prostate cancer (PCa) is the most frequently diagnosed malignancy and the second leading contributor to cancer-related death among males in the United States.53 GAS5 expression levels were reduced in the PCa tissues and cells.86,87 Also, the rs145204276 and rs55829688 SNPs of GAS5 may be used as key indicators for the diagnosis and prognosis prediction of PCa.88 Thus, GAS5 provides novel insights to identify potential diagnostic biomarker and therapeutic target for PCa in the clinic. Renal cell carcinoma (RCC) is the third most common urological tumor and represents about 3% of all cancers in adults.89 Among all RCC cases, clear cell renal cell carcinoma (ccRCC) is the major histological subtype, which accounts for more than 70% of all RCC types. According to previous studies, GAS5 expression was decreased in RCC tissues compared to adjacent non-tumor kidney tissues and RCC cells.34,35 Reduction of GAS5 indicated tumor progression and recurrence and was independently associated with DFS of ccRCC patients.34 RCC patients could be clustered by GAS5 and miR-34a co-expression profile, suggesting potential utilities of GAS5 and miR-34a for diagnostic and therapeutic roles in RCC.90 These studies provide evidence that a decrease in GAS5 expression is associated with RCC genesis and progression, and this provided a potential attractive diagnostic biomarker and therapeutic approach for RCC. Bladder cancer (BCa) is the most common malignancy of the urinary system with a low OS.91 GAS5 expression was commonly downregulated in BCa specimens and cells.92–95 For example, lower levels of GAS5 were found in 82 bladder transitional cell carcinomas (BTCC) tissues and cells and were correlated with high pathological grades and short DFS time for BTCC patients.94 Low GAS5 levels was also associated with invasive high-grade tumors, and high European Organization for Research and Treatment of Cancer (EORTC) risk in non-muscle-invasive bladder cancer (NMIBC) patients, and GAS5 loss was correlated with a higher risk for NMIBC short-term relapse and progression, making GAS5 a prognosis biomarker for NMIBC patients.95 Together, acting as a tumor suppressor, GAS5 provided a novel therapeutic strategy and a potential diagnostic and prognostic biomarker for BCa. Glioma is a type of neurologically destructive human brain tumors with a poor prognosis, which accounts for nearly 80% of malignant brain tumors and 30% of all central nervous system tumors.96 Low expression of GAS5 was observed in glioma tissues and cells compared with normal ones.97–99 Also, carriers of the GAS5 del allele were significantly associated with an elevated risk of glioma.100 Glioblastoma (GBM) is a frequently diagnosed aggressive tumor with poor clinical outcome, which accounts for over 60% of primary brain tumors in the human central nervous system.101 GAS5 was significantly reduced in GBM.90 Patients with high GAS5 levels were associated with increased probability of OS and DFS rates in patients with GBM.102 These results suggested that GAS5 might have great potential to be used as a therapeutic target and biomarkers for GBM. Head and neck cancer (HNC) is a heterogeneous group of cancers originating from the upper aero-digestive tract and one of the ten most frequent cancers worldwide. Among those, nasopharyngeal carcinoma (NPC) is a common malignancy of HNC. GAS5 rs2067079 carriers presented a more remarkable increased risk of severe myelosuppression and severe neutropenia in NPC patients after the treatment with chemoradiotherapy.103 Besides, compared with rs6790 AA genotype carriers, rs6790 GG and GA genotype carriers had increased incidence rate of severe myelosuppression and severe neutropenia in NPC patients.103 These results indicated a potential role of GAS5 polymorphisms rs2067079 and rs6790 as predictive biomarkers for chemoradiotherapy-induced toxicities in NPC. The prevalence of thyroid cancer (TC) has increased worldwide in recent decades. GAS5 was expressed less in TC tissues and cells104 and was significantly correlated with TNM staging, LNM and multiple cancer foci of TC.105 The survival rate with high GAS5 expression was higher than that of low GAS5 expression during the DFS and OS periods, and thus GAS5 could be considered as an independent risk factor for prognosis in TC patients.105 These findings suggested that GAS5 could also be potential for therapeutic target and diagnostic biomarker for TC. Skin cancer (SC) represents a major and growing public health problem. Low expression of GAS5 was observed in SC tissues compared to normal ones, and GAS5 could suppress cell proliferation and promote cell apoptosis in SC.106 Melanoma is the most common form of skin cancer as well as the leading cause of death in patients with SC. The GAS5 expression was significantly lower in melanoma tumors relative to that in adjacent noncancerous tissues and correlated with larger tumor size, advanced TNM stage and higher incidences of ulceration and metastasis,107 and significantly associated with DM and TNM stage.108 Osteosarcoma is the most common primary malignant bone tumor developing in childhood and adolescence with poor outcome and the 5-year OS rate of patients with metastatic osteosarcoma is less than 20%.109 GAS5 was down-regulated in MG-63 and OS732 osteosarcoma cells compared to normal hFOB1.19 cells.110 In addition, the genetic variant rs145204276 of GAS5 functioned as a protective factor in the incidence of OS possibly by upregulating the rate of methylation in the 7th CpG site of GAS5 promoter, which could further increase GAS5 levels in osteosarcoma.111 Thus, findings about GAS5 may offer a new potential therapeutic strategy for the patient with osteosarcoma.
Table 1.
The Expression And Clinical Relevance Of GAS5 In Human Cancer
| Cancer Type | Expression | Clinical Relevance | Reference |
|---|---|---|---|
| LC | Down | Small tumor size, early TNM stage and good differentiation, biomarker | 16,17,43,44,48–52 |
| BC | Down | Little LNM, small tumor size, early TNM stage and long OS time, biomarker | 18,19,45,55–58 |
| EC | Down | Early TNM stage | 22,23,60 |
| GC | Down | Small tumor size, early pathologic stage and good prognosis, biomarker | 24,25,63–65 |
| CRC | Down | Early clinical stage, little LNM, low DM rate, small tumor size and long OS time, biomarker | 26,27,70–75 |
| HCC | Down | Small tumor size, little LNM and good differentiation, biomarker | 20,21,36,76,90 |
| CC | Down | Early FIGO stage, biomarker | 30,31,84,85 |
| OC | Down | Small tumor size, early clinical stage, little LNM, long DFS and OS time, biomarker | 32,33,81–83 |
| PCa | Down | Long OS time, biomarker | 86–88 |
| RCC | Down | Long DFS time, biomarker | 34,35,90 |
| BCa | Down | Shallow invasive depth, low EORTC risk, high pathological grades, long DFS and RFS time, biomarker | 92–95,148 |
| GBM | Down | Long OS and DFS time, biomarker | 90,102 |
| TC | Down | Early TNM stage, little LNM, long DFS and OS time, biomarker | 104,105 |
| Melanoma | Down | Small tumor size, early TNM stage and low DM rate | 107,108 |
| Osteosarcoma | Down | Long OS time | 110,111 |
Notes: Clinical relevance includes but not limited to histological differentiation, tumor sizes, tumor node metastasis (TNM) stage, lymph node metastasis (LNM), infiltration depth, vascular invasion, overall survival (OS), recurrence-free survival (RFS), disease-free survival (DFS), and/or prognostic biomarkers.
Abbreviations: LC, lung cancer; BC, breast cancer; EC, esophageal carcinoma; GC, gastric cancer; CRC, colorectal cancer; HCC, hepatocellular carcinoma; CC, cervical cancer; OC, ovarian cancer; PCa, prostate cancer; RCC, renal cell carcinoma; BCa, bladder cancer; GBM, glioblastoma; OSCC, oral squamous cell carcinoma; TC, thyroid cancer; LNM, lymph node metastasis; DM, distance metastasis.
Biological Behaviors
GAS5 was closely involved in proliferation, cell cycle arrest, apoptosis, migration, invasion, chemo-resistance and EMT contribution to the tumorigenesis processes in various tumor cells (Table 2). GAS5 could significantly inhibit the proliferation, invasion, and induce the apoptosis in vitro and in vivo in NSCLC.16 Overexpression of GAS5 inhibited cell proliferation and invasion and promoted apoptosis in vitro and inhibited tumor growth in vivo.48 While silencing of GAS5 diminished the cell growth inhibition induced by cisplatin (DDP) in A549 cells, overexpression of GAS5 enhanced the DDP-inhibited cell viability in A549/DDP NSCLC cells.44 GAS5 was also found to induce cell apoptosis by enhancing DDP sensitivity in NSCLC.44 Overexpression of GAS5 could attenuate proliferation and enhance apoptosis in TNBC cells as well.19 Moreover, expression of GAS5 was found to be decreased in BC tissues of trastuzumab-treated patients and SKBR-3/Tr (trastuzumab-resistant) BC cells.112 Also, inhibition of GAS5 promoted proliferation SKBR-3 cells and reversed lapatinib-induced proliferation inhibition of SKBR-3/Tr BC cells.112 Moreover, the expression of GAS5 increased following the treatment of dendrosomal curcumin (DNC) and facilitated the antitumor effect of DNC in BC cells.41 Thus, the combination of DNC and GAS5 might provide a potential for treatment of drug resistance in BC cells.41 Bharangin, a natural agent obtained from the roots of pigmacopremna herbacea, suppressed the proliferation and induced apoptosis via inducing GAS5 expression in BC cells.113 Paclitaxel (PCT) and bleomycin (BLM) increased expression of GAS5 in BC cells, and the accumulation of GAS5 in exosomes was noticed and showed a prevalent event in apoptotic processes in the MCF-7 and MDA-MB-231 BC cells.114 Interestingly, part of the GAS5 function as anti-tumor activity was observed within the hormone response element mimic (HREM) area of the GAS5 molecule in BC cells.115–117 Similar to GAS5, the expression of GAS5 HREM increased ultraviolet-C-induced apoptosis, cell death upon DNA damage and decreased cell viability in both hormone-sensitive and hormone-insensitive BC cells.115 Moreover, as summarized in one recent review article, overexpression of GAS5 inhibited tumor proliferation, migration and progression via regulating miRNAs expression, blockade of EMT, inhibiting cell cycle progression, and regulating relevant signaling pathways in several digestive malignancies.39 GAS5 was found to be reduced in adriamycin (ADM)-resistant GC cells, and overexpression of GAS5 enhanced the anti-proliferation and pro-apoptosis activity of ADM in SGC-7901/ADM cells, suggesting that GAS5 could induce ADM sensitivity in GC cells.24 Functional studies demonstrated that GAS5 inhibited proliferation and induced G0/G1 cell cycle arrest and apoptosis in CRC cells.71 Overexpression of GAS5 and the small nucleolar RNU44 (SNORD44) by oncolytic adenovirus inhibited growth and induced apoptosis in SW620 and LS174T CRC cells, and suppressed tumor growth in vivo.118 GAS5 could also inhibit migration and invasion of HCC cells in vitro and could suppress tumor metastasis in vivo.20 Corylin, a flavonoid extracted from the nuts of Psoralea corylifolia L. (Fabaceae), which was a widely used anti-inflammatory and anti-cancer agent in China, inhibited the proliferation, EMT, migration and invasion of Huh7 and HepG2 HCC cells through upregulation of GAS5.40 Thus, the above findings suggested an important role of GAS5 in the occurrence, growth, and progression of HCC. Inhibition of GAS5 expression could also confer OC cells with faster proliferation and smaller percentage of apoptosis in vitro, and more aggressive tumor growth in vivo.82 GAS5 prohibited cell proliferation, colony formation, migration and invasion, and increased cell cycle arrest in Hela and Siha CC cells.119 Overexpression of GAS5 inhibited cell proliferation, migration and invasion, induced cell apoptosis, and arrested cell cycle in A498 RCC cells as well.35 Oral squamous cell carcinoma (OSCC) is the most common cancer of HNC. Expression of GAS5 was comparatively low in OSCC, and overexpression of GAS5 inhibited proliferation, migration and invasion in OSCC cells.120
Table 2.
The Effects Of GAS5 On Phenotype In Human Cancer
| Phenotype | Inhibition Or Promotion | Cancer Type |
|---|---|---|
| Proliferation | Inhibited | LC, BC, EC, GC, CRC, HCC, PC, CC, OC, PCa, RCC, BCa, glioma, OSCC, SC, melanoma, osteosarcoma |
| Apoptosis | Promoted | LC, BC, EC, GC, CRC, HCC, PC, ECa, CC, OC, RCC, BCa, glioma, SC, melanoma |
| Cell cycle arrest | Promoted | BC, EC, GC, CRC, PC, CC, PCa, RCC, BCa, melanoma |
| Migration | Inhibited | LC, BC, CRC, HCC, PC, CC, OC, RCC, glioma, OSCC, melanoma, osteosarcoma |
| Invasion | Inhibited | LC, EC, CRC, HCC, PC, CC, OC, RCC, glioma, OSCC, melanoma, osteosarcoma |
| EMT | Inhibited | PC, OSCC |
| Radio and drug therapy sensitivity | Promoted | LC, BC, GC, PC, CC, PCa, RCC, BCa, glioma |
| Angiogenesis | Inhibited | CRC |
Abbreviations: LC, lung cancer; BC, breast cancer; EC, esophageal carcinoma; GC, gastric cancer; CRC, colorectal cancer; HCC, hepatocellular carcinoma; PC, pancreatic cancer; ECa, endometrial cancer; CC, cervical cancer; OC, ovarian cancer; PCa, prostate cancer; RCC, renal cell carcinoma; BCa, bladder cancer; GBM, glioblastoma; OSCC, oral squamous cell carcinoma; TC, thyroid cancer; SC, skin cancer.
Molecular Mechanisms
Studies have shown the high expression pattern and tumor suppressor role of GAS5 in many types of cancer, and dysregulation of expression of GAS5 is involved in biological functions, such as cell proliferation, apoptosis, migration and invasion, through modulating downstream target genes via multiple molecular mechanisms (Tables 2 and 3 and Figure 2). GAS5 could affect biological functions through riborepression of steroid hormone, miRNA sponge or binding to mRNAs at transcriptional and translational levels (Figure 2). GAS5 may also regulate gene expression by binding protein to epigenetically modulate the promoter histone methylation of target gene expression, serving as competing endogenous RNA (ceRNA) to sponge microRNA (miRNA) and through kinase signaling regulatory pathways, among others. GAS5 could significantly inhibit the proliferation, invasion, and induce the apoptosis in vitro and in vivo via regulating p53 and E2F transcription factor 1 (E2F1) expression16 and by inhibiting miR-23a in NSCLC cells.48 GAS5 inhibited the high glucose (HG)-induced proliferation, anti-apoptosis, and migration of PC-9 and H1299 NSCLC cells through degradation of tribbles pseudokinase 3 (TRIB3) protein by ubiquitination, indicating that GAS5/TRIB3 might be novel targets for the prevention and treatment of diabetic NSCLC.121 In addition, exogenously expressed GAS5 repressed cell proliferation and invasion and enhanced the radiosensitivity of NSCLC cells in vitro and in vivo by suppressing miR-135b expression, which deepens our understanding of the mechanism of miRNA–lncRNA interaction and providing a potential therapeutic for patients with NSCLC.43 Moreover, GAS5 inhibited the proliferation and colony formation capability of NSCLC cells and induced the sensitivity of DDP in NSCLC via GAS5/miR-21/PTEN regulatory pathway.51 Also, GAS5 expression was significantly higher in gefitinib-sensitive cells than that in gefitinib-resistant cells.52 Overexpression of GAS5 was inversely correlated with the expression of the EGFR and insulin-like growth factor 1 receptor (IGF-1R) proteins and relevant signaling pathways, and reversed the gefitinib-resistance lung cancer cells in vitro and in vivo, indicating that GAS5 may overcome the resistance to EGFR-tyrosine kinase inhibitors (TKIs) in lung cancer.52 Conversely, knockdown of GAS5 resulted in decreased expression of carbamoyl phosphate synthetase-1 (CPS1) and aldo-keto reductase 1C2 (AKR1C2) target genes in lung cancer cells but not in normal cells, suggesting that GAS5 acted as a regulator in tumorigenesis without disturbing normal cell functions.122 The links of GAS5 and miRNAs in mediating the BC survival were also reported. Overexpression of GAS5 could attenuate proliferation and enhance apoptosis through directly binding to miR-196a-5p, thereby affecting forkhead box protein O1 (FOXO1)/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) regulatory axis in TNBC cells.19 MiR-221/222 acted as oncomiRs via inhibition of GAS5, resulting in enhancing tumor growth in BC.55 Conversely, restoration of GAS5 expression attenuated the anti-apoptotic effects of miR-221/222 in BC cells.55 GAS5 also promoted autophagy in vivo and in vitro via GAS5/miR-23a/autophagy-related 3 (ATG3) regulatory axes in BC cells.57 Likewise, GAS5 functioned as a tumor suppressor in vitro and in vivo in BC through the feedback GAS5/miR-21 regulatory mechanism.123 Additionally, GAS5 levels decreased in MCF-7R tamoxifen (TAM)-resistant cells and overexpression of GAS5 enhanced cell sensitivity to TAM in MCF-7R cells by serving as a molecular sponge for miR-222, thus suppressing PTEN expression.45 GAS5 suppressed the growth of HER2-positive BC cells by acting as a molecular sponge for miR-21, and this led to the de-repression of PTEN as well.112 Conversely, silencing of the putative tumor suppressor and apoptosis-promoting gene GAS5 attenuated cell responses to chemotherapeutic agents; interestingly, GAS5 promoted the apoptosis of triple-negative and oestrogen receptor-positive cells in the presence of dual PI3K/mTOR inhibitor, such as imatinib, which may increase GAS5 expression levels. Thus, dual PI3K/mTOR inhibitor could improve apoptotic responses to conventional chemotherapies via enhancing GAS5 expression in BC.124 GAS5 was increased during DNA damage-induced cell death using bleomycin (BLM) and c-radiation assays in caspase-3-deficient MCF-7 cells.125 Tumor promoter Notch-1 could promote cell proliferation by negatively regulating GAS5 expression in T47D BC cells.126 GAS5 functioned as a tumor suppressor in vitro and in vivo, and was regulated by miR-196a involved in RNA-induced silencing complex (RISC) in ESCC.22 Overexpression of GAS5 inhibited the proliferation and migration of EC9706 and KYSE510 ESCC cells by inactivating the PI3-K/Akt/mTOR signaling pathway.23 Likewise, GAS5 significantly inhibited cell proliferation and invasion, and induced cell cycle arrest by activating the ATM-p-checkpoint kinase 2 (CHK2) pathways and regulating EMT-associated proteins.60 The feedback loop between GAS5 and interferon (INF) signaling via the janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway played an important anti-tumor role of GAS5 in ESCC cells.127 These findings contribute to our understanding of the mechanisms of miRNA–lncRNA interaction and may provide insight for new avenues in diagnosis and treatment of ESCC. GAS5 could function as a competing endogenous RNA (ceRNA) for regulation of miR-23a by binding to its 3ʹ untranslated region (3ʹ UTR) thereby suppressing metallothionein-2A (MT2A), and thus potentially serve as a therapeutic target in GC cells.25 GAS5 induced growth arrest of GC cells by induction of cyclin-dependent kinase (CDK) inhibitor p21 and reduction of CDK6, 64 and through interacting with Y box binding protein 1(YBX1) and regulating expression in GC cells.63 GAS5 also suppressed proliferation via directly targeted and suppressed miR-222 expression, and thereby regulating PTEN/Akt/mTOR signaling pathway in GC cells.65 GAS5 decreased cell proliferation and induced apoptosis via regulating cell cycle activator E2F1 and p21 expressions in GC cells.37 Together, the results above could enhance our understanding of the important role of GAS5 played in the molecular etiology and new biomarker for prognosis and therapeutic target for GC intervention. Upregulated GAS5 also repressed invasion and migration in vitro and inhibited tumor growth, angiogenesis and metastases in vivo via inhibiting the Wnt/β-catenin pathway in CRC indicating a tumor-suppressor role.26 In addition, GAS5 inhibited proliferation, migration and invasion via suppressing miR-221 expression27 and targeting Akt/extracellular regulated protein kinases (ERK) signaling pathways.70 Also, GAS5/miR-182-5p/FOXO3a regulatory axis might play an important role in the regulation of cell growth and apoptosis, and serve as a target for therapeutic utilities in CRC.74 GAS5 inhibited vascular endothelial growth factor (VEGF-A) and cytokine interleukin-10 (IL-10) via NF-κB, and ERK1/2 pathways may provide a potential mechanism underlying the GAS5-based therapies for CRC.72 GAS5 functioned as a tumor suppressor through GAS5/miR-182/angiopoietin-related protein 1 (ANGPTL1) axis in HCC cells.20 Overexpression of GAS5 suppressed migration and invasion through negative regulation of miR-2121 and negatively regulating vimentin expression in HCC cells.76 GAS5 were down-regulated in natural killer (NK) cells of patients with HCC, and more specifically, GAS5 enhanced the killing effect of NK cell through regulating IFN-c secretion, NK cell cytotoxicity, the percentage of CD107a+NK cells on HCC via miR-544/runt-related transcription factor 3 (RUNX3) regulatory axis.128 Moreover, GAS5 was elevated after HCV infection Huh7 cells and upregulated GAS5 inhibited HCV viral replication by decoying HCV NS3 protein, and this led to inhibit HCC cell migration and invasion.129 GAS5 significantly inhibited cell growth of PC in vitro and in vivo.28 Additionally, GAS5 antagonized chemo-resistance through miR-181c-5p/Hippo signaling pathway in PC cells.28 Moreover, GAS5 inhibited cell proliferation and induced a cell cycle arrest by negatively regulating CDK6 expression in PC.29 GAS5 suppressed the proliferation, migration, gemcitabine resistance, stem cell self-renewal and EMT in vitro, and promoted gemcitabine-induced inhibition of tumor growth and metastasis in vivo via the miR-221/suppressor of cytokine signaling 3 (SOCS3) 42 and miR-32-5p/PTEN pathways in PC.78 Thus, GAS5 functions as a tumor suppressor and control proliferation, migration and invasion via multiple mechanisms in PC. Moreover, GAS5 inhibited proliferation and colony formation and promoted apoptosis by triggering shaping of inflammasomes in OC cells.33 Mechanistically, overexpression of GAS5 inhibited proliferation and induced apoptosis of OC via directly targeting miR-196a-5p and thereby reducing homeobox protein Hox-A5 (HOXA5) expression82 and through suppressing miR-21 expression and increasing subsequent sprouty homolog 2 (SPRY2) expression in OC cells.32 GAS5 could also suppress proliferation of OC cells via regulating p21, cyclin D1 and apoptosis protease activating factor 1 (APAF1) expressions.81 Moreover, exogenously expressed GAS5 inhibited proliferation and promoted apoptosis of OC cells by disrupting mitochondrial membrane potential and promoting BCL2 associated X (BAX), Bcl-2 homologous antagonist/killer (BAK), cleaved-caspase 3 and cleaved-caspase 9 expression.83 Together, these studies suggested that GAS5 depressed proliferation and induced apoptosis of OC via multiple mechanisms. Overexpression of GAS5 suppressed CC cell proliferation, invasion, and promoted apoptosis in vitro and hindered tumor growth in vivo through regulating miR-196a/FOXO1 and miR-205/PTEN pathways.31 Additionally, GAS5 could inhibit proliferation, migration, and enhance DPP sensitivity via regulating the phosphorylation of Akt in CC cells.84 Moreover, GAS5 enhanced the activity of DPP-induced cell growth inhibition and apoptosis in DPP-resistance cell via GAS5/miR-21/E2F transcription factor 3 (E2F3)/STAT pathway in CC, suggesting that GAS5 could be a novel target for CC patients with DPP-resistance in clinical.119 Overexpression of GAS5 enhanced radio-sensitivity in SiHa CC cells, while GAS5 knockdown decreased radio-sensitivity in ME180 CC cells.130 In-depth mechanism studies showed that GAS5 enhanced the radio-sensitivity of CC cells via inducing immediate early response 3 (IER3) through regulation of miR-106b expression.130 GAS5 was increased during DNA damage-induced cell death using BLM and c-radiation in HeLa cells.125 Interestingly, the induction of GAS5 was mainly restricted to irradiated cells, revealing a differential regulation of GAS5 during genotoxic stress.125 Endometrial carcinoma (ECa) is one of the most frequent gynecologic malignancy and is also the fourth most common cancer among females in the United States.131 GAS5 was down-regulated in ECa cells and promoted cell apoptosis through miR-103/PTEN axis in ECa. Thus, acted as a tumor suppressor, GAS5 could be an important mediator in the pathogenesis of ECa.132 Moreover, the GAS5/miR-21 programmed cell death protein 4 (PDCD4)/PTEN and GAS5/miR-1284/Akt signaling pathways might be closely related to OS rate and prognosis in patient with PCa.88 GAS5 inhibited cell proliferation and induced cell cycle arrest via interacting with E2F1 and thus enhancing the binding of E2F1 to the P27Kip1 promoter.86 GAS5 played a vital role in decelerating PCa development via mediating the Akt/mTOR signaling pathway through targeting miR-103.87 Overexpression of GAS5 could increase apoptosis and decrease cell survival in 22Rv1and PC-3 cells and could also augment cell death of 22Rv1 induced by UV-C irradiation and chemotherapeutic drugs.133 Cellular GAS5 expression can be enhanced by the use of mTOR inhibitors in androgen-dependent/sensitive PCa cells (LNCaP/22Rv1) but not in androgen-independent cells (PC-3 and DU145).134 Silencing of GAS5 decreased the sensitivity to mTOR inhibitors in LNCaP and 22Rv1 cells, while GAS5 overexpression sensitized PC-3 and DU145 cells to these agents.134 The refractoriness of androgen-independent cells might be due to their low expression levels of GAS5 since GAS5 positively regulated mTOR inhibitor action.134 Thus, potential therapeutic utilities such as increase in GAS5 expression in advanced PCa may be beneficial in promoting the effectiveness of chemotherapeutic and irradiation agents to enhance therapeutic outcomes of patients with PCa. Silencing of GAS5 also enhanced the tumorigenicity of A498 and 786-O RCC cells, and GAS5 might act as a ceRNA to regulate human ZRT, IRT-like protein 1 (hZIP1) by sponging miR-223 in ccRCC cells.34 Moreover, GAS5 overexpression conferred sorafenib sensitive to RCC through miR-21/sex-determining region Y-box protein 5 (SOX5) regulatory pathway, suggesting that GAS5 could be a potential therapeutic target for sorafenib treatment in RCC.135 Functionally, overexpression of GAS5 reduced viability and induced apoptosis via interacting with E2F transcription factor 4 (E2F4) and recruiting to enhancer of zeste homolog 2 (EZH2) promoter, which further repressed EZH2 transcription in T24 and EJ BCa cells.93 GAS5 suppressed proliferation and induced cell cycle arrest via suppressing chemokine ligand 1 (CCL1) expression136 and regulating CDK6 in BCa cells.92 Overexpression of GAS5 significantly inhibited proliferation, reduced the chemotherapy resistance to doxorubicin (DOX) and promoted apoptosis in T24/DOX cells via depressing the expression of anti-apoptosis protein B-cell lymphoma 2 (Bcl2).94 Together, acting as a tumor suppressor, GAS5 provided a novel therapeutic strategy and a potential diagnostic and prognostic biomarker for BCa. GAS5 could inhibit malignant phenotypes of proliferation, migration and invasion by repressing miR-18a-5p expression98 and directly targeting miR-222 and further increasing a pro-apoptotic factor Bcl-2-modifying factor (BMF) and Plexin C1 (PLXN C1) expression.99 GAS5 suppressed glioma stem cells (GSCs) malignancy through a miR196a-5p/FOXO1 feedback regulatory loop.137 The expression of GAS5 was lower in cisplatin-resistance cells than cisplatin-sensitive cells.97 Silencing of GAS5 enhanced cell resistance to DDP in U87 cells, while elevation of GAS5 enhanced cell sensitivity to DDP in U138 cells by suppressing excessive autophagy through the activation of mTOR signaling.97 The induction of GAS5 was observed during DNA damage-induced apoptosis in U251 and U87 cells using DOX, suggesting that the distinct regulation of GAS5 may be possibly involved in the process of cellular defense against genotoxic agents.138 It was also reported that GAS5 was up-regulated after EGFR-TKI erlotinib treatment in glioma cells.139 Intriguingly, silencing of GAS5 sensitized T98 glioma cells to erlotinib, suggesting that GAS5 played a role in the mechanism of erlotinib resistance of glioma cells under certain genetic contexts.139 One study found that DNA comet tail length of T98G was negatively correlated with the GAS5 levels, and miR-222/GAS5 might be involved in DNA damage and cytotoxic effects induced by an oral alkylating agent temozolomide (TMZ) in glioma cells.140 On the contrary, inhibition of GAS5 promoted proliferation, migration, invasion and EMT of OSCC cells through the miR-21/PTEN pathway.141 In addition, GAS5 acted as a ceRNA, effectively becoming a sink for miR-222-3p, activating PTEN/Akt pathway, to play its tumor-suppressive role in vitro and in vivo in papillary thyroid carcinoma (PTC).104 Knockdown of GAS5 increased proliferation through increase in cyclin D1, CDK4, and p27 expressions and inhibited apoptosis through an increase in Bcl-2 expression via oxidative status and redox balance signaling regulatory axis and interaction between GAS5 and the G6PD protein in melanoma cells.107 GAS5 suppressed proliferation, migration and invasion in vitro and inhibited growth in vivo via GAS5/miR-137 pathway in melanoma cells.108 Overexpressing GAS5 inhibited the migration and invasion by decreasing the matrix metalloproteinase-2 (MMP2) expression in SK-Mel-110 cells142 and MMP9 expressions in melanoma cells.143 Together, these studies open up new opportunities for designing innovative agents for improving prognosis by site-directed overexpressing of GAS5 in melanoma. In osteosarcoma cells, GAS5 suppressed the proliferation, migration and EMT via regulating miR-221/aplasia Ras homologue member I (ARHI) regulatory pathway.144 Silencing GAS5 significantly promoted viability, migration and invasion by acting as a sponge for miR-203a, sequestering miR-203a away from tissue inhibitor of metalloproteinases 2 (TIMP2) via deactivating PI3K/Akt/glycogen synthase kinase 3 beta (GSK3β) and activating NF-κB signaling pathways in MG-63 osteosarcoma cells.110 In addition, zinc finger and BTB domain-containing protein 7A (ZBTB7A) bound to the promoter of GAS5 and suppressed GAS5 expression, leading to a decline in endoplasmic reticulum (ER) stress-induced cell apoptosis by tunicamycin (TM) or thapsigargin (TG) in osteosarcoma cells, suggesting that miR-663a/ZBTB7A/GAS5 regulatory pathway might be essential for the growth osteosarcoma cells under ER stress.145 Nevertheless, the tumor-suppressor potential and clinical uses of GAS5 in various cancer types are still required to be further elucidated in the future.
Table 3.
The Potential Target Genes Of GAS5 In Human Cancer
| Cancer Type | Target Gene | Reference |
|---|---|---|
| LC | E2F1, TRIB3, EGFR, IGF1R, p53, miR-23a, miR-135b, miR-21 | 16,17,43,44,48–52,121,122 |
| BC | miR-196a-5p, miR-23a, miR-222, miR-21 | 18,19,41,45,55–57,112,115,116,123,124 |
| EC | miR-196a, miR-301a, PI3K, CHK2, JAK | 22,23,60,127 |
| GC | miR-23a, miR-222, p21, E2F1, YBX1, CDK6 | 24,25,63–65 |
| CRC | miR-221, miR-182-5p, Wnt, AKT, NF-κB, ERK | 26,27,70–74,118 |
| HCC | miR-544, miR-182, miR-21, vimentin | 21,36,40,76,90,128,129 |
| PC | miR-221, miR-181c-5p, miR-32-5p, CDK6 | 28,29,42,78,79 |
| ECa | miR-103 | 132 |
| CC | miR‐21, miR-106b, miR-196a, miR-205, AKT | 30,31,84,85,119,130 |
| OC | miR-196a-5p, miR-21, cyclin D1, p21, APAF1 | 32,33,81–83,149 |
| PCa | miR‐21, miR‐1284, miR-103, E2F1 | 86-88,133,134 |
| RCC | miR-21, miR-223 | 34,35,90,135 |
| BCa | E2F4, Bcl2, CCL1, CDK6 | 92–95,136,148,150 |
| Glioma | mTOR, miR-18a-5p, miR-196a-5p, miR222 |
97–99,137–139 |
| GBM | NA | 90,102,140 |
| OSCC | miR-21 | 120,141 |
| TC | miR-222-3p | 104,105 |
| SC | NA | 106 |
| Melanoma | miR-137, G6PD, MMP2, MMP9 | 107,108,142,143 |
| Osteosarcoma | miR-203a, miR-221 | 110,111,144 |
Discussion
LncRNAs have raised widespread attention in recent years as a potentially new and crucial player of fundamental biological functions. With the help of next-generation sequencing technology, computational prediction of lncRNAs from ultra-deep RNA sequencing, and lncRNA chip-based screening, hundreds of lncRNAs are found to be associated with the initiation and development of human cancers. LncRNAs affect cancer cellular processes, such as proliferation, apoptosis, migration and invasion. Clinical data demonstrated that aberrant expression of lncRNAs was related to the outcomes of cancer patients. Moreover, researchers have begun to develop lncRNA-targeting therapy based on the evidence for the role of specific lncRNAs and their specific action in tumor development.
GAS5 lncRNA is down-regulated in multiple cancers and related to both clinicopathological characteristics and patient prognosis. Low expression levels of GAS5 predicted poor OS and RFS, and patients with low GAS5 expression were more prone to LNM and advanced TNM stage in various cancers. Given the correlations between GAS5 levels and clinicopathological characteristics, GAS5 could be used as a diagnostic and prognostic biomarker in cancer patients. Moreover, it also has the potential for monitoring therapeutic responses. However, the clinic value of GAS5 in distinguishing benign lesions and malignancies, monitoring early recurrence and guiding therapeutic still need further exploration and validation.38 A large body of evidence has shown that modulation of GAS5 is capable of regulating cell proliferation, apoptosis, invasion, metastasis, EMT, therapeutic resistance in cancer and that these cellular mechanisms are likely to form the basis of its tumor-suppressor action. In preclinical observations, reductions in endogenous GAS5 expression may adversely impact the therapeutic responses in cancer. Advanced hormone-independent breast and prostate cancers often exhibit low levels of GAS5 expression.123,124,133 However, they can maintain the ability to respond to ectopic GAS5.124,133 Therefore, forced expression of GAS5 would be an attractive potential promising therapeutic strategy for these types of cancer. One simple way to achieve this goal may be to exploit the molecular mechanisms that control cellular GAS5 levels through the use of mTOR inhibitor as targeting mTOR are already in clinical use.146,147 Also, DNA methylation modification is another mechanism in modulating GAS5 expression level. Thus, we can achieve GAS5 enhancement by using DNA methyltransferase inhibitor, which is independent of inherent GAS5 expression level. Given the fact that the translation from basic researches to clinical utilities still needs more in-depth experiments and exploration, more efforts are needed in understanding of the mechanisms underlying the contribution of GAS5 inhibition, which may unveil novel therapeutic strategies.
The molecular mechanism of cancer is a complicated biological process, which may involve in stepwise accumulation of gene mutations, genomic instability, epigenetic changes and aberrant protein-coding and noncoding gene expressions. Instead of the traditional concepts that only protein coding genes can play vital roles in biological processes, lncRNAs such as GAS5 provide a novel insight into the understanding of cancer occurrence and development, and even therapeutic potential. Regardless, targeting of GAS5 as a novel diagnosis biomarker and therapeutic approach still require an in-depth understanding of the biological functions and molecular mechanisms.38 From a clinical perspective, more effort in understanding the role of GAS5 in cancer biology will provide opportunities of biological functions and molecular mechanisms for cancer occurrence, progression and treatment, and unveil a novel diagnostic biomarker and therapeutic target in improving patient outcome.
Conclusion And Prospective
Recent studies lead to significant progress in understanding the biology of lncRNA, including GAS5, and even in the development of biomarkers and therapeutic application. The present study is to assess the expression, function and mechanism of GAS5 and their clinical utilities in diagnosis and therapeutic potential in human cancer. Our current knowledge provides the basis that GAS5 may be a promising biomarker for cancer development and diagnosis and has potential utilities in cancer treatment. Moreover, GAS5 is associated with the clinical-pathological characteristics, prognosis of cancer, indicating that GAS5 may potentially be used as a predictive biomarker for cancer patients. It is necessary to systematically study with a larger patient cohort to accelerate its clinical application. In addition, the important role of SNP of GAS5 in cancer especially rs145204276 played in occurrence of several malignancies, such as hepatocarcinoma, colorectal cancer, and gastric cancer, and regulated the original tumor-suppressor effect of GAS5 may open a hallmark feature of cancer prevention and treatment in the future.39
Although GAS5 holds potential promise towards the discovery of novel diagnostic and therapeutic for cancer, there are still many challenges. Currently, the GAS5 research is still in its infancy, the biological functions and mechanism of GAS5 involved are more complicated than we can think and some still remain uncharacterized. The precise mechanism of down-regulation of GAS5 and its central downstream signaling pathway are still not clear and need further analysis and confirmation. Thus, more efforts are still needed to focus on understanding the mechanisms and regulation of GAS5 in cancer, such as the biochemical and structural nature of GAS5-miRNA and GAS5–protein interactions, all of which may provide an in-depth understanding of the role of GAS5 in cancer biology and offer evidence for the development of therapeutic strategies for cancer. Moreover, there are some unresolved issues to transfer the basic and translational areas into the clinical arena to benefit cancer patients. Thus, future well-designed, large-size and high-quality patient cohorts studies are required to explore not only functional characterization, but also optimize isolation procedures and tissue-specific delivery methods to confirm the clinical value for the use of GAS5 as a diagnostic and prognostic marker, and a therapeutic target in clinic in cancer.
Acknowledgments
This work was supported in part by the grants from the National Nature Scientific Foundation of China (No. 81871863) and the Major Program of National Natural Science Foundation of Guangdong (No. 2018B030311061).
Author Contributions
All authors (YY and SSH) contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Booton R, Lindsay MA. Emerging role of microRNAs and long noncoding RNAs in respiratory disease. Chest. 2014;146:193–204. [DOI] [PubMed] [Google Scholar]
- 2.Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145:178–181. [DOI] [PubMed] [Google Scholar]
- 3.Shi X, Sun M, Wu Y, et al. Post-transcriptional regulation of long noncoding RNAs in cancer. Tumour Biol. 2015;36:503–513. [DOI] [PubMed] [Google Scholar]
- 4.Li CH, Chen Y. Targeting long non-coding RNAs in cancers: progress and prospects. Int J Biochem Cell Biol. 2013;45:1895–1910. [DOI] [PubMed] [Google Scholar]
- 5.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Zou X, He J, Mao Y. Identification of long non-coding RNAs biomarkers associated with progression of endometrial carcinoma and patient outcomes. Oncotarget. 2017;8:52604–52613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Q, Liu H, Cheng H, Li Y, Li X, Zhu C. Downregulation of long noncoding RNA TUG1 inhibits proliferation and induces apoptosis through the TUG1/miR-142/ZEB2 axis in bladder cancer cells. Onco Targets Ther. 2017;10:2461–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–793. [DOI] [PubMed] [Google Scholar]
- 10.Smith CM, Steitz JA. Classification of gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member of the 5ʹ-terminal oligopyrimidine gene family reveals common features of snoRNA host genes. Mol Cell Biol. 1998;18:6897–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coccia EM, Cicala C, Charlesworth A, et al. Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol Cell Biol. 1992;12:3514–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming JV, Hay SM, Harries DN, Rees WD. Effects of nutrient deprivation and differentiation on the expression of growth-arrest genes (gas and gadd) in F9 embryonal carcinoma cells. Biochem J. 1998;330(Pt 1):573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mourtada-Maarabouni M, Hasan AM, Farzaneh F, Williams GT. Inhibition of human T-cell proliferation by mammalian target of rapamycin (mTOR) antagonists requires noncoding RNA growth-arrest-specific transcript 5 (GAS5). Mol Pharmacol. 2010;78:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tani H, Torimura M, Akimitsu N. The RNA degradation pathway regulates the function of GAS5 a non-coding RNA in mammalian cells. PLoS One. 2013;8:e55684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur J Biochem. 2000;267:6321–6330. [DOI] [PubMed] [Google Scholar]
- 16.Shi X, Sun M, Liu H, et al. A critical role for the long non-coding RNA GAS5 in proliferation and apoptosis in non-small-cell lung cancer. Mol Carcinog. 2015;54(Suppl 1):E1–E12. [DOI] [PubMed] [Google Scholar]
- 17.Liang W, Lv T, Shi X, et al. Circulating long noncoding RNA GAS5 is a novel biomarker for the diagnosis of nonsmall cell lung cancer. Medicine (Baltimore). 2016;95:e4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Zhou J, Wang Z, Wang P, Gao X, Wang Y. Long noncoding RNA GAS5 suppresses triple negative breast cancer progression through inhibition of proliferation and invasion by competitively binding miR-196a-5p. Biomed Pharmacother. 2018;104:451–457. [DOI] [PubMed] [Google Scholar]
- 20.Chen F, Li Y, Li M, Wang L. Long noncoding RNA GAS5 inhibits metastasis by targeting miR-182/ANGPTL1 in hepatocellular carcinoma. Am J Cancer Res. 2019;9:108–121. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Hu L, Ye H, Huang G, et al. Long noncoding RNA GAS5 suppresses the migration and invasion of hepatocellular carcinoma cells via miR-21. Tumour Biol. 2016;37:2691–2702. [DOI] [PubMed] [Google Scholar]
- 22.Wang K, Li J, Xiong G, et al. Negative regulation of lncRNA GAS5 by miR-196a inhibits esophageal squamous cell carcinoma growth. Biochem Biophys Res Commun. 2018;495:1151–1157. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Sun J, Zhao H, Li H. Long non-coding RNA (lncRNA) growth arrest specific 5 (GAS5) suppresses esophageal squamous cell carcinoma cell proliferation and migration by inactivating phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway. Med Sci Monit. 2018;24:7689–7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren S, Li G, Liu C, et al. Next generation deep sequencing identified a novel lncRNA n375709 associated with paclitaxel resistance in nasopharyngeal carcinoma. Oncol Rep. 2016;36:1861–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Jiao T, Wang Y, Su W, Tang Z, Han C. Long non-coding RNA GAS5 acts as a molecular sponge to regulate miR-23a in gastric cancer. Minerva Med. 2016;235:616–621. [PubMed] [Google Scholar]
- 26.Song J, Shu H, Zhang L, Xiong J. Long noncoding RNA GAS5 inhibits angiogenesis and metastasis of colorectal cancer through the Wnt/beta-catenin signaling pathway. J Cell Biochem. 2019;120:6937–6951. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Meng T, Yang XH, et al. Prognostic and predictive value of long non-coding RNA GAS5 and mircoRNA-221 in colorectal cancer and their effects on colorectal cancer cell proliferation, migration and invasion. Cancer Biomark. 2018;22:283–299. [DOI] [PubMed] [Google Scholar]
- 28.Gao ZQ, Wang JF, Chen DH, et al. Long non-coding RNA GAS5 antagonizes the chemoresistance of pancreatic cancer cells through down-regulation of miR-181c-5p. Biomed Pharmacother. 2018;97:809–817. [DOI] [PubMed] [Google Scholar]
- 29.Lu X, Fang Y, Wang Z, et al. Downregulation of gas5 increases pancreatic cancer cell proliferation by regulating CDK6. Cell Tissue Res. 2013;354:891–896. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Wan YP, Bai Y. Correlation between long strand non-coding RNA GASS expression and prognosis of cervical cancer patients. Eur Rev Med Pharmacol Sci. 2018;22:943–949. [DOI] [PubMed] [Google Scholar]
- 31.Yang W, Hong L, Xu X, Wang Q, Huang J, Jiang L. LncRNA GAS5 suppresses the tumorigenesis of cervical cancer by downregulating miR-196a and miR-205. Tumour Biol. 2017;39:1010428317711315. [DOI] [PubMed] [Google Scholar]
- 32.Ma N, Li S, Zhang Q, Wang H, Qin H, Wang S. Long non-coding RNA GAS5 inhibits ovarian cancer cell proliferation via the control of microRNA-21 and SPRY2 expression. Exp Ther Med. 2018;16:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Yang C, Li Y, Chen A, Li L, You Z. LncRNA GAS5 suppresses ovarian cancer by inducing inflammasome formation. Biosci Rep. 2017;38:BSR20171150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong X, Kong C, Liu X, et al. GAS5 functions as a ceRNA to regulate hZIP1 expression by sponging miR-223 in clear cell renal cell carcinoma. Am J Cancer Res. 2018;8:1414–1426. [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao HP, Gao WS, Huo JX, Yang ZS. Long non-coding RNA GAS5 functions as a tumor suppressor in renal cell carcinoma. Asian Pac J Cancer Prev. 2013;14:1077–1082. [DOI] [PubMed] [Google Scholar]
- 36.Tu ZQ, Li RJ, Mei JZ, Li XH. Down-regulation of long non-coding RNA GAS5 is associated with the prognosis of hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:4303–4309. [PMC free article] [PubMed] [Google Scholar]
- 37.Sun M, Jin FY, Xia R, et al. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma C, Shi X, Zhu Q, et al. The growth arrest-specific transcript 5 (GAS5): a pivotal tumor suppressor long noncoding RNA in human cancers. Tumour Biol. 2016;37:1437–1444. [DOI] [PubMed] [Google Scholar]
- 39.Lu S, Su Z, Fu W, Cui Z, Jiang X, Tai S. Altered expression of long non-coding RNA GAS5 in digestive tumors. Biosci Rep. 2019;39:BSR20180789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen CY, Chen CC, Shieh TM, et al. Corylin suppresses hepatocellular carcinoma progression via the inhibition of epithelial-mesenchymal transition, mediated by long noncoding RNA GAS5. Int J Mol Sci. 2018;19:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esmatabadi MJD, Motamedrad M, Sadeghizadeh M. Down-regulation of lncRNA, GAS5 decreases chemotherapeutic effect of dendrosomal curcumin (DNC) in breast cancer cells. Phytomedicine. 2018;42:56–65. [DOI] [PubMed] [Google Scholar]
- 42.Liu B, Wu S, Ma J, et al. lncRNA GAS5 reverses EMT and tumor stem cell-mediated gemcitabine resistance and metastasis by targeting miR-221/SOCS3 in pancreatic cancer. Mol Ther Nucleic Acids. 2018;13:472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue Y, Ni T, Jiang Y, Li Y. Long noncoding RNA GAS5 inhibits tumorigenesis and enhances radiosensitivity by suppressing miR-135b expression in non-small cell lung cancer. Oncol Res. 2017;25:1305–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang N, Yang GQ, Shao XM, Wei L. GAS5 modulated autophagy is a mechanism modulating cisplatin sensitivity in NSCLC cells. Eur Rev Med Pharmacol Sci. 2016;20:2271–2277. [PubMed] [Google Scholar]
- 45.Gu J, Wang Y, Wang X, et al. Downregulation of lncRNA GAS5 confers tamoxifen resistance by activating miR-222 in breast cancer. Cancer Lett. 2018;434:1–10. [DOI] [PubMed] [Google Scholar]
- 46.Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372:1700–1709. [DOI] [PubMed] [Google Scholar]
- 47.Tang S, Pan Y, Wang Y, et al. Genome-wide association study of survival in early-stage non-small cell lung cancer. Ann Surg Oncol. 2015;22:630–635. [DOI] [PubMed] [Google Scholar]
- 48.Mei Y, Si J, Wang Y, et al. Long noncoding RNA GAS5 suppresses tumorigenesis by inhibiting miR-23a expression in non-small cell lung cancer. Oncol Res. 2017;25:1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Y, He Y, Shi L, et al. Co-expression of AFAP1-AS1 and PD-1 predicts poor prognosis in nasopharyngeal carcinoma. Oncotarget. 2017;8:39001–39011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W, Huang K, Wen F, Cui G, Guo H, Zhao S. Genetic variation of lncRNA GAS5 contributes to the development of lung cancer. Oncotarget. 2017;8:91025–91029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao L, Chen J, Ou B, Liu C, Zou Y, Chen Q. GAS5 knockdown reduces the chemo-sensitivity of non-small cell lung cancer (NSCLC) cell to cisplatin (DDP) through regulating miR-21/PTEN axis. Biomed Pharmacother. 2017;93:570–579. [DOI] [PubMed] [Google Scholar]
- 52.Dong S, Qu X, Li W, et al. The long non-coding RNA, GAS5, enhances gefitinib-induced cell death in innate EGFR tyrosine kinase inhibitor-resistant lung adenocarcinoma cells with wide-type EGFR via downregulation of the IGF-1R expression. J Hematol Oncol. 2015;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 54.Levy AR, Bruen BK, Ku L. Health care reform and women’s insurance coverage for breast and cervical cancer screening. Prev Chronic Dis. 2012;9:E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zong Y, Zhang Y, Sun X, Xu T, Cheng X, Qin Y. miR-221/222 promote tumor growth and suppress apoptosis by targeting lncRNA GAS5 in breast cancer. Biosci Rep. 2019;39:BSR20181859. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Arshi A, Sharifi FS, Khorramian Ghahfarokhi M, et al. Expression analysis of MALAT1, GAS5, SRA, and NEAT1 lncRNAs in breast cancer tissues from young women and women over 45 years of age. Mol Ther Nucleic Acids. 2018;12:751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gu J, Wang Y, Wang X, et al. Effect of the LncRNA GAS5-MiR-23a-ATG3 axis in regulating autophagy in patients with breast cancer. Cell Physiol Biochem. 2018;48:194–207. [DOI] [PubMed] [Google Scholar]
- 58.Han L, Ma P, Liu SM, Zhou X. Circulating long noncoding RNA GAS5 as a potential biomarker in breast cancer for assessing the surgical effects. Tumour Biol. 2016;37:6847–6854. [DOI] [PubMed] [Google Scholar]
- 59.Hu D, Zhang M, Wang S, Wang Z. High expression of cyclooxygenase 2 is an indicator of prognosis for patients with esophageal squamous cell carcinoma after Ivor Lewis esophagectomy. Thorac Cancer. 2016;7:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ke K, Sun Z, Wang Z. Downregulation of long non-coding RNA GAS5 promotes cell proliferation, migration and invasion in esophageal squamous cell carcinoma. Oncol Lett. 2018;16:1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 62.Kagawa S, Shigeyasu K, Ishida M, et al. Molecular diagnosis and therapy for occult peritoneal metastasis in gastric cancer patients. World J Gastroenterol. 2014;20:17796–17803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Zhao J, Zhang W, et al. lncRNA GAS5 enhances G1 cell cycle arrest via binding to YBX1 to regulate p21 expression in stomach cancer. Sci Rep. 2015;5:10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo X, Deng K, Wang H, et al. GAS5 inhibits gastric cancer cell proliferation partly by modulating CDK6. Oncol Res Treat. 2015;38:362–366. [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Gu J, Lu H. The GAS5/miR-222 axis regulates proliferation of gastric cancer cells through the PTEN/Akt/mTOR pathway. Dig Dis Sci. 2017;62:3426–3437. [DOI] [PubMed] [Google Scholar]
- 66.Li QJ, Ma G, Guo HM, Sun SH, Xu Y, Wang BJ. The variant rs145204276 of GAS5 is associated with the development and prognosis of gastric cancer. J Gastrointestin Liver Dis. 2018;27:19–24. [DOI] [PubMed] [Google Scholar]
- 67.Li Q, Ma G, Sun S, Xu Y, Wang B. Polymorphism in the promoter region of lncRNA GAS5 is functionally associated with the risk of gastric cancer. Clin Res Hepatol Gastroenterol. 2018;42:478–482. [DOI] [PubMed] [Google Scholar]
- 68.Aminian K, Mashayekhi F, Mirzanejad L, Salehi Z. A functional genetic variant in GAS5 lncRNA (rs145204276) modulates p27(Kip1) expression and confers risk for gastric cancer. Br J Biomed Sci. 2018;76:1–3. [DOI] [PubMed] [Google Scholar]
- 69.Malvezzi M, Carioli G, Bertuccio P, et al. European cancer mortality predictions for the year 2017, with focus on lung cancer. Ann Oncol. 2017;28:1117–1123. [DOI] [PubMed] [Google Scholar]
- 70.Li J, Wang Y, Zhang CG, Xiao HJ, Hou JM, He JD. Effect of long non-coding RNA Gas5 on proliferation, migration, invasion and apoptosis of colorectal cancer HT-29 cell line. Cancer Cell Int. 2018;18:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang Y, Shen Z, Yan Y, et al. Long non-coding RNA GAS5 inhibits cell proliferation, induces G0/G1 arrest and apoptosis, and functions as a prognostic marker in colorectal cancer. Oncol Lett. 2017;13:3151–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu Z, Li G, Li Z, et al. Endogenous miRNA sponge LincRNA-ROR promotes proliferation, invasion and stem cell-like phenotype of pancreatic cancer cells. Cell Death Discov. 2017;3:17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang QR, Fan HN, Yin ZX, et al. Effect of curcumin on radiosensitization of CNE-2 cells and its mechanism. Zhongguo Zhong Yao Za Zhi. 2014;39:507–510. [PubMed] [Google Scholar]
- 74.Cheng K, Zhao Z, Wang G, Wang J, Zhu W. lncRNA GAS5 inhibits colorectal cancer cell proliferation via the miR1825p/FOXO3a axis. Oncol Rep. 2018;40:2371–2380. [DOI] [PubMed] [Google Scholar]
- 75.Zheng Y, Song D, Xiao K, et al. LncRNA GAS5 contributes to lymphatic metastasis in colorectal cancer. Oncotarget. 2016;7:83727–83734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang L, Li C, Lan T, et al. Decreased expression of long non-coding RNA GAS5 indicates a poor prognosis and promotes cell proliferation and invasion in hepatocellular carcinoma by regulating vimentin. Mol Med Rep. 2016;13:1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. [DOI] [PubMed] [Google Scholar]
- 78.Gao ZQ, Wang JF, Chen DH, et al. Long non-coding RNA GAS5 suppresses pancreatic cancer metastasis through modulating miR-32-5p/PTEN axis. Cell Biosci. 2017;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Permuth JB, Chen DT, Yoder SJ, et al. Linc-ing circulating long non-coding RNAs to the diagnosis and malignant prediction of intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2017;7:10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mizrahi A, Czerniak A, Levy T, et al. Development of targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences. J Transl Med. 2009;7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li J, Huang H, Li Y, Li L, Hou W, You Z. Decreased expression of long non-coding RNA GAS5 promotes cell proliferation, migration and invasion, and indicates a poor prognosis in ovarian cancer. Oncol Rep. 2016;36:3241–3250. [DOI] [PubMed] [Google Scholar]
- 82.Zhao H, Yu H, Zheng J, et al. Lowly-expressed lncRNA GAS5 facilitates progression of ovarian cancer through targeting miR-196-5p and thereby regulating HOXA5. Gynecol Oncol. 2018;151:345–355. [DOI] [PubMed] [Google Scholar]
- 83.Gao J, Liu M, Zou Y, et al. Long non-coding RNA growth arrest-specific transcript 5 is involved in ovarian cancer cell apoptosis through the mitochondria-mediated apoptosis pathway. Oncol Rep. 2015;34:3212–3221. [DOI] [PubMed] [Google Scholar]
- 84.Wen Q, Liu Y, Lyu H, et al. Long noncoding RNA GAS5, which acts as a tumor suppressor via microRNA 21, regulates cisplatin resistance expression in cervical cancer. Int J Gynecol Cancer. 2017;27:1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao S, Liu W, Li F, Zhao W, Qin C. Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int J Clin Exp Pathol. 2014;7:6776–6783. [PMC free article] [PubMed] [Google Scholar]
- 86.Luo G, Liu D, Huang C, et al. LncRNA GAS5 inhibits cellular proliferation by targeting P27(Kip1). Mol Cancer Res. 2017;15:789–799. [DOI] [PubMed] [Google Scholar]
- 87.Xue D, Zhou C, Lu H, Xu R, Xu X, He X. LncRNA GAS5 inhibits proliferation and progression of prostate cancer by targeting miR-103 through AKT/mTOR signaling pathway. Tumour Biol. 2016;37:16187–16197. [DOI] [PubMed] [Google Scholar]
- 88.Zhu L, Zhu Q, Wen H, Huang X, Zheng G. Mutations in GAS5 affect the transformation from benign prostate proliferation to aggressive prostate cancer by affecting the transcription efficiency of GAS5. J Cell Physiol. 2019;234:8928–8940. [DOI] [PubMed] [Google Scholar]
- 89.Slaby O, Jancovicova J, Lakomy R, et al. Expression of miRNA-106b in conventional renal cell carcinoma is a potential marker for prediction of early metastasis after nephrectomy. J Exp Clin Cancer Res. 2010;29:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Toraih EA, Alghamdi SA, El-Wazir A, et al. Dual biomarkers long non-coding RNA GAS5 and microRNA-34a co-expression signature in common solid tumors. PLoS One. 2018;13:e0198231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hedegaard J, Lamy P, Nordentoft I, et al. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell. 2016;30:27–42. [DOI] [PubMed] [Google Scholar]
- 92.Nie Y, Liu X, Qu S, Song E, Zou H, Gong C. Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013;104:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang M, Guo C, Wang L, et al. Long noncoding RNA GAS5 promotes bladder cancer cells apoptosis through inhibiting EZH2 transcription. Cell Death Dis. 2018;9:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang H, Guo Y, Song Y, Shang C. Long noncoding RNA GAS5 inhibits malignant proliferation and chemotherapy resistance to doxorubicin in bladder transitional cell carcinoma. Cancer Chemother Pharmacol. 2017;79:49–55. [DOI] [PubMed] [Google Scholar]
- 95.Avgeris M, Tsilimantou A, Levis PK, et al. Loss of GAS5 tumour suppressor lncRNA: an independent molecular cancer biomarker for short-term relapse and progression in bladder cancer patients. Br J Cancer. 2018;119:1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;205:613–621. [DOI] [PubMed] [Google Scholar]
- 97.Huo JF, Chen XB. Long noncoding RNA growth arrest-specific 5 facilitates glioma cell sensitivity to cisplatin by suppressing excessive autophagy in an mTOR-dependent manner. J Cell Biochem. 2019;120:6127–6136. [DOI] [PubMed] [Google Scholar]
- 98.Liu Q, Yu W, Zhu S, et al. Long noncoding RNA GAS5 regulates the proliferation, migration, and invasion of glioma cells by negatively regulating miR-18a-5p. J Cell Physiol. 2018;234:757–768. [DOI] [PubMed] [Google Scholar]
- 99.Zhao X, Wang P, Liu J, et al. Gas5 exerts tumor-suppressive functions in human glioma cells by targeting miR-222. Mol Ther. 2015;23:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yuan J, Zhang N, Zheng Y, Chen YD, Liu J, Yang M. LncRNA GAS5 indel genetic polymorphism contributes to glioma risk through interfering binding of transcriptional factor TFAP2A. DNA Cell Biol. 2018;37:750–757. [DOI] [PubMed] [Google Scholar]
- 101.Woehrer A, Bauchet L, Barnholtz-Sloan JS. Glioblastoma survival: has it improved? Evidence from population-based studies. Curr Opin Neurol. 2014;27:666–674. [DOI] [PubMed] [Google Scholar]
- 102.Shen J, Hodges TR, Song R, et al. Serum HOTAIR and GAS5 levels as predictors of survival in patients with glioblastoma. Mol Carcinog. 2018;57:137–141. [DOI] [PubMed] [Google Scholar]
- 103.Guo Z, Wang Y, Zhao Y, et al. Genetic polymorphisms of long non-coding RNA GAS5 predict platinum-based concurrent chemoradiotherapy response in nasopharyngeal carcinoma patients. Oncotarget. 2017;8:62286–62297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang XF, Ye Y, Zhao SJ. LncRNA Gas5 acts as a ceRNA to regulate PTEN expression by sponging miR-222-3p in papillary thyroid carcinoma. Oncotarget. 2018;9:3519–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo LJ, Zhang S, Gao B, et al. Low expression of long non-coding RNA GAS5 is associated with poor prognosis of patients with thyroid cancer. Exp Mol Pathol. 2017;102:500–504. [DOI] [PubMed] [Google Scholar]
- 106.Wang Q, Zhang W, Hao S. LncRNA CCAT1 modulates the sensitivity of paclitaxel in nasopharynx cancers cells via miR-181a/CPEB2 axis. Cell Cycle. 2017;16:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Chen L, Yang H, Yi Z, et al. LncRNA GAS5 regulates redox balance and dysregulates the cell cycle and apoptosis in malignant melanoma cells. J Cancer Res Clin Oncol. 2019;145:637–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bian D, Shi W, Shao Y, Li P, Song G. Long non-coding RNA GAS5 inhibits tumorigenesis via miR-137 in melanoma. Am J Transl Res. 2017;9:1509–1520. [PMC free article] [PubMed] [Google Scholar]
- 109.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, Kong D. LncRNA GAS5 represses osteosarcoma cells growth and metastasis via sponging MiR-203a. Cell Physiol Biochem. 2018;45:844–855. [DOI] [PubMed] [Google Scholar]
- 111.Xu L, Xia C, Xue B, Sheng F, Xiong J, Wang S. A promoter variant of lncRNA GAS5 is functionally associated with the development of osteosarcoma. J Bone Oncol. 2018;12:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li W, Zhai L, Wang H, et al. Downregulation of LncRNA GAS5 causes trastuzumab resistance in breast cancer. Oncotarget. 2016;7:27778–27786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Awasthee N, Rai V, Verma SS, Sajin Francis K, Nair MS, Gupta SC. Anti-cancer activities of Bharangin against breast cancer: evidence for the role of NF-kappaB and lncRNAs. Biochim Biophys Acta Gen Subj. 2018;1862:2738–2749. [DOI] [PubMed] [Google Scholar]
- 114.Koldemir O, Ozgur E, Gezer U. Accumulation of GAS5 in exosomes is a marker of apoptosis induction. Biomed Rep. 2017;6:358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pickard MR, Williams GT. The hormone response element mimic sequence of GAS5 lncRNA is sufficient to induce apoptosis in breast cancer cells. Oncotarget. 2016;7:10104–10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3:ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hudson WH, Pickard MR, de Vera IM, et al. Conserved sequence-specific lincRNA-steroid receptor interactions drive transcriptional repression and direct cell fate. Nat Commun. 2014;5:5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yuan S, Wu Y, Wang Y, Chen J, Chu L. An oncolytic adenovirus expressing SNORD44 and GAS5 exhibits antitumor effect in colorectal cancer cells. Hum Gene Ther. 2017;28:690–700. [DOI] [PubMed] [Google Scholar]
- 119.Yao T, Lu R, Zhang J, et al. Growth arrest-specific 5 attenuates cisplatin-induced apoptosis in cervical cancer by regulating STAT3 signaling via miR-21. J Cell Physiol. 2019;234:9605–9615. [DOI] [PubMed] [Google Scholar]
- 120.Yang M, Xiong X, Chen L, Yang L, Li X. Identification and validation long non-coding RNAs of oral squamous cell carcinoma by bioinformatics method. Oncotarget. 2017;8:107469–107476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ding CZ, Guo XF, Wang GL, et al. High glucose contributes to the proliferation and migration of non-small cell lung cancer cells via GAS5-TRIB3 axis. Biosci Rep. 2018;38:BSR20171014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou Y, Wu K, Jiang J, et al. Integrative analysis reveals enhanced regulatory effects of human long intergenic non-coding RNAs in lung adenocarcinoma. J Genet Genomics. 2015;42:423–436. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Z, Zhu Z, Watabe K, et al. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013;20:1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pickard MR, Williams GT. Regulation of apoptosis by long non-coding RNA GAS5 in breast cancer cells: implications for chemotherapy. Breast Cancer Res Treat. 2014;145:359–370. [DOI] [PubMed] [Google Scholar]
- 125.Ozgur E, Mert U, Isin M, Okutan M, Dalay N, Gezer U. Differential expression of long non-coding RNAs during genotoxic stress-induced apoptosis in HeLa and MCF-7 cells. Clin Exp Med. 2013;13:119–126. [DOI] [PubMed] [Google Scholar]
- 126.Pei J, Wang B. Notch-1 promotes breast cancer cells proliferation by regulating LncRNA GAS5. Int J Clin Exp Med. 2015;8:14464–14471. [PMC free article] [PubMed] [Google Scholar]
- 127.Huang J, Li Y, Lu Z, et al. Long non-coding RNA GAS5 is induced by interferons and plays an antitumor role in esophageal squamous cell carcinoma. Cancer Med. 2018;7:3157–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fang P, Xiang L, Chen W, et al. LncRNA GAS5 enhanced the killing effect of NK cell on liver cancer through regulating miR-544/RUNX3. Innate Immun. 2019;25:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qian X, Xu C, Zhao P, Qi Z. Long non-coding RNA GAS5 inhibited hepatitis C virus replication by binding viral NS3 protein. Virology. 2016;492:155–165. [DOI] [PubMed] [Google Scholar]
- 130.Gao J, Liu L, Li G, et al. LncRNA GAS5 confers the radio sensitivity of cervical cancer cells via regulating miR-106b/IER3 axis. Int J Biol Macromol. 2019;126:994–1001. [DOI] [PubMed] [Google Scholar]
- 131.Yeramian A, Moreno-Bueno G, Dolcet X, et al. Endometrial carcinoma: molecular alterations involved in tumor development and progression. Oncogene. 2013;32:403–413. [DOI] [PubMed] [Google Scholar]
- 132.Guo C, Song WQ, Sun P, Jin L, Dai HY. LncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in endometrial cancer cells. J Biomed Sci. 2015;22:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta. 2013;1832:1613–1623. [DOI] [PubMed] [Google Scholar]
- 134.Yacqub-Usman K, Pickard MR, Williams GT. Reciprocal regulation of GAS5 lncRNA levels and mTOR inhibitor action in prostate cancer cells. Prostate. 2015;75:693–705. [DOI] [PubMed] [Google Scholar]
- 135.Liu L, Pang X, Shang W, Xie H, Feng Y, Feng G. Long non-coding RNA GAS5 sensitizes renal cell carcinoma to sorafenib via miR-21/SOX5 pathway. Cell Cycle. 2019;18:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136.Cao Q, Wang N, Qi J, Gu Z, Shen H. Long noncoding RNAGAS5 acts as a tumor suppressor in bladder transitional cell carcinoma via regulation of chemokine (CC motif) ligand 1 expression. Mol Med Rep. 2016;13:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao X, Liu Y, Zheng J, et al. GAS5 suppresses malignancy of human glioma stem cells via a miR-196a-5p/FOXO1 feedback loop. Biochim Biophys Acta Mol Cell Res. 2017;1864:1605–1617. [DOI] [PubMed] [Google Scholar]
- 138.Liu Q, Sun S, Yu W, et al. Altered expression of long non-coding RNAs during genotoxic stress-induced cell death in human glioma cells. J Neurooncol. 2015;122:283–292. [DOI] [PubMed] [Google Scholar]
- 139.Garcia-Claver A, Lorente M, Mur P, et al. Gene expression changes associated with erlotinib response in glioma cell lines. Eur J Cancer. 2013;49:1641–1653. [DOI] [PubMed] [Google Scholar]
- 140.Huang H, Jiang R, Lian Z, Zhang W, Hu Z, Hu D. miR-222/GAS5 is involved in DNA damage and cytotoxic effects induced by temozolomide in T98G cell line. J Appl Toxicol. 2018;39:726–734. [DOI] [PubMed] [Google Scholar]
- 141.Zeng B, Li Y, Jiang F, et al. LncRNA GAS5 suppresses proliferation, migration, invasion, and epithelial-mesenchymal transition in oral squamous cell carcinoma by regulating the miR-21/PTEN axis. Exp Cell Res. 2019;374:365–373. [DOI] [PubMed] [Google Scholar]
- 142.Chen L, Yang H, Xiao Y, et al. Lentiviral-mediated overexpression of long non-coding RNA GAS5 reduces invasion by mediating MMP2 expression and activity in human melanoma cells. Int J Oncol. 2016;48:1509–1518. [DOI] [PubMed] [Google Scholar]
- 143.Chen L, Yang H, Xiao Y, et al. LncRNA GAS5 is a critical regulator of metastasis phenotype of melanoma cells and inhibits tumor growth in vivo. Onco Targets Ther. 2016;9:4075–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ye K, Wang S, Zhang H, Han H, Ma B, Nan W. Long noncoding RNA GAS5 suppresses cell growth and epithelial-mesenchymal transition in osteosarcoma by regulating the miR-221/ARHI pathway. J Cell Biochem. 2017;118:4772–4781. [DOI] [PubMed] [Google Scholar]
- 145.Zhang L, Wang Y, Zhang L, et al. ZBTB7A, a miR-663a target gene, protects osteosarcoma from endoplasmic reticulum stress-induced apoptosis by suppressing LncRNA GAS5 expression. Cancer Lett. 2019;448:105–116. [DOI] [PubMed] [Google Scholar]
- 146.Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tian T, Li X, Zhang J. mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. Int J Mol Sci. 2019;20:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Du L, Duan W, Jiang X, et al. Cell-free lncRNA expression signatures in urine serve as novel non-invasive biomarkers for diagnosis and recurrence prediction of bladder cancer. J Cell Mol Med. 2018;22:2838–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Song J, Zhang W, Wang S, Liu K, Song F, Ran L. A panel of 7 prognosis-related long non-coding RNAs to improve platinum-based chemoresistance prediction in ovarian cancer. Int J Oncol. 2018;53:866–876. [DOI] [PubMed] [Google Scholar]
- 150.Quan J, Pan X, Zhao L, et al. LncRNA as a diagnostic and prognostic biomarker in bladder cancer: a systematic review and meta-analysis. Onco Targets Ther. 2018;11:6415–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]