Abstract
Background
Fibrosis is deemed to be a pivotal determinant of the long-term prognosis in non-alcoholic fatty liver disease (NAFLD).
Objective
We aimed to develop a novel nomogram-based non-invasive model to accurately predict significant fibrosis in patients with NAFLD.
Methods
We designed a prospective cohort study including 207 patients with biopsy-proven NAFLD. Detailed anthropometric and fibrosis-related laboratory parameters were collected. A nomogram was established based on variables that were independently associated with significant fibrosis identified by the logistic regression model. Then it was compared with aspartate aminotransferase-to-platelet ratio index (APRI), NAFLD fibrosis score (NFS), FIB-4 and BARD score. Diagnostic accuracy was assessed according to area under the receiver operator characteristic curve (AUROC), sensitivity, specificity, positive and negative predictive values, and decision curve analysis.
Results
Variables included in the nomogram were: waist-to-height ratio, hyaluronic acid, procollagen-III-peptide, chitinase-3-like protein 1, and cytokeratine-18 neoepitope M65. The discrimination ability of the nomogram (AUROC = 0.829, 95%CI 0.755–0.904) was significantly superior to APRI (AUROC = 0.670, 95%CI 0.563–0.777), NFS (AUROC = 0.601, 95%CI 0.480–0.722), FIB-4 (AUROC = 0.624, 95%CI 0.511–0.736) and BARD (AUROC = 0.579, 95%CI 0.459–0.699) for significant fibrosis (all p < 0.05). The nomogram showed a larger net benefit to aid in decision-making as to whether biopsy is required.
Conclusions
This novel nomogram was more accurate, and achieved higher net benefit than APRI, NFS, FIB-4 and BARD to detect significant fibrosis. It can be useful as a non-invasive method to screen ≥F2 fibrosis in the overall population with NAFLD.
Keywords: Fibrosis, NAFLD, nomogram, diagnosis, liver biopsy
Background
Non-alcoholic fatty liver disease (NAFLD) is rapidly becoming a grave threat to public health; it is caused by hepatic steatosis without excessive alcohol consumption. The prevalence rate of this disease has reached 25% during the last decade, and it is considered to be one of the most common liver diseases in China.1 NAFLD has been associated with obesity, type 2 diabetes (T2DM), hyperlipidemia and insulin resistance, and is considered a component of metabolic syndrome. NAFLD encompasses non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH), which may result in liver cirrhosis and hepatocellular carcinoma.2 Previous research indicates that fibrosis stage, but not NASH, is the most powerful prognostic factor for mortality and time to develop end-stage liver disease in biopsy-proven NAFLD.3–6 Patients with higher stages of fibrosis are at greater risk of developing end-stage liver disease and should receive timely and appropriate intervention.
At present, liver biopsy has an irreplaceable position in the diagnosis of NAFLD as it remains the gold standard for distinguishing NASH from NAFL and assessing the stage of fibrosis. Besides its high costs, liver biopsy is an invasive examination associated with potential complications. However, a growing number of non-invasive diagnostic methods based on clinical and biochemical variables have been reported in recent years. These non-invasive scoring systems to predict advanced fibrosis, including aspartate aminotransferase (AST)-to-platelet ratio index (APRI), NAFLD fibrosis score (NFS), fibrosis index based on four factors (FIB-4), and body mass index, AST/ALT ratio, and diabetes (BARD) score, can be applied to exclude patients without advanced fibrosis.7–10
A well-designed retrospective study revealed that the hazard ratio (HR) for death or liver transplantation was 3.76 (95% CI 2.40–5.89) for F3 compared with F0 in patients with NAFLD, and a similar HR of 2.89 (95% CI 1.93–4.33) for F2 patients.6 Thus, detecting not only advanced fibrosis, but also patients with F2 fibrosis, presents an important clinical need for secondary prevention. The above well-known clinical models, namely APRI, NFS, FIB-4, and BARD score, had the greatest efficacies in detecting advanced fibrosis. However, distinguishing between significant fibrosis versus F0–F1 fibrosis remains unsolved.2,11 By identifying significant fibrosis with a non-invasive model, an earlier stage of fibrosis can be detected to avoid further liver injury.
A nomogram is a graphical depiction of prediction models for individuals; although it has been developed for other diseases,12 application in the field of hepatic steatosis is rare, with only one study regarding pediatric NAFLD.13 The objective of this study was to develop a novel nomogram-based non-invasive model to accurately detect significant fibrosis in patients with NAFLD.
Materials and methods
Study population
A prospective cohort design was used to develop a nomogram to detect NAFLD-related significant fibrosis. Consecutive NAFLD patients were recruited at the First Affiliated Hospital of Wenzhou Medical University from December 2016 to January 2018. For each patient, a complete medical history and blood samples were obtained, and a physical examination and liver biopsy were performed. Participants were eligible for this study if they met the following inclusion criteria: (1) adult patients aged 18–75 years; (2) fatty liver indicated by imaging, and/or abnormal liver function; (3) the participant was willing to provide written informed consent. Potential participants were excluded if they: (1) had history of alcohol consumption of >20 g/day over the past 2 years; (2) developed viral hepatitis (B or C), autoimmune hepatitis, primary biliary cholangitis or Wilson’s disease; (3) had history of a long-term consumption of non-steroid anti-inflammatory drugs, calcium channel blockers, tamoxifen, amiodarone, corticosterone, isoniazid and methotrexate; (4) were a pregnant or lactating woman; (5) developed hepatic carcinoma, or other benign and malignant tumor; (6) had missing records of important parameters. The study protocol was approved by the ethics committee of the First Affiliated Hospital of Wenzhou Medical University (2016-246, 1 December 2016) and registered in the Chinese Clinical Trial Registry (ChiCTR-EOC-17013562). Written informed consent was obtained from each participant.
Laboratory parameters
Venous blood samples were collected on the day of biopsy for hematological workup including a blood cell count and measurement of the following levels: fasting plasma glucose (FPG), fasting plasma C-peptide (FCP), fasting plasma insulin (FINS), glycated hemoglobin (HbA1c), aspartate aminotransferase (AST), alanine transaminase (ALT), alpha-fetal protein (AFP), alkaline phosphatase (ALP), γ-glutamyl transferase (GGT), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), blood urea nitrogen (BUN), hyaluronic acid (HA), procollagen-3 N-terminal peptide (P3NP), type IV collagen (IV-C), laminin (LN), triiodothyronine (T3), thyroxine (T4), thyroid stimulating hormone (TSH), albumin and uric acid (UA). All biochemical assessments were performed using standard laboratory methods.
Body measurements
Anthropometric measurements including height, weight, waist circumference, abdominal circumference, hip circumference, waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) were recorded in all subjects. Body mass index (BMI) was calculated as weight/height2. Visceral adiposity index (VAI) was calculated according to the published literature.14 Waist circumference was measured with a tape all around the body in the horizontal position at the mid-point between the lower rib margin and the iliac crest.
Diagnoses of concomitant diseases
Blood pressure was measured using standardized equipment in the sitting position. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg and diastolic blood pressure (DBP) ≥90 mmHg or utilizing antihypertensives. Insulin resistance was quantified by the homeostasis model assessment (HOMA-IR), which was equal to fasting insulin (μU/ml)*fasting glucose (mmol/l)/22.5. Diagnosis of T2DM was based on history of diabetes, use of antidiabetic medications, and/or FPG ≥ 7.0 mmol/l or 2-hour glucose ≥11.1 mmol/l. Hyperlipidemia was defined as TC ≥ 6.2 mmol/l, LDL-C ≥ 4.1 mmol/l, or TG ≥ 2.3 mmol/l.
Novel biomarkers related to NAFLD and/or fibrosis
Some novel biomarkers related to NAFLD and liver fibrosis were measured using sera stored at −80℃. Serum chitinase-3-like protein 1 (CHI3L1) concentration was determined with a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) kit (Proprium Biotech Company Limited, Hangzhou, China). Serum Golgi protein 73 (GP73) concentration was measured by ELISA kit from Hotgen Biotech Inc., Beijing, China. Cytokeratine-18 neoepitope M30 (CK-18 M30) and M65 were detected using ELISA kits of Herui Biomed Company Limited, Suzhou, China. They were performed according to the manufacturers’ instruction. All the tests were double blind and coefficient of variability was <15%.
Pathological evaluation of NAFLD
The disease progression of NAFLD was defined by the presence and pattern of specific histological abnormalities on liver biopsy. Ultrasound-guided percutaneous liver biopsy was performed using a 16-gauge Hepafix needle. Obtained specimens with hematoxylin and eosin and Masson’s trichrome staining were interpreted by an experienced hepatopathologist (Yang-Yang Li), who was blinded to clinical data and the sequence of the specimens at the same time.
The slips were evaluated and reported from two aspects, comprising NAFLD activity score (NAS, 0–8) and fibrosis stage (1–4). NAS calculates the unweighted sum of the scores for steatosis (0–3), lobular inflammation (0–3) and hepatocellular ballooning (0–2).15 Fibrosis is staged from 0–4: 0 = no fibrosis, 1 = perisinusoidal or portal fibrosis; 2 = perisinusoidal and portal/periportal fibrosis; 3 = bridging fibrosis; 4 = highly suspicious or definite cirrhosis.16 Significant liver fibrosis was defined as fibrosis stages 2 or above. Non-invasive fibrosis prediction models (APRI, NFS, FIB-4 and BARD score) were calculated according to published formulas.7–9,17
Statistical analyses
Continuous variables were expressed as mean ± standard deviation or median ± inter-quartile range, while categorical values were expressed using relative frequencies and proportions. Comparisons of parameters between two different groups were conducted with the Student’s t-test and the Mann–Whitney U-test for continuous variables with or without normal distribution and with the chi-square test for categorical variables. Univariate and multivariate analyses were then performed to define independent factors strongly associated with mortality. Logistic regression models were performed to estimate the odds ratio (OR) and 95% confidence interval (CI). The nomogram was developed based on a logistic regression model, which allowed us to obtain significant fibrosis probability estimations. The nomogram was calibrated graphically by a bootstrap re-sampling approach with 1000 replications. An area under the receiver operator characteristic curve (AUROC) was used as a measure of the diagnostic accuracy. We performed a decision curve analysis (DCA) to evaluate the net benefit, namely whether the application of the new model does more good (identification of significant fibrosis) than harm (unnecessary biopsy). The probability thresholds reflected the level of diagnostic certainty above which the patient would choose to have liver biopsy. The highest curve at a given threshold probability is the optimal decision-making strategy to maximize the net benefit. For all analyses, a p-value of less than 0.05 was considered statistically significant. All statistical analysis was performed on SPSS version 22.0 (SPSS, Chicago, IL, USA), R 3.3.1 (R Development Core Team, http://www.r-project.org) and MedCalc version 12.7 (MedCalc Software, Ostend, Belgium).
Results
Patients’ characteristics
For the study period, between December 2016 and January 2018, we enrolled a total of 250 patients who had previously been diagnosed with NAFLD and subsequently underwent a biopsy. Among them, 43 patients were excluded (15 for alcoholic fatty liver, 19 for missing records of important parameters, six for autoimmune hepatitis, and three for drug-induced hepatitis). Thus, 207 patients with biopsy-proven NAFLD were enrolled in this prospective cohort study. To explore useful markers, we screened a series of variables including routine as well as novel body measurements and biochemical tests. Table 1 shows a summary of patients’ anthropometric, clinical, laboratory and histological characteristics. Patients had a mean age of 41.8 ± 12.2 years, and 73.4% were male. Some 178 subjects (86% of all subjects) had biopsy-proven fibrosis stages 0–1, and 29 subjects (14% of all subjects) had fibrosis stages ≥2. Waist circumference, abdominal circumference, WHtR, AST, ALT, FINS, FCP, HOMA-IR, GP73, CHI3L1, HA, P3NP, IV-C, CK-18 M30, and CK-18 M65 levels were significantly higher in patients with fibrosis stages ≥2 versus patients with fibrosis stages 0–1 (all p < 0.05). In addition, prevalence of hyperlipidemia and T2DM were found to be associated with significant fibrosis.
Table 1.
Clinical and histological features of patients.
| Variables | Overall (n = 207) | Fibrosis stages 0–1 (n = 178) | Fibrosis stages ≥ 2 (n = 29) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 41.8 ± 12.2 | 41.5 ± 11.7 | 44.0 ± 15.3 | 0.18 |
| Gender (male) | 152 (73.4%) | 135 (75.8%) | 17 (58.6%) | 0.05 |
| Body measurements | ||||
| Height (cm) | 167.4 ± 9.4 | 167.8 ± 9.5 | 164.4 ± 8.1 | 0.07 |
| Weight (kg) | 75.7 ± 13.3 | 75.5 ± 13.1 | 76.5 ± 15.6 | 0.73 |
| BMI (kg/m2) | 27.0 ± 4.4 | 26.8 ± 4.4 | 28.1 ± 3.7 | 0.14 |
| Waist circumference (cm) | 91.2 ± 8.8 | 90.7 ± 8.7 | 94.1 ± 8.4 | 0.02 |
| Abdominal circumference (cm) | 94.5 ± 8.7 | 93.9 ± 8.5 | 97.7 ± 9.3 | 0.03 |
| Hip circumference (cm) | 98.8 ± 7.4 | 98.5 ± 7.3 | 100.7 ± 8.0 | 0.14 |
| VAI | 3.8 ± 3.0 | 3.9 ± 3.2 | 3.1 ± 1.5 | 0.20 |
| WHR | 0.93 ± 0.05 | 0.92 ± 0.06 | 0.93 ± 0.04 | 0.21 |
| WHtR | 0.55 ± 0.05 | 0.54 ± 0.05 | 0.57 ± 0.04 | 0.002 |
| SBP (mmHg) | 125.3 ± 15.1 | 125.7 ± 15.6 | 122.7 ± 12.0 | 0.33 |
| DBP (mmHg) | 78.6 ± 9.9 | 78.8 ± 9.9 | 77.7 ± 10.2 | 0.59 |
| Laboratory parameters | ||||
| AST (U/l) | 45.7 ± 32.3 | 42.9 ± 30.0 | 65.5 ± 42.0 | 0.01 |
| ALT (U/l) | 49.0 ± 20.8 | 46.0 ± 18.0 | 81.0 ± 42.5 | 0.01 |
| AST/ALT ratio | 0.8 ± 0.4 | 0.8 ± 0.3 | 0.9 ± 0.7 | 0.17 |
| GGT (U/l) | 52.0 ± 20.8 | 50.0 ± 19.0 | 62.0 ± 28.0 | 0.12 |
| ALP (U/l) | 88.4 ± 39.9 | 88.8 ± 42.0 | 85.6 ± 21.0 | 0.69 |
| Albumin (g/l) | 46.4 ± 3.3 | 46.5 ± 3.4 | 46.2 ± 3.2 | 0.67 |
| PLT (×109/l) | 249.9 ± 58.0 | 251.1 ± 57.9 | 242.1 ± 59.0 | 0.44 |
| Hb (g/l) | 147.3 ± 14.5 | 148.0 ± 14.2 | 142.4 ± 15.3 | 0.06 |
| FPG (mmol/l) | 5.7 ± 1.7 | 5.7 ± 1.6 | 6.2 ± 1.9 | 0.13 |
| HbA1c (%) | 6.1 ± 1.4 | 6.1 ± 1.4 | 6.4 ± 1.2 | 0.26 |
| FINS (pmol/l) | 104.1 ± 36.5 | 98.8 ± 33.1 | 156.1 ± 46.2 | 0.001 |
| FCP (pmol/l) | 980.4 ± 230.3 | 968.3 ± 239.6 | 1195.5 ± 258.6 | 0.008 |
| HOMA-IR | 3.4 ± 1.1 | 3.2 ± 1.0 | 5.0 ± 1.4 | 0.001 |
| BUN (mmol/l) | 4.9 ± 1.4 | 4.9 ± 1.4 | 4.9 ± 1.0 | 0.91 |
| Creatinine (µmol/l) | 67.7 ± 14.6 | 68.3 ± 14.8 | 62.9 ± 12.5 | 0.06 |
| eGFR | 114.7 ± 18.4 | 114.6 ± 18.0 | 115.4 ± 21.4 | 0.83 |
| INR | 0.96 ± 0.06 | 0.96 ± 0.06 | 0.96 ± 0.06 | 0.87 |
| Total bilirubin (µmol/l) | 14.1 ± 6.5 | 14.3 ± 6.7 | 12.8 ± 5.1 | 0.24 |
| TC (mmol/l) | 2.4 ± 1.5 | 2.5 ± 1.6 | 2.0 ± 0.8 | 0.12 |
| TG (mmol/l) | 5.0 ± 1.2 | 5.0 ± 1.2 | 4.9 ± 1.2 | 0.59 |
| LDL-C (mmol/l) | 3.0 ± 0.9 | 3.0 ± 0.9 | 3.1 ± 1.1 | 0.75 |
| HDL-C (mmol/l) | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.2 | 0.39 |
| UA (µmol/l) | 394.4 ± 102.8 | 395.7 ± 101.4 | 385.7 ± 113.6 | 0.62 |
| T3 (nmol/l) | 1.6 ± 0.3 | 1.6 ± 0.3 | 1.6 ± 0.2 | 0.69 |
| T4 (nmol/l) | 104.9 ± 18.4 | 104.2 ± 17.8 | 110.1 ± 21.8 | 0.11 |
| TSH (nmol/l) | 2.0 ± 4.8 | 2.0 ± 5.1 | 1.6 ± 0.9 | 0.66 |
| AFP (ng/ml) | 3.1 ± 1.6 | 3.2 ± 1.7 | 2.7 ± 1.1 | 0.17 |
| HA (ng/ml) | 43.7 ± 5.2 | 43.2 ± 4.8 | 48.6 ± 5.8 | 0.02 |
| P3NP (ng/ml) | 19.5 ± 3.7 | 18.9 ± 3.3 | 23.4 ± 4.1 | <0.001 |
| IV-C (ng/ml) | 19.4 ± 3.9 | 18.8 ± 3.7 | 23.7 ± 3.0 | <0.001 |
| LN (ng/ml) | 10.0 ± 3.9 | 9.7 ± 3.9 | 11.5 ± 2.0 | 0.04 |
| Novel biomarkers related to NAFLD and fibrosis | ||||
| GP73 (ng/ml) | 65.7 ± 15.4 | 64.4 ± 15.3 | 77.2 ± 19.2 | 0.02 |
| CHI3L1 (ng/ml) | 54.8 ± 12.4 | 52.0 ± 9.8 | 74.9 ± 22.2 | 0.002 |
| CK-18 M30 (U/l) | 134.0 ± 289.0 | 101.0 ± 203.0 | 343.0 ± 679.0 | 0.002 |
| CK-18 M65 (U/l) | 217.0 ± 273.0 | 174.0 ± 306.5 | 400.0 ± 871.5 | 0.002 |
| Concomitant diseases | ||||
| Hyperlipidemia (%) | 96 (46.6%) | 78 (43.8%) | 18 (64.3%) | 0.04 |
| Hypertension (%) | 42 (20.3%) | 37 (20.8) | 5 (17.2%) | 0.66 |
| T2DM (%) | 51 (24.6%) | 40 (22.5%) | 11 (37.9%) | 0.06 |
| Non-invasive models | ||||
| APRI | 0.49 ± 0.36 | 0.47 ± 0.35 | 0.65 ± 0.40 | 0.01 |
| NFS | –2.91 ± 1.37 | –2.98 ± 1.32 | –2.53 ± 1.62 | 0.10 |
| FIB-4 | 0.98 ± 0.53 | 0.94 ± 0.49 | 1.19 ± 0.73 | 0.02 |
| BARD score | 1.28 ± 1.19 | 1.24 ± 1.15 | 1.52 ± 1.38 | 0.24 |
| Histological characteristics | ||||
| Steatosis | 0.09 | |||
| 1 | 80 (38.6%) | 72 (40.4%) | 8 (27.6%) | |
| 2 | 80 (38.6%) | 70 (39.3%) | 10 (34.5%) | |
| 3 | 47 (22.7%) | 36 (20.2%) | 11(37.9%) | |
| Ballooning | 0.34 | |||
| 0 | 42 (20.3%) | 39 (21.9%) | 3 (10.3%) | |
| 1 | 124 (59.9%) | 105 (59.0%) | 19 (65.5%) | |
| 2 | 41 (19.8%) | 34 (19.1%) | 7 (24.1%) | |
| Lobular inflammation | 0.97 | |||
| 0 | 27 (13.0%) | 23 (12.9%) | 4 (13.8%) | |
| 1 | 141 (68.1%) | 122 (68.5%) | 19 (65.5%) | |
| 2 | 34 (16.4%) | 29 (16.3%) | 5 (17.2%) | |
| 3 | 5 (2.4%) | 4 (2.2%) | 1 (3.4%) | |
| NAS | 3.9 ± 1.4 | 3.8 ± 1.4 | 4.3 ± 1.3 | 0.07 |
| Fibrosis stage | ||||
| 0 | 99 (47.8%) | |||
| 1 | 79 (38.2%) | |||
| 2 | 21 (10.1%) | |||
| 3 | 8 (3.9%) | |||
| 4 | - | |||
Notes: AFP: alpha-fetal protein; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; BUN: blood urea nitrogen; CHI3L1: chitinase-3-like protein 1; CK-18 M30L: cytokeratine-18 neoepitope M30; CK-18 M65: cytokeratine-18 neoepitope M65; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; FCP: fasting plasma C-peptide; FIB-4: fibrosis index based on four factors; FINS: fasting plasma insulin; FPG: fasting plasma glucose; GGT: γ-glutamyl transferase; GP73: Golgi protein 73; HA: hyaluronic acid; Hb: hemoglobin; HbA1c: glycated hemoglobin; HDL-C: high-density lipoprotein cholesterol; HOMA-IR: homeostasis model assessment of insulin resistance; INR: international normalized ratio; IV-C: type IV collagen; LDL-C: low-density lipoprotein cholesterol; LN: laminin; NAS: NAFLD activity score; PLT: platelet count; P3NP: procollagen-3 N-terminal peptide; SBP: systolic blood pressure; TG: triglyceride; TC: total cholesterol; TSH: thyroid stimulating hormone; T2DM: type 2 diabetes; T3: triiodothyronine; T4: thyroxine; UA: uric acid; VAI: visceral adiposity index; WHR: waist-to-hip ratio; WHtR: waist-to-height ratio.
Presented at the bottom of Table 1 is a summary of histological features. There was no significant difference regarding steatosis, ballooning and lobular inflammation. The mean NAS score in patients with F ≥ 2 was 4.3 ± 1.3, which was higher than those with F0–F1 who had an average NAS of 3.8 ± 1.4, but fell short of statistical significance (p = 0.07).
Development of an individualized prediction nomogram
The results of logistic regression analysis among variables related to significant fibrosis are given in Table 2. CK-18 M65 showed a better performance (a higher AUROC and OR) than CK-18 M30. Also, WHtR was superior to either waist circumference, WHR or BMI in detecting significant fibrosis. GP73 has been not only suggested as a potential biomarker for the diagnosis of HCC, but also defined as a biomarker of fibrosis in chronic liver diseases including NAFLD.18 Our study also demonstrated that GP73 could predict significant fibrosis, with a moderate AUROC of 0.668. CHI3L1, another novel biomarker for liver fibrosis, also performed well with a higher AUROC of 0.672. To avoid collinearity and simplify the nomogram, we selected CHI3L1 as a variable in the final model. Five independent predictors with the best performance were incorporated into our model and presented as the nomogram (Figure 1).
Table 2.
Results of univariate and multivariate analysis for prediction of significant fibrosis.
| Variables |
Univariate
|
Multivariate
|
|||
|---|---|---|---|---|---|
| AUROC | OR (95%CI) | p | OR (95%CI) | p | |
| Age | 0.564 | 1.04 (1.00–1.07) | 0.03 | ||
| Gender | 0.421 | 0.37 (0.17–0.79) | 0.01 | ||
| Waist circumference | 0.649 | 1.05 (1.01–1.10) | 0.02 | ||
| Abdominal circumference | 0.669 | 1.07 (1.02–1.11) | 0.01 | ||
| WHtR | 0.728 | 1.14 (1.06–1.24)# | <0.001 | 1.15 (1.05–1.26)# | 0.002 |
| AST | 0.712 | 1.02 (1.01–1.03) | 0.001 | ||
| ALT | 0.660 | 1.00 (1.00–1.01) | 0.05 | ||
| FINS | 0.692 | 1.00 (0.99–1.01) | 0.14 | ||
| FCP | 0.640 | 1.00 (1.00–1.01) | 0.18 | ||
| HOMA-IR | 0.701 | 1.07 (0.91–1.25) | 0.44 | ||
| Creatinine | 0.382 | 0.98 (0.95–1.00) | 0.06 | ||
| GP73 | 0.668 | 1.01 (1.01–1.02) | 0.01 | ||
| CHI3L1 | 0.672 | 1.02 (1.00–1.03) | 0.01 | 1.01 (1.00–1.02) | 0.01 |
| HA | 0.631 | 1.02 (1.01–1.04) | 0.01 | 1.02 (1.01–1.04) | 0.02 |
| P3NP | 0.717 | 1.10 (1.05–1.16) | <0.001 | 1.10 (1.01–1.12) | 0.04 |
| IV-C | 0.727 | 1.10 (1.04–1.15) | <0.001 | ||
| LN | 0.611 | 1.04 (1.01–1.08) | 0.01 | ||
| CK-18 M30 | 0.703 | 1.06 (1.00–1.12)* | 0.03 | ||
| CK-18 M65 | 0.715 | 1.12 (1.05–1.19)* | <0.001 | 1.08 (1.01–1.15)* | 0.006 |
| T2DM | 0.585 | 2.55 (1.14–5.74) | 0.02 | ||
Notes: ALT: alanine aminotransferase; AST: aspartate aminotransferase; AUROC: area under the receiver operator characteristic curve; CHI3L1: chitinase-3-like protein 1; CI: confidence interval; CK-18 M30: cytokeratine 18 neoepitope M30; CK-18 M65: cytokeratine 18 neoepitope M65; FCP: fasting plasma C-peptide; FINS: fasting plasma insulin; GP73: Golgi protein 73; HA: hyaluronic acid; IV-C: Type IV collagen; LN: laminin; OR: odds ratio; P3NP: procollagen-3 N-terminal peptide; T2DM: Type 2 diabetes; WHtR: waist-to-height ratio. #Per 0.01 increase. *Per 100 U/L increase.
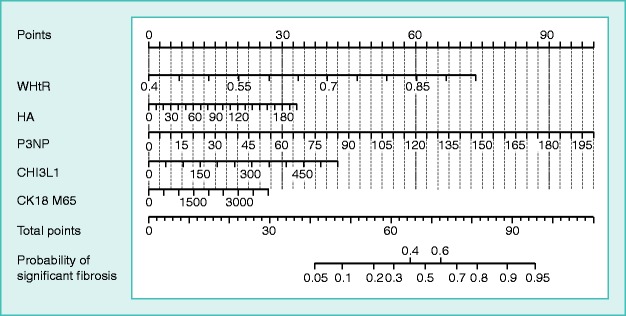
Figure 1.
Nomogram for predicting significant fibrosis.
Values for each variable are individually plotted and correspond to point values assigned from the point scale (top). These point values are then totaled and plotted on the total point scale (bottom), which is used to assign a corresponding value for risk of significant fibrosis.
To use the nomogram, the first variable was located. A straight line was then drawn upwards to the Points axis to determine the points obtained for the variable. This process was repeated for the other four variables and these points were then summated for each variable. The sum of these numbers was located on the Total Points axis, and a line was drawn downwards to the Probability of Significant Fibrosis axis to determine the likelihood of fibrosis stages ≥2. For example, a patient whose waist-to-hip ratio was 0.57, HA was 61.6 ng/ml, P3NP was 24 ng/ml, CK-18 M65 was 330 U/L and CHI3L1 was 93.6 ng/ml, and the total points scored was 54, significant fibrosis probability was approximately 20%.
The accuracy of the significant fibrosis diagnosis
We calculated the sensitivity and specificity of the scoring models differentiating NAFLD-related fibrosis stages 2–4 from NAFLD without significant fibrosis. Figure 2 shows the ROC curves for the nomogram, NFS, FIB-4 and BARD score. The cutoff points of the nomogram, APRI, NFS, FIB-4 and BARD score were 50, 0.6, −3.168, 0.89 and 2, respectively. Table 3 shows the performance of these models. The nomogram had the highest AUROC (0.829, 95% CI 0.755–0.904) in predicting the presence of F ≥ 2, compared with APRI (0.670, 95% CI 0.563–0.777, p = 0.049), NFS (0.601, 95% CI 0.480–0.722, p < 0.001), FIB-4 (0.624, 95% CI 0.511–0.736, p < 0.001) and BARD score (0.579, 95% CI 0.459–0.699, p < 0.001). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the nomogram with a cutoff of 50 for significant fibrosis were 69.0%, 81.8%, 79.1% and 72.5%. In comparison, these assessments for APRI, NFS, FIB-4 and BARD score were 62.1%/75.9%/65.5%/31.0%, 68.0%/46.1%/57.9%/84.3%, 66.0%/58.5%/60.8%/66.4% and 64.2%/65.7%/62.6%/55.0%, respectively.
Figure 2.
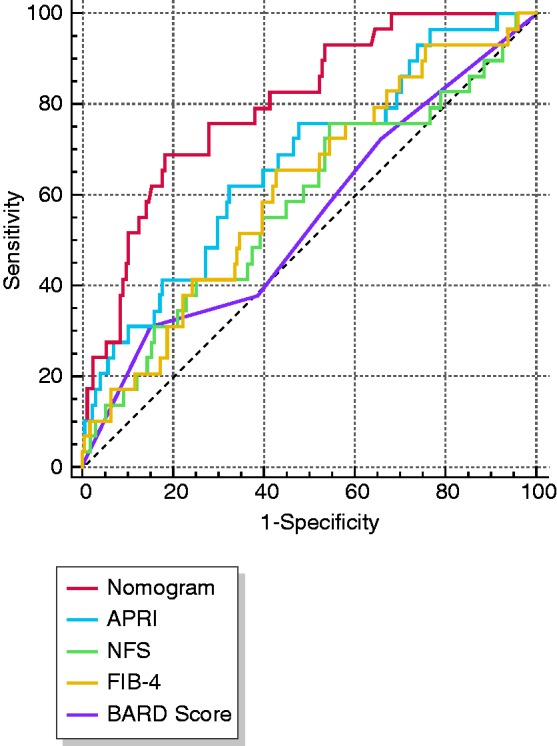
Receiver operating characteristics (ROC) curves for predicting F ≥ 2 fibrosis.
Table 3.
Performance assessment of our developed nomogram model and other scoring systems (FIB4 index, BARD score, and NFS) for the prediction of significant fibrosis.
| Models | AUROC (95%CI) | p | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| nomogram | 0.829 (0.755–0.904) | <0.001 | 69.0 | 81.8 | 79.1 | 72.5 |
| APRI | 0.670 (0.563–0.777) | 0.002 | 62.1 | 68.0 | 66.0 | 64.2 |
| NFS | 0.601 (0.480–0.722) | 0.17 | 75.9 | 46.1 | 58.5 | 65.7 |
| FIB-4 | 0.624 (0.511–0.736) | 0.04 | 65.5 | 57.8 | 60.8 | 62.6 |
| BARD score | 0.579 (0.459–0.699) | 0.39 | 31.0 | 84.3 | 66.4 | 55.0 |
Notes: APRI: AST-to-platelet ratio index; AUROC: area under the receiver operator characteristic curve; BARD score: body mass index, AST/ALT ratio and diabetes score; CI: confidence interval; NFS: NAFLD fibrosis score; FIB-4: fibrosis index based on four factors; NPV: negative predictive value; PPV: positive predictive value.
The calibration curve of the nomogram to predict significant fibrosis risk in NAFLD patients demonstrated relatively good agreement in this cohort (Figure 3). An ideal model would result in a plot where the actual and predicted probabilities fall along the 45° line.
Figure 3.

The calibration curves for nomogram (1000 bootstrap resamples).
Nomogram-predicted probability of significant fibrosis is plotted on the x-axis; actual probability is plotted on the y-axis.
DCA for clinical utility of the nomogram
The DCA used to assess the nomogram is presented in Figure 4. This analyzes the clinical utility of the nomogram in indicating liver biopsy compared with NFS, FIB-4 and BARD score. The DCA revealed that, from a threshold probability of >10%, we could obtain more net benefit by employing the nomogram. In particular, if the threshold probability of a patient is >10% and <60%, application of this nomogram to predict significant fibrosis risk adds a lot more benefit than the referenced strategies (APRI, NFS, FIB-4 and BARD score).
Figure 4.
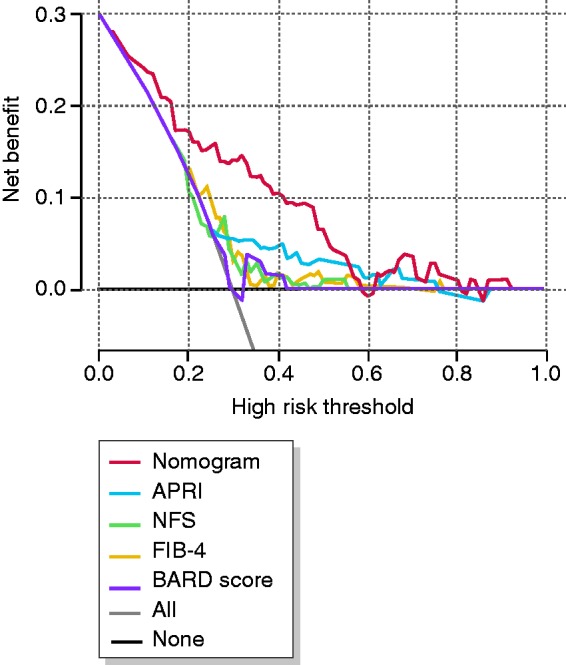
Decision curve analysis (DCA) for the nomogram.
The y-axis represents net benefits, calculated by subtracting the relative harms (true positives) from the benefits (false positives). The x-axis measures the threshold probability. A screening strategy is superior if it has the highest value compared with other models, including two simple strategies, such as performing liver biopsy for all patients (sloping solid line) or no patients (horizontal solid line).
Discussion
To the best of our knowledge, this study was the first to apply a nomogram in the adult NAFLD population. Nomograms are widely used as a user-friendly decision-making tool in oncology and precision medicine. In a similar manner, our novel prediction device for significant fibrosis risk among biopsy-proven NAFLD patients was developed to better suit the needs of an individual. This nomogram showed a better performance of AUROC and made a larger net benefit in screening ≥F2 patients than other non-invasive models tailored to exclude ≥F3 liver fibrosis.
More specifically, by incorporating WHtR, HA, P3NP, CHI3L1 and CK-18 M65, this easy-to-use nomogram facilitated individualized and optimal liver fibrosis prediction of ≥F2. Our study showed that WHtR was a better predictor of fibrosis than waist circumference and BMI, which is in accordance with multiple findings that demonstrated WHtR’s stronger association with NAFLD.19,20 Whether VAI is associated with significant fibrosis in NAFLD remains controversial,21,22 but VAI was not related to significant fibrosis in our cohort. Traditionally, serum biomarkers HA, P3NP, LN and IV-C have been routinely tested to diagnose liver fibrosis or cirrhosis.23 In other studies, CHI3L1, also known as YKL-40, is a novel biomarker associated with liver fibrosis in NAFLD in that CHI3L1 plays a vital role in inflammation and tissue remodeling.24 Serum levels of CHI3L1 are also increased in cancers and many inflammatory diseases.25
CK-18 is the major intermediate filament protein in the liver. Hepatocyte apoptotic pathways are activated in the pathogenesis of NASH and fibrosis, while the presence of CK-18 caspase-generated cleavage fragments is readily tested in the serum.26 CK-18 is decomposed by caspase 3 and exposes its specific Asp396 binding sites; CK18-M30 antibody can identify the released protein fragment, reflecting the level of apoptosis. CK-18 M65 antibody can identify the uncleaved fragment of CK18, reflecting autophagy and necrosis.27 German researchers recommended the CK18-M65 assay to differentiate between low fibrosis stages, as it had a higher AUROC than the M30 assay and was independent of ALT levels.28 However, most subjects in their studies were patients with viral hepatitis, and only 22 were patients with NAFLD. In our research, CK-18 M65 was also found to be a better biomarker than CK-18 M30 for detecting F2 fibrosis in a relatively large NAFLD cohort.
In addition, a recent study demonstrated that APRI could better distinguish fibrosis stages F2/F3 versus F0/F1 than FIB-4 and NFS, which was in accordance with our results.29 In our study, these existing models showed only a mediocre performance in detecting ≥F2 patients in a cohort where most patients had mild fibrosis (F0–F1). Since recent guidelines recommend a close follow-up and early intervention in patients with NASH and/or liver fibrosis,2,30 more F2 patients can be identified and treated at an earlier stage by using our nomogram.
One strength of our study is that we proposed an individualized risk prediction model generated from a biopsy-proven NAFLD cohort. It is undeniable that liver biopsy has an irreplaceable role in the diagnosis of NAFLD. Also, due to the prospective nature of our cohort, we had great access to various novel biomarkers for investigative purposes. We acknowledge several limitations that merit comments. Though it did not affect our prediction model, this cohort predominantly consisted of patients with fibrosis in the earlier stages, and only 24.6% were patients with T2DM, which contributed to the decreased number of patients with significant fibrosis. In addition, the cost and accessibility of some biomarkers in our model may limit clinical application, though novel biomarkers can improve the diagnostic efficiency. Another shortcoming is the lack of validation for this new scoring system. To remedy both the limited number of patients with higher fibrosis as well as the absence of a validation study, a multicenter investigation in both Asian and Western populations may be implemented in the future. Although the net benefit of the nomogram was higher than other scoring systems, further examination by external validation is warranted.
In conclusion, we developed a novel nomogram with a relatively good accuracy to help clinicians assess the risk of significant fibrosis in patients with NAFLD. With an estimate of individual risk, clinicians and patients can take necessary measures in lifestyle monitoring and medical interventions at an earlier stage, before progression to advanced fibrosis.
Acknowledgments
The authors thank Kenneth I. Zheng for linguistic assistance. We also thank Prof. Ji-Min Liu, a pathologist from McMaster University, who conducted quality control of pathology data. This work is a part of the PERSONS study.
Declaration of conflicting interests
All authors: no conflicts. The abstract had been presented as a poster in 2018 EASL NAFLD SUMMIT, which was held in Geneva, Switzerland from 20 to 23 September 2018. The authors also thank Proprium Biotech Company Limited (Hangzhou, China), Hotgen Biotech Inc. (Beijing, China), and Herui Biomed Company Limited (Suzhou, China) for providing ELISA kits.
Ethical approval
Ethical approval for the study was obtained from the ethics committee of the First Affiliated Hospital of Wenzhou Medical University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81500665), High Level Creative Talents from Department of Public Health in Zhejiang Province and Project of New Century 551 Talent Nurturing in Wenzhou.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol 2017; 67: 862–873. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67: 328–357. [DOI] [PubMed] [Google Scholar]
- 3.Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017; 67: 1265–1273. [DOI] [PubMed] [Google Scholar]
- 4.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015; 61: 1547–1554. [DOI] [PubMed] [Google Scholar]
- 5.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: A multi-national cohort study. Gastroenterology 2018; 155: 443–457. [DOI] [PubMed] [Google Scholar]
- 6.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149: 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angulo P, Hui J, Marchesini G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology (Baltimore, MD) 2007; 45: 846–854. [DOI] [PubMed] [Google Scholar]
- 8.Sterling R, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology (Baltimore, MD) 2006; 43: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 9.Harrison S, Oliver D, Arnold H, et al. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008; 57: 1441–1447. [DOI] [PubMed] [Google Scholar]
- 10.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38: 518–526. [DOI] [PubMed] [Google Scholar]
- 11.Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol 2018; 68: 305–315. [DOI] [PubMed] [Google Scholar]
- 12.Balachandran V, Gonen M, Smith J, et al. Nomograms in oncology: More than meets the eye. Lancet Oncol 2015; 16: e173–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eng K, Lopez R, Liccardo D, et al. A non-invasive prediction model for non-alcoholic steatohepatitis in paediatric patients with non-alcoholic fatty liver disease. Digest Liver Dis 2014; 46: 1008–1013. [DOI] [PubMed] [Google Scholar]
- 14.Amato M, Giordano C, Galia M, et al. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010; 33: 920–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology (Baltimore, MD) 2005; 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 16.Stal P. Liver fibrosis in non-alcoholic fatty liver disease – diagnostic challenge with prognostic significance. World J Gastroenterol 2015; 21: 11077–11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Z, Xin Y, Dong Q, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology (Baltimore, MD) 2011; 53: 726–736. [DOI] [PubMed] [Google Scholar]
- 18.Yao M, Wang L, Leung PSC, et al. The clinical significance of GP73 in immunologically mediated chronic liver diseases: Experimental data and literature review. Clin Rev Allerg Immunol 2018; 54: 282–294. [DOI] [PubMed] [Google Scholar]
- 19.Motamed N, Rabiee B, Hemasi GR, et al. Body roundness index and waist-to-height ratio are strongly associated with non-alcoholic fatty liver disease: A population-based study. Hepatitis Month 2016; 16: e39575–e39575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pimenta NM, Cortez-Pinto H, Melo X, et al. Waist-to-height ratio is independently related to whole and central body fat, regardless of the waist circumference measurement protocol, in non-alcoholic fatty liver disease patients. J Hum Nutr Dietet 2017; 30: 185–192. [DOI] [PubMed] [Google Scholar]
- 21.Petta S, Amato MC, Di Marco V, et al. Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2012; 35: 238–247. [DOI] [PubMed] [Google Scholar]
- 22.Vongsuvanh R, George J, McLeod D, et al. Visceral adiposity index is not a predictor of liver histology in patients with non-alcoholic fatty liver disease. J Hepatol 2012; 57: 392–398. [DOI] [PubMed] [Google Scholar]
- 23.Rossi E, Adams LA, Bulsara M, et al. Assessing liver fibrosis with serum marker models. Clin Biochem Rev 2007; 28: 3–10. [PMC free article] [PubMed] [Google Scholar]
- 24.Kumagai E, Mano Y, Yoshio S, et al. Serum YKL-40 as a marker of liver fibrosis in patients with non-alcoholic fatty liver disease. Sci Rep 2016; 6: 35282–35282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libreros S, Garcia-Areas R, Iragavarapu-Charyulu V. CHI3L1 plays a role in cancer through enhanced production of pro-inflammatory/pro-tumorigenic and angiogenic factors. Immunol Res 2013; 57: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wieckowska A, Zein NN, Yerian LM, et al. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 2006; 44: 27–33. [DOI] [PubMed] [Google Scholar]
- 27.Yagmur E, Trautwein C, Leers MP, et al. Elevated apoptosis-associated cytokeratin 18 fragments (CK18Asp386) in serum of patients with chronic liver diseases indicate hepatic and biliary inflammation. Clin Biochem 2007; 40: 651–655. [DOI] [PubMed] [Google Scholar]
- 28.Joka D, Wahl K, Moeller S, et al. Prospective biopsy-controlled evaluation of cell death biomarkers for prediction of liver fibrosis and nonalcoholic steatohepatitis. Hepatology 2012; 55: 455–464. [DOI] [PubMed] [Google Scholar]
- 29.Polyzos SA, Slavakis A, Koumerkeridis G, et al. Noninvasive liver fibrosis tests in patients with nonalcoholic fatty liver disease: An external validation cohort. Horm Metab Res 2019; 51: 134–140. [DOI] [PubMed] [Google Scholar]
- 30.Chitturi S, Wong VW, Chan WK, et al. The Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 2: Management and special groups. J Gastroenterol Hepatol 2018; 33: 86–98. [DOI] [PubMed] [Google Scholar]