Abstract
Background
Recurrence remains a challenge in Clostridium difficile infection (CDI), and in this field fecal microbiota transplantation (FMT) has attracted significant interest. Network meta-analysis (NWM) has been established as an evidence-synthesis tool that incorporates direct and indirect evidence in a collection of randomized controlled trials. So far no NWM exists concerning therapeutic interventions for recurrent CDI (rCDI).
Objective
In this NWM we assessed the comparative effectiveness of various therapies for rCDI to examine the efficacy rank order and determine the optimum therapeutic approach.
Methods
A Bayesian network meta-analysis was performed to investigate the efficacy rank order of rCDI interventions.
Results
Six eligible RCTs were entered into an NWM. They included 348 rCDI patients, in whom seven therapeutic interventions were used, i.e. donor fecal microbiota transplantation (DFMT), vancomycin, fidaxomicin, vancomycin + DFMT, vancomycin + bowel lavage, autologous FMT and placebo. DFMT showed the highest efficacy in comparison with vancomycin [odds ratio (95% credible interval), 20.02 (7.05–70.03)] and fidaxomicin (22.01 (4.38–109.63)).
Conclusion
This NWM showed that DFMT is the optimum therapeutic approach for rCDI, as it was the most efficacious among various therapeutic interventions, particularly in comparison with commonly used antibiotics such as vancomycin or fidaxomicin.
Keywords: Fecal microbiota transplantation, network meta-analysis, recurrent Clostridium difficile infection, treatment
Key summary
Established knowledge on this subject
A substantial percentage of patients with Clostridium difficile infection (CDI) experience recurring symptoms.
Usually recurrent CDI (rCDI) is treated using a regimen of metronidazole or vancomycin. Owing to poor treatment results, efforts for new therapeutic approaches are still being sought. Among these attempts, fecal microbiota transplantation (FMT) has attracted significant interest.
So far conventional pairwise meta-analyses have included some randomized controlled trials (RCTs) comparing donor FMT (DFMT) with various other therapies for rCDI. However, some of these RCTs have more than two branches in their design and additionally new relevant RCTs are available, thus a reexamination of the current evidence is warranted.
Network meta-analysis (NWM) is an evidence-synthesis tool for comparing RCTs with multiple treatments. No NWM exists concerning rCDI.
Significant and/or new findings
This NWM suggests that among various therapeutic interventions tested in RCTs, DFMT is the best intervention for rCDI.
DFMT holds considerable promise as a therapy for rCDI, a finding expected to be taken into account in future therapeutic guidelines.
Introduction
Clostridium difficile (currently called Clostridioides difficile) infection (CDI) is today considered the most common hospital-acquired cause of diarrhea, and it is estimated that over recent decades the global incidence of this infection has greatly increased. Additionally, CDI outbreaks have been related to dire consequences concerning morbidity and mortality. The burden of CDI has become a challenge for health care policies, and it is estimated that in the United States, per year, CDI accounts for almost US$5 billion in health care costs and almost 29,000 deaths.1–3
The most accepted theory for the pathogenesis of CDI supports the notion that antibiotic use might alter intestinal microbiota composition, thus permitting pathogenic toxin-producing C. difficile strains to colonize the intestine. Initial CDI episodes are usually treated with metronidazole or vancomycin, but a substantial percentage of these patients, after an initial amelioration, experience recurring CDI (rCDI), which is usually treated with a regimen of antibiotics.1–3 However, taking into account their poor treatment results for rCDI, efforts for new treatments are still being sought.4–7 Among these attempts, the utilization of gut microbiota from a healthy donor to restore the patient's microbiota by infusing a liquid suspension of the donor's stool, the so-called fecal microbiota transplantation (FMT), has attracted significant interest. It is noteworthy that despite the fact that FMT was first performed in humans many decades ago,8 this therapy has not been widely accepted as a treatment modality, probably for reasons related to acceptability and safety. However, there is growing interest throughout the world, and apart from CDI, its utility has been extended to other conditions including inflammatory bowel disease9,10 and irritable bowel syndrome,11 two diseases that are considered to have pathogenetic links to intestinal microbiota.
There are existing systematic reviews examining FMT effectiveness in CDI,12–16 but the majority of them have concentrated on case series evidence and not randomized controlled trials (RCTs). Recently two conventional pairwise meta-analyses17,18 included RCTs comparing donor FMT (DFMT) with various other therapies for rCDI. However, some of the RCTs have more than two branches in their design, and additional new, relevant RCTs are available, meaning that a reexamination of the current evidence is warranted. Network meta-analysis (NWM) has been used as an evidence-synthesis tool that, in a collection of RCTs with multiple treatments, provides information concerning the relative effects of three or more interventions competing for a similar result.19–21 Since there is no NWM concerning rCDI, the aim of this NWM was to assess the relative efficacies of various rCDI therapeutic interventions evaluated in relevant RCTs.
Methods
Identification of studies and data extraction
In this NWM for identification of studies and data extraction, we followed steps as described in our earlier publications.22 Thus, PubMed /MEDLINE and Embase databases were searched up to December 2018 to identify English-language human studies using the following search text and/or Medical Subject Heading terms: “clostridium infections” OR “clostridium” AND “infections” OR “clostridium infections” OR “clostridium” AND “difficile” AND “infection” OR “clostridium difficile infection” AND “therapy” [Subheading] OR “therapy” OR “treatment” OR “therapeutics” OR “therapeutics” AND “fecal microbiota transplantation” OR “fecal” AND “microbiota” AND “transplantation” OR “fecal microbiota transplantation.” In addition a manual search of all review articles, published editorials and retrieved original studies was performed. Two authors (T.R. and J.P.G.) independently extracted data from each study. Any disagreement was settled after discussion with a third author until consensus was reached. This NWM was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension statement for interventions,23 whereas the rating of the quality of RCTs was achieved by using the Grading of Recommendations Assessment, Development and Evaluation Working Group modality.24 Furthermore, we appraised the confidence in estimates derived from the NWM.25 In this process the construction of a contributions matrix depicting the contribution of each direct piece of evidence to the NWM results and also the construction of a bar graph depicting the risk of bias (RoB) for each network estimate, as well as the entire network, aids in assessing the quality of evidence in the NWM.
Selection criteria
We defined the inclusion and exclusion criteria prior to commencement of the study search; thus, suitable studies were included in the meta-analysis provided that the following criteria were met:
(1) They were published as articles or abstracts or presented at a congress of a recognized medical society, (2) they were written in English and (3) they were RCTs comparing FMT with other rCDI therapeutic interventions. The primary outcome assessment in these RCTs was the resolution of CDI-related symptoms, without the need for additional CDI treatment during the follow-up period. Studies that did not fulfill these inclusion criteria were excluded.
Statistical analysis
The κ coefficient was used to evaluate the study selection process between the two reviewers. For pairwise meta-analyses and assessment of heterogeneity, we followed the methodology as we have previously described.22 In addition to heterogeneity we assessed inconsistency, since this is crucial when conducting an NWM.25 We constructed comparison-adjusted funnel plots and checked their symmetry to evaluate whether small-size trials influence the efficacy results. Surface under cumulative ranking (SUCRA) values were used in rankograms for the rCDI intervention network to examine the cumulative rank probability for each intervention concerning the efficacy achieved by this intervention in comparison to an ideal intervention that shows the best efficacy without doubt, i.e. SUCRA = 1 or 100% when expressed as a percentage.19–21 Data were processed by using software suitable for Bayesian NMA, i.e. Stata 13.2 (StataCorp, College Station, TX, USA)19,20 and NetMetaXL.21 A p value < 0.05 reflected significance for all tests, except for heterogeneity, for which the respective value was 0.1.
Results
Characteristics of studies
A flowchart depicting the study selection is illustrated in Figure 1. Thus, out of 643 titles yielded by the initial search, six RCTs26–31 were in the end eligible for meta-analysis. Reviewers' agreement concerning the selection of studies was high (κ = 0.97; 95% confidence interval (CI) 0.94–1). The characteristics of the six involved RCTs are shown in Table 1. They were conducted in different geographic locations and when combined included 348 patients. The network and intervention characteristics together with direct pairwise comparisons are shown in Table 2. Briefly, there were two RCTs with three branches26,31 and four RCTs with two branches.27–30 In these RCTs there were seven therapeutic interventions, i.e. A = donor FMT (DFMT), B = vancomycin, C = vancomycin plus bowel lavage (BL), D = autologous FMT (AFMT), E = vancomycin plus DFMT, F = placebo, and G = fidaxomicin. There were eight pairwise comparisons with 10 sets of direct data and a total of 21 possible pairwise comparisons (Table 2). In the eight pairwise comparisons with direct data, DFMT proved to be superior to other interventions, i.e. vancomycin, fidaxomicin, DFMT plus vancomycin, AFMT and placebo. There was significant heterogeneity across studies (Q value = 8.66, I2 = 88.45%, p = 0.003), whereas there was no statistically significant publication bias. One of the causes of the observed heterogeneity might be related to delivery modalities that had been used for FMT, i.e. nasogastric or nasojejunal tube, upper gastrointestinal (GI) endoscopy, colonoscopy and enemas. Concerning quality, overall the RCTs were assessed at the low to moderate end of the rating scale mainly because of lack of blinding, as four out of six (67%) were unblinded (open label) and in addition were underpowered.
Figure 1.
Flowchart of studies included in the network meta-analysis.
Table 1.
Main characteristics of studies included in the network meta-analysis.
| Reference | Year/Country | Study type/ total no included patients | Disease characteristics | FMT donor/ delivery method | Intervention/ no patients included | Comparator/ no patients included | Definition of cure | Follow-up | DFMT-related events |
|---|---|---|---|---|---|---|---|---|---|
| 26 | 2013/ Netherlands | RCT/n = 42 | CDI recurrence after adequate antibiotic treatment (vancomycin, metronidazole) | Healthy volunteer/ nasoduodenal tube | Vancomycin (500 mg qid, 4 days), followed by bowel lavage with 4 l Macrogol solution (Klean-Prep) on final day of antibiotic treatment and subsequent infusion of FMT solution (n = 16) | Vancomycin (500 mg qid, 14 days), or vancomycin with bowel lavage on day 4 or 5 (n = 26) | Absence of diarrhea, or persistent diarrhea explained by other causes, with three consecutive negative stool tests for C. difficile toxin | 10 weeks | Belching, nausea, abdominal cramps, diarrhea |
| 27 | 2015/Italy | RCT/n = 39 | Recurrent CDI after antibiotic treatment | Healthy volunteer/ colonoscopy | Vancomycin (125 mg qid, three days), followed by one or more infusions of feces (diluted in 500 ml sterile saline) (n = 20) | Vancomycin (125 mg qid, 10 days), followed by 125–500 mg/day every two to three days for at least three weeks (n = 19) | Disappearance of diarrhea, or persistent diarrhea explicable by other causes, with two negative stool tests for C. difficile toxin | 10 weeks | Diarrhea, bloating, abdominal cramps |
| 28 | 2016/USA | RCT/n = 46 | Recurrent CDI after antibiotic treatment | Healthy volunteer/ colonoscopy | 300 ml fecal suspension infused into terminal ileum or cecum (n = 22) | Autologous FMT: 300 ml fecal suspension from own stool infused into terminal ileum or cecum (n = 24) | Resolution of diarrhea without need for further anti-CDI therapy for eight weeks | Eight weeks | Αbdominal pain, bloating, nausea, vomiting, flatulence, anorexia, constipation, fatigue, diarrhea Four serious side effects reported, none directly related to FMT |
| 29 | 2017/Canada | RCT/n = 30 | Two or more recurrences of CDI; at least one treatment with oral vancomycin. | Sixteen fresh donations from healthy donors/ enema | Oral vancomycin (125 mg qid, 14 days) followed by single 500 ml FMT (n = 16) | Oral tapering vancomycin for six weeks: 125 mg, qid, 14 days; then twice daily, once daily, every second day, every third day; each one-week duration (n = 14) | No recurrence of CDAD within 120 days (primary) or no recurrence of CDAD symptoms (not laboratory confirmed) (secondary outcome) | 17 weeks | Abdominal pain, tenderness, bloating |
| 30 | 2018/Canada | RCT/n = 127 | At least two CDI recurrences after antibiotic treatment | Commercially prepared fecal microbiota suspension/ enema | RBX2660: one or two doses, seven days apart (n = 83) | Two doses of placebo, seven days apart (n = 44) | Clinical resolution of diarrhea without relapse at 13 weeks, without need for antibiotics | Eight weeks | Diarrhea, abdominal pain, flatulence, constipation, nausea |
| 31 | 2019/ Denmark | RCT/n = 64 | Recurrent CDI (≥2 prior CDI episodes; median number of episodes = 4) | Healthy donors recruited and screened at Aarhus University Hospital/ colonoscopy or nasojejunal tube | Frozen-thawed single-donor feces (50 g solution). FMT administered after 4–10 days of vancomycin) (n = 24) | Fidaxomicin (200 mg twice per day for 10 days); n = 24 or standard vancomycin (125 mg qid for 10 days) (n = 16). | Composite of clinical resolution and negative C. difficile stool toxin PCR test eight weeks after treatment) | Eight weeks | Abdominal pain, bloating, constipation, diarrhea. A 50-year-old woman developed sepsis-like clinical picture (i.e. fever, convulsions, vomiting and diarrhea) with complete recovery within 24 hours without further treatment. |
CDAD: Clostridium difficile–associated diarrhea; CDI: Clostridium difficile infection; FMT: fecal microbiota transplantation; qid: four times per day; PCR: polymerase chain reaction; RCT: randomized controlled trial; USA: United States of America.
Table 2.
Network characteristics (a), intervention characteristics (b) and direct pair-wise comparisons (c).
| (a) Network characteristics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of interventions | 7 | ||||||||||||
| Number of studies | 6 | ||||||||||||
| Total number of patients in network | 348 | ||||||||||||
| Total number of treated patients in network | 193 | ||||||||||||
| Total possible pairwise comparisons | 21 | ||||||||||||
| Number of pairwise comparisons with direct data | 8 | ||||||||||||
| Number of two-arm studies | 4 | ||||||||||||
| Number of multiarm studies | 2 | ||||||||||||
| Number of studies with no zero events | 6 | ||||||||||||
| Number of studies with at least one zero event | 0 | ||||||||||||
| Number of studies with all zero events | 0 | ||||||||||||
AFMT: autologous fecal microbiota transplantation; BL: bowel lavage; CI: confidence interval; DFMT: donor fecal microbiota transplantation; NA: not applicable; OR: odds ratio.
Network meta-analysis
(a) Network map
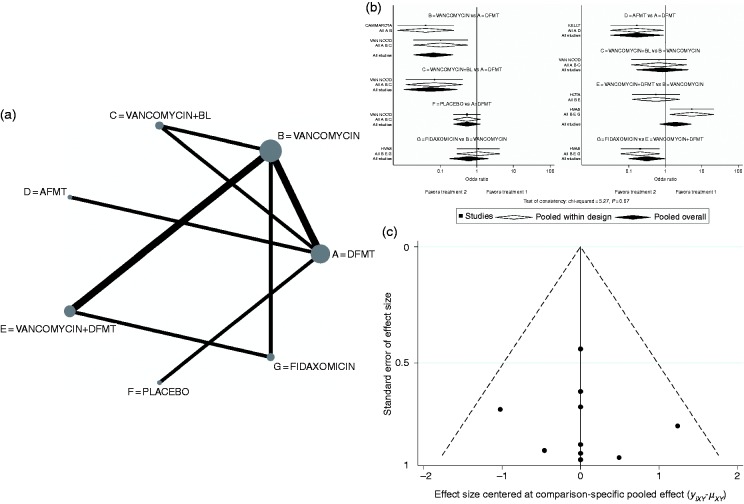
The network map of the rCDI therapeutic interventions included in the six RCT studies is depicted in Figure 2(a). In this map the node size reflects the number of patients allocated to each treatment, whereas the edge thickness is in proportion to the precision, i.e. the inverse of variance of each direct comparison.25
Figure 2.
(a) Network map of recurrent Clostridium difficile infection interventions included in the randomized controlled trials. Node size reflects the number of patients randomly assigned to each treatment. Edge thickness is in proportion to the precision, i.e. the inverse of variance of each direct comparison. (b) Network forest plot. There was no significant inconsistency (p = 0.7). (c) Comparison-adjusted funnel plot.
Treatment labels: A: DFMT (donor fecal microbiota transplantation); B: vancomycin; C: vancomycin + BL (bowel lavage); D: AFMT (autologous fecal microbiota transplantation); E: vancomycin + DFMT; F: placebo; G: fidaxomicin.
(b) Network plots
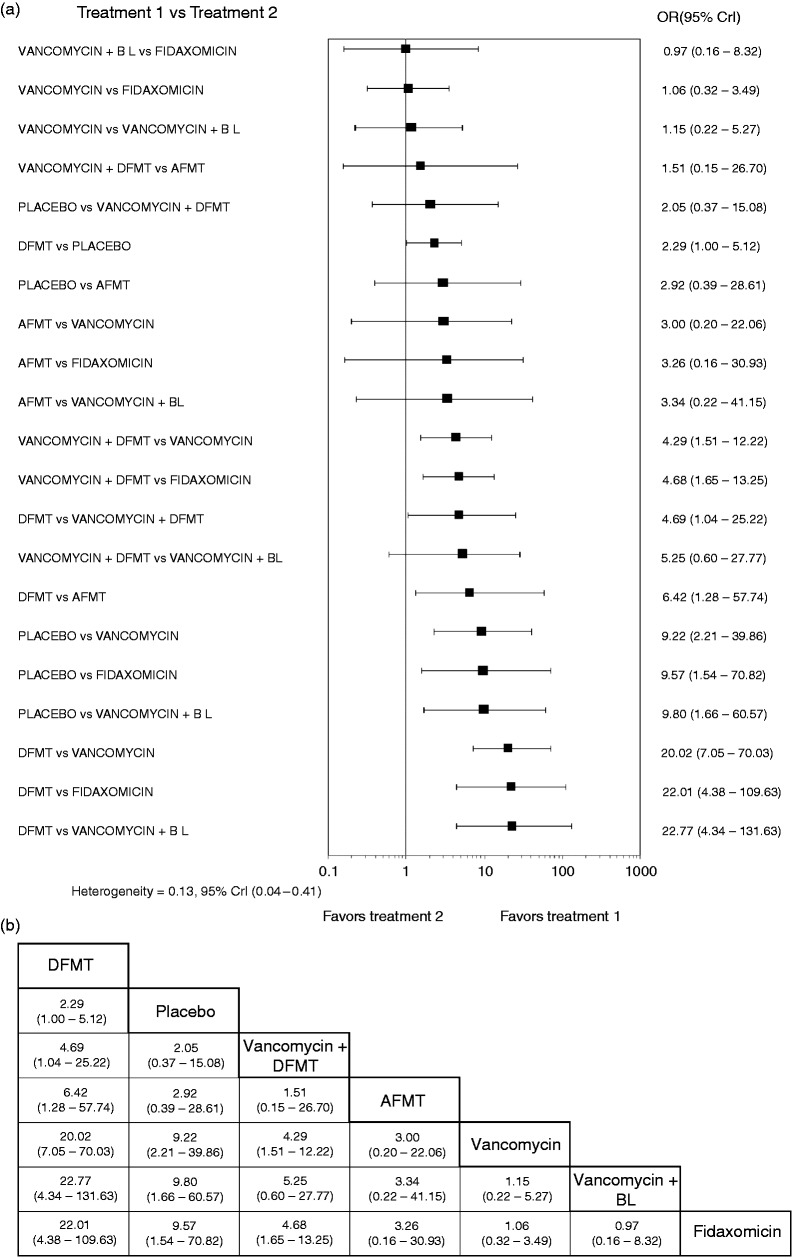
The network forest plot is shown in Figure 2(b), which also shows that the evaluation of inconsistency yielded insignificant overall results, meaning that the comparative effect sizes that were obtained by direct and indirect comparisons are consistent. The relevant funnel plot (Figure 2(c)) appeared symmetric, reflecting the lack of effects from small studies in the network. The contribution of each comparison in the network is demonstrated in the constructed contribution plot (Figure 3(a)). This plot shows that the comparison of DFMT vs vancomycin had the greatest contribution to the entire network (20.1%). The associated bar graph (Figure 3(b)) depicts the bias risk for each network assessment, demonstrating the volume of information originating from high, unclear and low risk of bias studies (Figure 3(c)). The comparative efficacy of different therapeutic interventions was checked by conducting a total of 21 possible pairwise comparisons (direct and indirect) as shown in the forest plot of Figure 4(a). There was no significant heterogeneity [Q = 0.13, 95% credible interval(CrI) 0.04–0.41]. Among all the therapeutic interventions evaluated, DFMT showed the highest efficacy in comparison with vancomycin, AFMT and fidaxomicin (odds ratios (ORs) (95% CrI), 20.02 (7.05–70.03), 6.42 (1.28–57.74) and 22.01 (4.38–109.63), respectively). Efficacy comparison between vancomycin and fidaxomicin showed no statistically significant difference (1.06 (0.32–3.49)).
Figure 3.
(a) Contribution plot for the comparisons network. The numbers represent the percentage contribution of the column showing direct comparisons to the row defining network meta-analysis estimates. (b) Bar graph depicting the risk of bias (RoB) for each network estimate. Light gray = low RoB, dark gray = unclear RoB, black = high RoB. (c) Quality matrix depicting the summary of RoB for each included study. Studies are identified by the number in the reference list. Treatment labels: A: DFMT (donor fecal microbiota transplantation); B: vancomycin; C: vancomycin + BL (bowel lavage); D: AFMT (autologous fecal microbiota transplantation); E: vancomycin + DFMT; F: placebo; G: fidaxomicin.
Figure 4.
(a) Forest plot illustrating all possible pairwise comparisons of recurrent Clostridium difficile (rCDI) interventions according to their efficacies. The horizontal lines represent credible intervals (CrI). (b) League table showing the comparative efficacies of rCDI interventions.
AFMT: autologous fecal microbiota transplantation; BL: bowel lavage; DFMT: donor fecal microbiota transplantation; OR: odds ratio.
(c) League table and rankogram
The superiority of DFMT, in comparison with other interventions, is characteristically shown in the league table of the comparative efficacies of rCDI therapeutic modalities (Figure 4(b)) and also in the rankogram of Figure 5(a) together with SUCRA values (Figure 5(b)). Thus, the SUCRA value for DFMT was 99.1%, whereas the respective SUCRA value for fidaxomicin represented the least efficacious regimen (Figure 5(b)).
Figure 5.
(a) Rankograms for the recurrent Clostridium difficile intervention network showing the cumulative rank order for each intervention. (b) SUCRA: surface under cumulative ranking. Values for the six therapeutic interventions. AFMT: autologous fecal microbiota transplantation; BL: bowel lavage; DFMT: donor fecal microbiota transplantation.
Discussion
The significance of the gut microbiome in human inflammation and disease has attracted considerable international interest in recent decades. In particular, DFMT has gained attention as a valuable treatment option for patients with CDI, and conventional pairwise meta-analyses have highlighted its efficacy in treating CDI. However, the majority of pairwise analyses have focused on evidence from case series. The present NWM included all relevant RCTs investigating treatments for rCDI and showed that, among the various therapeutic interventions, DFMT was the most efficacious, particularly in comparison with antibiotics commonly used, i.e. the older vancomycin treatment and the newer fidaxomicin regimen. In this context there was no therapeutic superiority of either of the two antibiotics, i.e. vancomycin versus fidaxomicin, in their pairwise comparison. For acute diarrhea, however, a relevant RCT7 showed that fidaxomicin and vancomycin had equal results concerning its resolution, but more sustained resolution was achieved with fidaxomicin. This finding might be due to lesser impairment of the intestinal microbiome during treatment of the infection.
As the RCTs included in this NWM differ in their design, the results should be interpreted with caution. Thus, the use of bowel lavage before FMT26 or repeated FMT27 may influence the effect of FMT. In addition the results of the comparison of vancomycin vs DFMT should also be interpreted carefully, especially in the context of overall low to moderate quality of evidence. It is noteworthy that one RCT28 showed a high clinical cure rate after AFMT in the patients from New York as opposed to Rhode Island. One of the possible explanations might be the higher use of fidaxomicin in the New York group, which may cause less dysbiosis, so AFMT may not be “inert” because of changes in the FMT during processing. In addition to the above, the good placebo response finding in another RCT30 should be noted. This finding was unexpected and may suggest that in a number of enrolled patients rCDI may not have been active. However, since there is no obvious explanation, further exploratory studies are needed to interpret possible underlying reasons for this placebo performance.
While this NWM suggests DFMT is the best intervention for rCDI after antibiotics have failed, there are still unanswered problems to be addressed before this therapeutic modality can be recommended as the routine therapeutic standard, despite being reported decades ago. Thus, across the existing reports there are challenges related to classification, research definitions, delivery routes and in addition matters connected to FMT donor type, i.e. unrelated vs patient-selected (traditionally close relative or spouse). Some of the above challenges were addressed recently in some RCTs and in a pairwise meta-analysis.18 Thus, it seems that frozen/thawed fecal material, which is an easier method to reduce the pressure on a donor to provide feces on the day required, is as effective as fresh FMT.32 Toward this, capsulated forms of frozen or lyophilized material might be the solution.33 However, some early RCT findings34 are not as promising as expected, and therefore more RCT data are needed. Concerning delivery route, it seems that the nasoduodenal or colonoscopic routes might be more effective than enemas. It must be said, however, that despite all the problems, for some patients FMT may be the only therapeutic option.
One important matter that frequently comes to light in relation to FMT concerns safety. From this point of view in this NWM, as shown in Table 1, only mild transient events, such as abdominal pain, bloating, nausea, vomiting, flatulence, anorexia, constipation, fatigue and diarrhea were reported in all RCTs, which were similar among treatment groups. However, FMT safety remains an important issue when FMT is considered as a therapeutic option.
The lack of significant inconsistency strengthens our results. However, despite the positive and optimistic messages concerning DFMT effectiveness in rCDI that emerge from this NWM, some limitations should be stressed. The main limitation is related to the fact that the studies involved in this NWM were assessed as being of low to moderate quality when considering factors such as blindness and power. These reflect some risk of bias through the overall appraisal of confidence in estimates. Other limitations are related to different FMT protocols used in the involved RCTs. For example, there was heterogeneity concerning the delivery modalities that had been used for FMT, i.e. nasogastric or nasojejunal tube, upper GI endoscopy, colonoscopy and enemas. From this point of view, standardized methodology is important concerning research and clinical perspectives alike. An additional potentially important limitation may be related to the heterogeneity of enrolled patients infected, according to C. difficile ribotype. For example, one of the six meta-analyzed RCTs31 did not include patients infected with C. difficile ribotype 027 and therefore their results may not be extrapolated to settings with a high frequency of this ribotype. It is noteworthy that the trials in this NWM mostly enrolled patients with less-severe disease on the rCDI spectrum (mostly diarrhea) and therefore the question is pending as to whether DFMT is effective in patients with severe CDI. Toward this, Ianiro and colleagues35 recently published an RCT comparing single vs multiple FMT infusions and showed that in refractory-to-antibiotics, severe (pseudomembrane-driven) CDI, multiple infusions and concomitant vancomycin were more effective in comparison with a single fecal transplant followed by vancomycin.
In conclusion, the results of this NWM show that DFMT holds a high position in the therapeutic armamentarium for rCDI. This finding is expected to be taken into account in future therapeutic guidelines. However, since there are a number of questions still to be answered, well-designed RCTs and long-term follow-up registries are needed to ensure the efficacy and safety profile of DFMT.
Acknowledgments
Author contributions include the following: T.R. and J.P.G. conceived and designed the network meta-analysis, performed eligibility screening, carried out the data extraction, analyzed the data and wrote the original draft. A.G., G.L.H., H.T., P.M., F.M., C.O. interpreted the data and revised the draft critically. All authors approved the final version of the paper, including the authorship list.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
Not applicable.
Ethics approval
Not applicable.
References
- 1.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015; 372: 1539–1548. [DOI] [PubMed] [Google Scholar]
- 2.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 2012; 55(Suppl 2): S88–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372: 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009; 15: 285–289. [DOI] [PubMed] [Google Scholar]
- 5.Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol 2009; 15: 1554–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer MP, van Dissel JT. Alternative strategies for Clostridium difficile infection. Int J Antimicrob Agents 2009; 33(Suppl 1): S51–S56. [DOI] [PubMed] [Google Scholar]
- 7.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364: 422–431. [DOI] [PubMed] [Google Scholar]
- 8.Eiseman B, Silen W, Bascom GS, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44: 854–859. [PubMed] [Google Scholar]
- 9.Paramsothy S, Paramsothy R, Rubin DT, et al. Faecal microbiota transplantation for inflammatory bowel disease: A systematic review and meta-analysis. J Crohns Colitis 2017; 11: 1180–1199. [DOI] [PubMed] [Google Scholar]
- 10.McIlroy J, Ianiro G, Mukhopadhya I, et al. Review article: The gut microbiome in inflammatory bowel disease—avenues for microbial management. Aliment Pharmacol Ther 2018; 47: 26–42. [DOI] [PubMed] [Google Scholar]
- 11.Wen W, Zhang H, Shen J, et al. Fecal microbiota transplantation for patients with irritable bowel syndrome: A meta-analysis protocol. Medicine (Baltimore) 2018; 97: e12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moayyedi P, Marshall JK, Yuan Y, et al. Canadian Association of Gastroenterology position statement: Fecal microbiota transplant therapy. Can J Gastroenterol Hepatol 2014; 28: 66–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drekonja D, Reich J, Gezahegn S, et al. Fecal microbiota transplantation for Clostridium difficile infection: A systematic review. Ann Intern Med 2015; 162: 630–638. [DOI] [PubMed] [Google Scholar]
- 14.Rossen NG, MacDonald JK, de Vries EM, et al. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J Gastroenterol 2015; 21: 5359–5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman BC, Moore HB, Overby DM, et al. Fecal microbiota transplant in patients with Clostridium difficile infection: A systematic review. Acute Care Surg 2016; 81: 756–764. [DOI] [PubMed] [Google Scholar]
- 16.Kassam Z, Lee CH, Yuan Y, et al. Fecal microbiota transplantation for Clostridium difficile infection: Systematic review and meta-analysis. Am J Gastroenterol 2013; 108: 500–508. [DOI] [PubMed] [Google Scholar]
- 17.Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: The efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther 2017; 46: 479–493. [DOI] [PubMed] [Google Scholar]
- 18.Moayyedi P, Yuan Y, Baharith H, et al. Faecal microbiota transplantation for Clostridium difficile-associated diarrhoea: A systematic review of randomised controlled trials. Med J Aust 2017; 207: 166–172. [DOI] [PubMed] [Google Scholar]
- 19.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J Clin Epidemiol 2011; 64: 163–171. [DOI] [PubMed] [Google Scholar]
- 20.Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown S, Hutton B, Clifford T, et al. A Microsoft-Excel–based tool for running and critically appraising network meta-analyses—an overview and application of NetMetaXL. Syst Rev 2014; 3: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rokkas T, Gisbert JP, Niv Y, et al. The association between Helicobacter pylori infection and inflammatory bowel disease based on meta-analysis. United European Gastroenterol J 2015; 3: 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions checklist and explanations. Ann Intern Med 2015; 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 24.Puhan MA, Schuenamann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2008; 336: 924–926. [DOI] [PubMed] [Google Scholar]
- 25.Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS One 2014; 9: e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368: 407–415. [DOI] [PubMed] [Google Scholar]
- 27.Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther 2015; 41: 835–843. [DOI] [PubMed] [Google Scholar]
- 28.Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: A randomized trial. Ann Intern Med 2016; 165: 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hota SS, Sales V, Tomlinson G, et al. Oral vancomycin followed by fecal transplantation versus tapering oral vancomycin treatment for recurrent Clostridium difficile infection: An open-label randomized controlled trial. Clin Infect Dis 2017; 64: 265–271. [DOI] [PubMed] [Google Scholar]
- 30.Dubberke ER, Lee CH, Orenstein R, et al. Results from a randomised, double-blinded, placebo-controlled trial of a RBX2660—a microbiota-based drug for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis 2018; 67: 1198–1204. [DOI] [PubMed] [Google Scholar]
- 31.Hvas CL, Dahl Jørgensen SM, Jørgensen SP, et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent Clostridium difficile infection. Gastroenterology 2019; 156: 1324–1332.e3. [DOI] [PubMed] [Google Scholar]
- 32.Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: A randomized clinical trial. JAMA 2016; 315: 142–149. [DOI] [PubMed] [Google Scholar]
- 33.Youngster I, Mahabamunuge J, Systrom HK, et al. Oral, frozen fecal microbiota transplant (FMT) capsule for recurrent Clostridium difficile infection. BMC Med 2016; 14: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang ZD, Ajami NJ, Petrosino JF, et al. Randomized clinical trial: Fecal microbiota transplantation for recurrent Clostridium difficile—fresh, or frozen, or lyophilized microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol Ther 2017; 45: 899–908. [DOI] [PubMed] [Google Scholar]
- 35.Ianiro G, Masucci L, Quaranta G, et al. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory Clostridium difficile infection-single versus multiple infusions. Aliment Pharmacol Ther 2018; 48: 152–159. [DOI] [PubMed] [Google Scholar]