Abstract
Background
Irritable bowel syndrome (IBS) is highly prevalent and presents a clinical challenge. Gelsectan is a medical device containing xyloglucan (XG), pea protein and tannins (PPT) from grape seed extract, and xylo-oligosaccharides (XOS), which act together to protect and reinforce the intestinal barrier.
Objective
The objective of this study is to evaluate the efficacy and safety of XG + PPT + XOS in patients with diarrhoea-predominant IBS (IBS-D).
Methods
In this double-blind study, 60 patients were randomly assigned to receive XG + PPT + XOS or placebo for 28 days, then crossed over to the alternative treatment. Patients were followed for 60 days.
Results
At Day 28, a significantly higher proportion of patients starting treatment with XG + PPT + XOS than placebo (87 vs 0%; p = 0.0019) presented normal stools (Bristol Stool Form Scale type 3−4). At Day 56, a significantly higher proportion of patients who crossed over to XG + PPT + XOS than placebo (93% vs 23%; p = 0.0001) presented normal stools. In the group allocated to receive XG + PPT + XOS after placebo, benefits of XG + PPT + XOS were maintained during follow-up. Subjective assessments of abdominal pain, bloating, quality of life and general health indicated significant improvement with XG + PPT + XOS over placebo. There were no related adverse events.
Conclusion
XG + PPT + XOS effectively controlled diarrhoea and alleviated clinical symptoms in patients with IBS-D, and was well tolerated.
Keywords: Diarrhoea-predominant irritable bowel syndrome, Gelsectan, pea protein and tannins, prebiotics, mucoprotectants, xyloglucan, xylo-oligosaccharide
Key summary
Established knowledge:
Irritable bowel syndrome characterised by diarrhoea (IBS-D) has a high prevalence and is associated with substantial health, social and economic costs.
The aetiology of IBS-D is complex and no standard treatment protocol exists.
Gelsectan is a medical device containing xyloglucan (XG), pea protein and tannins (PPT) from grape seed extract, and xylo-oligosaccharides (XOS) with intestinal mucosal protective properties.
Significant and/or new findings:
XG + PPT + XOS induced remission of diarrhoea in most patients with IBS-D.
Clinical symptoms of abdominal pain and bloating were significantly improved with XG + PPT + XOS compared with placebo.
Patients' quality of life and general health were better under treatment with XG + PPT + XOS than placebo.
XG + PPT + XOS was well tolerated.
XG + PPT + XOS represents a valuable nonpharmacological option for effective management of IBS-D.
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder with a chronic evolution, characterised by recurrent episodes of abdominal pain or discomfort and disordered bowel function (e.g. constipation, diarrhoea, or alternating constipation and diarrhoea).1 The current accepted aetiological explanation for IBS is a biopsychosocial model which describes interaction between psychological, behavioural, psychosocial and environmental factors.2
IBS can affect up to one in five people at some point in their lives1 and has a significant impact on quality of life and health resource utilisation.1,2 Indeed, IBS is the most commonly diagnosed gastrointestinal condition1 and the most frequent reason for referral to gastroenterology clinics.3
Approximately 40% of patients with IBS have diarrhoea as the predominant bowel symptom (IBS-D subtype).4 Patients with IBS-D typically present abdominal pain associated with frequent loose stools, cramping, urgency not relieved by defecation, and mucus in the stool; acute diarrhoea is a common symptom.5 A multidisciplinary approach and an ongoing management strategy are generally required to maintain symptom control.2
Gelsectan (Noventure, Barcelona, Spain) is a Class IIa European Conformity–marked medical device indicated for symptomatic relief and prevention of chronic or relapsing diarrhoea, abdominal tension, pain, bloating and flatulence. It has a protective effect on the intestinal mucosa which is attributable to the properties of its active components: xyloglucan (XG), pea protein and tannins (PPT) from grape seed extract, and xylo-oligosaccharides (XOS). XG is a nonionic, neutral polysaccharide that is present in the primary cell walls of all vascular plants and thus a natural part of the human diet. It has a cellulose-like backbone with side chains containing xylose and galactosyl substituents.6 This mucin-like molecular structure confers mucoadhesive properties, allowing XG-containing formulations to form a physical barrier that protects mucosal cells against damage from microorganisms, allergens and proinflammatory compounds.6 XG has documented efficacy for treatment of acute diarrhoea in adults and children.7,8 PPT is also mucoprotective, and XOS is a prebiotic known to exert a beneficial effect via a bifidogenic effect in the colon.9 In a rat model of IBS, XG + PPT + XOS was shown to inhibit stress-induced visceral hypersensitivity and gut hyperpermeability, providing a preclinical rationale for its use in patients with IBS-D.10
The aim of this study is to assess the efficacy and safety of XG + PPT + XOS in patients with IBS-D.
Materials and methods
This multicentre, double-blind, placebo-controlled, randomised, crossover clinical trial was conducted to evaluate the efficacy and safety of XG + PPT + XOS in patients with diarrhoea-predominant IBS.
Patients age 18–65 years with a confirmed diagnosis of IBS-D according to the Rome III criteria11 were enrolled at five accredited community-based gastroenterology clinics in Romania. Patients were excluded if they were pregnant or breastfeeding; diabetic; unwilling to sign the informed consent form; unable to attend the study visits; had an allergy to any of the product ingredients; or whose health status precluded participation.
A computer-generated randomisation list was used to allocate patients to treatment with XG + PPT + XOS or placebo, one capsule twice daily before breakfast and before dinner, for 28 days, followed by crossover to the alternate treatment for 28 days. Follow-up assessments were performed at 30 and 60 days after the end of crossover treatment. Patients received lots of study medication on Day 1 and Day 28 based on codes (two per patient) generated during randomisation. Patients and investigators were blinded to treatment and treatment sequence.
Assessments were conducted at screening and on study days 1, 15, 28, 56, 86 and 116 (end of study). General physical (vital signs), clinical (abdominal pain and bloating) and biochemical and haematological (creatinine, glucose, glutamic-pyruvic transaminase/alanine transaminase/aspartate transaminase, alkaline phosphatase, haemoglobin, and erythrocyte sedimentation rate) assessments were performed. Patients completed the IBS Quality of Life (IBS QoL) questionnaire12,13 and EQ-5D-3L questionnaire.14 Patients used a diary to record daily stools emissions, evolution of clinical symptoms, frequency and severity of adverse events, and use of rescue medication. Concomitant medication was assessed at each study visit. Concomitant antidiarrhoeal medication was not allowed without permission of the investigators, and then only in nonresponders or for aggravation of diarrhoea. Treatment adherence was evaluated at the end of first-line and crossover treatment using drug accountability forms.
Efficacy was assessed according to the clinical remission rate, which defined clinical remission as the disappearance of diarrhoea, that is, two or fewer nonwatery stools per day (less than type 5 on the Bristol Stool Form Scale (BSFS))15; subjective improvement of abdominal pain and bloating on a seven-point Likert scale (1 = totally unacceptable to 7 = perfectly acceptable); and change from baseline in scores on the IBS QoL and EQ-5D-3L instruments. Safety was assessed according to the occurrence of adverse events (frequency, intensity and relationship with treatment) and by vital signs and routine clinical laboratory tests.
Ethics and registration
This study was performed in accordance with Good Clinical Practice for clinical trials and the Declaration of Helsinki regarding the Ethical Principles for Medical Research. Ethics committee approval was obtained 2 February 2017. All patients provided signed informed consent, and were free to withdraw from the study at any time.
The study was performed in compliance with the requirements of the National Agency of Medicine and Medical Devices of Romania and the National Ethical Committee for Biomedical Research. The study was registered with EudraCT Number 2016-004832-40.
Statistical analysis
A sample size of 60 patients (30 per treatment arm) was calculated to ensure an 80% or greater power of rejecting the null hypothesis that no difference exists between treatments at a 5% significance level (two-sided test), factoring in a dropout rate of 10%.
Results are reported using descriptive statistics. Continuous variables are presented as mean ± standard deviation (SD) and categorical variables are presented as number (%). Statistical differences in mean values between treatment groups were assessed with the t-test.
Results
Baseline characteristics of study population
A total of 60 patients were recruited from five gastroenterology clinics in Romania (Bucharest, Iasi and Oradea) between 28 June 2017 and 5 January 2018. All patients received study medication and all patients completed the study.
Patients' demographic and clinical characteristics at baseline are presented in Table 1. There were 16 (27%) men and 44 (73%) women, and mean age was 35 years. Treatment arms did not differ significantly with respect to baseline characteristics except for a higher mean body weight (74.4 ± 17.5 kg vs 62.6 ± 8.1 kg; p = 0.002) and a higher (more acceptable) mean abdominal pain score (3.0 ± 1.6 vs 2.2 ± 1.4; p = 0.03) in the XG + PPT + XOS-placebo arm vs placebo-XG + PPT + XOS arm.
Table 1.
Patients' baseline demographic and clinical characteristics.
| XG+PPT+XOS- placebo (n = 30) | Placebo- XG+PPT+XOS (n = 30) | p value | |
|---|---|---|---|
| Sex M:F, n (%) | 5 (17): 25 (83) | 11 (37): 19 (63) | 0.08 |
| Age (years), mean (SD) | 35.0 (7.8) | 34.5 (8.1) | 0.82 |
| Weight (kg), mean (SD) | 74.4 (17.5) | 62.6 (8.1) | 0.002 |
| Height (cm), mean (SD) | 167.9 (8.6) | 167.2 (7.0) | 0.75 |
| Temperature (℃), mean (SD) | 36.4 (0.3) | 36.5 (0.3) | 0.37 |
| Pulse (beats/min), mean (SD) | 74.5 (8.8) | 74.7 (9.4) | 0.95 |
| SBP (mmHg), mean (SD) | 127.2 (9.5) | 124.0 (9.7) | 0.21 |
| DBP (mmHg), mean (SD) | 75.5 (11.8) | 73.2 (13.2) | 0.47 |
| IBS-D symptoms, mean (SD) | |||
| Abdominal paina | 3.0 (1.6) | 2.2 (1.4) | 0.03 |
| Bloatinga | 2.7 (1.4) | 2.1 (1.4) | 0.23 |
| Stools, mean (SD) | |||
| No. of stools/day | 4.6 (0.9) | 4.7 (1.1) | 0.70 |
| Type of stoolb | 5.7 (0.8) | 6.0 (0.8) | 0.12 |
| IBS QoL score, mean | 34 | 34 | − |
| EQ-5D-3L score, mean | 30 | 40 | − |
DBP: diastolic blood pressure; F: female; IBS-D: diarrhoea-predominant irritable bowel syndrome; IBS-QoL: IBS Quality of Life questionnaire; M: male; PPT: pea protein and tannins from grape seed extract; SBP: systolic blood pressure; XG: xyloglucan; XOS: xylo-oligosaccharides.
Likert scale: = totally unacceptable; 7 = perfectly acceptable (Likert scale).
Bristol scale: Type 1 = separate hard lumps, like nuts (hard to pass); Type 7 = watery, no solid pieces (entirely liquid).
Thirteen patients had concomitant diseases at baseline: arterial hypertension (six cases), respiratory virosis (three), rheumatoid arthritis (two), lumbar discopathy (one) and spondylosis (one). Patients continued currently recommended treatment for their condition throughout the study.
Clinical remission
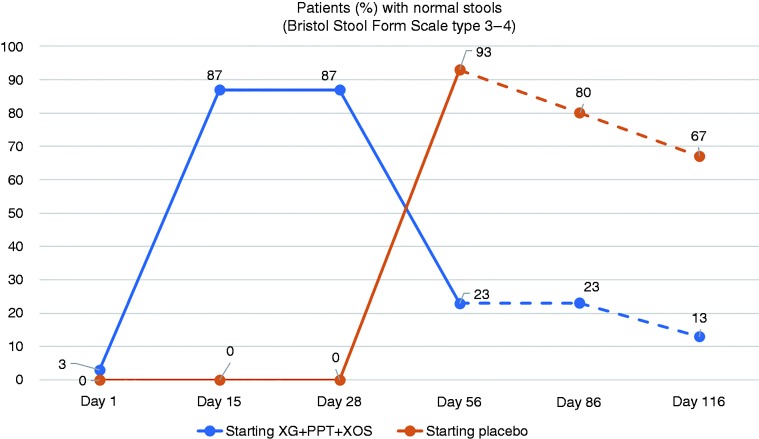
Figure 1 shows the clinical remission rate (i.e. the proportion of patients with normal stools: BSFS types 3–4) from treatment start to end of follow-up. On Day 28 at the end of first-line treatment, the proportion of patients with normal stools increased from 3% to 87% in the group that began treatment with XG + PPT + XOS and was unchanged (0% and 0%, respectively) in the group that began treatment with placebo (p = 0.0019). On Day 56, the proportion of patients with normal stools increased from 0% to 93% in the group that crossed over from placebo to XG + PPT + XOS, and decreased from 87% to 23% in the group that crossed over from XG + PPT + XOS to placebo (p = 0.0001). A higher proportion of patients who completed the second treatment period with XG + PPT + XOS than placebo presented normal stools on Day 86 (80 vs 23%) and Day 116 (67 vs 13%).
Figure 1.
Clinical remission rate: proportion of patients with normal stools (Bristol Stool Form Scale type 3–4) at evaluation time points. Day 28: end of first-line treatment; Day 56: end of crossover treatment; Day 86: after 30 days' follow-up; Day 116: after 60 days' follow-up. Broken lines indicate follow-up period. PPT: pea protein and tannins from grape seed extract; XG: xyloglucan; XOS: xylo-oligosaccharides.
Abdominal pain and bloating
With respect to evolution in abdominal pain, from Day 1 to Day 28, the number (%) of patients with totally to slightly unacceptable abdominal pain (Likert scale 1–3) decreased from 20 (67%) patients to 0 (0%) patients in the group that began treatment with XG + PPT + XOS, and decreased from 25 (83%) patients to 18 (60%) patients in the group that began treatment with placebo. The number (%) of patients with totally to slightly unacceptable abdominal pain decreased from 18 (60%) patients on Day 28 to 0 (0%) patients on Day 56 in the group that crossed over to XG + PPT + XOS, and increased from 0 (0%) patients to 9 (30%) patients, respectively, in the group that crossed over to placebo.
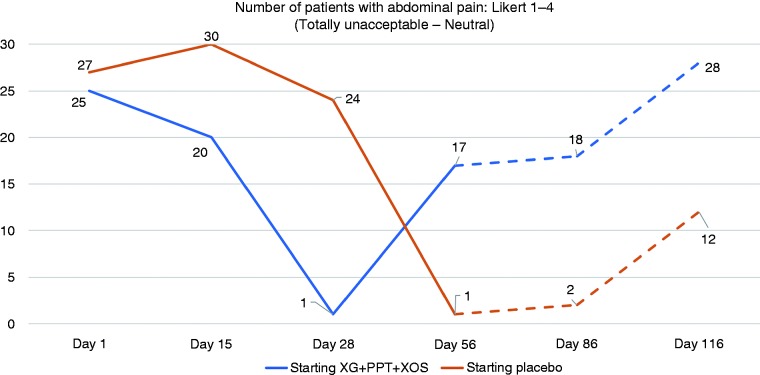
The number (%) of patients reporting totally unacceptable to neutral abdominal pain (Likert scale 1–4) is shown in Figure 2. On Day 28, values were significantly lower in the XG + PPT + XOS-placebo arm than placebo-XG + PPT + XOS arm (1 (3%) vs 24 (80%) patients; p = 0.002). On Day 56, values were significantly lower in the placebo-XG + PPT + XOS arm than XG + PPT + XOS-placebo arm (1 (3%) vs 17 (57%) patients; p = 0.027). At follow-up Day 86, fewer patients who had completed the second treatment phase with XG + PPT + XOS than placebo (2 (7%) vs 18 (60%) patients) reported totally unacceptable to neutral abdominal pain, although the difference was not statistically significant (p = 0.066). A trend toward a better outcome with XG + PPT + XOS was also apparent at follow-up Day 116 (12 (40%) vs 28 (93%) patients; p = 0.078).
Figure 2.
Number of patients with unacceptable to neutral abdominal pain (Likert scale 1–4) at evaluation time points. Day 28: end of first-line treatment; Day 56: end of crossover treatment; Day 86: after 30 days' follow-up; Day 116: after 60 days' follow-up. Broken lines indicate follow-up period. PPT: pea protein and tannins from grape seed extract; XG: xyloglucan; XOS: xylo-oligosaccharides.
With respect to evolution in bloating, from Day 1 to Day 28, the number (%) of patients with totally to slightly unacceptable bloating (Likert scale 1–3) decreased from 22 (73%) patients to 1 (3%) patient in the group allocated to begin treatment with XG + PPT + XOS, and decreased from 24 (80%) patients to 16 (53%) patients in the group allocated to begin treatment with placebo. After crossover, the number (%) of patients with totally to slightly unacceptable bloating decreased from 16 (53%) patients on Day 28 to 0 (0%) patients on Day 56 in the group that crossed over to XG + PPT + XOS, and increased from 1 (3%) patient on Day 28 to 11 (37%) patients on Day 56 in the group that crossed over to placebo.
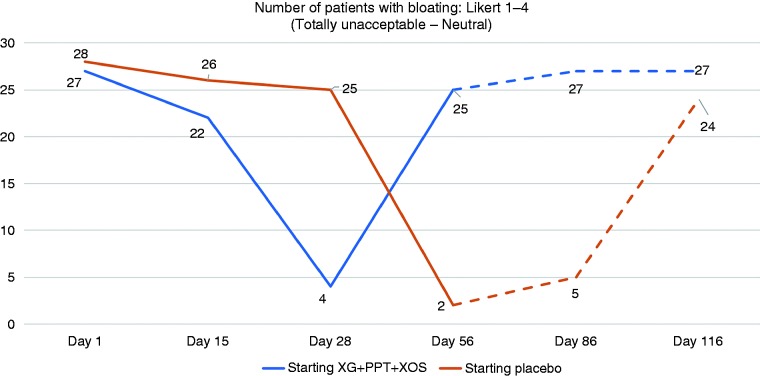
The number (%) of patients reporting totally unacceptable to neutral bloating (Likert scale 1–4) is shown in Figure 3. On Day 28, values were significantly lower in the XG + PPT + XOS-placebo arm vs placebo-XG + PPT + XOS arm (4 (13%) vs 25 (83%) patients; p = 0.028). On Day 56, values were significantly lower in the placebo-XG + PPT + XOS arm vs XG + PPT + XOS-placebo arm (2 (7%) vs 25 (83%) patients; p = 0.041). At follow-up Day 86, significantly fewer patients who had completed treatment with XG + PPT + XOS than placebo reported totally unacceptable to neutral bloating (5 (17%) vs 27 (90%) patients; p = 0.009). At follow-up Day 116, the difference between treatment arms was not statistically significant (24 (80%) vs 27 (90%) patients; p = 0.26).
Figure 3.
Number of patients with unacceptable to neutral bloating (Likert scale 1–4) at evaluation time points. Day 28: end of first-line treatment; Day 56: end of crossover treatment; Day 86: after 30 days' follow-up; Day 116: after 60 days' follow-up. Broken lines indicate follow-up period. PPT: pea protein and tannins from grape seed extract; XG: xyloglucan; XOS: xylo-oligosaccharides.
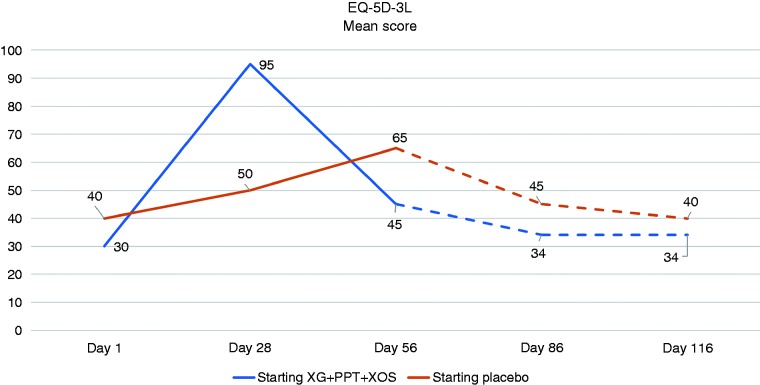
Evolution in IBS-QoL and EQ-5D-3L scores
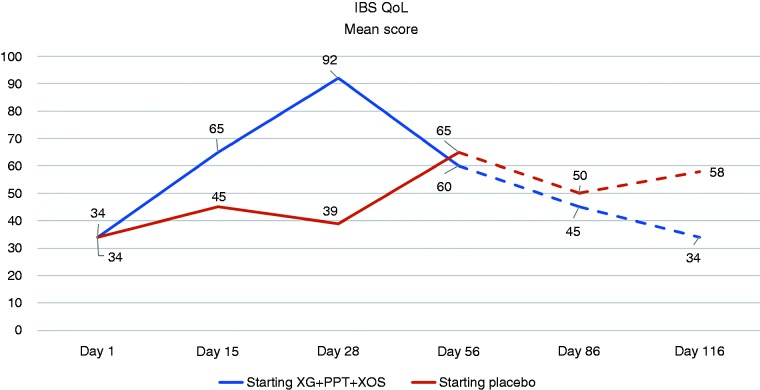
The change from baseline in the mean IBS-QoL score was greater, compared with placebo, on Day 28 in the group allocated to initial treatment with XG + PPT + XOS, and on Day 56 in the group that crossed over to treatment with XG + PPT + XOS (Figure 4). Results for evolution in the mean EQ-5D-3L health score were similar (Figure 5).
Figure 4.
Evolution in the mean score on the Irritable Bowel Syndrome Quality of Life (IBS QoL) questionnaire at evaluation time points. Day 28: end of first-line treatment; Day 56: end of crossover treatment; Day 86: after 30 days' follow-up; Day 116: after 60 days' follow-up. Broken lines indicate follow-up period. PPT: pea protein and tannins from grape seed extract; XG: xyloglucan; XOS: xylo-oligosaccharides.
Figure 5.
Evolution in the mean score on the EQ-5D-3L questionnaire at evaluation time points. Day 28: end of first-line treatment; Day 56: end of crossover period; Day 86: after 30 days' follow-up; Day 116: after 60 days' follow-up. Broken lines indicate follow-up treatment. PPT: pea protein and tannins from grape seed extract; XG: xyloglucan; XOS: xylo-oligosaccharides.
Safety
On Day 15 while receiving XG + PPT + XOS, one female patient presented back pain which the investigator considered to be related to her medical history (not study medication), as it had appeared after some physical effort. No other adverse events were reported.
Monitoring of vital signs and clinical laboratory parameters indicated no values outside normal ranges.
None of the 13 patients with a concomitant medical condition reported disease progression or unacceptable symptoms during the study.
Discussion
In this study, significantly more patients with IBS-D who began treatment with XG + PPT + XOS than placebo (87 vs 0%; p = 0.0019) had their stools normalised (BSFS type 3 or 4). After crossover, significantly more patients allocated to receive XG + PPT + XOS after placebo achieved stool normalisation compared with those allocated to receive placebo after XG + PPT + XOS (93 vs 23%; p = 0.0001). In most cases, remission from diarrhoea symptoms was apparent within 15 days of starting treatment with XG + PPT + XOS and was maintained for the 28-day active treatment period. In conjunction with the disappearance of diarrhoea, patients' self-assessed rating of abdominal pain and bloating symptoms, measured on a seven-point Likert scale, evolved from unacceptable to acceptable categories under treatment with XG + PPT + XOS but not placebo. Likewise, mean IBS QoL and EQ-5D-3L scores improved substantially under treatment with XG + PPT + XOS but not placebo.
In the group that began treatment with placebo and crossed over to XG + PPT + XOS, improvements in diarrhoea and clinical symptoms were maintained during follow-up. In contrast, in the group that began treatment with XG + PPT + XOS, improvements in diarrhoea and clinical symptoms under active treatment deteriorated after crossover to placebo and outcomes at follow-up were worse. Since the aetiologies of IBS-D among enrolled patients are unknown and, in general, are complex and multifactorial, the differences observed at Day 86 between the XG + PPT + XOS-placebo arm and placebo-XG + PPT + XOS arm may have multiple and varied explanations. However, the potential for XG + PPT + XOS to provide sustained benefit warrants further investigation.
The high prevalence of IBS carries with it significant health, social and economic repercussions. Frequent medical visits, diagnostic tests and therapeutic prescriptions involve considerable consumption of health care resources.1 IBS-D is a leading cause of absenteeism from work16 and, although IBS-D does not jeopardise life, it significantly undermines quality of life,17,18 imposing a substantial burden on patients and underscoring an urgent need to identify effective treatments.
There is currently no standard treatment algorithm for IBS-D. Therapeutic options involve lifestyle and dietary modifications, medical foods, over-the-counter medications, prescription medications and psychological therapies.18 Novel pharmacological therapies for IBS are under development.19 However, the complexity and diversity of IBS pathophysiology demands flexibility and variety in the approach to treatment, including lifestyle changes, dietary adjustments, probiotics, pharmacotherapy and alternative approaches targeted at mucosal function and integrity.20
Nonpharmacological approaches to the management of diseases associated with mucosal barrier disruption and tight junction alterations, such as IBS, are becoming more common. The preclinical activities and clinical use of XG-containing medical devices has been extensively reviewed.6 Since an altered intestinal barrier associated with immune activation and clinical symptoms is a key feature in IBS-D,21 film-forming mucosal protective agents in combination with prebiotics may offer a valuable nonpharmacological alternative for effective symptom control in patients with IBS-D.6 In a controlled clinical trial, XG formulated with tyndallised Lactobacillus reuteri and Bifidobacterium brevis significantly reduced abdominal extension and flatulence in patients with functional bloating.22
According to Rome IV guidelines for the design of treatment trials for functional gastrointestinal disorders,23 the double-blind, randomised, placebo-controlled, parallel-group design is the accepted standard for evaluating the efficacy of new treatments. Crossover designs are also popular as they reduce variability and may increase sensitivity to detect change, allowing a smaller sample size for the desired statistical power. However, crossover trials have drawbacks relative to parallel-group studies in terms of the impact of patient dropouts, the potential for carryover effects and the risk of unmasking due to adverse effects. At the time the current study was designed, the Rome IV guidelines were not yet available and we had opted for a crossover design to increase sensitivity. The absence of patient dropouts and treatment-related adverse effects in either group strengthens the findings. However, we cannot dismiss the possibility of a carryover effect as there was no washout period between the first and second treatment phases. A washout period was deemed unnecessary for safety and compliance reasons. In addition, we believe that the potential for carryover effects was reduced by the relatively short half-life of XG + PPT + XOS and the extended follow-up after the end of crossover treatment. Nevertheless, we acknowledge this as a potential relevant limitation of our study and advise readers to interpret the results accordingly.
The study was conducted in accredited gastroenterology centres equipped to carry out clinical studies with therapeutic benefits for patients and able to present valid authorisation. Authorisation is granted by local health agencies to units with the requisite logistics (qualified personnel, infrastructure, able to fulfil quality standards). The centres are community based and provide a full range of health care services. In view of the naturalistic setting and minimal exclusion criteria, we consider that the results are widely applicable to adult patients of sufficient health status with a diagnosis of IBS-D.
The present study supports the efficacy and safety of XG + PPT + XOS for controlling diarrhoea, abdominal pain and bloating in adult patients with IBS-D. XG + PPT + XOS can be considered a valuable tool in the array of interventions available for effective management of IBS-D.
Acknowledgements
Medical writing assistance was provided by Jon Monk and Kerry Dechant on behalf of Content Ed Net (Madrid, Spain), with funding by Noventure SL, Barcelona, Spain.
Declaration of conflicting interests
A. Trifan has received fees from Noventure for conducting research studies and for scientific advisory. O. Burta, N. Tiuca and D.C. Petrisor have nothing to declare. A. Lenghel has received fees from Noventure for conducting research studies and for scientific advisory. J. Santos has received fees from Noventure for conducting research studies and for scientific advisory. The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Funding
This study was sponsored by Novintethical Pharma SA, Switzerland.
Ethics approval
This study was performed in accordance with Good Clinical Practice for clinical trials and the Declaration of Helsinki regarding the Ethical Principles for Medical Research. Ethics committee approval was obtained 2 February 2017. The study was performed in compliance with the requirements of the National Agency of Medicine and Medical Devices of Romania and the National Ethical Committee for Biomedical Research. The study was registered with EudraCT Number 2016-004832-40.
Informed consent
All patients provided signed informed consent, and were free to withdraw from the study at any time.
References
- 1.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. JAMA 2015; 313: 949–958. [DOI] [PubMed] [Google Scholar]
- 2.Cashman MD, Martin DK, Dhillon S, et al. Irritable bowel syndrome: A clinical review. Curr Rheumatol Rev 2016; 12: 13–26. [DOI] [PubMed] [Google Scholar]
- 3.Saha L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol 2014; 20: 6759–6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 712–721.e4. [DOI] [PubMed] [Google Scholar]
- 5.Lacy BE. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int J Gen Med 2016; 9: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piqué N, Gómez-Guillén MDC, Montero MP. Xyloglucan, a plant polymer with barrier protective properties over the mucous membranes: An overview. Int J Mol Sci 2018; 19: E673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnessi L, Bacarea V, Marusteri M, et al. Xyloglucan for the treatment of acute diarrhea: Results of a randomized, controlled, open-label, parallel group, multicentre, national clinical trial. BMC Gastroenterol 2015; 15: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pleş ea Condratovici C, Bacarea V, Piqué N. Xyloglucan for the treatment of acute gastroenteritis in children: Results of a randomized, controlled, clinical trial. Gastroenterol Res Pract 2016; 2016: 6874207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finegold SM, Li Z, Summanen PH, et al. Xylooligosaccharide increases bifidobacteria but not lactobacilli in human gut microbiota. Food Funct 2014; 5: 436–445. [DOI] [PubMed] [Google Scholar]
- 10.Eutamene H, Placide F, Tondereau V, et al. Protective effect of mucoprotectants and prebiotic combination on gut barrier impairment and visceral hypersensitivity induced by an acute stress in rat. Gastroenterology 2018; 154(6 Suppl 1): S916. [Google Scholar]
- 11.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 2006; 15: 237–241. [PubMed] [Google Scholar]
- 12.Lee J, Lee EH, Moon SH. A systematic review of measurement properties of the instruments measuring health-related quality of life in patients with irritable bowel syndrome. Qual Life Res 2016; 25: 2985–2995. [DOI] [PubMed] [Google Scholar]
- 13.Andrae DA, Patrick DL, Drossman DA, et al. Evaluation of the Irritable Bowel Syndrome Quality of Life (IBS-QOL) questionnaire in diarrheal-predominant irritable bowel syndrome patients. Health Qual Life Outcomes 2013; 11: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 15.Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2016; 44: 693–703. [DOI] [PubMed] [Google Scholar]
- 16.Buono JL, Carson RT, Flores NM. Health-related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes 2017; 15: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballou S, Bedell A, Keefer L. Psychosocial impact of irritable bowel syndrome: A brief review. World J Gastrointest Pathophysiol 2015; 6: 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucak S, Chang L, Halpert A, et al. Current and emergent pharmacologic treatments for irritable bowel syndrome with diarrhea: Evidence-based treatment in practice. Therap Adv Gastroenterol 2017; 10: 253–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam Y, Min YS, Sohn UD. Recent advances in pharmacological research on the management of irritable bowel syndrome. Arch Pharm Res 2018; 41: 955–966. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri M. Management options for irritable bowel syndrome. Mayo Clin Proc 2018; 93: 1858–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vicario M, González-Castro AM, Martínez C, et al. Increased humoral immunity in the jejunum of diarrhoea-predominant irritable bowel syndrome associated with clinical manifestations. Gut 2015; 64: 1379–1388. [DOI] [PubMed] [Google Scholar]
- 22.Burta O, Iacobescu C, Mateescu RB, et al. Efficacy and safety of APT036 versus simethicone in the treatment of functional bloating: A multicentre, randomised, double-blind, parallel group, clinical study. Transl Gastroenterol Hepatol 2018; 3: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irvine EJ, Tack J, Crowell MD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology 2016; 150: 1469–1480.e1. [DOI] [PubMed] [Google Scholar]