Abstract
Background and aims
Endoscopic submucosal dissection is the reference treatment for early oesophageal squamous cell carcinoma. However, data from Western centres are scarce.
Methods
We conducted a retrospective study from a prospectively collected database at a tertiary care centre in France. All consecutive patients undergoing endoscopic submucosal dissection for oesophageal squamous cell carcinoma were included. The main outcome was the curative resection rate. Secondary outcomes were en-bloc resection rates, histologically complete resection rates, morbidity, recurrence-free and overall survival.
Results
Fifty-six cases of oesophageal squamous cell carcinoma (49 patients; mean age 61.5 ± 10 years; 36 men) were included. En-bloc, histologically complete and curative resection rates were 98%, 86% and 71%, respectively. Fifteen (30%) patients received an additional treatment after endoscopic submucosal dissection, nine treated by chemoradiotherapy, four by surgery and two by further endoscopic submucosal dissection. Within a mean follow-up of 21 ± 15 months, recurrences occurred in 14 (29%) patients (four local, eight metachronous and three distant recurrences). Eight patients died during follow-up, of which two (4%) patients died from oesophageal squamous cell carcinoma. Factors significantly associated with mortality in this series were: moderate or poor differentiation of oesophageal squamous cell carcinoma (p = 0.02) and recurrence of oesophageal squamous cell carcinoma (p = 0.028).
Conclusion
Moderately or poorly differentiated cancer is a major prognostic factor and should probably be taken into account when indicating an additional treatment after endoscopic submucosal dissection. Close endoscopic follow-up is essential considering the high recurrence rate.
Keywords: Superficial oesophageal cancer, early squamous cell carcinoma, high grade dysplasia, endoscopic submucosal dissection
Key summary
- 1. Summarise the established knowledge on this subject
- Endoscopy submucosal dissection (ESD) is the gold standard for the management of early oesophageal squamous cell carcinoma (ESCC) as a staging procedure and curative treatment.
- Little information is available on the long-term outcomes after ESD, and particularly on the role of additional treatments.
- The rate of local recurrence after ESD for ESCC ranges from 0–14%.
- 2. What are the significant and/or new findings of this study?
- Even when resection is not curative, distant recurrence seems to be limited by additional treatments.
- Close endoscopic follow-up is essential considering the high local and metachronous recurrence rate.
Introduction
Oesophageal cancer is the eighth most common cancer around the world: in 2008, its incidence was estimated at 482,000 new cases, and 407,000 related deaths were reported.1 With the improvement of diagnostic methods and implementation of screening programmes, oesophageal neoplasia (adenocarcinoma and squamous cell carcinoma) can be diagnosed in their early stages.2
Endoscopic mucosal resection (EMR) of early neoplastic lesions of the oesophagus has gained momentum in the 1990s, allowing us to avoid the high morbidity and mortality rates of oesophagectomy for superficial tumours with a low risk of lymph node involvement.3–5
Endoscopic submucosal dissection (ESD) allows en-bloc resection of lesions irrespective of their size5 and significantly decreases the rate of local recurrence when compared to EMR.6 Furthermore, ESD enables an optimal assessment of the risk of lymph node metastasis based on histological quantitative parameters such as the depth of mural invasion, and qualitative parameters such as the grade of tumour differentiation and the presence of lymphovascular involvement.3,7 For these reasons, ESD has become the gold standard for the management of early oesophageal squamous cell carcinoma (ESCC), as staging procedure and potentially curative treatment. However, the vast majority of published articles are retrospective case series from expert centres in Asia,3,7–10 which can raise issues about their applicability in Western populations, since the epidemiology and carcinogenetic determinants of Squamous cell carcinoma (SCC) are different. Until now, outcomes of ESD for ESCC have been only scarcely reported in Western series.11–15 This study aimed to report the long-term results of ESD in early ESCC at a tertiary care centre in France.
Methods
Patient selection
We conducted a retrospective study of a prospectively collected database conducted at a tertiary referral centre (Cochin Hospital, Paris, France). All consecutive patients treated by ESD for ESCC between January 2012–November 2016 were included. Patients with a diagnosis of oesophageal adenocarcinoma, squamous low-grade dysplasia, and other types of oesophageal neoplasm were excluded from the analysis. Pretherapeutic workup included thoraco-abdomino-pelvic computed tomography (CT) scan in all patients, and an endoscopic ultrasonography (EUS) when biopsies suggested invasive or poorly differentiated carcinoma or whenever worrisome endoscopic features, such a ulcerated or protruded lesions, or type V-N intrapapillary capillary loops.16 EUS was performed before resection in order to exclude suspicious periesophageal lymph nodes and muscularis propria extension of the tumour (T2 stage) contraindicating endoscopic resection. Subsequent management decisions, such as surveillance, radiochemotherapy or surgery, were made during a multidisciplinary team meeting dedicated to early gastrointestinal cancers.
Endoscopic procedures
A diagnostic staging endoscopy was performed immediately before ESD, using a high-definition endoscope with lugol staining, in order to delineate the lesion and confirm resectability. ESD procedures were conducted under general anaesthesia with endotracheal intubation and CO2 insufflation. Antiplatelet agents (other than aspirin) and anticoagulant therapy were discontinued before the procedure. ESDs were performed by one of three experienced operators (FP, SL, SC), each of whom had a previous experience of more than 50 rectal, gastric and oesophageal ESDs. High-definition upper gastrointestinal (GI) endoscopes with narrow-band-imaging (GIF-H180J or GIF-HQ190, Olympus, Japan) or blue-light-imaging (EG-L590ZW, Fujifilm, Japan) were used. Procedures were carried out with a distal attachment cap. ESD knives included the 1.5 mm Dual knife (Olympus, Japan) and 1.5 mm Flush knife (Fujifilm, Japan), using a VIO200 or 300D electrosurgery unit (Erbe Medizin, Germany) with standard settings (Endocut I for incision, swift coagulation for submucosal dissection and soft coagulation for vessel occlusion). The ESD procedure was carried out as previously described,5 using an indigo-carmine-stained lifting solution made of 5% fructose and 10% glycerol mixed with saline. Haemostasis of submucosal vessels was achieved with the ESD knife or coagulation forceps (Coagrasper, Olympus, Japan). Most lesions were resected following the ‘tunnel' technique in which distal and proximal incisions are made before antegrade submucosal tunnelling followed by cutting both lateral margins.17 In prevention of post ESD stricture, steroids could be administered to patients.18 Only liquids were allowed during the periods of 24 and 18 h after ESD, and 80 mg esomeprazole were administered before discharge followed by oral proton pump inhibitor (PPI) during four weeks. Oral soft food was resumed and patients were usually discharged the next day.
Histological assessment
Resected specimens were fixed in 10% formalin histological slides and were assessed by pathologists with expertise in digestive pathology and endoscopic resection specimen (FB, BT). The following data were assessed: en-bloc resection, tumour and specimen size, minimal vertical and lateral margins, depth of tumour invasion (µm), measured from the muscularis mucosae in case of submucosal invasion, tumour differentiation and lymphovascular involvement.
Adverse events
Adverse events were graded and classified as early or late according to the American Society for Gastrointestinal Endoscopy (ASGE) lexicon.19 Bleeding was defined as a either a two-point haemoglobin drop within 24 h or haematemesis, melaena requiring either blood cell transfusion or control endoscopy. Per-procedural bleeding managed with haemostatic forceps was not recorded as a complication. Oesophageal perforation was defined as a visible hole in the oesophageal muscle layer and/or postoperative collection. Oesophageal strictures were recorded as a narrowing of the oesophageal lumen either associated with dysphagia or too small to admit a 10 mm endoscope.
Follow-up
All patients were followed-up with an oesophagogastroduodenoscopy with lugol staining three months after the ESD (or earlier in case of dysphagia), and annually thereafter. In cases of ESCC with high risk of lymph node metastasis, the thoracoabdominopelvic CT-scanner and EUS were carried out every six months. In case of symptomatic oesophageal stricture, oesophageal dilatation was conducted until sustained resolution of the symptoms was obtained.
Definitions
En-bloc resection was defined by the resection of the neoplastic lesion in a single piece.
Histologically complete (R0) resection of neoplasia was defined as en-bloc resection of neoplasia with vertical and lateral margins free of carcinoma and dysplasia.
The curative resection rate of neoplasia was defined as a histologically complete resection of a well differentiated tumour without any sign of lymphovascular involvement, and a submucosal infiltration of less than 200 µm.3
A recurrence was considered local when occurring at the site of the initial resection, metachronous if it occurred at another location of the oesophagus, or distant in case of lymph node or visceral metastasis.
Data collection, endpoints and outcome measurements
The following data were collected: patient characteristics (age, sex, history of previous neoplasia, main comorbidity, Charlson comorbidity score, alcohol and tobacco intake, antiplatelet or anticoagulant prescriptions); endoscopic procedural data (preoperative evaluation with endoscopic description including Paris classification and visible extent after lugol staining, resection procedure characteristics, early adverse events); histological data (histological classification, depth of parietal infiltration (mucosa m1-m2-m3, superficial submucosa below 200 µm, deep submucosa beyond 200 µm), lateral and deep resection margins, grade of differentiation, presence of lymphatic or vascular involvement); and follow-up data (findings at endoscopic follow-up, late adverse events, recurrences with their location, extent and management, mortality and cause of death).
The main study endpoint was the efficacy of ESD as a curative treatment of early ESCC (i.e. curative resection rate). The secondary endpoints were early and distant ESD-related morbidity, technical success of ESD in terms of en-bloc and complete resection rates, and long-term outcomes in terms of progression and recurrence, and outcomes of additional treatments.
Our main outcome measurement was the rate of curative resection of early ESCC. Secondary outcome measurements were the rates of en-bloc and histologically complete resection, the rates of adverse events, recurrences and death 30 days after ESD and during follow-up, and risk factors for oesophageal stricture, recurrence and death.
Statistical analysis and ethical aspects
Descriptive statistics were used to analyse patients’ characteristics. Data were expressed as mean ± standard deviation (SD) or median with interquartile (IQR 25–75). The depth of mural infiltration was categorised as low risk (in situ/m1 and m2), middle risk (m3 and superficial submucosa <200 µm) or high risk (deep submucosal infiltration >200 µm)) in order to carry out a univariate analysis of the risk of recurrence also including other previously cited histoprognostic factors. Student’s t test for quantitative variables and Chi-square test or Fisher’s exact tests for qualitative variables were used in the univariate analysis. All statistical analyses were performed using the SPSS software program (version 18.0) (IBM, Armonk, New York, USA). A two-tailed p-value <0.05 determined a statistically significant result.
Written informed consent was obtained from patients before each endoscopic procedure. The data used were anonymised and collected from the computer file which is declared to the Commission Nationale Informatique et Liberté (French National Commission for Data Protection). The study received approval from our local institutional ethical review board in July 2017 (CLEP Decision: AAA-2017-05007). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Results
Patient characteristics
From January 2012–November 2016, 136 ESDs were performed in our department. After exclusion of 80 lesions (76 adenocarcinomas, two granular cell tumours, one leiomyoma, one squamous low-grade dysplasia with parakeratosis), 49 patients with 56 early ESCCs were included. The mean age of patients was 61.5 ± 10 years and 36 (73%) patients were males. The most frequent comorbidities were: history of oropharyngeal cancer (n = 11, 22%), history of thoracic radiotherapy (n = 15, 30%; oropharyngeal cancer in nine patients, breast cancer in two patients, and oesophageal cancer in three patients) and cirrhosis (n = 12, 24%). Four of the latter had portal hypertension with grade I–II oesophageal varices. The characteristics of the patients are summarised in Table 1.
Table 1.
Characteristics of the patients.
| Patients – n | 49 |
| Lesions – n | 56 |
| Age – mean ± SD | 61.5 ± 10 |
| Sex – n (%) | |
| Male | 36 (74%) |
| Female | 13 (26%) |
| Charlson comorbidity score – mean ± SD | 5 ± 2 |
| History of – n (%) | |
| Cirrhosis | 12 (24%) |
| ENT cancer | 11 (22%) |
| Antiplatelet therapy – n (%) | 8 (14%) |
| Chronic alcohol abuse – n (%) | 20 (45%) |
| Tobacco user – n (%) | 22 (52%) |
| History of thoracic radiotherapy – n (%) | 15 (31%) |
| Pre-ESD histology – n (%) | |
| Low grade dysplasia | 2 (4%) |
| High grade dysplasia | 15 (27%) |
| Squamous cell carcinoma | 39 (69%) |
ENT: ear, nose, and throat; ESCC: early squamous cell carcinoma; ESD: endoscopic submucosal dissection; SD: standard deviation
On pretherapeutic CT scan, five (9%) patients had a thickening of the oesophagus and non-specific mediastinal lymph nodes were seen in two (4%) patients. EUS was performed in 52 (93%) cases. Lesions were classified as usT1N0 in 94% of cases and EUS findings were inconclusive in 6% of the cases. EUS found peri-oesophageal lymph nodes in one patient and us T2 stage in none of the patients. However, the lymph nodes were not found to be suspicious and ESD was carried out.
Procedural data
The endoscopic findings and procedural characteristics of the 56 ESDs are presented in Table 2. All ESD procedures were successful, however en-bloc resection failed in one lesion. Eighty percent of the patients had a Paris 0-IIa lesion, and the predominant tumour location was the middle third of oesophagus (57%). Finally, 48% of the lesions were resected with an apparent mucosal defect exceeding 75% of the oesophageal circumference.
Table 2.
Endoscopic findings and procedural characteristics of the 56 endoscopic submucosal dissections (ESDs) for early squamous cell carcinoma of the esophagus.
| Localization of the lesion in the esophagus – n (%) | |
| Upper third | 6 (11%) |
| Middle third | 32 (57%) |
| Lower third | 18 (32%) |
| Paris classification – n (%) | |
| 0–IIa | 21 (38%) |
| 0–IIb | 10 (18%) |
| 0–IIc | 1 (2%) |
| IIa–IIb | 12 (21%) |
| IIa–IIc | 10 (18%) |
| IIb–IIc | 1 (2%) |
| 0–Is | 1 (2%) |
| En bloc resection – n (%) | 55 (98%) |
| Circumference of the mucosal defect after ESD – n (%) | |
| <50% | 17 (31%) |
| 50%–75% | 12 (21%) |
| 75%–<100% | 21 (38%) |
| Circumferential | 6 (11%) |
Adverse events
The median (IQR) length of hospital stay was three (2.7–3) days. Early adverse events occurred in five cases (9%), including three oesophageal perforations diagnosed during ESD and immediately closed by haemoclips, with subsequent conservative treatment, one aspiration pneumonia and one giant rash attributed to the anaesthetic agent. No significant bleeding occurred. All three perforations were graded as moderate and the last two early adverse events as mild. The level of 30-day mortality was 0%.
The overall stricture rate was 20%, with an overall good response to dilatation: seven patients had one dilation, two patients had <5 dilations, one patient had >5 dilations. Steroids were administered orally to one patient with circumferential ESD of the upper third of the oesophagus, who eventually developed a stricture requiring over five dilatations. No patient received local injection or application of steroids for oesophageal stricture prevention.
Three factors were significantly associated with the development of a post-ESD stricture in the univariate analysis: largest lesion diameter >41 mm (p = 0.001), the surface of the histological specimen >13 cm2 (p = 0.002) and a circumferential dissection (p = 0.0001). Histological data or a history of radiotherapy was not associated with the development of a post-ESD stricture in univariate analysis. In multivariate analysis, only circumferential dissection was associated with post-ESD stricture (p = 0.006; odds ratio (OR) = 8.1, 95% confidence interval (CI) (1.8–35.8)). The stricture rate was nil for mucosal defects of less than 50% of circumference, against 19% for defects of 50 to less than 100%, and 100% for circumferential mucosal defects.
Histological findings
Of the 56 lesions resected by ESD, 21 (37.5%) were T1am1, 13 (23%) T1am2, four (7%) T1am3, 12 (21.5%) were superficial T1b and five (10%) were deep T1b and one (1%) T2. Poor qualitative histoprognostic factors were found in 16 (29%) lesions in 14 patients, including eight (14%) cases of lymphovascular involvement and 11 (22%) moderately or poorly differentiated tumours. Of these, nine (56%) had one poor histoprognostic factor (lymphovascular involvement or moderately/poorly differentiated tumours or deep submucosal invasion or positive margins), while four (25%) had two, and three (19%) had all poor histoprognostic factors. The R0 resection and curative resection rates were 86% (48/56 lesions), and 71% (40/56 lesions), respectively. Detailed histological outcomes are presented in Table 3.
Table 3.
Histological outcomes after endoscopic submucosal dissection of 56 esophageal early squamous cell carcinomas.
| Surface of the histological specimen, cm2 – mean ± SD | 13.5 ± 10 |
| Maximal diameter of lesion, mm – mean ± SD | 41 ± 19 |
| Depth of tumour infiltration – n (%) | |
| High grade dysplasia or in situ carcinoma (m1) | 21 (37.5%) |
| m2 | 13 (23%) |
| m3 | 4 (7%) |
| Superficial submucosa (<200 µm) | 12 (21%) |
| Deep submucosa (>200 µm) or T2 | 6 (11%) |
| Grade of tumour differentiation – n (%) | |
| Well-differentiated | 45 (80%) |
| Moderately differentiated | 6 (11%) |
| Poorly differentiated | 5 (9%) |
| Lymphatic or vascular involvement – n (%) | 8 (14%) |
| R0 resection rate – n (%) | 48 (86%) |
| Positive lateral margins | 5 (9%) |
| Positive vertical margins | 4 (7%) |
| Positive vertical and lateral margins | 1 (2%) |
| Curative resection rate | 40 (71%) |
R0 resection: histologically complete resection; SD: standard deviation.
Table 4 displays outcomes depending on depth of ESCC invasion. Factors significantly associated with deep submucosal invasion of ESCC in univariate analysis were: absence of histologically complete resection (p = 0.001), poorly differentiated cancer (p < 0.001) and lymphatic or vascular invasion (p = 0.001).
Table 4.
Outcomes depending on the depth of mural invasion of the 56 resected oesophageal early squamous cell carcinomas (ESCCs).
| Depth of invasion | Low risk group : m1–2 (n = 33) | Intermediate risk group: m3, superficial sm (n = 16) | High risk group: > superficial sm (n = 7) | p a |
|---|---|---|---|---|
| Female sex (n = 13) | 7 (21%) | 2 (12%) | 4 (57%) | 0.11 |
| Age <60 years (n = 25) | 16 (48%) | 7 (44%) | 2 (28%) | 0.63 |
| Piecemeal resection (n = 1) | 0 (0%) | 0 (0%) | 1 (14%) | 0.125 |
| Moderately or poorly differentiated cancer (n = 11) | 0 (0%) | 7 (44%) | 4 (57%) | <0.001 |
| Lymphatic or vascular invasion (n = 8) | 1(3%) | 3 (19%) | 4 (57%) | 0.001 |
| Positive vertical margin (R1) (n = 4) | 0 (0%) | 0 (0%) | 4 (43%) | 0.001 |
| Local recurrence (n = 5) | 3 (9%) | 2 (12%) | 0 (0%) | 0.99 |
| Cancer related death (n = 2) | 0 (0%) | 2 (4%)b | 0 (0%) | 0.17 |
Superficial sm: tumour infiltration of the submucosa below 200 µm.
Deep sm: tumour infiltration of the submucosa beyond 200 µm.
Univariate analysis; bboth cases were poorly differentiated tumours.
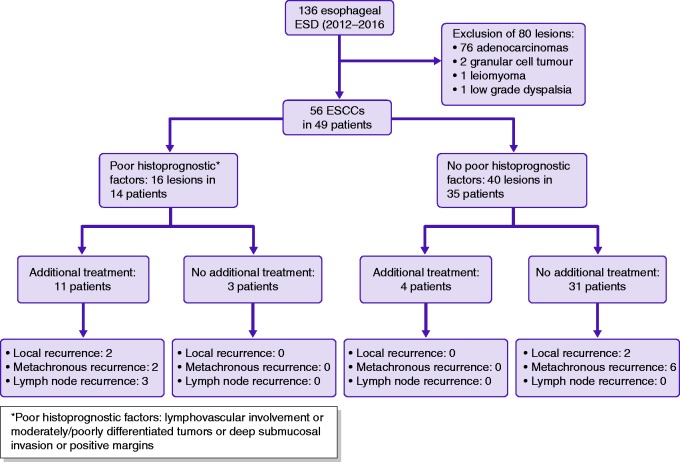
Need for additional treatments (Figure 1)
Overall, 16 lesions in 14 patients presented poor histoprognostic features suggesting a higher than acceptable risk of lymph node involvement (>10%) and potentially requiring an additional treatment. Additional treatments were discussed for all of these patients in dedicated multidisciplinary meetings, but only 11 actually received additional treatments. Oesophagectomy was carried out in three patients, chemoradiotherapy in five patients, chemotherapy alone in one patient, and a second ESD in two patients. Three patients had no additional treatment because refusal or inability to receive further treatment. Conversely, four patients had an additional treatment for m3 or superficial submucosal invasion without poor histoprognostic factor (one had surgery, two radiotherapy and one radiochemotherapy). They had no tumour recurrence.
Figure 1.
Additional treatment after a first endoscopic submucosal dissection (ESD) for oesophageal squamous cell carcinoma (ESCC) and recurrence.
Finally, 15 (30%) patients actually received an additional treatment, of whom six (33%) developed tumour recurrence (two local, two metachronous and three distant, one patient had a local and a lymph node recurrence) and four died (two of ESCC):
Six patients were treated with chemoradiotherapy: among these, two presented a tumour recurrence, one metachronous and one distant (this patient died because of ESCC), nine and 11 months after the treatment, respectively.
One patient was treated by chemotherapy alone and had no recurrence after a six-month follow-up.
Two patients were treated by a second ESD (for a local and a metachronous recurrence) of poorly differentiated tumours. They declined any additional treatment except a second ESD. One presented a local recurrence and the other a local and a distant recurrence. One died of ESCC. The second patient died without the cause of death being known.
Two patients were treated by radiotherapy alone, of which one developed a metachronous lesion.
Four patients were treated by oesophagectomy, of which one presented a distant tumour recurrence 19 months after treatment, one died from non-cancer related death.
Mortality
Eight (16%) patients died during follow-up, two of oesophageal cancer, while the others died of ENT malignancies (n = 3), cardiac disease (n = 2) and decompensated cirrhosis (n = 1).
Factors significantly associated with mortality in the study population (in univariate analysis) were a moderately differentiated or undifferentiated ESCC (p = 0.02) and a distant recurrence of ESCC (p = 0.028).
Local, metachronous and distant recurrences
After a mean follow-up of 21 ± 15 months, 35 (71%) patients had normal follow-up endoscopy. Sixteen recurrences of ESCC occurred in 14 (29%) patients:
• Three (6%) patients had a distant recurrence. Two patients with visceral metastases died of oesophageal cancer (one of them after chemoradiotherapy). The patient with lymph node recurrence was operated on. The cancer-specific survival rate was 96%.
• Four (8%) patients had a local recurrence (among them, one had also a metachronous recurrence) after ESD, occurring after a mean time of 15 ± 16 months. Among these recurrences, three were treated by a second ESD, one by chemoradiotherapy and oesophagectomy, and the other by a palliative oesophageal stent.
• Eight patients had a metachronous recurrence occurred after a mean time of 12 ± 10 months. Among these, four were treated by a second ESD, one had chemoradiotherapy, three were not treated because of another serious condition.
No risk factors (among age, sex, Charlson comorbidity score, history of ENT cancer or radiotherapy, tobacco consumption, size of the resected lesion, adverse events, poorly differentiated tumours, lymphovascular involvement, positive margins) were significantly associated with all type of recurrence in the study population in univariate analysis
Discussion
In this study, the curative resection rate by ESD of early ESCC was 71%, and the cancer-specific survival rate was 96% after 21 ± 15 months follow-up. These figures are comparable to those of Eastern centres.3,8,20,21 This study is to our knowledge the largest Western series of ESCC treated by ESD. As compared to previously published series, we reported the rate of curative endoscopic resections and performed a comprehensive analysis of prognostic factors and stricture-associated factors (Table 5). The limitations of our work include its retrospective and monocentric nature and the relatively small patient numbers limiting the possibilities of multivariate analyses.
Table 5.
Data on Western series of endoscopic submucosal dissection for oesophageal squamous cell carcinoma in the literature.
| First author (year) | No. lesions | Mean patient age | Mean lesion size | R0 resection rate | Curative resection rate | Early adverse event | Stricture | Mean follow-up (months) | Local recurrence | Distant recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| Repici (2010)11 | 20 | 64 (46–81) | 32 (15–60) | 18 (90%) | NA | 2 (10%) | 1 (5%) | 18 | 0 | 0 |
| Chaves (2010)13 | 6 | 50.8 (48–55) | 17.8 (6–30) | 5 (83%) | NA | 2 | 0 | 9 | 0 | 0 |
| Probst (2015)14 | 24 | 68 ± 8 | 25 (15–45) | 92% | 45.8% | 0.9% | 11.7% | 38 | 0 | 1 |
| Barret (2017)12 | 35 | 62 ± 10 | 40 (15–90) | 28(80%) | 24 (69%) | 2 (6%) | 5 (14%) | 4.8 ± 5 | 2 (6%) | 0 |
| Lorenzo (2019) | 56 | 61.5 ± 10 | 40.9 ± 19 | 48 (86%) | 40 (71%) | 3 (5%) | 10 (18%) | 21.2 ± 14.8 | 5 (8.9%) | 3 (5%) |
NA: not available.
The efficacy and safety of ESD for ESCC has been demonstrated in many studies (more than 970 cases published) and a technical success rate of 99%.3,8 The rate of local recurrence after ESD for ESCC ranges from 0–14%, 9,11,22,23,24,25–27 with up to 11% metachronous recurrences.29 We found a 7% (4/56) local recurrence rate, predictable from the 86% histologically complete resection rate, and a 14% metachronous recurrence rate, in keeping with larger monocentric studies.28,29 These data highlight the importance of close endoscopic monitoring, especially since most of these lesions, both local and metachronous, are accessible to a second endoscopic resection by ESD.
Little information is available on the long-term outcomes after ESD, and particularly on the role of additional treatments, such as surgery, radiotherapy or/and chemotherapy after potentially curative or non-curative ESD.27,28 In our work, nearly a third of the patients had an additional treatment, and three out of these 15 patients experienced distant recurrence.
Factors significantly associated with all causes of mortality in the study population in univariate analysis were a moderately differentiated or undifferentiated ESCC and a distant recurrence of ESCC. These results suggest that patients with more aggressive ESCC are also most likely to die from co-morbidities associated with alcohol and tobacco consumption.
Our study highlights the connection between the degree of intramural extension and the poor histoprognostic factors such as poor differentiation or lymphovascular involvement. While several works have underlined the importance of each parameter taken separately,7,24,30,31 our study shows that they actually coexist in a majority of the cases and correlate to survival.
To improve R0 and curative resection rates, ESD for ESCC should be performed in expert centres with a high case load, although no specific cut-off level has been suggested to date in Europe. Finally, an final endoscopic assessment before resection with a high-definition gastroscope to search for worrisome endoscopic features, such an ulcerated or protruded morphology, and virtual chromoendoscopy associated with zoom or magnification to search for type V-N intrapapillary capillary loops (associated with deep invasive or poorly differentiated carcinoma) and better definition of the tumour margins are needed. A pretherapeutic EUS should be included in the work-up in case of invasive cancer, to search for suspicious lymph nodes or muscularis propria infiltration with two caveats: first, the significance of small mediastinal lymph nodes in patients with frequent chronic obstructive pulmonary disease is limited, and the mediastinal infracentimetric lymph nodes are rarely seen on PET CT. Second, EUS is best performed during a pretherapeutic diagnostic procedure, since performing EUS immediately before the resection can make a careful assessment of the mucosa difficult.
The prediction of deep submucosal invasion can be attempted using the analysis of the intrapapillary capillary loops (IPCL) and the overall tumour morphology (ulcerated, Paris 0–III or protruded, Paris 0–I types) that should be searched for systematically.33 Furthermore, the use of high frequency EUS mini-probes could improve pretherapeutic staging of ESCC and potentially improve R0 and curative resection rates, since high frequency EUS mini-probes is the only way to allow us to precisely assess submucosal involvement of an oesophageal cancer.
Several works have reported high stricture rates after endoscopic resections exceeding 75% of the oesophageal circumference.32 However, despite a high proportion of resections exceeding 75% of the oesophageal circumference and the absence of specific stricture prevention method, we mostly observed oesophageal strictures in patients with circumferential defects. This finding suggests that the circumferential extent of the lesion before resection is likely to be a better predictor of stricture than the size of the mucosal defect at the end of the resection, which overestimates the circumferential extension and thereby the risk for oesophageal stricture.
Conclusion
ESD of early oesophageal squamous cell cancer is the recommended treatment option and seems possible at a Western centre with acceptable technical and oncological outcomes. Endoscopic monitoring is paramount given the high risk of metachronous recurrence. Additional treatments after potentially curative or non-curative resections are likely to limit the rate of distant metastasis, but only prospective studies could clarify the role of such additional treatments, especially in a patient unfit for surgery.
Declaration of conflicting interests
The authors have no conflict of interest or financial ties to disclose in relation to this study.
Ethics approval
The study received approval from our local institutional ethical review board (CLEP Decision: AAA-2017-05007).
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Informed consent
Patients provided written informed consent for all the procedures and only then were they included in the database.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2.Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: A multicenter randomized controlled trial. J Clin Oncol Of J Am Soc Clin Oncol 2010; 28: 1566–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47: 829–854. [DOI] [PubMed] [Google Scholar]
- 4.McMillan RR, Berger A, Sima CS, et al. Thirty-day mortality underestimates the risk of early death after major resections for thoracic malignancies. Ann Thorac Surg 2014; 98: 1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto H, Kawata H, Sunada K, et al. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy 2003; 35: 690–694. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc 2010; 72: 255–264. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Hashimoto S, Mizuno K-I, et al. Management decision based on lymphovascular involvement leads to favorable outcomes after endoscopic treatment of esophageal squamous cell carcinoma. Endoscopy 2018; 50: 662–670. [DOI] [PubMed]
- 8.Kim JS, Kim B-W, Shin I-S. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: A meta-analysis. Dig Dis Sci 2014; 59: 1862–1869. [DOI] [PubMed] [Google Scholar]
- 9.Joo DC, Kim GH, Park DY, et al. Long-term outcome after endoscopic submucosal dissection in patients with superficial esophageal squamous cell carcinoma: A single-center study. Gut Liver 2014; 8: 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsujii Y, Nishida T, Nishiyama O, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: A multicenter retrospective cohort study. Endoscopy 2015; 47: 775–783. [DOI] [PubMed] [Google Scholar]
- 11.Repici A, Hassan C, Carlino A, et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: Results from a prospective Western series. Gastrointest Endosc 2010; 71: 715–721. [DOI] [PubMed] [Google Scholar]
- 12.Barret M, Lepilliez V, Coumaros D, et al. The expansion of endoscopic submucosal dissection in France: A prospective nationwide survey. United Eur Gastroenterol J 2017; 5: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaves DM, Maluf Filho F, de Moura EGH, et al. Endoscopic submucosal dissection for the treatment of early esophageal and gastric cancer–initial experience of a Western center. Clin Sao Paulo Braz 2010; 65: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Probst A, Aust D, Märkl B, et al. Early esophageal cancer in Europe: Endoscopic treatment by endoscopic submucosal dissection. Endoscopy 2015; 47: 113–121. [DOI] [PubMed] [Google Scholar]
- 15.Farhat S, Chaussade S, Ponchon T, et al. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 2011; 43: 664–670. [DOI] [PubMed] [Google Scholar]
- 16.Inoue H, Kaga M, Ikeda H, et al. Magnification endoscopy in esophageal squamous cell carcinoma: A review of the intrapapillary capillary loop classification. Ann Gastroenterol 2015; 28: 41–48. [PMC free article] [PubMed] [Google Scholar]
- 17.Pioche M, Mais L, Guillaud O, et al. Endoscopic submucosal tunnel dissection for large esophageal neoplastic lesions. Endoscopy 2013; 45: 1032–1034. [DOI] [PubMed] [Google Scholar]
- 18.Hanaoka N, Ishihara R, Takeuchi Y, et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: A controlled prospective study. Endoscopy 2012; 44: 1007–1011. [DOI] [PubMed] [Google Scholar]
- 19.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc 2010; 71: 446–454. [DOI] [PubMed] [Google Scholar]
- 20.Nagami Y, Ominami M, Shiba M, et al. The five-year survival rate after endoscopic submucosal dissection for superficial esophageal squamous cell neoplasia. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver 2017; 49: 427–433. [DOI] [PubMed] [Google Scholar]
- 21.Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2006; 4: 688–694. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Zhao Y, Zhao X, et al. Clinical outcomes of endoscopic submucosal dissection for early esophageal squamous cell neoplasms: A retrospective single-center study in China. Gastroenterol Res Pract 2016; 2016: 3741456–3741456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JS, Youn YH, Park JJ, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal squamous neoplasms. Clin Endosc 2016; 49: 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Du J, Li H, et al. Clinicopathologic analysis of lymph node status in superficial esophageal squamous carcinoma. World J Surg Oncol 2016; 12; 14: 259–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011; 25: 2541–2546. [DOI] [PubMed] [Google Scholar]
- 26.Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008; 68: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu Y, Kato M, Yamamoto J, et al. EMR combined with chemoradiotherapy: A novel treatment for superficial esophageal squamous-cell carcinoma. Gastrointest Endosc 2004; 59: 199–204. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda A, Hoshi N, Yoshizaki T, et al. Endoscopic submucosal dissection (ESD) with additional therapy for superficial esophageal cancer with submucosal invasion. Intern Med 2015; 54: 2803–2813. [DOI] [PubMed] [Google Scholar]
- 29.Yamashina T, Ishihara R, Nagai K, et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol 2013; 108: 544–551. [DOI] [PubMed] [Google Scholar]
- 30.Moriya H, Ohbu M, Kobayashi N, et al. Lymphatic tumor emboli detected by D2-40 immunostaining can more accurately predict lymph-node metastasis. World J Surg 2011; 35: 2031–2037. [DOI] [PubMed] [Google Scholar]
- 31.Merkow RP, Bilimoria KY, Keswani RN, Chung J, Sherman KL, Knab LM, et al. Treatment trends, risk of lymph node metastasis, and outcomes for localized esophageal cancer. J Natl Cancer Inst. juill 2014; 106(7). [DOI] [PubMed]
- 32.Mizumoto T, Hiyama T, Quach DT, et al. Magnifying endoscopy with narrow band imaging in estimating the invasion depth of superficial esophageal squamous cell carcinomas. Digestion 2018; 98: 249–256. [DOI] [PubMed] [Google Scholar]
- 33.Barret M, Beye B, Leblanc S, et al. Systematic review: The prevention of oesophageal stricture after endoscopic resection. Aliment Pharmacol Ther 2015; 42: 20–39. [DOI] [PubMed] [Google Scholar]