Abstract
Background
Data on the efficacy and safety of the long-acting somatostatin analogue lanreotide (LAN) for postoperative dumping syndrome are lacking.
Objective
We performed a double-blind, randomised and placebo-controlled crossover study of LAN Autogel® 90 mg in postoperative dumping.
Methods
Adults with a positive prolonged oral glucose tolerance test or spontaneous hypoglycaemia and total dumping score (DS) ≥ 10 despite dietary measures were treated with three monthly injections of LAN or placebo in a randomised crossover fashion with an eight-week wash-out period. Primary outcome was the effect of LAN on total DS versus placebo. Secondary outcomes were the effect on early and late DS, treatment assessment, quality of life and safety.
Results
Of 24 included patients (66.7% female; age 49.1 ± 2.1 years), 12 were randomised to LAN first. Pooled DS after three injections were lower compared to baseline after LAN (median=14 (interquartile range (IQR) 11.5–23) vs. median = 22 (IQR 16–27); p = 0.03) but not placebo (median = 20 (IQR 15–27) vs. median = 23 (IQR 13–29); p = 0.15). Improvement of early (median = 7.5 (IQR 4.5–13) vs. median = 12 (IQR 9–16); p = 0.03) but not late (median = 7 (IQR 6–10.3) vs. median = 9 (IQR 6–13); p = 0.26) DS was seen. Overall treatment assessment correlated with change in DS (r = –0.69, p = 0.004). Symptom improvement was not associated with changes in quality of life. Of the 81 reported adverse events, 44 occurred on LAN compared to 37 on placebo (p > 0.05), with seven serious adverse events on LAN.
Conclusions
LAN is effective for treating early postoperative dumping symptoms, although side effects are common and quality of life is not significantly affected.
Keywords: Dumping syndrome, postoperative dumping, lanreotide, somatostatin analogue, randomised controlled trial
Introduction
Dumping syndrome is a common and debilitating complication of oesophageal and gastric surgery, with a reported incidence of up to 50%.1 The characteristic alimentary and systemic manifestations can be separated into an early and late phase, depending on the occurrence of symptoms in relation to the postprandial timing.2 Early dumping is caused by rapid delivery of undigested nutrients in the small bowel which causes a fluid shift with gastrointestinal (GI) and vasomotor symptoms. Late dumping is caused by a rapid increase of the glycaemia and insulin secretion with reactive hypoglycaemia. Dumping syndrome results from an impaired storage function of the stomach and/or the pyloric emptying mechanism characterised by accelerated early gastric emptying.3
Although the majority of patients with mild symptoms respond to dietary measures, including frequent small meals with low concentration of mono- and disaccharides and avoiding liquids during the meal, a significant subset will require medical therapy.3 Somatostatin analogues decrease the meal-induced release of insulin, preventing the occurrence of late hypoglycaemia. In addition, the concentrations of several GI peptide hormones responsible for the vasodilatation and activation of the renin–angiotensin system are decreased through activation of the somatostatin receptor.3 Both short- and long-acting release (LAR) forms of octreotide (OCT), a first-generation somatostatin analogue, have demonstrated efficacy in improving hypoglycaemia and early and late dumping symptoms, with higher quality of life (QoL) on LAR therapy.4,5 However, long-term treatment discontinuation up to 60% has been reported, mainly due to side effects including pain at the injection site and steatorrhea, and secondary loss of efficacy.6
One of the main problems is the high burden and cost of daily subcutaneous (SC) administration of first-generation somatostatin analogue for patients. In addition, OCT LAR requires intramuscular (IM) injections which also limit its use in some patients. Somatuline® Autogel® is a slow-release formulation that requires monthly deep SC injections and supplies high-dose and stable serum levels of lanreotide (LAN). However, no placebo-controlled trials are available for the use of LAN in postoperative dumping syndrome. Therefore, the aim of the present study was to assess the efficacy and safety of LAN for postoperative dumping in a multi-centre Phase II, randomised and placebo-controlled crossover trial.
Methods
Participants and sites
Adult patients (>18 years old) with postoperative dumping syndrome and a total dumping score (DS) of ≥ 10 (see below) were included following a positive oral glucose tolerance test (OGTT) or history of hypoglycaemia (<60 mg/dL) despite standard dietary measures (six meals a day with low concentrations of mono- and disaccharides for at least one month before inclusion). OGGT was performed by measuring glycaemia, haematocrit and pulse rate every 30 minutes during 180 minutes after drinking a 200 mL solution containing 75 g of glucose. The test was considered positive if there was an early (30 minutes) rise in haematocrit of >3% or pulse rate of 10 bpm and/or a late (between 120 and 180 minutes) hypoglycaemia of <60 mg/dL.2,3 Baseline investigations also included abdominal ultrasound, routine blood tests and oesophagogastroduodenoscopy with biopsies within six months before inclusion.
Patients with evidence of gallstones, diabetes mellitus, coeliac disease, Giardia lamblia infection, erosive oesophagitis (>Los Angeles grade B) and gastric or duodenum ulcers were excluded. Female patients of childbearing potential were only eligible if a pregnancy test was negative and contraception was used throughout the trial. Other exclusion criteria were past treatment with LAN or OCT LAR and known hypersensitivity to LAN or a compound of the study drug. Forbidden concomitant medications during the trial were acarbose, buspirone, guar gum and pectin. Patients were recruited from the outpatient clinic from three Belgian expert motility centres: University Hospitals Leuven, AZ Sint-Lucas Brugge and ZOL Genk. Written informed consent was obtained from all patients, and the study was performed according to the Declaration of Helsinki after approval by the Ethics Committee of University Hospitals Leuven (number S50966).
Study design and treatment
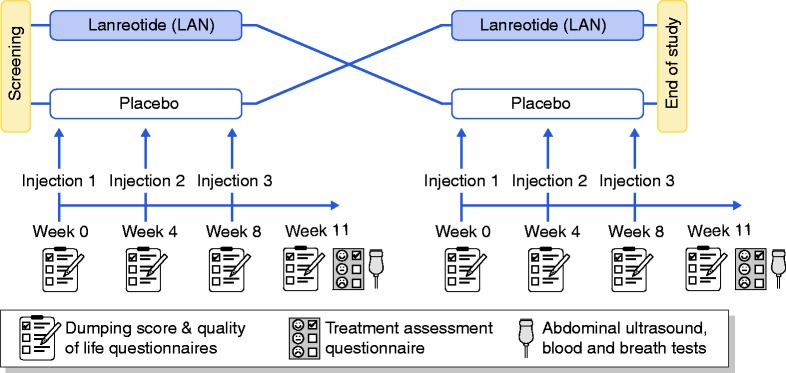
The study design is summarised in Figure 1. In this multi-centre Phase II trial, patients were included after screening and randomised to LAN or placebo first. Participants received three monthly deep SC injections of LAN (Autogel® 90 mg) or placebo (NaCl 0.9%) with assessment of dumping symptoms and QoL. As steady-state concentrations of LAN are achieved after three injections, treatment evaluation was planned three weeks after the last injection (week 11 of the first period) with follow-up DS, QoL and treatment assessment. After a washout period of eight weeks, patients were crossed over to the other treatment (week 0), with three monthly injections and treatment evaluation three weeks after the last injection (week 11 of the second period). Randomisation was performed by the funding source (Ipsen) with block sizes of four. Subjects were assigned unique consecutive identification numbers and allocated to the corresponding study treatment after inclusion. LAN and placebo were injected by an independent injector using the same type of syringe. LAN Autogel® comes in a prefilled syringe, while placebo (NaCl 0.9%) needed to be drawn in an empty similar syringe. All other study personnel were blinded. Unblinding was only performed after database lock. The trial was registered on clinicaltrials.gov (NCT01923649) with EudraCt-number 2008-000643-34.
Figure 1.
Study design. LAN: lanreotide.
Study outcomes
The primary outcome was the efficacy of LAN compared to placebo using the pooled (first and second treatment periods combined) total DS, calculated as the sum of eight early and six late dumping symptoms, as described by Arts et al.5 Early dumping symptoms include sweating, flushes, dizziness, palpitations, abdominal pain, diarrhoea, bloating and nausea occurring within one hour after a meal. Late dumping symptoms include sweating, palpitations, hunger, drowsiness to unconsciousness, shaking and aggression occurring an hour or more after a meal. In case of immediate postprandial symptoms lasting more than an hour, both early and late DS are included. Symptoms were scored using a four-point Likert scale (0 = none, 1 = mild, 2 = moderate or 3 = severe). Pooled total DS before (week 0) and after (week 11) each treatment were compared within and between groups.
Secondary outcomes were the effect of LAN compared to placebo on the pooled early and late dumping sub-scores, the treatment assessment scale, QoL and the safety data. Overall treatment assessment was done as an overall treatment evaluation by asking the question ‘How do you feel compared to your situation before starting the study?’ at each treatment evaluation and using a seven-point Likert scale ranging from 1 (a lot worse) to 7 (a lot better). QoL was assessed using the Short Form Health Survey (SF-36), consisting of eight scaled scores which are the weighted sums of the questions in their section with a score of 0 equivalent to maximum disability and a score of 100 equivalent to no disability.
Safety and tolerability
Safety and tolerability were assessed at each visit by recording (serious) adverse events (AE). Abdominal ultrasound and laboratory tests were performed after each treatment evaluation to assess development of gallstones. Blood tests included haematology, electrolytes and liver tests (aminotransferases, gamma-glutamyl transferase, alkaline phosphatases and bilirubin). In addition, a mixed triglyceride breath test to assess lipid digestion was performed at home within 7–12 days before each treatment evaluation, as previously described.7 Briefly, breath samples were collected at baseline and every 30 minutes during six hours after consumption of a test meal consisting of two slices of white bread with a chocolate paste (30 g) containing 250 mg of 13C-substrate (1,3-distearyl,2[13C-carboxyl]octanoyl glycerol). Results are reported as the cumulative percentage of the administered dose excreted at six hours. Decreased lipase activity, which may occur with the use of somatostatin analogues, is determined as a cumulative percentage of 13C recovery < 23% of the administered dose at six hours, corresponding to a lipase output of <90 kIU/h.7
Statistical analysis
Sample size was calculated based on an anticipated 30% difference in total DS on active treatment as compared to a previous placebo-controlled study on OCT LAR5 with a statistical power of 85% and alpha 0.05 in a crossover setting (paired t-test). Inclusion of 24 patients would therefore allow a drop-out of 30%. Continuous data are presented as the median with interquartile range (IQR) and proportions as frequencies. Non-parametric analyses were performed for pooled results (both treatment periods combined) on LAN or placebo treatment compared with the respective baseline for each period using the Wilcoxon signed rank test (within-group comparisons). Between-group comparisons for pooled results were performed using the Mann–Whitney U-test. Total DS were also compared between treatment periods on LAN or placebo to test for an order effect in the crossover design. Pearson correlation analysis was performed between the overall treatment assessment scale and change in total DS. Proportions were compared using the chi-square test. The primary and secondary analyses were based on the full-analysis set (patients receiving one or more dose of study treatment) according to the intention-to-treat (ITT) principle for proportions or a modified per-protocol (PP) analysis for continuous variables including only patients completing baseline and week 11 of at least one treatment period. All analyses were performed in GraphPad Prism v8 (GraphPad Software, Inc., La Jolla, CA) with two-tailed p-values and significance set at 0.05.
Results
Patient flow
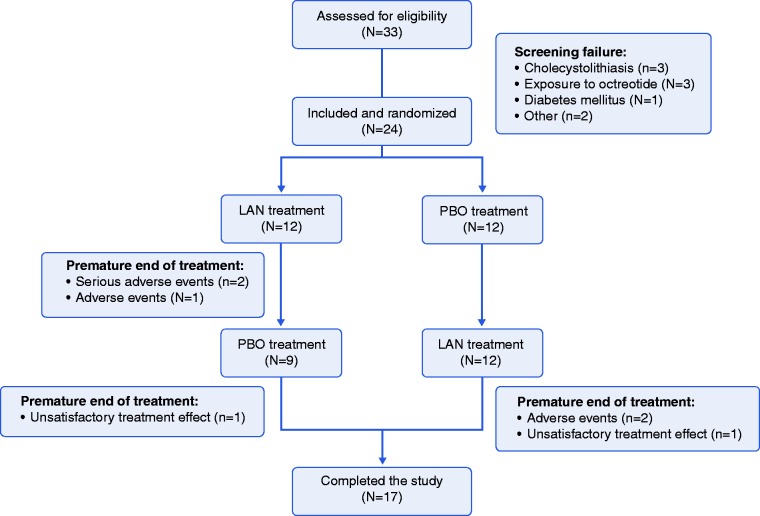
In total, 33 patients were assessed for eligibility, with nine screening failures (three with gallstones, three previous exposure to OCT, one diabetes mellitus, one pregnancy and one with DS <10), resulting in 24 patients included and randomised in the trial (12 to LAN and 12 placebo first; Figure 2). Premature study termination occurred in three patients on LAN in the first treatment period, two due to serious AE (one epilepsy and one hypoglycaemia) and one due to AE (vomiting). After crossover, two patients dropped out on LAN in the second treatment period due to AE (one with diarrhoea and vomiting and one abdominal cramps, diarrhoea and hypoglycaemia). In addition, one patient on placebo and one on LAN (both in the second period) withdrew consent due to unsatisfactory treatment effect, resulting in 17 patients who completed the study.
Figure 2.
Consort flow diagram for the current study.
Study population
Baseline patient characteristics are shown in Table 1. There were no significant differences between both groups regarding patient demographics (including age, sex, weight and body mass index) or clinical variables (including disease duration and type of surgery). Overall, gastrectomy and gastric bypass were the most common (42%), followed by non-resective oesophageal surgery (e.g. Nissen fundoplication; 33%) and oesophagectomy (21%). One patient in the placebo-LAN group had both a partial gastrectomy and oesophagectomy. In addition, four patients in the LAN-placebo and five in the placebo-LAN group had a history of cholecystectomy (33% vs. 42%; p = 0.67). Baseline DS were similar for total, early and late dumping symptoms.
Table 1.
Baseline patient characteristics.
| Characteristic | LAN-placebo (N = 12) | Placebo-LAN (N = 12) | p-Value |
|---|---|---|---|
| Age, years | 48 (45–52) | 47 (36–59) | 0.62 |
| Female sex (%) | 6 (50%) | 10 (83%) | 0.08 |
| Weight, kg | 69.5 (58.3–84) | 60 (54.8–66.4) | 0.10 |
| BMI, kg/m2 | 24 (22–29) | 22 (20–24) | 0.14 |
| Disease duration, years | 1.6 (0.7–4.1) | 2.7 (1.1–13.1) | 0.16 |
| Type of surgery, % Gastrectomy/bypass Oesophagectomy Non-resective oesophageal surgery | 3 (25) 3 (25) 6 (50) | 7 (58) 2 (17) 2 (17) | 0.10 0.62 0.08 |
| Baseline OGTT, % Early criteria Late criteria | 6 (50) 5 (42) | 9 (75) 4 (33) | 0.21 0.67 |
| History of hypoglycaemia, % | 2 (17) | 4 (33) | 0.35 |
| Baseline DS Total DS Early DS Late DS | 24 (17–27) 13 (9–16) 10 (8–13) | 23 (18–29) 13 (12–19) 8 (6–12) | 1 0.7 0.4 |
Data presented as median (interquartile range) or percentage.
LAN: lanreotide; BMI: body mass index; DS: dumping score; OGTT: oral glucose tolerance test.
Primary outcome
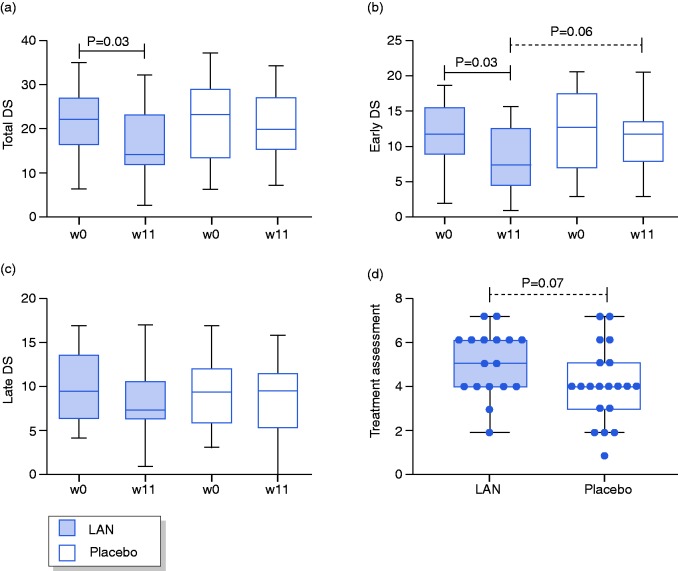
The pooled total DS was significantly reduced after three injections of LAN compared to baseline (14 (11.5–23) vs. 22 (16–27); p = 0.03) but not placebo (20 (15–27) vs. 23 (13–29); p = 0.15; Figure 3(a)). Between-group comparisons showed no significant differences for total DS between LAN and placebo after three injections (p = 0.21) or at baseline (p = 0.99). There were no significant differences in total DS on LAN or placebo between each treatment period at week 0, 4, 8, or 11 (Table 2), indicating the lack of a significant order effect.
Figure 3.
Change in pooled dumping scores (DS) for total (a), early (b) and late (c) symptoms and treatment assessment (d) after three monthly injections of LAN or placebo.
Table 2.
Changes in total DS before and during LAN or placebo in the first or second treatment period.
| LAN treatment | LAN-placebo (first period) | Placebo-LAN (second period) | p value |
|---|---|---|---|
| Week 0 | 23.5 (17.3–27) | 18 (15–27) | 0.50 |
| Week 4 | 15 (12–24) | 20.5 (10.5–30.5) | 0.77 |
| Week 8 | 16.5 (9–24.5) | 17.5 (6.8–25.3) | 0.87 |
| Week 11 | 14 (12–22.5) | 14 (7–23) | 0.71 |
| Placebo treatment |
LAN-placebo (second period) |
Placebo-LAN (first period) |
p value |
| Week 0 | 24 (9–30) | 22.5 (18–29.3) | 0.69 |
| Week 4 | 22 (7.5–27) | 20 (13.3–25) | 0.85 |
| Week 8 | 19.5 (11.5–27.8) | 22 (9.5–25.5) | 0.84 |
| Week 11 | 20 (19–28) | 18.5 (12.8–25.5) | 0.37 |
Data presented as median (interquartile range).
Secondary outcomes
The pooled early DS was significantly reduced after LAN (7.5 (4.5–13) vs. 12 (9–16); p = 0.03) but not placebo (12 (8–14) vs. 13 (7–18); p = 0.09) compared to baseline (Figure 3(b)). A trend was found for lower early DS after LAN compared to placebo (p = 0.06), with similar scores at baseline (p = 0.40). No differences were found for pooled late DS after LAN (7 (6–10.3) vs. 9 (6–13); p = 0.26) or placebo (9 (5–11) vs. 9 (5.5–11.5); p = 0.72) compared to baseline and after LAN compared to placebo (p = 0.48) or at baseline (p = 0.90; Figure 3(c)). A trend was also found for a higher overall treatment assessment scores on LAN compared to placebo (5 (4–6) vs. 4 (3–5); p = 0.07; Figure 3(d)), with a higher proportion of patients with an overall treatment score ≥5 (83% LAN vs. 50% placebo; p = 0.08). In addition, a negative correlation was found between treatment assessment scores and the change in total DS (r = –0.69, p = 0.004; Supplemental Figure S1). No differences were seen in any domain of the SF-36 after LAN compared to baseline, but vitality scored higher after placebo compared to baseline (40 (30–50) vs. 25 (15–33); p < 0.01; Table 3). There were no significant differences in any domain of the SF-36 after LAN compared to placebo.
Table 3.
Quality of life (SF-36) before and after lanreotide (LAN) or placebo treatment.
| Domain | LAN week 0 | LAN week 11 | p value | Placebo week 0 | Placebo week 11 | p value |
|---|---|---|---|---|---|---|
| Physical function | 60 (36-70) | 55 (43-70) | 0.94 | 65 (53-80) | 60 (50-79) | 0.67 |
| Role physical | 0 (0-19) | 0 (0-50) | 0.43 | 0 (0-25) | 25 (0-63) | 0.34 |
| Role emotion | 0 (0-100) | 0 (0-100) | 0.99 | 0 (0-50) | 33 (0-92) | 0.13 |
| Body pain | 27 (22-41) | 31 (22-41) | 0.81 | 32 (17-41) | 32 (22-41) | 0.25 |
| Social function | 38 (25-59) | 38 (25-53) | 0.61 | 38 (25-50) | 50 (38-63) | 0.06 |
| Mental health | 44 (37-64) | 52 (44-64) | 0.40 | 52 (42-58) | 52 (37-66) | 0.29 |
| Vitality | 25 (16-44) | 30 (25-35) | 0.99 | 25 (15-33) | 40 (30-50) | 0.009 |
| General health | 34 (20-47) | 25 (20-41) | 0.49 | 30 (20-48) | 33 (20-47) | 0.55 |
Data presented as median (interquartile range).
Safety
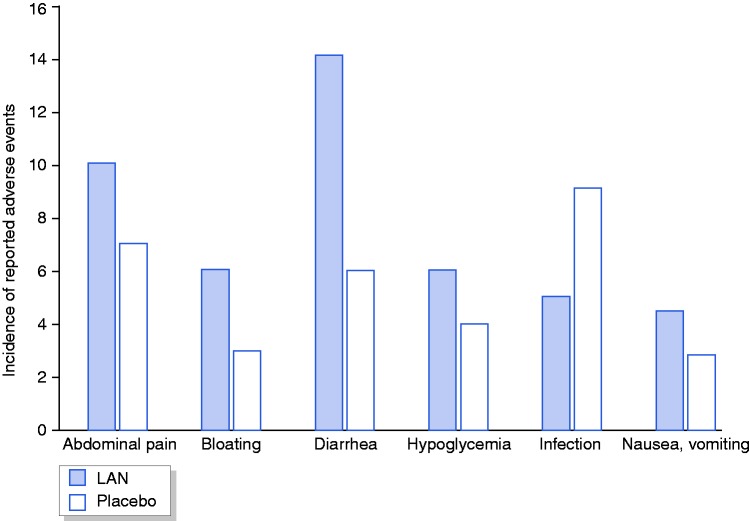
Of the 81 reported AE (all patients ≥ 1 AE), 44 (54%) occurred on LAN compared to 37 (46%) placebo (p > 0.05), with treatment-related AE on LAN in 33 (75%). Diarrhoea was the most common AE (12 LAN vs. 6 placebo; p > 0.05), followed by infection (5 LAN vs. 9 placebo) and hypoglycaemia (5 LAN vs. 5 placebo; p > 0.05). Physician assessment of diarrhoea on LAN was compatible with steatorrhea in 5/8 (63%) patients. Painful injection site was reported in five (42%) patients on LAN. Proportions of individual AE are shown in Figure 4.
Figure 4.
Incidence of adverse event on LAN or placebo treatment.
Overall, seven serious AE were reported (all on LAN treatment), of which one was evaluated as unrelated to the treatment (epilepsy) and one possibly related (hypoglycaemia) by the investigator, and both led to drop-out. There were four (sub)obstructions, of which one was treated surgically (i.e. repair of internal herniation after gastric bypass) and was evaluated as unlikely related to the treatment by the investigator and did not lead to drop-out. Several patients dropped out due to AE including diarrhoea, hypoglycaemia, abdominal cramps and vomiting (3 on LAN) or unsatisfactory treatment effect (1 on LAN and 1 placebo).
Abdominal ultrasound was reported normal after each treatment period on LAN and placebo, with no evidence for newly developed gallstones. Mixed triglyceride breath tests showed no difference in the pooled cumulative percentage of 13C recovery at six hours after LAN compared to placebo (24.1 (22.1–34.2) vs. 22.8 (12.1–28.6); p = 0.29). However, the proportion of patients with a normal result (>23%) was higher after LAN compared to placebo after the first (83% vs. 42%; p = 0.03) but not second (67% vs. 75%; p > 0.05) treatment period.
Discussion
Postoperative dumping syndrome is a frequent complication of gastric and/or oesophageal surgery with invalidating symptoms. Due to the complex and heterogeneous pathophysiology of early and late dumping, current medical treatments are often of limited benefit. The results of this multi-centre Phase II, randomised and placebo-controlled crossover trial suggest the efficacy of LAN, a novel generation somatostatin analogue, for the treatment of postoperative dumping. A beneficial effect was found for the improvement in total and early but not late dumping symptoms when comparing LAN to baseline. Although only a trend was observed for early and not total DS with LAN compared to placebo after three injections and hence the primary end point of the study was not met, within-treatment assessments were in favour of LAN, and the treatment assessment scores were higher on LAN compared to placebo. The treatment assessment scores also correlated with the change in dumping symptoms measured with the DS, supporting the use of this score and its subscales for assessing treatment responses of total, early and late dumping.5 QoL was not significantly altered by LAN treatment, and although side effects such as diarrhoea and hypoglycaemia were commonly reported, they were also frequent on placebo treatment.
In a previous open-label study, we demonstrated the efficacy of monthly IM LAR compared to daily SC OCT injections using the total DS, with higher overall treatment efficacy compared to OCT SC (83% vs. 52%; p = 0.01).5 The proportion of patients scoring the treatment assessment as (partly) better on OCT LAR (31% partly and 52% better) was similar compared to the present randomised placebo-controlled trial using monthly LAN injections (17% slightly better and 67% (a lot) better). In addition, no initial overlap with short-acting somatostatin analogue (e.g. daily SC OCT) is needed, as LAN treatment provides high-dose and stable serum levels with a rapid onset of effect.8 Therefore, use of LAN is potentially more attractive to patients due to the ability to start immediately with monthly SC administration. However, higher postprandial plasma peaks may be required for effective treatment of late dumping, and this could partially explain the lack of effect of LAN on late DS in the present study. Retrospective studies with OCT have indeed confirmed that compared to OCT LAR, OCT SC may provide better control of late dumping.4,6 Pasireotide, a second-generation somatostatin analogue with a broader receptor affinity, was shown to improve signs and symptoms of both early and late dumping.9,10 Other treatments including GLP-1 agonists have also shown beneficial effects on postprandial hypoglycaemia following gastric surgery,11–13 although late dumping is frequently managed with dietary measures and acarbose.3 The effect of LAN on total and early DS in the present study is promising, as early dumping is often the most challenging to manage in clinical practice.5
Long-term follow-up studies on the use of OCT have reported a treatment discontinuation up to 60%, mainly due to side effects and secondary loss of efficacy.6 LAN injections were not frequently reported as painful, and no gallstone formation or major hepatobiliary complications were evident on abdominal ultrasound and blood tests. Of all reported AE in the present study, diarrhoea was the most common. Nausea and/or vomiting were more frequent in LAN compared to placebo, and this may also be explained by the delay in GI transit.6 However, many of these symptoms are also common in postoperative dumping, with similar frequency in placebo compared to LAN (e.g. hypoglycaemia), indicating potential confounding from persisting dumping symptoms. Moreover, the results of the mixed triglyceride breath test were similar after LAN and placebo, except for an unexpected higher proportion of patients with a normal result after LAN compared to placebo in the first treatment period. Although this test is commonly used to diagnose pancreatic insufficiency, a clear influence of gastric emptying on the rate of intraluminal lipolysis has been described for subjects without pancreatic insufficiency, limiting its use in postoperative dumping with rapid GE.14 The higher proportion of normal results on LAN may thus reflect a positive effect on transit, and no definite conclusions on exocrine pancreatic functions can be made at this time. Although all serious AE occurred on LAN treatment, these did not always lead to drop-out.
The strengths of the present study include the double-blind, randomised and placebo-controlled design including only postoperative dumping patients after standardised diagnosis and exclusion of other GI abnormalities on routine examinations. Both early and late dumping symptoms were systematically evaluated, as well as treatment assessment scores, QoL and safety with follow-up ultrasound and blood tests. Similar to a previous study on OCT,5 we used the DS as a primary end point and showed that it correlates with the overall treatment evaluation, indicating lower treatment assessment scores with an increase in DS. The SF-36 was used as a general QoL measure, as no dumping-specific questionnaires exist to date. Analyses were done on pooled results, as no significant order effects (differences in total DS on LAN or placebo between treatment periods) were found. A potential carry-over effect was also minimised by a long washout period of eight weeks. The limitations include the small sample size with a relevant number of drop-outs and the use of only one dose of LAN. Outcomes were subjective, using recall questionnaires rather than daily diaries. No follow-up OGTT or gastric emptying tests were performed on LAN, although in other studies in dumping syndrome this does not always correlate with symptoms. Continuous variables were analysed PP, as missing data were not included in paired analyses (e.g. in case of drop-outs). However, the favourable treatment assessment on LAN was also evident in an ITT-analysis, with a high percentage of patients indicating overall treatment evaluation ≥5. The lack of validated DS and knowledge of minimal clinically important difference thresholds prohibited a responder analysis for the primary outcome or total DS.
In conclusion, although the primary end point was not met, LAN Autogel® 90 mg seems to be partially effective for treating postoperative dumping symptoms, with improvement in both total and early dumping symptoms after LAN compared to baseline but not placebo after three injections. Overall treatment evaluation scored high on LAN, and treatment assessment scores correlated with changes in total dumping symptoms, supporting the use of the DS. Side effects were common in both LAN and placebo treatment, possibly resulting from under-treatment in a subset of patients. QoL was not significantly affected. Future studies should focus on optimal dosing to enhance tolerability while maintaining efficacy.
Supplementary Material
Acknowledgements
LW is a research fellow of the Flanders Research Foundation (FWO Vlaanderen). JT is supported by a Methusalem grant from Leuven University. RB and TV are senior clinical investigators supported by the Flanders Research Foundation (FWO Vlaanderen).
Declaration of conflicting interests
None declared.
Funding
The study was supported by a research grant from Ipsen. The active and placebo treatment was also supplied by Ipsen. The funding source reviewed the protocol and the manuscript, but did not suggest changes and was not involved in the decision to publish the data.
Ethics approval
The study was approved by the Ethics Committee of University Hospitals Leuven (number S50966).
Informed consent
Written, informed consent was obtained from each patient included in the study before any study-related procedure was performed.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Wauters L, Vanuytsel T. Applications of peptide hormone ligands for the treatment of dumping and short bowel syndrome. Curr Opin Pharmacol 2018; 43: 118–123. [DOI] [PubMed] [Google Scholar]
- 2.van Beek AP, Emous M, Laville M, et al. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes Rev 2017; 18: 68–85. [DOI] [PubMed] [Google Scholar]
- 3.Tack J, Arts J, Caenepeel P, et al. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol 2009; 6: 583–590. [DOI] [PubMed] [Google Scholar]
- 4.Penning C, Vecht J, Masclee AAM. Efficacy of depot long-acting release octreotide therapy in severe dumping syndrome. Aliment Pharmacol Ther 2005; 22: 963–969. [DOI] [PubMed] [Google Scholar]
- 5.Arts J, Caenepeel P, Bisschops R, et al. Efficacy of the long-acting repeatable formulation of the somatostatin analogue octreotide in postoperative dumping. Clin Gastroenterol Hepatol 2009; 7: 432–437. [DOI] [PubMed] [Google Scholar]
- 6.Didden P, Penning C, Masclee AAM. Octreotide therapy in dumping syndrome: analysis of long-term results. Aliment Pharmacol Ther 2006; 24: 1367–1375. [DOI] [PubMed] [Google Scholar]
- 7.Vantrappen G, Rutgeerts P, Ghoos Y, et al. Mixed triglyceride breath test: a noninvasive test of pancreatic lipase activity in the duodenum. Gastroenterology 1989; 96: 1126–1134. [DOI] [PubMed] [Google Scholar]
- 8.Hu M, Tomlinson B. Pharmacokinetic evaluation of lanreotide. Expert Opin Drug Metab Toxicol 2010; 6: 1301–1312. [DOI] [PubMed] [Google Scholar]
- 9.Deloose E, Bisschops R, Holvoet L, et al. A pilot study of the effects of the somatostatin analog pasireotide in postoperative dumping syndrome. Neurogastroenterol Motil 2014; 26: 803–809. [DOI] [PubMed] [Google Scholar]
- 10.Tack J, Aberle J, Arts J, et al. Safety and efficacy of pasireotide in dumping syndrome-results from a phase 2, multicentre study. Aliment Pharmacol Ther 2018; 47: 1661–1672. [DOI] [PubMed] [Google Scholar]
- 11.Miholic J, Hoffmann M, Holst JJ, et al. Gastric emptying of glucose solution and associated plasma concentrations of GLP-1, GIP, and PYY before and after fundoplication. Surg Endosc 2007; 21: 309–314. [DOI] [PubMed] [Google Scholar]
- 12.Abrahamsson N, Engstrom BE, Sundbom M, et al. GLP1 analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication?. Eur J Endocrinol 2013; 169: 885–889. [DOI] [PubMed] [Google Scholar]
- 13.Kataria R, Linn S, Malik Z, et al. Post-fundoplication dumping syndrome: a frequent ‘rare’ complication. ACG Case Reports J 2017; 5: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maes B, Ghoos Y, Geypens B, et al. Relation between gastric emptying rate and rate of intraluminal lipolysis. Gut 1996; 38: 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.