Abstract
Background
To assess the comparative efficacy and safety of elective nodal irradiation (ENI) and involved-field irradiation (IFI) in patients with esophageal cancer (EC) receiving neoadjuvant chemoradiotherapy plus surgery (nCRTS).
Material and methods
PubMed, Embase, Cochrane Library, Web of Science and major meetings were searched for randomized controlled trials (RCTs) that compared at least two of the following treatment regimens: nCRTS, neoadjuvant chemotherapy plus surgery (nCTS), and surgery (S) alone. Overall survival (OS) was the primary outcomes of interest, reported as hazard ratio (HR) and 95% confidence intervals (CIs). A Bayesian network meta-analysis was performed to compare all regimens simultaneously.
Results
Twenty-nine RCTs with a total of 5212 patients were included in the meta-analysis. Both nCRTS adopting ENI (nCRTS-ENI) (HR = 0.63, 95% CI: 0.48–0.83) and nCRTS adopting IFI (nCRTS-IFI) (HR = 0.75, 95% CI: 0.66–0.86) significantly improved OS compared to S alone. No significant differences in OS, locoregional recurrence, distant metastases, R0 resection and postoperative mortality were observed between nCRTS-ENI and nCRTS-IFI. In subgroup analyses, nCRTS-IFI showed a significant OS advantage over nCTS (HR = 0.78, 95% CI: 0.63–0.96) and S alone (HR = 0.50, 95% CI: 0.38–0.68) for esophagus squamous cell carcinoma (ESCC), but nCRTS-ENI did not; nCRTS-ENI using three-dimensional radiotherapy (3D-RT) resulted in an improved OS compared to that with 2D-RT (HR = 0.58, 95% CI: 0.34–0.99). Based on treatment ranking in term of OS, nCRTS-IFI (0.90) and nCRTS-ENI (0.96) was ranked the most effective treatment for ESCC and esophagus adenocarcinoma (EAC), respectively.
Conclusion
Either adopting ENI or IFI, nCRTS is likely to be the optimal treatment for resectable EC, and nCRTS-IFI and nCRTS-ENI seem to be more effective for patients with ESCC and EAC, respectively. Future head to head comparison trials are needed to confirm these findings.
Keywords: Esophagus cancer, Neoadjuvant chemoradiotherapy, Elective nodal irradiation, Involved-field irradiation, Network meta-analysis
Introduction
Esophagus cancer (EC) is the eighth most common cancer worldwide and the sixth most common cause of cancer-related deaths [1, 2]. Surgery is still considered as a major component of treatment for all resectable cases. However, surgery alone (S alone) showed poor long-term outcomes, and the 5-year survival rate was rarely > 30% even after curative resection [3, 4]. Some recent randomized control trials (RCTs) have demonstrated the survival benefit of neoadjuvant chemoradiotherapy followed by surgery (nCRTS) compared with S alone [5–8]. While, there are also trials reporting negative results [9–22].
It should be noted that radiation fields used for patients receiving nCRTS are inconsistent in trials, which might affect the outcomes. Some trials adopted elective nodal irradiation (ENI, nodal target volume covering both metastatic lymph nodes and regional nodes) [17–22], and others adopted involved-field irradiation (IFI, nodal target volume including only the metastatic nodes) [5–16]. Efficacy of ENI and IFI has been compared in patients with locally advanced EC undergoing radical CRT in some retrospective studies [23–26], but with different results. At present, no trials have compared the two radiation fields directly in patients undergoing nCRTS, and therefore, there are still questions around which is more superior, and what is the suitable patient population for adopting ENI or IFI.
In light of these issues, we performed a network meta-analysis to assess the comparative effectiveness and safety of ENI and IFI, attempting to identify the best radiation field in patients receiving nCRT.
Materials and methods
Literature search strategy
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria [27] (Additional file 1: Tables S1). PubMed, Embase, Cochrane Library, Web of Science were searched for the available studies published before April 1, 2019, using the strategy as shown in Additional file 1: Tables S2. The reference lists of retrieved studies were manually scanned for relevant additional studies missed by the electronic search.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (1) types of studies: RCTs; (2) types of participants: resectable EC; (3) types of interventions: compared at least two of the following treatments: nCRTS, neoadjuvant chemotherapy plus surgery (nCTS), and S alone; and (4) outcomes: overall survival (OS), locoregional recurrence (LR), distant metastases (DM), R0 resection, and postoperative mortality (POM) data. Studies which failed to meet the above criteria were excluded from the network meta-analysis.
Data extraction
The data were extracted by two investigators independently. The following data were extracted from each study: first author or name of individual RCT, years of publication, duration of the study, country of origin, treatments, numbers of patients, pathologic type, and data of OS, LR, DM, R0 resection, and POM.
Quality assessment
The methodological quality of RCTs was assessed by Cochrane risk of bias tool [28], which consists of the following five domains: sequence generation, allocation concealment, blinding, incomplete data, and selective reporting. A RCT was finally rated as “low risk of bias” (all key domains indicated as low risk), “high risk of bias” (one or more key domains indicated as high risk), and “unclear risk of bias”.
Statistical analysis
The primary outcome was OS, and the secondary outcomes were LR, DM, R0 resection, and POM. Hazard ratios (HRs) or odds ratios (ORs) and their 95% confidence intervals (CIs) were used as summary statistics. For direct comparisons, standard pairwise meta-analysis was performed. A statistical test for heterogeneity was performed using the chi-square (χ2) and I-square (I2) tests with the significance set at I2 > 50% or P < 0.10. If significant heterogeneity existed, a random-effects analysis model was used; otherwise, a fixed-effects model was used.
The Bayesian network-meta analysis (NMA) was performed in a random-effect model using Markov chain Monte Carlo methods [29, 30] in JAGS and the GeMTC package in R (https://drugis.org/software/r-packages/gemtc). For each outcome measure, four independent Markov chains were simultaneously run for 20,000 burn-ins and 100,000 inference iterations per chain to obtain the posterior distribution. The traces plot and Brooks-Gelman-Rubin method were used to assess the convergence of model [31]. Treatment effects were estimated by HR/OR and corresponding 95% CI. Network consistency was assessed with node-split models by statistically testing between direct and indirect estimates within treatment loop [32]. To rank probabilities of all available treatments, the surfaces under the cumulative ranking curve (SUCRAs) were calculated [33]. SUCRA equals one if the treatment is certain to be the best and zero if it’s certain to be the worst [33]. In addition, we conducted subgroup analyses according to histologic type, RT dose, and RT technique. Lastly, comparison-adjusted funnel plot was used to detect the presence of small-study effects or publication bias [34].
Results
Literature search results and characteristics of included studies
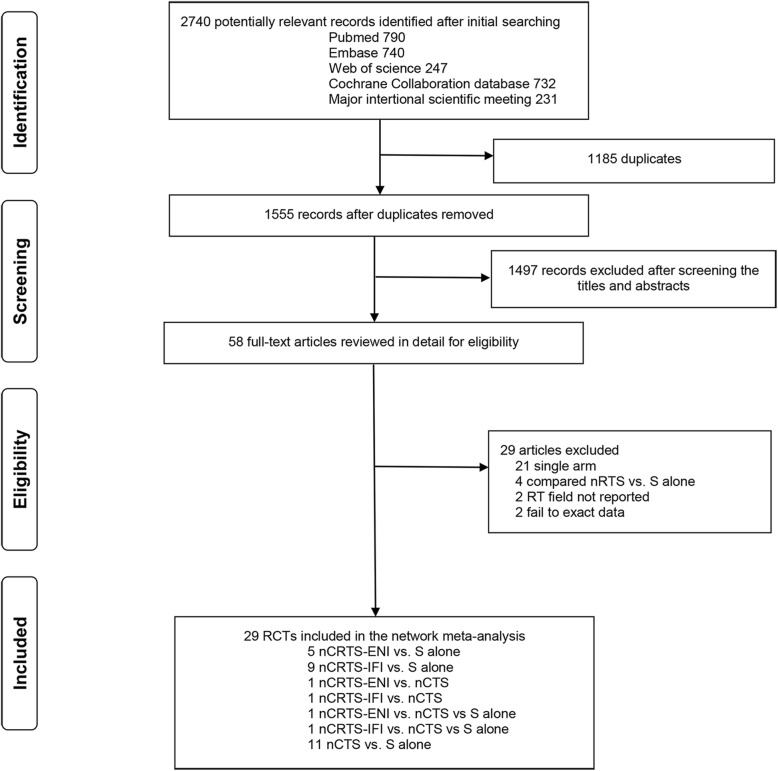
The literature search results and study selection process are shown in Fig. 1. The initial search retrieved 2740 studies. After removing the duplicates, 1555 citations were identified, and 1497 of them were excluded through an abstract review. The remaining 58 studies were screened through a full-text review for further eligibility. Finally, 29 RCTs [5–22, 35–50] with 5212 patients were included in the meta-analysis. Among them, 5 compared nCRTS using ENI (nCRTS-ENI) with S alone [17–21], 9 compared nCRTS using IFI (nCRTS-IFI) with S alone [5–15], 11 compared nCTS with S alone [38–50], 1 compared nCRTS-ENI and nCTS with S alone [22], 1 compared nCRTS-IFI and nCTS with S alone [16], 1 compared nCRTS-ENI with nCTS [35, 36], and 1 compared nCRTS-IFI with nCTS [37]. The study characteristics are shown in Table 1. Details of radiation fields are shown in Additional file 1: Tables S3.
Fig. 1.
Literature search and selection. RCTs, randomized control trials; nCRTS, neoadjuvant chemoradiotherapy plus surgery; nCTS, neoadjuvant chemotherapy plus surgery; nRTS, neoadjuvant radiotherapy plus surgery; S, surgery; RT, radiotherapy; ENI, elective nodal irradiation; IFI, involved-field irradiation
Table 1.
Characteristics of included trials
| Trial | Time Range |
Region | Treatment | Sample size |
Median follow-up |
Median Age |
pStage | Histology | CT | RT | RT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| regimen | dose (Gy) | technique | |||||||||
| NEOCRTEC5010/2018 [5] | 2007–2014 | China | nCRTS-IFI | 224 | 41 m | 56 | I-IV | SCC | NP | 40 | 3D |
| S alone | 227 | 58 | |||||||||
| CROSS/2011 [6, 7] | 2004–2008 | Netherlands | nCRTS-IFI | 178 | 84 m | 60 | I-III | SCC/AC | PC | 41.4 | 3D |
| S alone | 188 | 60 | |||||||||
| Lv/2010 [8] | 1997–2004 | China | nCRTS-IFI | 80 | 45 m | NR | I-III | SCC | PC | 40 | 2D |
| S alone | 80 | ||||||||||
| FFCD9901/2014 [9] | 2000–2009 | France | nCRTS-IFI | 98 | 94 m | 58.1 | I-III | SCC/AC | FP | 45 | 3D |
| S alone | 97 | 57.6 | |||||||||
| IG9401/2005 [10] | 1994–2000 | Australia | nCRTS-IFI | 128 | 65 m | 61 | NR | SCC/AC | FP | 35 | 2D |
| S alone | 128 | 62 | |||||||||
| Urba/2001 [11] | 1985–1987 | America | nCRTS-IFI | 50 | 98 m | 62 | NR | SCC/AC | FP + Vin | 45 | 3D |
| S alone | 50 | 64 | |||||||||
| Bosset/1997 [12] | 1989–1995 | France | nCRTS-IFI | 143 | 55 m | 56.6 | I-III | SCC | Cis | 37 | 3D |
| S alone | 139 | 56.7 | |||||||||
| Walsh/1996 [13, 14] | 1990–1995 | Ireland | nCRTS-IFI | 58 | 10 m | 65 | I-IV | AC | FP | 40 | 2D |
| S alone | 55 | 65 | |||||||||
| Apinop/1994 [15] | 1986–1992 | Thailand | nCRTS-IFI | 35 | NR | 59.6 | NR | SCC | FP | 40 | 2D |
| S alone | 34 | 59.8 | |||||||||
| Cao/2009 [16] | 1991–2000 | China | nCRTS-IFI | 118 | NR | NR | II-IV | SCC | FP | 40 | 2D |
| nCTS | 119 | ||||||||||
| S alone | 118 | ||||||||||
| Yanagi/2018 [17] | 1997–2001 | Japan | nCRTS-ENI | 20 | 90 m | 61.5 | I-IV | SCC | FP | 40 | NR |
| S alone | 21 | 60 | |||||||||
| CALGB9781/2008 [18] | 1997–2000 | America | nCRTS-ENI | 30 | 72 m | 59.9 | NR | SCC/AC | FP | 50.4 | 3D |
| S alone | 26 | 62.2 | |||||||||
| Natsugoe/2006 [19] | 1997–2001 | Japan | nCRTS-ENI | 22 | 24 m | NR | II-IV | SCC | FP | 40 | NR |
| S alone | 23 | ||||||||||
| Lee/2004 [20] | 1999–2002 | Korea | nCRTS-ENI | 51 | 25 m | 63 | I-IV | SCC | FP | 45.6 | 2D |
| S alone | 50 | 63 | |||||||||
| Le Prise/1994 [21] | 1988–1991 | France | nCRTS-ENI | 41 | 16 m | 56 | NR | SCC | FP | 20 | 2D |
| S alone | 45 | 59 | |||||||||
| Nygaard/1992 [22] | 1983–1988 | Norway | nCRTS-ENI | 53 | NR | 60.1 | NR | SCC | Cis + Ble | 35 | 2D |
| nCTS | 56 | 62.9 | |||||||||
| S alone | 50 | 61.4 | |||||||||
| Stahl/2009 [35, 36] | 2000–2005 | Germany | nCRTS-ENI | 60 | 126 m | 60.6 | I-IV | AC | PLF | 30 | 3D |
| nCTS | 59 | 56 | |||||||||
| Burmeister/2011 [37] | 2000–2006 | Australia | nCRTS-IFI | 39 | 94 m | 60 | I-III | AC | FP | 35 | 3D |
| nCTS | 36 | 63 | |||||||||
| Boonstra/2011 [38] | 1989–1996 | Netherlands | nCTS | 85 | 15 m | 60 | I-IV | SCC | EP | ||
| S alone | 84 | 14 m | 60 | ||||||||
| Ychou/2011 [39] | 1995–2003 | Multicenter | nCTS | 84 | NR | NR | NR | AC | FP | ||
| S alone | 85 | ||||||||||
| OEO2/2002 [40, 41] | 1992–1998 | UK | nCTS | 400 | 73 m | 63 | NR | SCC/AC | FP | ||
| S alone | 402 | 63 | |||||||||
| MAGIC/2006 [42] | 1994–2002 | Multicenter | nCTS | 65 | NR | NR | NR | AC | ECF | ||
| S alone | 66 | ||||||||||
| RTOG8911/2007 [43, 44] | 1990–1995 | Multicenter | nCTS | 233 | NR | 61 | NR | SCC/AC | FP | ||
| S alone | 234 | 62 | |||||||||
| Ancona/2001 [45] | 1992–1997 | Italy | nCTS | 47 | NR | 58 | NR | NR | FP | ||
| S alone | 47 | 58 | |||||||||
| Baba/2000 [46] | 1993–1995 | Japan | nCTS | 21 | NR | 63.6 | I-IV | SCC | PLF | ||
| S alone | 21 | 60.1 | |||||||||
| Law/1997 [47] | 1989–1995 | China | nCTS | 74 | 17 m | 64 | I-III | SCC | FP | ||
| S alone | 73 | 63 | |||||||||
| Schlag/1992 [48] | NR | Germany | nCTS | 35 | 8 m | NR | NR | SCC | FP | ||
| S alone | 42 | ||||||||||
| Maipang/1994 [49] | 1988–1990 | Thailand | nCTS | 24 | NR | 64.2 | NR | SCC | Cis + Ble | ||
| S alone | 22 | 64.8 | |||||||||
| Roth/1988 [50] | 1982–1986 | America | nCTS | 19 | 30 m | NR | NR | NR | NP + Ble | ||
| S alone | 20 |
Abbreviations: m Months, UK United Kingdom, nCRTS Neoadjuvant chemoradiotherapy plus surgery, nCTS Neoadjuvant chemotherapy plus surgery, S Surgery, CT Chemotherapy, RT Radiotherapy, ENI Elective nodal irradiation, IFI Involved-field irradiation, Cis Cisplatin, Vin Vinblastine, FP Fluorouracil/cis, PC Paclitaxel/cis, NP Vinorelbine/cis, PLF Fluorouracil/leucovorin/cis, Ble Bleomycin, ECF Epirubicin/cisplatin/fluorouracil, SCC Squamous cell carcinoma, AC Adenocarcinoma, 2D Two-dimensional RT, 3D Three-dimensional RT, NR Not reported
Assessment of included trial
The risk of bias in included RCTs was summarized in Additional file 1: Figure S1. Seven trials [13–16, 21, 22, 48, 49] were judged to be unclear risk of bias, as they had more than three domains indicating as unclear risk. The remaining trials were rated with a low risk of bias. Funnel plot analysis in term of OS did not indicate any evident risk of publication bias (Additional file 1: Figure S2).
Conventional pairwise meta-analysis
Results of direct comparison meta-analysis are shown in Table 2. nCRTS-ENI (HR = 0.70, 95% CI: 0.54–0.92, I2 = 8%), nCRTS-IFI (HR = 0.74, 95% CI: 0.66–0.83, I2 = 10%), and nCTS (HR = 0.86, 95% CI: 0.76–0.98, I2 = 40%) showed significant OS advantage over S alone. Compared to S alone, nCRTS-IFI and nCTS showed a significant decrease in LR (OR = 0.43, 95% CI: 0.33–0.57, I2 = 0% and OR = 0.79, 95% CI: 0.62–0.99, I2 = 26%), and a trend of decrease in DM (OR = 0.79, 95% CI: 0.62–1.00, I2 = 0% and OR = 0.83, 95% CI: 0.68–1.01, I2 = 37%). nCRTS-ENI (OR = 5.75, 95% CI: 2.19–15.13, I2 = 0%), nCRTS-IFI (OR = 5.17, 95% CI: 1.95–13.67, I2 = 68%), and nCTS (OR = 1.71, 95% CI: 1.39–2.10, I2 = 0%) significantly increased R0 resection compared to S alone. nCRTS-ENI also increased R0 resection than nCTS (OR = 4.71, 95% CI: 1.98–11.24, I2 = 0%). nCRTS-IFI resulted in a significantly higher POM than S alone (OR = 1.79, 95% CI: 1.14–2.82, I2 = 27%).
Table 2.
Results of direct comparsions
| Outcome | Treatment | No. of studies |
No. of patients |
HR/OR(95%CI) | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2(%) | P | |||||
| OS | nCRTS-ENI vs S alone | 6 | 432 | HR 0.70(0.54–0.92) | 8 | 0.37 |
| nCRTS-IFI vs S alone | 10 | 2228 | HR 0.74(0.66–0.83) | 10 | 0.35 | |
| nCTS vs S alone | 13 | 2526 | HR 0.86(0.76–0.98) | 40 | 0.06 | |
| LR | nCRTS-ENI vs S alone | 4 | 288 | OR 0.69(0.35–1.35) | 46 | 0.13 |
| nCRTS-IFI vs S alone | 6 | 1221 | OR 0.43(0.33–0.57) | 0 | 0.50 | |
| nCTS vs S alone | 7 | 2176 | OR 0.79(0.62–0.99) | 26 | 0.23 | |
| DM | nCRTS-ENI vs S alone | 4 | 288 | OR 0.87(0.35–2.21) | 57 | 0.07 |
| nCRTS-IFI vs S alone | 6 | 1221 | OR 0.79(0.62–1.00) | 0 | 0.43 | |
| nCTS vs S alone | 7 | 2176 | OR 0.83(0.68–1.01) | 37 | 0.15 | |
| R0 resection | nCRTS-ENI vs S alone | 2 | 155 | OR 5.75(2.19–15.13) | 0 | 0.61 |
| nCRTS-IFI vs S alone | 4 | 1119 | OR 5.17(1.95–13.67) | 68 | 0.02 | |
| nCTS vs S alone | 7 | 1705 | OR 1.71(1.39–2.10) | 0 | 0.75 | |
| nCRTS-ENI vs nCT | 2 | 166 | OR 4.71(1.98–11.24) | 0 | 0.85 | |
| POM | nCRTS-ENI vs S alone | 5 | 324 | OR 1.52(0.66–3.52) | 0 | 0.85 |
| nCRTS-IFI vs S alone | 8 | 1704 | OR 1.79(1.14–2.82) | 27 | 0.21 | |
| nCTS vs S alone | 11 | 2453 | OR 1.02(0.75–1.38) | 0 | 0.87 | |
Abbreviations: No. Number, HR Hazard ratio, CI Confidence interval, OR Odds ratio, OS Overall survival, LR Locoregional recurrence, DM Distant metastases, POM Post-operative mortality, nCRTS Neoadjuvant chemoradiotherapy plus surgery, nCTS Neoadjuvant chemotherapy plus surgery, S Surgery, ENI Elective nodal irradiation, IFI Involved-field irradiation
Significant results are in bold
Network meta-analysis
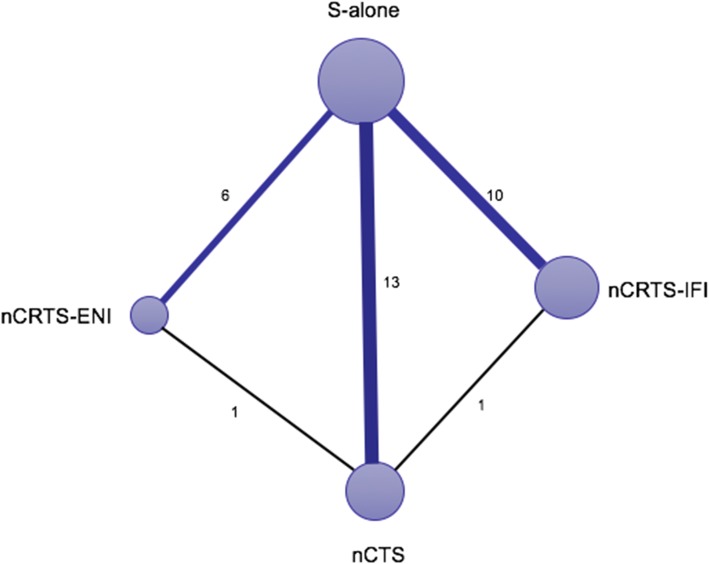
Figure 2 shows the network plot established for NMA for OS. Results of the NMA are presented in Table 3a. nCRTS-ENI (HR = 0.63, 95% CI: 0.48–0.83, P = 0.001), nCRTS-IFI (HR = 0.75, 95% CI: 0.66–0.86, P < 0.001), and nCTS (HR = 0.87, 95% CI: 0.77–0.97, P = 0.012) significantly improved OS compared to S alone; nCRTS-ENI also showed a significant OS advantage over nCTS (HR = 0.73, 95% CI: 0.55–0.97, P = 0.03). nCRTS-IFI significantly decreased LR compared to nCTS (OR = 0.59, 95% CI: 0.37–0.94, P = 0.03) and S alone (OR = 0.43, 95% CI: 0.30–0.60, P < 0.001). S alone and nCTS showed a lower R0 resection than nCRTS-ENI (OR = 0.16, 95% CI: 0.07–0.34, P < 0.001 and OR = 0.29, 95% CI: 0.13–0.59, P < 0.001) and nCRTS-IFI (OR = 0.16, 95% CI: 0.09–0.28, P < 0.001 and OR = 0.28, 95% CI: 0.14–0.53, P < 0.001). S alone had a lower POM than nCRTS-IFI (OR = 0.56, 95% CI: 0.33–0.92, P = 0.02). No significant difference in OS, LR, DM, R0 resection, and POM were observed between nCRTS-ENI and nCRTS-IFI.
Fig. 2.
Network of eligible comparisons for the Bayesian network meta-analysis. The size of the nodes is proportional to the number of patients (in parentheses) randomized to receive the treatment. The width of the lines is proportional to the number of trials (beside the line) comparing the connected treatments. nCRTS, neoadjuvant chemoradiotherapy plus surgery; nCTS, neoadjuvant chemotherapy plus surgery; S, surgery; ENI, elective nodal irradiation; IFI, involved-field irradiation
Table 3.
Network meta-analysis results
| a. Network meta-analysis results for five outcomes | |||||
| OS | |||||
| nCRTS-ENI | |||||
| 0.84(0.62–1.1) | nCRTS-IFI | ||||
| 0.73(0.55–0.97) | 0.87(0.73–1.0) | nCTS | |||
| 0.63(0.48–0.83) | 0.75(0.66–0.86) | 0.87(0.77–0.97) | S-alone | ||
| LR | |||||
| nCRTS-IFI | |||||
| 0.74(0.37–1.5) | nCRTS-ENI | ||||
| 0.59(0.37–0.94) | 0.61(0.30–1.3) | nCTS | |||
| 0.43(0.30–0.60) | 0.58(0.31–1.1) | 0.79(0.59–1.1) | S-alone | ||
| DM | |||||
| nCRTS-IFI | |||||
| 1.0(0.54–1.9) | nCRTS-ENI | ||||
| 0.92(0.60–1.4) | 0.90(0.50–1.6) | nCTS | |||
| 0.79(0.57–1.1) | 0.76(0.44–1.3) | 0.85(0.64–1.2) | S-alone | ||
| POM | |||||
| S-alone | |||||
| 0.99(0.68–1.4) | nCTS | ||||
| 0.56(0.33–0.92) | 0.56(0.30–1.0) | nCRTS-IFI | |||
| 0.56(0.27–1.1) | 0.56(0.27–1.2) | 1.0(0.41–2.4) | nCRTS-ENI | ||
| R0 resection | |||||
| S-alone | |||||
| 0.57(0.40–0.80) | nCTS | ||||
| 0.16(0.09–0.28) | 0.28(0.14–0.53) | nCRTS-IFI | |||
| 0.16(0.07–0.34) | 0.29(0.13–0.59) | 1.0(0.39–2.6) | nCRTS-ENI | ||
| b. Network meta-analysis results of OS for four subgroups | |||||
| ESCC | |||||
| nCRTS-IFI | |||||
| 0.83(0.47–1.5) | nCRTS-ENI | ||||
| 0.78(0.63–0.96) | 0.80(0.43–1.5) | nCTS | |||
| 0.50(0.38–0.68) | 0.61(0.35–1.0) | 0.76(0.57–1.0) | S-alone | ||
| EAC | |||||
| nCRTS-ENI | |||||
| 0.70(0.37–1.3) | nCRTS-IFI | ||||
| 0.65(0.38–1.1) | 0.93(0.71–1.3) | nCTS | |||
| 0.50(0.28–0.87) | 0.72(0.58–0.91) | 0.78(0.62–0.93) | S-alone | ||
| RT with dose of ≥40Gy/<40Gy | |||||
| nCRTS-ENI ≥ 40Gy | |||||
| 0.90(0.59–1.4) | nCRTS-IFI ≥ 40Gy | ||||
| 0.89(0.54–1.5) | 0.99(0.70–1.4) | nCRTS-ENI < 40Gy | |||
| 0.71(0.48–1.1) | 0.79(0.65–0.96) | 0.80(0.58–1.1) | nCTS | ||
| 0.68(0.43–1.1) | 0.76(0.56–1.0) | 0.76(0.51–1.1) | 0.96(0.72–1.3) | nCRTS-IFI < 40Gy | |
| 0.62(0.43–0.92) | 0.70(0.59–0.82) | 0.70(0.51–0.96) | 0.88(0.78–0.99) | 0.92(0.71–1.2) | S-alone |
| RT with technique of 3DRT/2DRT | |||||
| nCRTS-ENI-3DRT | |||||
| 0.74(0.46–1.2) | nCRTS-IFI-2DRT | ||||
| 0.68(0.42–1.1) | 0.92(0.68–1.2) | nCRTS-IFI-3DRT | |||
| 0.58(0.34–0.99) | 0.87(0.57–1.3) | 0.94(0.63–1.4) | nCRTS-ENI-2DRT | ||
| 0.61(0.39–0.94) | 0.83(0.64–1.1) | 0.90(0.72–1.1) | 0.96(0.66–1.4) | nCTS | |
| 0.53(0.34–0.80) | 0.72(0.57–0.88) | 0.78(0.64–0.94) | 0.82(0.58–1.2) | 0.86(0.76–0.98) | S-alone |
Abbreviations: OS Overall survival, LR Locoregional recurrence, DM Distant metastases, POM Post-operative mortality, nCRTS Neoadjuvant chemoradiotherapy plus surgery, nCTS Neoadjuvant chemotherapy plus surgery, S Surgery, RT Radiotherapy, ENI Elective nodal irradiation, IFI Involved-field irradiation, ESCC Esophagus squamous cell carcinoma, EAC Esophagus adenocarcinoma, 2D Two-dimensional, 3D Three-dimensional
Significant results are in bold
Inconsistency assessment and treatment ranking
There were two independent closed loops in the network for OS, LR, DM, and R0 resection: nCRTS-ENI/nCTS/S alone and nCRTS-IFI/nCTS/S alone; one independent closed loop for POM: nCRTS-ENI/nCTS/S alone. Analysis of inconsistency showed that the NMA results were similar to the PWMA results for the five outcomes, which suggested the consistency between the direct and indirect evidence (Additional file 1: Figure S3).
Results of the treatment rankings based on SUCRA are shown in Table 4a. In term of OS, nCRTS-ENI (0.93) was ranked the most effective treatment in term of OS, followed by nCRTS-IFI (0.71). nCRTS-IFI (0.95) was ranked the most effective treatment in term of LR, followed by nCRTS-ENI (0.62). With regard to DM, POM, and R0 resection, SUCRA values were similar between nCRTS-ENI and nCRTS-IFI.
Table 4.
SUCRA values
| a. SUCRA values for five outcomes | |||||||||
| OS | LR | DM | POM | R0 resection | |||||
| Treatment | SUCRA | Treatment | SUCRA | Treatment | SUCRA | Treatment | SUCRA | Treatment | SUCRA |
| nCRTS-ENI | 0.93 | nCRTS-IFI | 0.95 | nCRTS-IFI | 0.69 | S alone | 0.83 | S alone | 1.00 |
| nCRTS-IFI | 0.71 | nCRTS-ENI | 0.62 | nCRTS-ENI | 0.67 | nCTS | 0.79 | nCTS | 0.67 |
| nCTS | 0.36 | nCTS | 0.39 | nCTS | 0.53 | nCRTS-IFI | 0.20 | nCRTS-IFI | 0.19 |
| S alone | 0.00 | S alone | 0.04 | S alone | 0.11 | nCRTS-ENI | 0.19 | nCRTS-ENI | 0.15 |
| b. SUCRA values of OS for four subgroups | |||||||||
| ESCC | EAC | RT dose | RT-technique | ||||||
| Treatment | SUCRA | Treatment | SUCRA | Treatment | SUCRA | Treatment | SUCRA | ||
| nCRTS-IFI | 0.90 | nCRTS-ENI | 0.96 | nCRTS-ENI- ≥ 40Gy | 0.86 | nCRTS-ENI-3DRT | 0.98 | ||
| nCRTS-ENI | 0.68 | nCRTS-IFI | 0.63 | nCRTS-IFI- ≥ 40Gy | 0.75 | nCRTS-IFI-3DRT | 0.69 | ||
| nCTS | 0.34 | nCTS | 0.41 | nCRTS-ENI- < 40Gy | 0.73 | nCRTS-IFI-2DRT | 0.54 | ||
| S alone | 0.08 | S alone | 0.00 | nCTS | 0.35 | nCRTS-ENI-2DRT | 0.42 | ||
| nCRTS-IFI- < 40Gy | 0.25 | nCTS | 0.34 | ||||||
| S alone | 0.05 | S alone | 0.03 | ||||||
Abbreviations: SUCRA Surface under the cumulative ranking curve, OS Overall survival, LR Locoregional recurrence, DM Distant metastases, POM Post-operative mortality, nCRTS Neoadjuvant chemoradiotherapy plus surgery, nCTS Neoadjuvant chemotherapy plus surgery, S Surgery, RT Radiotherapy, ENI Elective nodal irradiation, IFI Involved-field irradiation, ESCC Esophagus squamous cell carcinoma, EAC Esophagus adenocarcinoma, 2D Two-dimensional, 3D Three-dimensional
Subgroup analyses
NMA results of subgroup analyses are shown in Table 3b (SUCRA values are shown in Table 4b). Subgroup analyses for esophagus squamous cell carcinoma (ESCC) and esophagus adenocarcinoma (EAC) were conducted in 23 trials with 3164 patients and 11 trials with 1997 patients, respectively. With regard to ESCC, nCRTS-IFI showed significant OS advantage over S alone and a trend OS advantage over nCTS, and was ranked the most effective treatment (0.90); nCRTS-ENI had a trend OS benefit over S alone. As for EAC, both nCRTS-ENI and nCRTS-IFI significantly improved OS compared to S alone, and nCRTS-ENI was ranked the best treatment (0.96).
In subgroup analysis according to RT dose (18 trials with 2860 patients), nCRTS-IFI with dose of ≥40Gy significantly improved OS compared to S alone, while nCRTS-IFI with dose of <40Gy did not; both nCRTS-ENI with dose of ≥40Gy and < 40Gy showed a significant OS advantage over S alone; and nCRTS-ENI with dose of ≥40Gy was ranked the most effective regimen (0.86).
In subgroup analysis according to RT technique (16 trials with 2774 patients), nCRTS-ENI adopting three-dimensional radiotherapy (3D-RT) significantly improved OS compared to nCRTS-ENI adopting 2D-RT, nCTS, and S alone, and was ranked the most effective regimen (0.99); nCRTS-IFI was more effective than S alone regardless RT technique adopted.
Discussion
Currently, nCRTS has been the most common treatment approach for patients with resectable EC, but the optimal radiation field remains unidentified. EC is characterized as an aggressive disease, and lymph node metastasis, particularly regional lymph node involvement, usually occurs early. Taking into consideration microscopic spread, some trials adopted ENI instead of IFI for patients receiving nCRTS. In CALGB 9781 trials [18], nCRTS adopting ENI followed by surgery showed a long-term survival advantage over S alone for patients with EC. Nevertheless, there are also trials of a series of cases treated with IFI. Recently, two large phase III trials [5–7] also showed that nCRTS improved survival over surgery alone among patients with esophageal or junctional cancer, while IFI was adopted in RT. To date, there are still no trials that have compared efficacy of the two radiation fields directly in EC patients receiving nCRTS, and which is more effective remains unclear.
To our knowledge, this is the first network meta-analysis assessing the comparative efficacy and safety of nCRTS-ENI and nCRTS-IFI for patients with EC. It showed that both nCRTS-ENI and nCRTS-IFI significantly improved OS compared to S alone. nCRTS-ENI also showed significant OS advantage over nCTS. No significant difference in OS, LR, DM, and POM was observed between nCRTS-ENI and nCRTS-IFI. Based on treatment ranking in term of OS, nCRTS-ENI had the highest probability of being the most effective treatment (93%), followed by nCRTS-IFI (71%) and nCTS (36%).
However, in subgroup analysis according to pathologic type, nCRTS-IFI (90%) was ranked the most effective treatment for ESCC, followed by nCRTS-ENI (68%). nCRTS-IFI showed significant and a trend OS advantage over S alone and nCTS, respectively. While nCRTS-ENI only had a trend OS benefit compared to S alone. In the CROSS trial [6, 7], nCRTS-IFI resulted in improved OS for both ESCC and EAC, but the magnitude of this benefit was greater for ESCC patients (HR for ESCC vs. EAC were 0.48 vs. 0.73 respectively). These results suggested that nCRTS-IFI seemed to be more effective than nCRTS-ENI for patients with ESCC. Future head to head comparison trials are needed to confirm this finding and explore the mechanism.
RT dose and technique used in individual trials were various, which might also affect the outcomes. In our NMA, although nCRTS-ENI and nCRTS-IFI with dose of ≥40Gy seemed to be superior to those with dose of <40Gy based on treatment ranking, there were no significant difference in OS between the two dose group. Moreover, common dose in subgroup of ≥40Gy was only 40–41.4Gy. With developments in RT technique, whether a rather higher dose might be more reasonable needs further investigation.
In subgroup analysis of RT technique, we found that nCRTS-ENI adopting 3D-RT had a significant OS benefit compared to nCRTS-ENI adopting 2D-RT. Compared with 2D-RT, 3D-RT delivered a high dose to the tumor target volume while potentially minimizing the dose to the organ at risk. The results suggested that 3D-RT was more important for EC patients receiving nCRTS-ENI.
Treatment-related toxicities between ENI and IFI have been compared for EC patients receiving radical CRT in several retrospective studies. Results of two small meta-analysis [51, 52] showed that the incidences of esophageal and lung toxicities were significantly higher in ENI group. However, most of trials comparing nCRTS with S alone did not reported CRT-related toxicities in detail, and therefore, indirect comparison of CRT-related toxicities between nCRTS-ENI and nCRTS-IFI could not be performed. In our NMA, nCRTS seemed to had a higher POM than S alone, but no significant difference was observed between nCRTS-ENI and nCRTS-IFI.
There are several limitations in our meta-analysis. Firstly, in common with other meta-analyses, data were collected and analyzed in aggregate on the basis of results reported from trials, instead of individual patient data. Secondly, different operative techniques and CT regimens were adopted in individual trials, which might lead to heterogeneity. Thirdly, most of the studies included patients with mixed stage and tumor location and could not be extracted separately, subgroup analyses according to stage and tumor location could not be performed. Finally, majority of trials comparing nCRTS with surgery alone did not reported RT related toxicities. Thus, the comparison of RT related toxicities between nCRTS-ENI and nCRTS-IFI could not be performed.
Conclusions
Either adopting ENI or IFI, nCRTS is likely to be the optimal treatment for resectable EC, and nCRTS-IFI and nCRTS-ENI seem to be more effective for patients with ESCC and EAC, respectively. 3D-RT seems to be more important for patients receiving nCRTS-ENI. nCRTS with RT dose of ≥40Gy seems to be superior to that with radiation dose of <40Gy, while the optimal dose remains unclear. Future head to head comparison trials are needed to confirm these findings.
Supplementary information
Additional file 1: Figure S1. Assessment of risk of bias. A: Methodological quality graph: authors’ judgment about each methodological quality item presented as percentages across all included studies; B: Methodological quality summary: authors’ judgment about each methodological quality item for each included study, “+” low risk of bias; “?” unclear risk of bias; “-” high risk of bias. Figure S2. Comparison-adjusted funnel plots of publication bias test for overall survival. nCRTS, neoadjuvant chemoradiotherapy plus surgery; nCTS, neoadjuvant chemotherapy plus surgery; S, surgery; ENI, elective nodal irradiation; IFI, involved-field irradiation. Figure S3. Inconsistency evaluation by node-splitting analyses. (a) overall survival; (b) locoregional recurrence; (c) distant metastases; (d) R0 resection; (e) post-operative mortality. nCRTS, neoadjuvant chemoradiotherapy plus surgery; nCTS, neoadjuvant chemotherapy plus surgery; S, surgery; ENI, elective nodal irradiation; IFI, involved-field irradiation. Table S1. PRISMA NMA Checklist. Table S2. Search strategy. Table S3. Details of radiation fields.
Acknowledgements
None.
Abbreviations
- 3D-RT
Three-dimensional radiotherapy
- Cis
Confidence intervals
- DM
Distant metastases
- EAC
Esophagus adenocarcinoma
- EC
Esophagus cancer
- ENI
Elective nodal irradiation
- ESCC
Esophagus squamous cell carcinoma
- HRs
Hazard ratios
- IFI
Involved-field irradiation
- LR
locoregional recurrence
- nCRTS
Neoadjuvant chemoradiotherapy followed by surgery
- nCRTS-ENI
nCRTs using ENI
- nCRTS-IFI
nCRTS using IFI
- nCTS
Neoadjuvant chemotherapy plus surgery
- NMA
Network-meta analysis
- ORs
Odds ratios
- OS
Overall survival
- POM
Postoperative mortality
- PWMA
Pairwise meta-analysis
- RCTs
Randomized control trials
- S alone
Surgery alone
- SUCRA
Surfaces under the cumulative ranking curve
Authors’ contributions
JD had full access to all the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JD, TL, SD. Acquisition of data: TL, SD, HW, JC. Analysis and interpretation of data: TL, SD, HW, JC. Drafting of the manuscript: TL, SD, GL. Critical revision of the manuscript for important intellectual content: JD. Statistical analysis: TL, SD. All authors read and approved the final manuscript.
Funding
Present study did not receive any funding.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
There was no ethics approval necessary because in this meta-analysis we were pulling numbers from the published manuscripts and pooling results.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tingting Liu and Silu Ding contributed equally to this work.
Contributor Information
Tingting Liu, Email: tingting504@163.com.
Silu Ding, Email: dsllnsy@163.com.
Jun Dang, Email: dangjunsy@163.com.
Hui Wang, Email: cindydyt@163.com.
Jun Chen, Email: cjsyxkyy@163.com.
Guang Li, Email: gl1963516@yahoo.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13014-019-1388-8.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Kumagai K, Rouvelas I, Tsai JA, Mariosa D, Klevebro F, Lindblad M, et al. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg. 2014;101:321–338. doi: 10.1002/bjs.9418. [DOI] [PubMed] [Google Scholar]
- 3.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 4.Hsu PK, Wu YC, Chou TY, Huang CS, Hsu WH. Comparison of the 6th and 7th editions of the American joint committee on Cancer tumor-node-metastasis staging system in patients with resected esophageal carcinoma. Ann Thorac Surg. 2010;89:1024–1031. doi: 10.1016/j.athoracsur.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, AME Thoracic Surgery Collaborative Group et al. Neoadjuvant Chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical Trial. J Clin Oncol. 2018;36:2796–2803. doi: 10.1200/JCO.2018.79.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, CROSS Group et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, CROSS study group et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 8.Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol. 2010;16:1649–1654. doi: 10.3748/wjg.v16.i13.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32:2416–2422. doi: 10.1200/JCO.2013.53.6532. [DOI] [PubMed] [Google Scholar]
- 10.Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, Trans-Tasman Radiation Oncology Group. Australasian Gastro-Intestinal Trials Group et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 11.Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 12.Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161–167. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 13.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 14.Walsh TN, Grennell M, Mansoor S, Kelly A. Neoadjuvant treatment of advanced stage esophageal adenocarcinoma increases survival. Dis Esophagus. 2002;15:121–124. doi: 10.1046/j.1442-2050.2002.00214.x. [DOI] [PubMed] [Google Scholar]
- 15.Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology. 1994;41:391–393. [PubMed] [Google Scholar]
- 16.Cao XF, He XT, Ji L, Xiao J, Lv J. Effects of neoadjuvant radiochemotherapy on pathological staging and prognosis for locally advanced esophageal squamous cell carcinoma. Dis Esophagus. 2009;22:477–481. doi: 10.1111/j.1442-2050.2008.00910.x. [DOI] [PubMed] [Google Scholar]
- 17.Yanagi M, Sasaki K, Uchikado Y, Omoto I, Arigami T, Kurahara H, et al. Effect of neoadjuvant Chemoradiotherapy on lymph node micrometastases in thoracic esophageal Cancer. Anticancer Res. 2018;38:893–900. doi: 10.21873/anticanres.12299. [DOI] [PubMed] [Google Scholar]
- 18.Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Setoyama T, Yokomakura N, et al. Randomized controlled study on preoperative chemoradiotherapy followed by surgery versus surgery alone for esophageal squamous cell cancer in a single institution. Dis Esophagus. 2006;19:468–472. doi: 10.1111/j.1442-2050.2006.00615.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee JL, Park SI, Kim SB, Jung HY, Lee GH, Kim JH, et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol. 2004;15:947–954. doi: 10.1093/annonc/mdh219. [DOI] [PubMed] [Google Scholar]
- 21.Le Prise E, Etienne PL, Meunier B, Maddern G, Ben Hassel M, Gedouin D, et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer. 1994;73:1779–1784. doi: 10.1002/1097-0142(19940401)73:7<1779::AID-CNCR2820730702>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.Nygaard K, Hagen S, Hansen HS, Hatlevoll R, Hultborn R, Jakobsen A, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16:1104–1110. doi: 10.1007/BF02067069. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Zhu S, Li S, Deng W. Elective nodal irradiation provides a superior therapeutic modality for lymph node positivity esophageal squamous cell carcinoma patients receiving definitive radiotherapy versus involved-field irradiation. Medicine (Baltimore) 2019;98:e14080. doi: 10.1097/MD.0000000000014080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita H, Takenaka R, Omori M, Imae T, Okuma K, Ohtomo K, et al. Involved-field radiotherapy (IFRT) versus elective nodal irradiation (ENI) in combination with concurrent chemotherapy for 239 esophageal cancers: a single institutional retrospective study. Radiat Oncol. 2015;10:171. doi: 10.1186/s13014-015-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jing W, Zhu H, Guo H, Zhang Y, Shi F, Han A, et al. Feasibility of elective nodal irradiation (ENI) and involved field irradiation (IFI) in radiotherapy for the elderly patients (aged ≥ 70 years) with esophageal squamous cell Cancer: a retrospective analysis from a single institute. PLoS One. 2015;10:e0143007. doi: 10.1371/journal.pone.0143007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu M, Zhao K, Chen Y, Jiang GL. Evaluation of the value of ENI in radiotherapy for cervical and thoracic esophageal cancer: a retrospective analysis. Radiat Oncol. 2014;9:232. doi: 10.1186/s13014-014-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomized trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelman A, Rubin D. Inference from iterative simulation using multiplesequences. Stat Sci. 1992;7:457–511. doi: 10.1214/ss/1177011136. [DOI] [Google Scholar]
- 30.Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages. PLoS One. 2014;9:e115065. doi: 10.1371/journal.pone.0115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks S, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–455. [Google Scholar]
- 32.van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7:80–93. doi: 10.1002/jrsm.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–856. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 36.Stahl M, Walz MK, Riera-Knorrenschild J, Stuschke M, Sandermann A, Bitzer M, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer. 2017;81:183–190. doi: 10.1016/j.ejca.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 37.Burmeister BH, Thomas JM, Burmeister EA, Walpole ET, Harvey JA, Thomson DB, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer. 2011;47:354–360. doi: 10.1016/j.ejca.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Boonstra JJ, Kok TC, Wijnhoven BP, van Heijl M, van Berge Henegouwen MI, Ten Kate FJ, et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomized controlled trial. BMC Cancer. 2011;11:181. doi: 10.1186/1471-2407-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 40.Medical Research Council Oesophageal Cancer Working Group Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 41.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 42.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, MAGIC Trial Participants et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 43.Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–1984. doi: 10.1056/NEJM199812313392704. [DOI] [PubMed] [Google Scholar]
- 44.Kelsen DP, Winter KA, Gunderson LL, Mortimer J, Estes NC, Haller DG, Radiation Therapy Oncology Group. USA Intergroup et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25:3719–3725. doi: 10.1200/JCO.2006.10.4760. [DOI] [PubMed] [Google Scholar]
- 45.Ancona E, Ruol A, Santi S, Merigliano S, Sileni VC, Koussis H, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91:2165–2174. doi: 10.1002/1097-0142(20010601)91:11<2165::AID-CNCR1245>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 46.Baba M, Natsugoe S, Shimada M, Nakano S, Kusano C, Fukumoto T, et al. Prospective evaluation of preoperative chemotherapy in resectable squamous cell carcinoma of the thoracic esophagus. Dis Esophagus. 2000;13:136–141. doi: 10.1046/j.1442-2050.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- 47.Law S, Fok M, Chow S, Chu KM, Wong J. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. J Thorac Cardiovasc Surg. 1997;114:210–217. doi: 10.1016/S0022-5223(97)70147-8. [DOI] [PubMed] [Google Scholar]
- 48.Schlag PM. Randomized trial of preoperative chemotherapy for squamous cell cancer of the esophagus. The Chirurgische Arbeitsgemeinschaft Fuer Onkologie der Deutschen Gesellschaft Fuer Chirurgie study group. Arch Surg. 1992;127:1446–1450. doi: 10.1001/archsurg.1992.01420120080015. [DOI] [PubMed] [Google Scholar]
- 49.Maipang T, Vasinanukorn P, Petpichetchian C, Chamroonkul S, Geater A, Chansawwaang S, et al. Induction chemotherapy in the treatment of patients with carcinoma of the esophagus. J Surg Oncol. 1994;56:191–197. doi: 10.1002/jso.2930560314. [DOI] [PubMed] [Google Scholar]
- 50.Roth JA, Pass HI, Flanagan MM, Graeber GM, Rosenberg JC, Steinberg S. Randomized clinical trial of preoperative and postoperative adjuvant chemotherapy with cisplatin, vindesine, and bleomycin for carcinoma of the esophagus. J Thorac Cardiovasc Surg. 1988;96:242–248. [PubMed] [Google Scholar]
- 51.Cheng YJ, Jing SW, Zhu LL, Wang J, Wang L, Liu Q, et al. Comparison of elective nodal irradiation and involved-field irradiation in esophageal squamous cell carcinoma: a meta-analysis. J Radiat Res. 2018;59:604–615. doi: 10.1093/jrr/rry055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Miao C, Chen Z, Li W, Yuan S, Yu J, et al. Can involved-feld irradiation replace elective nodal irradiation in chemoradiotherapy for esophageal cancer? A systematic review and meta-analysis. Onco Targets Ther. 2017;10:2087–2095. doi: 10.2147/OTT.S130285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Assessment of risk of bias. A: Methodological quality graph: authors’ judgment about each methodological quality item presented as percentages across all included studies; B: Methodological quality summary: authors’ judgment about each methodological quality item for each included study, “+” low risk of bias; “?” unclear risk of bias; “-” high risk of bias. Figure S2. Comparison-adjusted funnel plots of publication bias test for overall survival. nCRTS, neoadjuvant chemoradiotherapy plus surgery; nCTS, neoadjuvant chemotherapy plus surgery; S, surgery; ENI, elective nodal irradiation; IFI, involved-field irradiation. Figure S3. Inconsistency evaluation by node-splitting analyses. (a) overall survival; (b) locoregional recurrence; (c) distant metastases; (d) R0 resection; (e) post-operative mortality. nCRTS, neoadjuvant chemoradiotherapy plus surgery; nCTS, neoadjuvant chemotherapy plus surgery; S, surgery; ENI, elective nodal irradiation; IFI, involved-field irradiation. Table S1. PRISMA NMA Checklist. Table S2. Search strategy. Table S3. Details of radiation fields.
Data Availability Statement
Not applicable.