Abstract
Background
According to the 2018 NIA-AA research framework, Alzheimer’s disease (AD) is not defined by the clinical consequences of the disease, but by its underlying pathology, measured by biomarkers. Evidence of both amyloid-β (Aβ) and phosphorylated tau protein (p-tau) deposition—assessed interchangeably with amyloid-positron emission tomography (PET) and/or cerebrospinal fluid (CSF) analysis—is needed to diagnose AD in a living person. Our aim was to test the new NIA-AA research framework in a large cohort of cognitively impaired patients to evaluate correspondence between the clinical syndromes and the underlying pathologic process testified by biomarkers.
Methods
We retrospectively analysed 628 subjects referred to our centre in suspicion of dementia, who underwent CSF analysis, together with neuropsychological assessment and neuroimaging, and were diagnosed with different neurodegenerative dementias according to current criteria, or as cognitively unimpaired. Subjects were classified considering CSF biomarkers, and the prevalence of normal, AD-continuum and non-AD profiles in each clinical syndrome was calculated. The positivity threshold of each CSF biomarker was first assessed by receiver operating characteristic analysis, using Aβ-positive/negative status as determined by amyloid-PET visual reads. The agreement between CSF and amyloid-PET data was also evaluated.
Results
Among patients with a clinical diagnosis of AD, 94.1% were in the AD-continuum, whereas 5.5% were classified as non-AD and 0.4% were normal. The AD-continuum profile was found also in 26.2% of frontotemporal dementia, 48.6% of Lewy body dementia, 25% of atypical parkinsonism and 44.7% of vascular dementia. Biomarkers’ profile did not differ in amnestic and not amnestic mild cognitive impairment. CSF Aβ levels and amyloid-PET tracer binding negatively correlated, and the concordance between the two Aβ biomarkers was 89%.
Conclusions
The examination of the 2018 NIA-AA research framework in our clinical setting revealed a good, but incomplete, correspondence between the clinical syndromes and the underlying pathologic process measured by CSF biomarkers. The AD-continuum profile resulted to be a sensitive, but non-specific biomarker with regard to the clinical AD diagnosis. CSF and PET Aβ biomarkers were found to be not perfectly interchangeable to quantify the Aβ burden, possibly because they measure different aspects of AD pathology.
Keywords: Alzheimer’s disease, Dementia, CSF, Clinical neurology, Biomarkers, PET
Introduction
The diagnosis of probable Alzheimer’s disease (AD) requires core clinical criteria [1]. Cerebrospinal fluid (CSF) and neuroimaging biomarkers enhance the specificity of the criteria in clinical and research settings [1]. In 2018, the National Institute of Age-Alzheimer’s Association shifted the definition of AD to a biological construct [2]. According to the new research framework (2018-NIA-AA-RF), AD is not defined by its clinical consequences, but by its underlying pathology as measured during lifetime by biomarkers [2]. The amyloid/tau/neurodegeneration (AT(N)) classification is used to divide biomarkers into those measuring β-amyloid (Aβ) deposition (A) [CSF Aβ levels or Aβ-positron emission tomography (PET)], pathologic phosphorylated tau (T) [CSF phospho-tau (p-tau) levels or tau-PET], and neurodegeneration (N) [18F-fluorodeoxyglucose-PET (FDG-PET), magnetic resonance imaging (MRI), or CSF total tau (t-tau) levels] [3]. Regardless of the presence of clinical symptoms, both Aβ and p-tau pathology are required for classification as AD, whereas Aβ deposition alone is an early sign, labelled AD pathologic change [2]. CSF and PET biomarkers are considered interchangeable in demonstrating AD pathology, assuming that they provide the same information [2]. Nevertheless, while Aβ-PET tracers bind to Aβ fibrils and give both quantitative and qualitative data about the topology of Aβ deposition, CSF biomarkers do not provide any topological information. Moreover, Aβ-PET can be easily interpreted as positive or negative by visual inspection, whereas general cut-offs for CSF biomarkers are lacking and the existing ones show high variability among centres [4–6]. Standardisation of measurements and protocols are ongoing [7].
The 2018-NIA-AA-RF retains also a staging for clinical severity, ranging from cognitively unimpaired (CU) to mild cognitive impairment (MCI) and dementia [2]. However, the criteria are currently supposed to be used in research setting only, and their validity needs to be examined before adopted into clinical practice [2].
The aim of this work was to test the 2018-NIA-AA-RF in the clinical scenario of a large cohort of cognitively impaired patients to evaluate possible correspondence between the clinical syndromes and the underlying pathologic process testified by biomarkers. Due to the availability of our centre, we used only CSF data to classify subjects accordingly to the 2018-NIA-AA-RF. CSF biomarker positivity thresholds for subject dichotomization were first assessed in a subgroup of subjects who underwent both CSF analysis and 18F-florbetapir-PET (amyloid-PET).
Methods
Design of the study
We tested the 2018-NIA-AA-RF by retrospectively analysing all subjects who underwent lumbar puncture (LP) for diagnostic purpose in the AD Unit of the Ospedale Maggiore Policlinico, University of Milan, Italy, from June 2011 to December 2017. Participants were referred to our centre in suspicion of dementia, and they all received—in addition to LP—a complete neurological examination, neuropsychological assessment and neuroimaging (brain MRI and/or FDG-PET). All the exams were performed within a 365-day interval from subjects’ first visit. After the diagnostic work-up, subjects were diagnosed by expert neurologists with MCI, or dementia, according to the specific criteria of each syndrome [1, 8–17]. A few individuals were instead CU. Regarding AD, from 2011 to 2014, clinical diagnoses were supported by CSF (Aβ1-42, p-tau and t-tau) and/or neuroimaging (brain MRI and/or FDG-PET) biomarkers, according to previous criteria [1, 9]. After the publication of the IWG-2 criteria in 2014 [10], CSF analysis or amyloid PET were used to support the diagnosis of probable AD in our clinical setting. Conversely, in line with their current criteria [11–17], all the other dementia syndromes were diagnosed combining clinical, neuropsychological and neuroimaging profiles, and CSF biomarkers were used for excluding AD.
According to 2018-NIA-AA-RF, CSF biomarkers were used to classify subjects into three binary categories: A+/−, T+/− and N+/−, respectively. Participants had three possible biomarker profiles and eight combinations: (1) normal [A−T− (N−)]; (2) AD-continuum ([A+T−(N−)], [A+T+(N−)], [A+T+(N+)] and [A+T−(N+)]); and (3) non-AD pathologic change (non-AD) ([A−T+(N−)], [A−T−(N+)] and [A−T+(N+)]) [2]. The positivity thresholds for subject dichotomization were first assessed in a subgroup of our cohort who underwent both LP and amyloid-PET within a 365-day interval in our unit for research purpose. In line with previous literature [4, 5, 10, 18, 19], we used Aβ-positive/negative status—as determined by amyloid-PET visual reads—to define our CSF positivity thresholds. We considered amyloid-PET the most suitable surrogate in vivo marker for determining the amyloid burden due to its high correlation with neuropathological results [4, 20–23].
CSF analysis
CSF samples were collected by LP in the L3/L4 or L4/L5 interspace and centrifuged in 8000 rpm for 10 min. The supernatants were aliquoted in polypropylene tubes and stored at − 80 °C until use. CSF Aβ1-42, p-tau and t-tau were measured by using the commercially available sandwich enzyme-linked immunosorbent assay kits (Fujirebio, Ghent, Belgium). We also calculated the ratios of t-tau/Aβ1-42 and p-tau/Aβ1-42.
Amyloid-PET imaging
Amyloid-PET scans were obtained with a Biograph Truepoint 64 PET/CT scanner (Siemens, Erlangen, Germany). All patients underwent PET scanning at rest after intravenous injection of 370 MBq. Amyloid-PET data were first qualitatively analysed by a trained physiologist using a binary method of interpretation for relating “positive” or “negative” scans to neuropathologically defined categories of Aβ plaque density. Structural MRI was also acquired, and FLAIR-weighted images and PET images were co-registered to individual volumetric T1-weighted images. After PET/MRI co-registration, ImcCalc of Statistical Parametric Mapping (SPM12, Wellcome Department of Cognitive Neurology, London, UK) was used to derive standardised uptake value (SUV) PET maps as SUV = AC/(radiotracer dose/BW). AC represents activity concentration in a given voxel [kBq/ml], radiotracer dose is the injected tracer dose corrected for residual activity in the syringe [MBq] and BW is the body weight [kg]. SUV maps were calculated in the grey matter (GM) of anterior cingulate gyrus, frontal lobe, parietal lobe, posterior cingulate gyrus, precuneus, temporal lobe, and of the average of these six regions (GM mean). The whole cerebellum was the reference region for the SUV relative ratio (SUVR). For the GM mean, we applied the validated threshold for amyloid-PET SUVR (1.11) [24, 25].
Statistical analysis
All statistical analyses were conducted with Microsoft-Excel 2011, SPSS 21.0 (SPSS Inc., Chicago, IL, USA), or Graph-Pad-PRISM 6.0 (GraphPad Software, La Jolla, CA). Comparisons between amyloid-PET-positive (aP+) and amyloid-PET-negative (aP−) subjects were performed using non-parametric unpaired t tests (Mann-Whitney U test), except sex difference between aP+ and aP− that was tested by Χ2 test. Using receiver operating characteristic (ROC) analysis, we calculated the area under the curve (AUC) of the CSF analyte and ratio that best distinguished aP+ from aP− subjects. For each parameter, sensitivity was defined as the positivity rate in aP+ subjects, and specificity as the negativity rate in aP− subjects. The value of each CSF analyte or ratio with the highest Youden index (sensitivity + specificity − 1) was selected as the cut-off. The prevalence of normal, AD-continuum and non-AD in each clinical syndrome and in aP+ and aP− subjects was calculated and compared by Χ2 test. Correlations between amyloid-PET SUVR and CSF biomarkers were performed using Spearman coefficient, assuming a non-normal distribution of data. The concordance between amyloid-PET profile and CSF profile was defined as the sum of aP+ with pathologic CSF analyte or ratio levels and aP− with normal CSF analyte or ratio levels, divided by the entire cohort size. For all the analyses, we set the statistical threshold at p < 0.05.
Determination of positivity thresholds for CSF biomarkers
Forty-four subjects underwent amyloid-PET and LP within a 365-day period for research purpose and were considered to identify positivity thresholds for CSF biomarkers. Amyloid-PET was visually assessed as positive in 37 patients (aP+) and negative in 7 (aP−). Accordingly, GM mean SUVR was > 1.11 and ≤ 1.11 in all aP+ and aP− participants, respectively. The AUC for the detection of amyloid-PET positivity was 0.86 (95% CI 0.71–1.00, p = 0.002) for Aβ1-42 and 0.80 (95% CI 0.58–1.03, p = 0.01) for p-tau/Aβ1-42. The following cut-offs that maximised the Youden index for predicting amyloid-PET positivity were selected: Aβ1-42 < 660 pg/ml (sensitivity 0.89, specificity 0.71) and p-tau/Aβ1-42 > 0.09 (sensitivity 0.81, specificity 0.71, Additional file 1). In line with the 2018-NIA-AA-RF [2], Aβ1-42 (cut-off < 660 pg/ml) resulted a better biomarker of β-amyloidosis (A)—as compared with p-tau/Aβ1-42, due to the higher accuracy, sensitivity and specificity in detection of amyloid-PET positivity. Conversely, ROC analyses showed that t-tau and p-tau (and t-tau/Aβ1-42) had an insufficient accuracy in distinguishing aP+ from aP− subjects (AUC 0.52, 0.61, 0.68, respectively; data not significant). Thus, we decided to not calculate cut-offs for these biomarkers, but to use standardly used cut-offs [26] due to the (1) lack of tau-PET, FDG-PET and/or quantitative MRI data and (2) inaccuracy we found in the detection of amyloid-PET positivity of these biomarkers. In conclusion, the following thresholds were applied for patient dichotomization: Aβ1-42 < 660 pg/ml (A), p-tau > 61 pg/ml (T) and t-tau > 500 pg/ml (N). The main characteristics of aP+ and aP− subjects are summarised in Table 1.
Table 1.
Characteristics of subjects who underwent 18F-florbetapir-positron emission tomography (amyloid-PET) and lumbar puncture (LP) within a 365-day interval
| Amyloid-PET positive* | Amyloid-PET negative° | p value | |
|---|---|---|---|
| n | 37 | 7 | |
| Age | 71.4 ± 7.5 | 72.9 ± 3.6 | 0.76 |
| M:F | 13:24 | 3:4 | 0.69 |
| Interval LP/amyloid-PET (days) | 168.6 ± 119.5 | 246.4 ± 115.1 | 0.35 |
| Aβ1-42 (pg/ml) | 542.2 ± 119.2 | 842.3 ± 341.9 | 0.001 |
| t-tau (pg/ml) | 700.5 ± 493.3 | 681.3 ± 593.1 | 0.83 |
| p-tau (pg/ml) | 83.9 ± 38.5 | 67 ± 31.8 | 0.34 |
| t-tau/Aβ1-42 | 1.30 ± 0.9 | 0.90 ± 1.0 | 0.13 |
| p-tau/Aβ1-42 | 0.15 ± 0.07 | 0.09 ± 0.06 | 0.01 |
| Amyloid-PET SUVR GM mean | 1.47 ± 0.21 | 0.98 ± 0.10 | < 0.0001 |
| Amyloid-PET SUVR Ant Cing | 1.49 ± 025 | 0.99 ± 0.16 | < 0.0001 |
| Amyloid-PET SUVR frontal | 1.36 ± 0.25 | 0.89 ± 0.19 | < 0.0001 |
| Amyloid-PET SUVR parietal | 1.28 ± 0.19 | 1.04 ± 0.15 | 0.005 |
| Amyloid-PET SUVR post Cing | 1.55 ± 0.22 | 0.97 ± 0.20 | < 0.0001 |
| Amyloid-PET SUVR precuneus | 1.61 ± 0.27 | 1.02 ± 0.22 | < 0.0001 |
| Amyloid-PET SUVR temporal | 1.50 ± 0.19 | 1.04 ± 0.15 | < 0.0001 |
Data are expressed as mean ± SD, unless otherwise specified. p values by unpaired t test. *Among amyloid-PET-positive patients, 14 were diagnosed of Alzheimer’s disease, 2 cerebral amyloid angiopathy, 20 mild cognitive impairment (MCI) and 1 mixed dementia. °Four amyloid-PET-negative patients had a diagnosis of MCI, 1 frontotemporal dementia, 1 mixed dementia and 1 dysthymic dementia
All significant data (p value < 0.05) are presented in italic
Abbreviations: M males, F females, Aβ1-42 amyloid-β1-42, t-tau total tau, p-tau phosphorylated tau, SUVR standardised uptake value relative ratio, GM grey matter
Results
Application of the 2018-NIA-AA-RF into clinical practice
CSF data from 628 subjects were retrospectively analysed (Table 2). Among the 229 patients with a clinical diagnosis of AD [1, 8–10], 162 had AD typical core criteria, 36 logopenic primary progressive aphasia (PPA) [11], 23 posterior cortical atrophy (PCA) [27] and 8 frontal variant AD (fv-AD) [10]. Among the 107 subjects diagnosed as frontotemporal dementia (FTD), 82 had behavioural variant (bv)-FTD [12], 11 non-fluent PPA [11] and 14 semantic PPA [11]. We included also 37 Lewy body dementia (LBD) [13], 12 atypical parkinsonism [progressive supranuclear palsy (PSP) [16] or corticobasal syndrome (CBS) [14]], 5 idiopathic Parkinson’s diseases [15], 67 vascular/mixed dementia (VaD/mixed) [17] and 30 other dementia (see Table 2 for details). MCI (n = 132) were divided in amnestic (n = 99; aMCI) and not amnestic (n = 33; naMCI) [28, 29]. Nine individuals were diagnosed as CU and remained CU after at least 24-month follow-up.
Table 2.
Demographic data and AT(N) cerebrospinal fluid biomarker profiles of all included patients, divided according to their clinical syndrome
| Normal^ (n = 132) | AD-continuum^ (n = 389) |
Non-AD^ (n = 107) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age* | M:F | A−T−(N−) | A+T−(N−) AD pathologic change | A+T+(N−) AD | A+T+(N+) AD | A+T−(N+) AD and non-AD pathologic change |
A−T+(N−) | A−T−(N+) | A−T+(N+) | |
| AD | 229 | 72 ± 8 | 96:133 | 1 (0.4) | 56 (24.5) | 34 (14.9) | 118 (51.6) | 7 (3.1) | 5 (2.1) | 1 (0.4) | 7 (3.0) |
| FTD | 107 | 73 ± 7 | 61:46 | 39 (36.5) | 11 (10.4) | 7 (6.5) | 9 (8.4) | 1 (0.9) | 15 (14.0) | 4 (3.7) | 21 (19.6) |
| LBD | 37 | 76 ± 5 | 20:17 | 13 (35.1) | 9 (24.3) | 4 (10.8) | 3 (8.1) | 2 (5.4) | 2 (5.4) | 1 (2.7) | 3 (8.1) |
| PSP/CBS | 12 | 69 ± 7 | 6:6 | 9 (75.0) | 1 (8.3) | – | 2 (16.7) | – | – | – | – |
| PD | 5 | 62 ± 18 | 2:3 | 4 (80.0) | 1 (20%) | – | – | – | – | – | – |
| VaD/mixed | 67 | 76 ± 6 | 37:30 | 17 (25.4) | 16 (23.9) | 6 (8.9) | 8 (11.9) | – | 10 (14.9) | – | 10 (14.9) |
| Others° | 30 | – | – | 15 (50.0) | 7 (23.3) | 1 (3.3) | 2 (6.7) | – | 1 (3.3) | 1 (3.3) | 3 (10.0) |
| MCI | 132 | 73 ± 7 | 62:70 | 27 (20.4) | 35 (26.5) | 18 (13.6) | 25 (18.9) | 4 (3.0) | 11 (8.3) | – | 12 (9.2) |
| CU | 9 | 69 ± 6 | 8:1 | 7 (77.8) | 2 (22.2) | – | – | – | – | – | – |
| Total | 628 | 132 (21.0) | 138 (22.0) | 70 (11.1) | 167 (26.6) | 14 (2.2) | 44 (7.0) | 7 (1.1) | 56 (9.0) | ||
*Data are expressed as mean ± standard deviation. ^Data are expressed as number/total (percentage). °Among the 30 patients classified as “others” dementia, 4 had a diagnosis of sporadic cerebral amyloid angiopathy, 12 dysthymic dementia, 3 prion diseases, 1 Huntington disease, 1 Nasu-Hakola disease, 7 normal pressure hydrocephalus and 2 metabolic dementia
Abbreviations: AD Alzheimer’s disease, M males, F females, FTD frontotemporal dementia, LBD Lewy body dementia, PSP progressive supranuclear palsy, CBS corticobasal syndrome, PD idiopathic Parkinson’s disease, VaD vascular dementia, MCI mild cognitive impairment, CU cognitively unimpaired
According to their CSF data, participants were divided in normal (n = 132), AD-continuum (n = 389) and non-AD (n = 107) [2, 3]. The demographic data and a detailed list of biomarker profiles are given in Table 2.
Correspondence between biological biomarkers and clinical syndromes
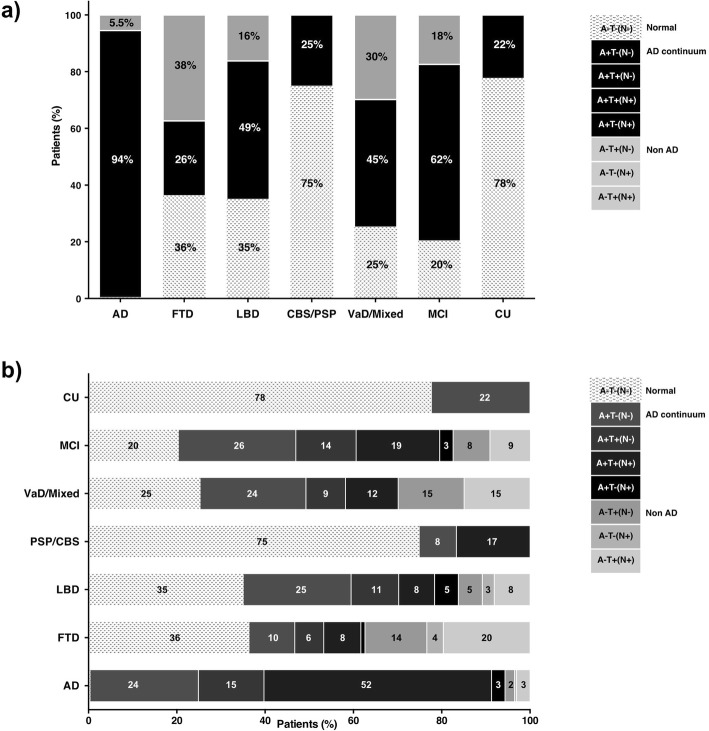
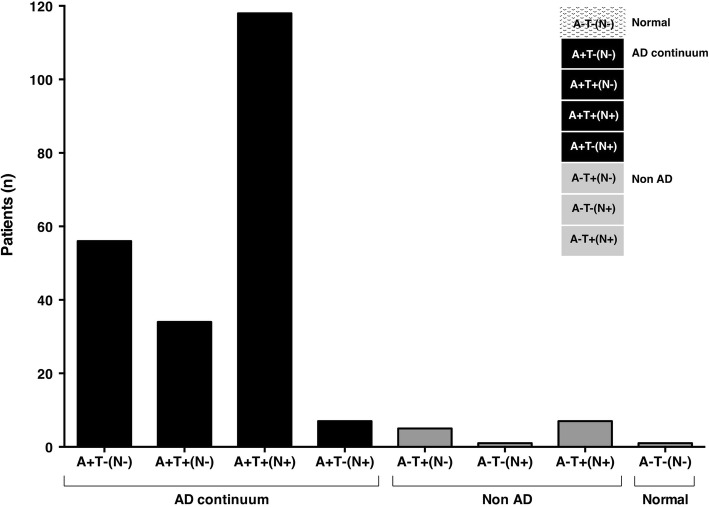
The percentages of the three main biomarker profiles (normal, AD-continuum and non-AD) in each clinical syndrome are summarised in Fig. 1a, whereas Fig. 1b and Table 2 provide a detailed representation of the percentages of all the eight biomarker profiles in the different clinical syndromes considered. Among patients diagnosed with AD, 94.1% were in the AD-continuum according to 2018-NIA-AA-RF [2], whereas 5.5% were classified as non-AD and 0.4% were normal. Particularly, the AD profile was the most common in AD-diagnosed patients (A+T+(N−) = 14.9% and A+T+(N+) = 51.6%), followed by the AD pathologic change profile (A+T−(N−) = 24.5%). Only 3.1% displayed an AD and non-AD pathologic change profile. As concern AD-diagnosed patients with a non-AD profile, 2.1% was A−T+(N−), 0.4% A−T−(N+) and 3% A−T+(N+) (Fig. 2). The AD-continuum profile was significantly higher in patients with clinically diagnosed AD as compared with all the other diagnostic groups (Χ2 = 175.1; df = 4; p < 0.0001). When considering all the eight biomarker subgroups, the same trend was shown, although the significance threshold was not reached (p > 0.05).
Fig. 1.
a Percentages of the three AT(N) biomarker profiles (AD-continuum, non-AD and normal) in each clinical syndrome. b Percentages of all the eight AT(N) biomarker profiles in each clinical syndrome. Percentages < 1% are not shown. AD Alzheimer’s disease (n = 229), FTD frontotemporal dementia (n = 107), LBD Lewy body dementia (37), PSP progressive supranuclear palsy (n = 3), CBS corticobasal syndrome (n = 9), VaD/mixed vascular/mixed dementia (n = 67), MCI mild cognitive impairment (n = 132), CU cognitively unimpaired (n = 9)
Fig. 2.
Number of Alzheimer’s disease-diagnosed patients (n tot = 229) for all the eight AT(N) biomarker profiles
Similar percentages were found when considering only typical AD [AD-continuum = 95%; non-AD = 5%] and logopenic PPA [AD-continuum = 92%; non-AD = 5.5%; normal = 2.5%]. PCA resulted AD-continuum and non-AD in 87% and 13% of cases, respectively. All fv-AD were AD-continuum.
37.3% of FTD-diagnosed patients were non-AD (A−T+(N−): 14%, A−T−(N+): 3.7%, A−T+(N+): 19.6%), and 36.5% were normal (36.5%). Nevertheless, 26.2% of FTD had an AD-continuum profile (A+T−(N−) = 10.4%, A+T+(N−) = 6.5%, A+T+(N+) = 8.4%, A+T−(N+) = 0.9%).
Among LBD patients, 48.6% was AD-continuum (A+T−(N−) = 24.3%, A+T+(N−) = 10.8%, A+T+(N+) = 8.1%, A+T−(N+) = 5.4%), 35.1% normal and 16.2% non-AD (A−T+(N−) = 5.4% A−T−(N+) = 2.7%, A−T+(N+) = 8.1%); 75% of CBS/PSP patients was normal, and 25% AD-continuum (A+T−(N−) = 8.3%, A+T+(N+) = 16.7%). Between VaD/mixed patients, 44.7% was AD-continuum (A+T−(N−) = 23.9%, A+T+(N−) = 8.9%, A+T+(N+) = 11.9%), 29.8% non-AD (A−T+(N−) = 14.9%, A−T+(N+) = 14.9%) and 25.4% normal. Seven out of the 9 CU had a normal profile (77.8%), but 2 CU subjects had AD pathologic change profile (A+T−(N− 22.2%)) [2], without clinical symptoms after 48-month follow-up.
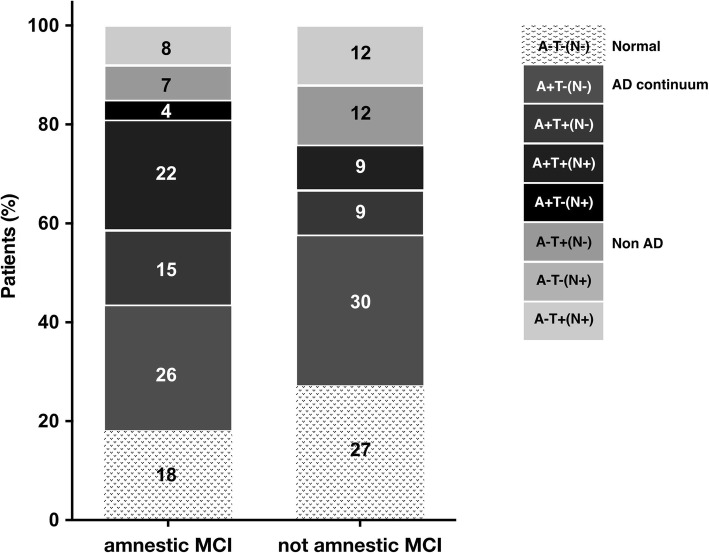
MCI-diagnosed patients displayed more often an AD-continuum profile (62%; A+T−(N−) = 26.5%, A+T+(N−) = 13.6%, A+T+(N+) = 18.9%, A+T−(N+) = 3%), but 20.4% was normal, and 17.5% non-AD (A−T+(N−) = 8.3%, A−T+(N+) = 9.2%). When dividing MCI patients in aMCI and naMCI (Fig. 3), the AD-continuum was more common in aMCI (67% versus 49%; X2 not significant; p > 0.05). According to 2018-NIA-AA-RF [2], 37% of aMCI and 18% of naMCI were classified as AD with MCI (A+T+(N−)/A+T+(N+)), whereas 25% of aMCI and 30% of naMCI displayed the AD pathologic change profile (A+T−(N−)). The prevalence of non-AD was 15% in aMCI and 24% in naMCI, whereas 18% of aMCI and 27% of naMCI had the normal profile (Fig. 3).
Fig. 3.
Percentages of all the eight AT(N) biomarker profiles in amnestic (n = 99) and not amnestic (n = 33) mild cognitive impairment (MCI) (n tot = 132)
Correlations between amyloid biomarkers
CSF Aβ1-42 levels and p-tau/Aβ1-42 ratio were significantly different in aP+ and aP− groups (Table 1). CSF Aβ1-42 levels—but not p-tau/Aβ1-42 ratio—negatively correlated with amyloid-PET SUVR in GM mean (r = − 0.31, p = 0.039) and precuneus (r = − 0.30, p = 0.04). No correlation between CSF and PET data and no significant differences in aP+ and aP− patients were found for t-tau, p-tau and t-tau/Aβ1-42 (p > 0.05).
When CSF biomarkers were dichotomized according to the previously determined cut-offs, Aβ1-42 displayed the higher concordance between amyloid-PET and CSF data (89%), compared to p-tau/Aβ1-42 ratio (73%). Among aP+ patients, 89% (n = 33/37) was classified as AD-continuum by their CSF Aβ1-42 levels, 8% (n = 3/37) as non-AD and 3% (n = 1/37) as normal. Conversely, 43% (n = 3/7) and 28.5% (n = 2/7) of aP− participants had non-AD and normal CSF profiles, respectively, but 28.5% (n = 2/27) was AD-continuum (Additional file 2).
Correlation between Aβ1-42 and p-tau/Aβ1-42
According to our previously determined cut-off, 382 out of 628 subjects had a pathologic p-tau/Aβ1-42 ratio (> 0.09), as compared with the 389 participants with pathologic CSF Aβ1-42 levels. When considering p-tau/Aβ1-42 ratio—instead of Aβ1-42 levels—to classify patients according to the 2018-NIA-AA-RF [2], 90.4% of clinically diagnosed AD patients resulted AD-continuum (n = 207/229), 7.9% normal (n = 18/229) and 1.7% non-AD (n = 4/229). As concerns the other forms of dementia, when using p-tau/Aβ1-42 ratio, the percentage of AD-continuum was 40.1% in FTD patients (n = 43/107), 37.8% in LBD (n = 14/37), 16.7% in CBS/PSP (n = 2/12), 0% in PD (n = 0/5), 43.3% in VaD/mixed (n = 29/67), 61.4% in MCI (n = 81/132) and 0% in CU (n = 0/9; Additional file 3).
Discussion
The 2018-NIA-AA-RF promotes a biological definition of AD based on its underlying pathologic process as measured by biomarkers. Evidence of both Aβ and pathologic p-tau deposition—assessed interchangeably with PET and/or CSF analysis—is needed to define AD in a living person [2, 4].
We first used amyloid-PET to calculate the CSF biomarker positivity thresholds for patient dichotomization as required by the AT(N) classification [3]. ROC analyses revealed that the optimal Aβ1-42 cut-off to predict PET positivity was < 660 pg/ml (sensitivity 89%, specificity 71%). This is in line with Palmqvist and colleagues who described an optimal cut-off for CSF Aβ1-42 of < 647 pg/ml (sensitivity 95%, specificity 90%) [18]. Aβ1-42 presented the best AUC, the major concordance with PET data, and was the only biomarker that correlated with PET SUVR. As already described, we found that the accuracy of CSF Aβ1-42 to predict cortical Aβ deposition status did not increase if the ratio p-tau/Aβ1-42 was used [5, 18, 30]. Given all these reasons, low CSF Aβ1-42 levels resulted the best biomarker of β-amyloidosis. Conversely, p-tau, t-tau and t-tau/Aβ1-42 showed a bad diagnostic value in our ROC analyses, which is in accordance with some previous studies [18, 31, 32], but not with others [19, 33]. A possible explanation for such discrepancies may derive from differences in staging or severity between cohorts studied.
When we applied the 2018-NIA-AA-RF to our cohort of patients, we found a good—but incomplete—correspondence between the diagnoses of AD made according to the clinical follow-up and those made with CSF biomarker profiles. Among all AD patients, 94.1% displayed an AD-continuum profile, but 5.5% were classified as non-AD, and 0.4% as normal. Similar percentages were found when considering both typical AD and logopenic PPA. PCA had AD-continuum and non-AD CSF profiles in 87% and 13% respectively, in line with the knowledge that multiple pathologies can underlie PCA [27]. One reason for this partial correspondence could be that from 2011 to 2014, AD clinical diagnoses in our centre were supported by both CSF and neuroimaging biomarkers, according to previous criteria [1, 9]. Thus, some patients with neuroimaging findings highly suggestive of AD (i.e. decreased FDG-PET in temporo-parietal cortex and/or atrophy on MRI in medial, basal and lateral temporal lobe, and medial parietal cortex) have been diagnosed with probable AD, even with a normal CSF profile. After the publication of the IWG-2 criteria in 2014 [10], CSF analysis or amyloid PET were used to support the diagnosis of probable AD in our clinical setting.
Our analysis revealed a low specificity of the AD-continuum profile, since it was found in a significant number of other forms of dementia, such as 25% of CBS/PSP, 44.7% of VaD/mixed, 48.6% of LBD and 26.2% of FTD. Among the AD-continuum, the most common CSF biomarker profile in all these syndromes was the AD pathologic change (A+T−(N−)). The lack in specificity of CSF Aβ1-42 levels is in accordance with previous findings [34], suggesting that brain β-amyloidosis may be a common comorbidity in other neurodegenerative syndromes [34]. Aware that only neuropathological studies could help in understanding these findings, our analysis suggests that the AT(N)) classification has a limited utility for differential diagnosis in the clinic setting. Notably, as compared to CSF Aβ1-42 levels, p-tau/Aβ1-42 ratio revealed a lower sensitivity with regard to the clinical AD diagnosis, but a slight higher specificity in differentiating the other forms of dementia, except FTD. This is in line with previous studies, showing that p-tau/Aβ1-42 ratio has a higher accuracy in AD differential diagnosis, as compared to Aβ1-42 levels [6, 35]. However, differently from what was reported by De Souza et al., p-tau/Aβ1-42 ratio did not appear a useful tool to distinguish AD from FTD [36].
As regards MCI, aMCI represents an early stage of AD, especially when associated with low CSF Aβ levels [37]. In our analysis, even though the AD-continuum profile was more common in aMCI compared to naMCI, data did not reach statistical significance and CSF profiles did not allow a substantial distinction between the two subgroups. Notably, AD pathologic change (A+/T−(N−))—that reflects Aβ deposition alone, thus representing an early sign of brain amyloidosis—did not appear the most represented profile in the aMCI group. Follow-up is needed to confirm the evolution of these patients, according to their baseline biomarker profile.
Recently, Jack et al. estimated in a large cohort of subjects the prevalence of three biomarker-based definitions of the AD-continuum from the NIA-AA-RF and compared it with the prevalence of three clinically defined diagnostic entities commonly associated with AD (MCI, dementia, and clinically defined probable AD). They found that biologically defined AD is more prevalent than clinically defined probable AD, possibly due to the asymptomatic individuals with biological AD [38]. Authors concluded that the two definitions create potential confusion around the term AD [38].
The last consideration regards the correspondence between CSF and PET Aβ biomarkers. As previously described [4, 19, 25, 31, 32, 39, 40], we confirmed a slight inverse correlation between CSF Aβ1-42 levels, and the binding of amyloid-PET tracer in total GM and precuneus. As speculated by Palmiqvist et al., this could indicate that CSF Aβ1-42 reflects the total aggregation status of Aβ1-42 in the whole brain [39]. When we applied the CSF Aβ1-42 cut-off based on amyloid-PET positivity, concordance was present in 89% of the subjects. Many different studies had previously investigated the agreement between PET and CSF data, reporting rates ranging from 72 to 92.4% [4, 5, 18, 25, 30, 33, 41]. The variable concordance between the two biomarkers has different explanations. Illàn-Gala et al. recently described a variable correlation between CSF Aβ1-42 levels and amyloid-PET data that was good in CU and MCI, but negligible in dementia [42]. Toledo and colleagues demonstrated a non-linear correlation between CSF and PET Aβ biomarkers [42, 43]. Recent studies have demonstrated that aP−/CSF+ subjects have increased rates of Aβ accumulation, and are likely to become aP+ [39, 44], suggesting that the two biomarkers measure AD pathology at different stages [4, 42]. CSF reflects soluble forms of Aβ that precede fibrillary deposition, and possibly becomes abnormal prior to PET [4, 39, 45]. In line with this observation, our two aP−/CSF+ patients had a diagnosis of aMCI and developed a clinically defined AD at follow-up. aP+/CSF− individuals have also been reported among both CU and CI [5, 7, 25, 46]. However, these subjects have usually elevated CSF t-tau and p-tau levels, and Aβ1-42 close to the cut-off, and are often diagnosed with MCI or AD. Different methodologies may also highly influence the proportions of CSF+ and/or aP+ subjects. Among the three aP+/CSF− participants we had in our study, two had elevated CSF t-tau and p-tau levels and one had nearly pathologic CFS Aβ1-42 levels (665 pg/ml). All of them had a clinical diagnosis of AD or mixed/VaD dementia. Lastly, the application of the 2018-NIA-AA-RF to those patients who performed both PET and CSF analysis confirmed a larger discordance in aP− participants, suggesting that the chosen CSF and neuroimaging markers of Aβ deposition are not perfectly interchangeable.
This work has several limitations. First, a gold standard for the assessment of cortical Aβ burden is lacking. In line with previous literature, we chose amyloid-PET as the best surrogate in vivo marker for determining the Aβ load because of its easy interpretation by visual inspection and its high correlation with neuropathological studies [5, 18, 20–23]. Even though ROC analyses and group comparisons are statistically significant, we are aware that there were relatively few aP− subjects, in comparison to aP+. The subgroup of participants with both CSF and PET data was small, and the average time interval between LP and amyloid-PET was of approximately 6 months. All these aspects might have influenced our findings. Moreover, the CSF positivity thresholds were defined based on maximising the discrimination between aP+ and aP− subjects, without an independent test set. Thus, the correspondence we described between CSF and PET data likely represents an upper limit. It should also be noted that the incorporation of biomarkers into some AD diagnosis may have biased the concordance between biomarker- and clinically based diagnoses. Due to lack of data, patients were classified considering CSF data only and Aβ deposition was measured without considering Aβ42/40 ratio, which is recently considered as having an improved diagnostic performance compared to Aβ42 alone [19]. Illàn-Gala et al. evaluated the consistency of the AT(N) classification with different biomarker combinations, finding important divergences and concluding that it does not achieve the required consistency to be used in clinical settings [42].
Conclusions
The application of the new criteria to a large cohort of patients revealed a good, but incomplete, correspondence between the clinical syndromes and the underlying pathologic process as measured by CSF biomarkers. The AD-continuum profile resulted to be a sensitive, but non-specific biomarker with regard to the clinical AD diagnosis. The incomplete agreement we found between CSF and PET Aβ biomarkers suggests that they are not perfectly interchangeable to quantify the Aβ burden, possibly because they measure different features of AD pathology.
Supplementary information
Additional file 1. Receiver operator characteristic (ROC) curves for Aβ1-42 levels, and p-tau/Aβ1-42 ratio compared to amyloid-PET tracer binding. Individuals were dichotomized into amyloid-PET positive and amyloid-PET negative as determined by PET visual reads. For each CSF biomarker, the table indicates the cut-off value and associated sensitivity (SE), specificity (SP), and area under the ROC curve (AUC) for the measure compared to amyloid-PET status. 95% confidence intervals are included in the parentheses.
Additional file 2. a) Column plot representing the 3 cerebrospinal fluid (CSF) biomarkers profiles in amyloid-PET positive (n = 37) and amyloid-PET negative (n = 7) subjects (n tot = 44). b) Column plot representing the percentage of amyloid-PET positive and amyloid-PET negative subjects in AD-continuum (n = 35), non-AD (n = 6), and Normal (n = 3) profiles, as assessed by CSF analyses.
Additional file 3. Comparison between the different percentages of subjects with an AD-continuum profile identified by using either cerebrospinal Aβ1-42 levels (black) or p-tau/Aβ1-42 ratio (white). [AD: Alzheimer’s disease; FTD: frontotemporal dementia; LBD: Lewy bodies dementia; PSP: progressive supranuclear palsy; CBS: corticobasal syndrome; PD: Parkinson’s disease; VaD/Mixed: vascular/mixed dementia; Other: other dementia syndromes; MCI: mild cognitive impairment; CU: cognitively unimpaired].
Acknowledgements
GGF was supported by Associazione Italiana Ricerca Alzheimer ONLUS (AIRAlzh Onlus)-COOP Italia.
Abbreviations
- 2018-NIA-AA-RF
2018 National Institute of Age-Alzheimer’s Association research framework
- AD
Alzheimer’s disease
- aMCI
Amnestic mild cognitive impairment
- Amyloid-PET
18F-florbetapir-positron emission tomography
- aP−
Amyloid-PET negative
- aP+
Amyloid-PET positive
- AT(N)
Amyloid/tau/(neurodegeneration)
- AUC
Area under the curve
- Aβ
β-amyloid
- bv-FTD
Behavioural variant frontotemporal dementia
- CBS
Corticobasal syndrome
- CSF
Cerebrospinal fluid
- CU
Cognitively unimpaired
- FDG-PET
18F-fluorodeoxyglucose-positron emission tomography
- FTD
Frontotemporal dementia
- fv-AD
Frontal variant AD
- GM
Grey matter
- LBD
Lewy body dementia
- LP
Lumbar puncture
- MCI
Mild cognitive impairment
- MRI
Magnetic resonance imaging
- naMCI
Not amnestic mild cognitive impairment
- Non-AD
Non-AD pathologic change
- PCA
Posterior cortical atrophy
- PET
Positron emission tomography
- PPA
Primary progressive aphasia
- PSP
Progressive supranuclear palsy
- p-tau
Phosphorylated tau protein
- ROC
Receiver operating characteristic
- SUV
Standardised uptake value
- SUVR
Standardised uptake value relative ratio
- t-tau
Total tau protein
- VaD/mixed
Vascular/mixed dementia
Authors’ contributions
TC and AA designed the study, analysed and interpreted the data and drafted the manuscript. LS collected and analysed the PET data. GGF and AMP contributed to the analysis and interpretation of the data. CF performed CSF analyses. LG, AC, MS and MAD added a minor contribution to the analysis of the data. GM acquired and analysed the PET data. EAS, GM and DG drafted and revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the Italian Ministry of Health (Ricerca Corrente to ES).
Availability of data and materials
The datasets used in this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
This retrospective study was approved by the Institutional Review Board of the Fondazione IRCCS Cà Granda, Ospedale Policlinico (Milan, Italy). All subjects (or their caregivers) gave their written informed consent.
Consent for publication
Written informed consent was obtained from all the participants for the publication of their individual details and accompanying images in this manuscript. The consent form is held by the authors’ institution.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elio Scarpini and Daniela Galimberti contributed equally to this work.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13195-019-0543-7.
References
- 1.McKhann Guy M., Knopman David S., Chertkow Howard, Hyman Bradley T., Jack Clifford R., Kawas Claudia H., Klunk William E., Koroshetz Walter J., Manly Jennifer J., Mayeux Richard, Mohs Richard C., Morris John C., Rossor Martin N., Scheltens Philip, Carrillo Maria C., Thies Bill, Weintraub Sandra, Phelps Creighton H. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack Clifford R., Bennett David A., Blennow Kaj, Carrillo Maria C., Dunn Billy, Haeberlein Samantha Budd, Holtzman David M., Jagust William, Jessen Frank, Karlawish Jason, Liu Enchi, Molinuevo Jose Luis, Montine Thomas, Phelps Creighton, Rankin Katherine P., Rowe Christopher C., Scheltens Philip, Siemers Eric, Snyder Heather M., Sperling Reisa, Elliott Cerise, Masliah Eliezer, Ryan Laurie, Silverberg Nina. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & Dementia. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack Clifford R., Bennett David A., Blennow Kaj, Carrillo Maria C., Feldman Howard H., Frisoni Giovanni B., Hampel Harald, Jagust William J., Johnson Keith A., Knopman David S., Petersen Ronald C., Scheltens Philip, Sperling Reisa A., Dubois Bruno. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen Ann D., Landau Susan M., Snitz Beth E., Klunk William E., Blennow Kaj, Zetterberg Henrik. Fluid and PET biomarkers for amyloid pathology in Alzheimer's disease. Molecular and Cellular Neuroscience. 2019;97:3–17. doi: 10.1016/j.mcn.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Zwan MD, Rinne JO, Hasselbalch SG, Nordberg A, Lleó A, Herukka SK, et al. Use of amyloid-PET to determine cutpoints for CSF markers. Neurology. 2016;86:50–58. doi: 10.1212/WNL.0000000000002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmqvist S, Zetterberg H, Mattsson N, Johansson P, Minthon L, Blennow K, et al. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 2015;85:1240–1249. doi: 10.1212/WNL.0000000000001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattsson N, Andreasson U, Persson S, Carrillo MC, Collins S, Chalbot S, et al. CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimers Dement. 2013;9:251–261. doi: 10.1016/j.jalz.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease. Rep NINCDS-ADRDA Work Group* under auspices Dep Heal Hum Serv Task Force Alzheimer’s Dis. 1984;34:939. [DOI] [PubMed]
- 9.Dubois Bruno, Feldman Howard H, Jacova Claudia, DeKosky Steven T, Barberger-Gateau Pascale, Cummings Jeffrey, Delacourte André, Galasko Douglas, Gauthier Serge, Jicha Gregory, Meguro Kenichi, O'Brien John, Pasquier Florence, Robert Philippe, Rossor Martin, Salloway Steven, Stern Yaakov, Visser Pieter J, Scheltens Philip. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS–ADRDA criteria. The Lancet Neurology. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 10.Dubois Bruno, Feldman Howard H, Jacova Claudia, Hampel Harald, Molinuevo José Luis, Blennow Kaj, DeKosky Steven T, Gauthier Serge, Selkoe Dennis, Bateman Randall, Cappa Stefano, Crutch Sebastian, Engelborghs Sebastiaan, Frisoni Giovanni B, Fox Nick C, Galasko Douglas, Habert Marie-Odile, Jicha Gregory A, Nordberg Agneta, Pasquier Florence, Rabinovici Gil, Robert Philippe, Rowe Christopher, Salloway Stephen, Sarazin Marie, Epelbaum Stéphane, de Souza Leonardo C, Vellas Bruno, Visser Pieter J, Schneider Lon, Stern Yaakov, Scheltens Philip, Cummings Jeffrey L. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. The Lancet Neurology. 2014;13(6):614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 11.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rascovsky K, Hodges JRJR, Knopman D, Mendez MFMF, Kramer JHJH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKeith IG, Boeve BF, DIckson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies. Neurology. 2017;4;89(1):88–100. [DOI] [PMC free article] [PubMed]
- 14.Armstrong MJ, Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postuma Ronald B., Berg Daniela, Stern Matthew, Poewe Werner, Olanow C. Warren, Oertel Wolfgang, Obeso José, Marek Kenneth, Litvan Irene, Lang Anthony E., Halliday Glenda, Goetz Christopher G., Gasser Thomas, Dubois Bruno, Chan Piu, Bloem Bastiaan R., Adler Charles H., Deuschl Günther. MDS clinical diagnostic criteria for Parkinson's disease. Movement Disorders. 2015;30(12):1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 16.Litvan I, Agid Y, Calne DB, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/WNL.47.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Gorelick Philip B., Scuteri Angelo, Black Sandra E., DeCarli Charles, Greenberg Steven M., Iadecola Costantino, Launer Lenore J., Laurent Stephane, Lopez Oscar L., Nyenhuis David, Petersen Ronald C., Schneider Julie A., Tzourio Christophe, Arnett Donna K., Bennett David A., Chui Helena C., Higashida Randall T., Lindquist Ruth, Nilsson Peter M., Roman Gustavo C., Sellke Frank W., Seshadri Sudha. Vascular Contributions to Cognitive Impairment and Dementia. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid β-amyloid 42. JAMA Neurol. 2014;71:1282. doi: 10.1001/jamaneurol.2014.1358. [DOI] [PubMed] [Google Scholar]
- 19.Schindler SE, Gray JD, Gordon BA, Xiong C, Batrla-Utermann R, Quan M, et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimers Dement. 2018;14:1460–1469. doi: 10.1016/j.jalz.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–678. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 21.Curtis C, Gamez JE, Singh U, Sadowsky CH, Villena T, Sabbagh MN, et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 2015;72:287–294. doi: 10.1001/jamaneurol.2014.4144. [DOI] [PubMed] [Google Scholar]
- 22.Sabri O, Sabbagh MN, Seibyl J, Barthel H, Akatsu H, Ouchi Y, et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement. 2015;11:964–974. doi: 10.1016/j.jalz.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Driscoll I, Troncoso JC, Rudow G, Sojkova J, Pletnikova O, Zhou Y, et al. Correspondence between in vivo 11C-PiB-PET amyloid imaging and postmortem, region-matched assessment of plaques. Acta Neuropathol. 2012;124:823–831. doi: 10.1007/s00401-012-1025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, et al. Comparing PET imaging and CSF measurements of Aβ. Ann Neurol. 2013;74:826–836. doi: 10.1002/ana.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pietroboni AM, Scarioni M, Carandini T, Basilico P, Cadioli M, Giulietti G, et al. CSF β-amyloid and white matter damage: a new perspective on Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2018;89:352–357. doi: 10.1136/jnnp-2017-316603. [DOI] [PubMed] [Google Scholar]
- 27.Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, Van Der Flier WM, et al. Consensus classification of posterior cortical atrophy on behalf of the Alzheimer’s Association ISTAART Atypical Alzheimer’s Disease and Associated Syndromes Professional Interest Area. Alzheimer’s Dement. 2017;13(8):870–84. [DOI] [PMC free article] [PubMed]
- 28.Petersen R. C. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 29.Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment report of the Guideline Development, Dissemination, and Implementation. Neurology. 2018;90:126–135. doi: 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zwan M, van Harten A, Ossenkoppele R, Bouwman F, Teunissen C, Adriaanse S, et al. Concordance between cerebrospinal fluid biomarkers and [11C] PIB PET in a memory clinic cohort. J Alzheimers Dis. 2014;41:801–807. doi: 10.3233/JAD-132561. [DOI] [PubMed] [Google Scholar]
- 31.Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tolboom N, van der Flier WM, Yaqub M, Boellaard R, Verwey NA, Blankenstein MA, et al. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med. 2009;50:1464–1470. doi: 10.2967/jnumed.109.064360. [DOI] [PubMed] [Google Scholar]
- 33.Bouallègue F Ben, Mariano-Goulart D, Payoux P. Comparison of CSF markers and semi-quantitative amyloid PET in Alzheimer’s disease diagnosis and in cognitive impairment prognosis using the ADNI-2 database. Alzheimer’s Res Ther. 2017;26;9(1):32. [DOI] [PMC free article] [PubMed]
- 34.Schoonenboom NSM, Reesink FE, Verwey NA, Kester MI, Teunissen CE, Van De Ven PM, et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78:47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- 35.Santangelo R, Dell Edera A, Sala A, Cecchetti G, Masserini F, Caso F et al. The CSF p-tau181/Aβ42 ratio offers a good accuracy “in vivo” in diagnosis of Alzheimer’s dementia. Curr Alzheimer Res. 2019;16(7):587–95. [DOI] [PubMed]
- 36.De Souza LC, Lamari F, Belliard S, Jardel C, Houillier C, De Paz R, et al. Cerebrospinal fluid biomarkers in the differential diagnosis of Alzheimer’s disease from other cortical dementias. J Neurol Neurosurg Psychiatry. 2011;82:240–246. doi: 10.1136/jnnp.2010.207183. [DOI] [PubMed] [Google Scholar]
- 37.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 38.Jack Clifford R., Therneau Terry M., Weigand Stephen D., Wiste Heather J., Knopman David S., Vemuri Prashanthi, Lowe Val J., Mielke Michelle M., Roberts Rosebud O., Machulda Mary M., Graff-Radford Jonathan, Jones David T., Schwarz Christopher G., Gunter Jeffrey L., Senjem Matthew L., Rocca Walter A., Petersen Ronald C. Prevalence of Biologically vs Clinically Defined Alzheimer Spectrum Entities Using the National Institute on Aging–Alzheimer’s Association Research Framework. JAMA Neurology. 2019;76(10):1174. doi: 10.1001/jamaneurol.2019.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmqvist S, Mattsson N, Hansson O. Cerebrospinal fluid analysis detects cerebral amyloid-β accumulation earlier than positron emission tomography. Brain. 2016;139:1226–1236. doi: 10.1093/brain/aww015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta;42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 41.Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, et al. Decreased β-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. J Am Med Assoc. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 42.Illán-Gala I, Pegueroles J, Montal V, Vilaplana E, Carmona-Iragui M, Alcolea D, et al. Challenges associated with biomarker-based classification systems for Alzheimer’s disease. Alzheimer’s Dement Diagnosis, Assess Dis Monit. 2018;10:346–357. doi: 10.1016/j.dadm.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toledo JB, Bjerke M, Da X, Landau SM, Foster NL, Jagust W, et al. Nonlinear association between cerebrospinal fluid and florbetapir F-18 β-amyloid measures across the spectrum of Alzheimer disease. JAMA Neurol. 2015;72:571–581. doi: 10.1001/jamaneurol.2014.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlassenko AG, McCue L, Jasielec MS, Su Y, Gordon BA, Xiong C, et al. Imaging and cerebrospinal fluid biomarkers in early preclinical Alzheimer disease. Ann Neurol. 2016;80:379–387. doi: 10.1002/ana.24719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nordberg A, High PIB. Retention in Alzheimer’s disease is an early event with complex relationship with CSF biomarkers and functional parameters. Curr Alzheimer Res. 2009;999:1–13. doi: 10.2174/1567209198607192050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Receiver operator characteristic (ROC) curves for Aβ1-42 levels, and p-tau/Aβ1-42 ratio compared to amyloid-PET tracer binding. Individuals were dichotomized into amyloid-PET positive and amyloid-PET negative as determined by PET visual reads. For each CSF biomarker, the table indicates the cut-off value and associated sensitivity (SE), specificity (SP), and area under the ROC curve (AUC) for the measure compared to amyloid-PET status. 95% confidence intervals are included in the parentheses.
Additional file 2. a) Column plot representing the 3 cerebrospinal fluid (CSF) biomarkers profiles in amyloid-PET positive (n = 37) and amyloid-PET negative (n = 7) subjects (n tot = 44). b) Column plot representing the percentage of amyloid-PET positive and amyloid-PET negative subjects in AD-continuum (n = 35), non-AD (n = 6), and Normal (n = 3) profiles, as assessed by CSF analyses.
Additional file 3. Comparison between the different percentages of subjects with an AD-continuum profile identified by using either cerebrospinal Aβ1-42 levels (black) or p-tau/Aβ1-42 ratio (white). [AD: Alzheimer’s disease; FTD: frontotemporal dementia; LBD: Lewy bodies dementia; PSP: progressive supranuclear palsy; CBS: corticobasal syndrome; PD: Parkinson’s disease; VaD/Mixed: vascular/mixed dementia; Other: other dementia syndromes; MCI: mild cognitive impairment; CU: cognitively unimpaired].
Data Availability Statement
The datasets used in this study are available from the corresponding author upon reasonable request.