Abstract
Background
Hybridization has been widely practiced in plant and animal breeding as a means to enhance the quality and fitness of the organisms. In domestic equids, this hybrid vigor takes the form of improved physical and physiological characteristics, notably for strength or endurance. Because the offspring of horse and donkey is generally sterile, this widely recognized vigor is expressed in the first generation (F1). However, in the absence of recombination between the two parental genomes, F1 hybrids can be expected to be phenotypically intermediate between their parents which could potentially restrict the possibilities of an increase in overall fitness. In this study, we examine the morphology of the main limb bones of domestic horses, donkeys and their hybrids to investigate the phenotypic impact of hybridization on the locomotor system. We explore bone shape variation and covariation to gain insights into the morphological and functional expressions of the hybrid vigor commonly described in domestic equids.
Results
Our data reveal the occurrence of transgressive effects on several bones in the F1 generation. The patterns of morphological integration further demonstrate that the developmental processes producing covariation are not disrupted by hybridization, contrary to functional ones.
Conclusions
These results suggest that an increase in overall fitness could be related to more flexibility in shape change in hybrids, except for the main forelimb long bones of which the morphology is strongly driven by muscle interactions. More broadly, this study illustrates the interest of investigating not only bone shape variation but also underlying processes, in order to contribute to better understanding how developmental and functional mechanisms are affected by hybridization.
Keywords: Appendicular skeleton, Bone morphology, Domestic equids, Hybridization, Three-dimensional geometric morphometrics
Background
Although hybridization may lead to the production of less competitive phenotypes [1–3], cases of an increase in fitness and a selective advantage over the parents have been documented in many taxa [4–6]. This phenomenon, generally considered as the result of heterozygosis in hybrids [7, 8], is known as hybrid vigor, or heterosis, and is usually measured by the capacity of hybrids to expand their ecological range and outperform their parent species under natural conditions [9–11]. Heterotic effects can also be artificially targeted in human-mediated hybridizations. This is especially the case in agricultural yield in which hybrids between cultivated and wild forms are created in order to increase crop yield with hybrid plants producing more seeds or fruits than their parents [12, 13]. Similarly, the production of hybrids is also common in animal breeding, as a way to increase the physical, physiological or even cognitive characteristics of the livestock [14, 15]. Among the most familiar examples are the hybrids between domestic equids, mules and hinnies. Mules, the offspring of a male donkey and a female horse (Equus asinus x Equus caballus), are renowned for the fact that they are taller, faster and more powerful than donkeys as well as for their increased endurance, hardiness and longevity in comparison with horses [16]. These hybrids have also been described as displaying greater cognitive capacities than either parent [17]. Hinnies, the product of mating a male horse with a female donkey (Equus caballus x Equus asinus) are less common and usually reported to not demonstrate the hybrid vigor generally described for mules [16], mainly because of their smaller stature supposed to be partly related to the mother’s size [18].
Although the hybrid vigor attributed to equid hybrids is widely recognized, especially for mules, its phenotypic expression remains unclear, particularly from an osteological point of view. The fact that mules and hinnies are (with only a few exceptions) sterile [19, 20] probably contributes to the low attention paid to the morphological consequences of hybridization in domestic equids. Indeed, evolutionary studies usually focus on this process in a context of speciation or introgression [21–24]. Moreover, no macroscopic feature specific to hybrid bones has been, for now, identified (in a context in which horse and donkey bones are themselves hard to distinguish) [25], which would suggest the absence of strongly transgressive morphologies related to hybridization in domestic equids.
In a standard polygenic additive model (i.e. one trait controlled by multiple genes with alleles having a similar and additive effect), hybrids are expected to be phenotypically intermediate between their parents [5, 26], especially in the F1 generation, due to the absence of recombination between the two parental genomes [27, 28]. However, non-additive genetic effects can also be involved. They can result in dominance effects (corresponding to the production of hybrid organisms phenotypically closer to one of the parents [29]) or contribute to produce morphologies falling outside the range of variation of both parent species, called transgressive phenotypes [27, 30]. Transgressive effects have already been demonstrated in F1 hybrids [31, 32] with an increased shape disparity and the production of original morphologies prone to play a role in the ability for hybrids to exhibit a wider range of fitness than their parents. The impact of hybridization on morphological traits, coupled with the frequently observed heterotic effect on size, can then induce mechanical or functional changes with respect to parental species which could provide fitness advantages (or disadvantages).
In hybrids from domestic equids, a large part of the widely described hybrid vigor is related to physical performance which is targeted to fit human needs and uses. In that respect, limb bones, even though they are rarely investigated for studying consequences of hybridization, are of particular interest. Indeed, they are the main anatomical elements involved in equid locomotion and their shape can thus be used as a skeletal indicator of functional specificities. Moreover, knowing that performance and functional efficiency of the locomotor system largely results from the mechanical interactions among elements [33, 34], exploring patterns of interactions between bones could provide insights into the functional expression of hybrid vigor. For this reason, we here do not only explore the shape variation but also covariation between bones. The tendency of morphological traits to covary is called morphological integration [35–37] and is produced by various mechanisms such as the sharing of a common function (e.g. modules of within-limb adjacent bones sharing common articulations or muscles) or the sharing of a same developmental origin (i.e. modules of serial homologous bones between fore- and hind limb; Hall, [38]). This incongruence between functional and developmental modules in the appendicular skeletons of tetrapods provides the opportunity to better understand what underlies the shape variation of a bone [39–45]. It is also a way to explore the functional characteristics of an animal but also the degree of conservation of patterns due to developmental processes. This is especially interesting in the study of hybridization knowing that hybridization has been suspected to sometimes disrupt developmental regulation [46, 47]. This issue is moreover particularly relevant in the case of the horse and the donkey, known to show (in comparison with other hybridizing mammals) a high genetic distance [24].
In the present study, we quantify, using 3D geometric morphometrics, the consequences of hybridization on the shape variation of the major limb bones in hybrids from domestic equids. One of the main limitations for characterizing the effect of hybridization in domestic equids is the absence, for these taxa, of available osteological samples including parents and offspring from a same lineage (as well as the near absence of hybrids of known descent which would have, at least, permitted comparisons with the parental breeds used to produce them). In that respect, in order to minimize the inevitable bias related to the sample composition, we tried to characterize at best the morphological variability within each species by including a large diversity of specimens in terms of breed, size, and conformation. We aim to examine the manner in which the “vigor”, defined according to human requirements, is expressed in morphological traits, from first hybrid generation (F1) onwards. To do that, we try to identify potential shape and size differences between hybrids and their parent species and to detect markers of phenotypic transgression. We also investigate the impact of hybridization on phenomena such as allometry (the influence of size on shape, expected to possibly contribute to transgressive effects on shape) and patterns of integration, in order to better understand the underlying causes of morphological variation in hybrids and their parent species.
Results
The analyses involves the bones of 101 complete or sub-complete skeletons of adult (with fully fused epiphyses) domestic horses of various breeds (n = 42), domestic donkeys and wild asses (n = 38) and their hybrids (n = 21; Table 1, see Additional file 1 for more details). In the absence of significant differences in shape between mules and hinnies [48], they were grouped in following analyses due to the small size of the hybrid sample (13 mules and 8 hinnies).
Table 1.
List and sample sizes of the breeds included in the analyses
| Species | Breed | Sample size |
|---|---|---|
| E. caballus | Arabian | 5 |
| E. caballus | Thoroughbred | 2 |
| E. caballus | Selle Français | 3 |
| E. caballus | Trotteur français | 1 |
| E. caballus | Lusitano | 1 |
| E. caballus | Unknown (riding horse) | 1 |
| E. caballus | Boulonnais | 1 |
| E. caballus | Percheron | 2 |
| E. caballus | Nordiker | 2 |
| E. caballus | Clydesdale | 2 |
| E. caballus | Shire | 1 |
| E. caballus | Unknown (pony) | 4 |
| E. caballus | Shetland pony | 4 |
| E. caballus | Icelandic | 4 |
| E. caballus | Camargue | 1 |
| E. caballus | Pottok | 3 |
| E. caballus | Mongol | 4 |
| E. caballus | Konik | 1 |
| E. asinus | Egyptian | 2 |
| E. asinus | Poitou | 6 |
| E. asinus | Asinara | 1 |
| E. asinus | Unknown | 29 |
| E. asinus x E. caballus | Unknown | 13 |
| E. caballus x E. asinus | Unknown | 8 |
Shape analyses
Pairwise comparisons show significant shape differences between the three groups in all the bones (MANOVA, p < 0.05). The two-way MANOVA performed on the shape data does not indicate an interaction between species-specific and sexual differences in our sample except for the coxal bone. Consequently, coxal shape variation was explored in males and females independently plotting first PCs. Trangression and dominance degrees were also computed to compare with results obtained on the whole dataset but the limited size of our sample does not enable us to perform statistical tests for each sex separately (see Additional file 3).
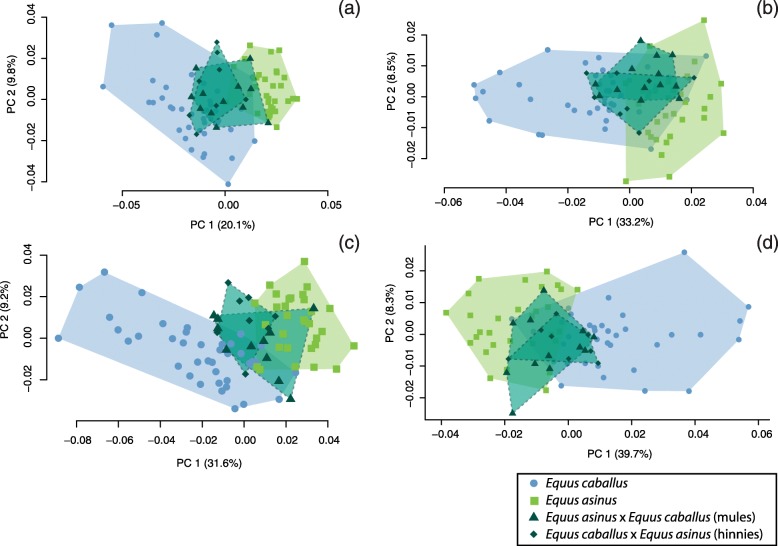
The distribution of the specimens along the first axes of the PCA reveals that, for all the bones, most of the hybrid individuals overlap with parental species (Fig. 1; see Additional file 4). Hybrids systematically display an intermediate position along the axis expressing differentiation between parental species (in most cases the PC1, in few instances the PC2) and generally do not exceed the variation of parental groups.
Fig. 1.
Scatter plot of the two first PCs of the PCA performed on the shape data of the humerus (a), radio-ulna (b), femur (c), tibia (d)
No significant difference in morphological disparity was observed between hybrid and donkey bones except for the middle posterior phalanx in which Procrustes variance is significantly lower in hybrids (Table 2). Concerning the comparison with horses, three bones display a significant difference in morphological disparity corresponding systematically to lower Procrustes variances in hybrids than in horses (metacarpal bone, femur and middle posterior phalanx).
Table 2.
Estimation of morphological disparity within each group (Procrustes variances), in bold when significantly different from hybrids (p < 0.05)
| Scapula | Humerus | Radio-ulna | Metacarpal bone | Proximal anterior phalanx | Middle anterior phalanx | Distal anterior phalanx | Coxal bone | |
| Variance | Variance | Variance | Variance | Variance | Variance | Variance | Variance | |
| Donkeys | 5,23E-03 | 1,96E-03 | 9,28E-04 | 5,97E-04 | 3,35E-03 | 1,04E-02 | 2,10E-02 | 6,97E-03 |
| Horses | 5,39E-03 | 2,24E-03 | 1,11E-03 | 9,73E-04 | 1,87E-02 | 3,07E-02 | 3,45E-02 | 1,44E-02 |
| Hybrids | 7,34E-03 | 1,66E-03 | 6,90E-04 | 4,82E-04 | 2,20E-03 | 7,30E-03 | 1,56E-02 | 5,09E-03 |
| Femur | Tibia | Talus | Calcaneus | Metatarsal bone | Proximal posterior phalanx | Middle posterior phalanx | Distal posterior phalanx | |
| Variance | Variance | Variance | Variance | Variance | Variance | Variance | Variance | |
| Donkeys | 2,48E-03 | 1,03E-03 | 2,17E-02 | 1,53E-02 | 4,56E-04 | 3,89E-03 | 1,30E-02 | 1,82E-02 |
| Horses | 3,04E-03 | 1,23E-03 | 7,79E-03 | 6,96E-03 | 6,50E-04 | 4,47E-03 | 1,18E-02 | 1,60E-02 |
| Hybrids | 1,62E-03 | 7,65E-04 | 6,77E-03 | 6,77E-03 | 3,22E-04 | 2,39E-03 | 7,48E-03 | 1,35E-02 |
Size and allometry
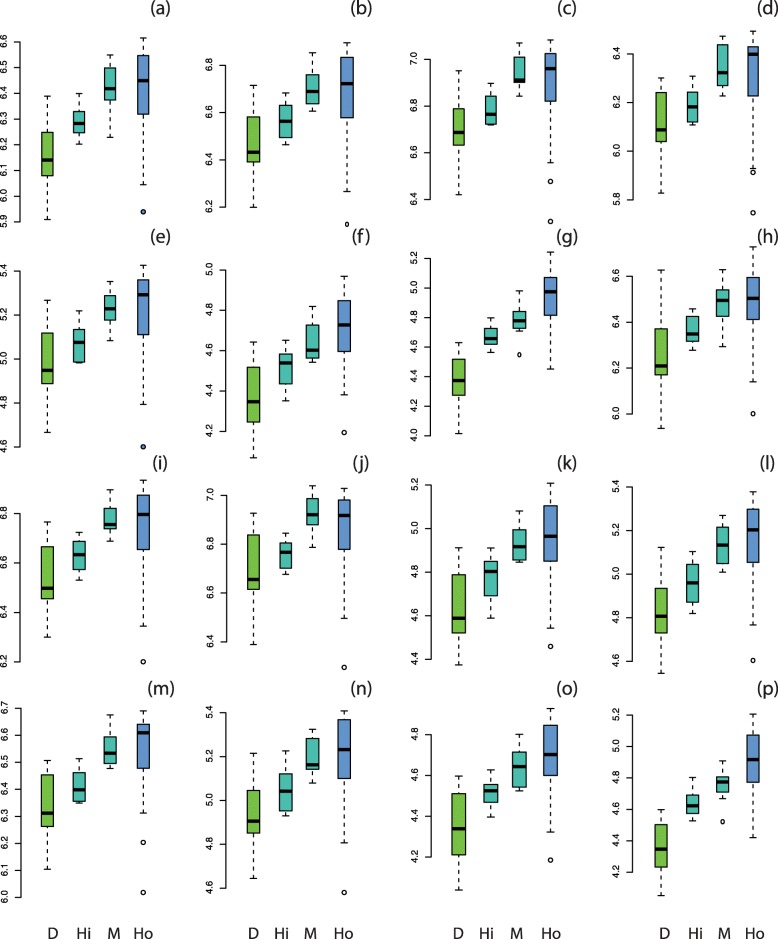
The results of the ANOVA analyses on size data reveal significant differences between donkeys and horses and between donkeys and hybrids for all the bones (p < 0.05), corresponding to smaller bone size in donkeys (Fig. 2). However, bone size is generally not significantly different between horses and hybrids except for distal phalanges (smaller in hybrids than in horses; Fig. 2). The small number of hybrid specimens does not enable us to statistically test potential differences in size between the two hybrid groups but the boxplots suggest that the bone centroid size is higher in mules than in hinnies.
Fig. 2.
Boxplots of the variation in log-transformed centroid size of the scapula (a), humerus (b), radio-ulna (c), metacarpal bone (d), proximal anterior phalanx (e), middle anterior phalanx (f), distal anterior phalanx (g), coxal bone (h), femur (i), tibia (j), talus (k), calcaneus (l), metatarsal bone (m), proximal posterior phalanx (n), middle posterior phalanx (o), distal posterior phalanx (p). Abbreviations: D, donkeys; Hi, hinnies; M, mules; Ho, horses
For all the bones, allometry is significant (see Additional file 5). Slope parallelism between groups was supported for half of the bones: metacarpal bone, anterior phalanges, talus, calcaneus and proximal posterior phalanx. However, the percentage of shape variance related to size is low except for distal phalanges and talus.
Shape transgression and dominance
The girdles (scapula and coxal bone) reveal the highest degrees of transgression (> 50%) followed by the talus (46%, Fig. 3a). This transgression observed for the coxal bone is reinforced in the analyses computed on males and females separately (see Additional file 3). The forelimb proximal long bones (humerus and radio-ulna, respectively 38 and 27%) are more transgressive than the hind limb ones (femur and tibia, respectively 15 and 19%). Concerning the distal bones (metapodials and phalanges), they generally display the lowest degrees of transgression (< 20%) except for the middle phalanges which appear as more transgressive in both limbs.
Fig. 3.
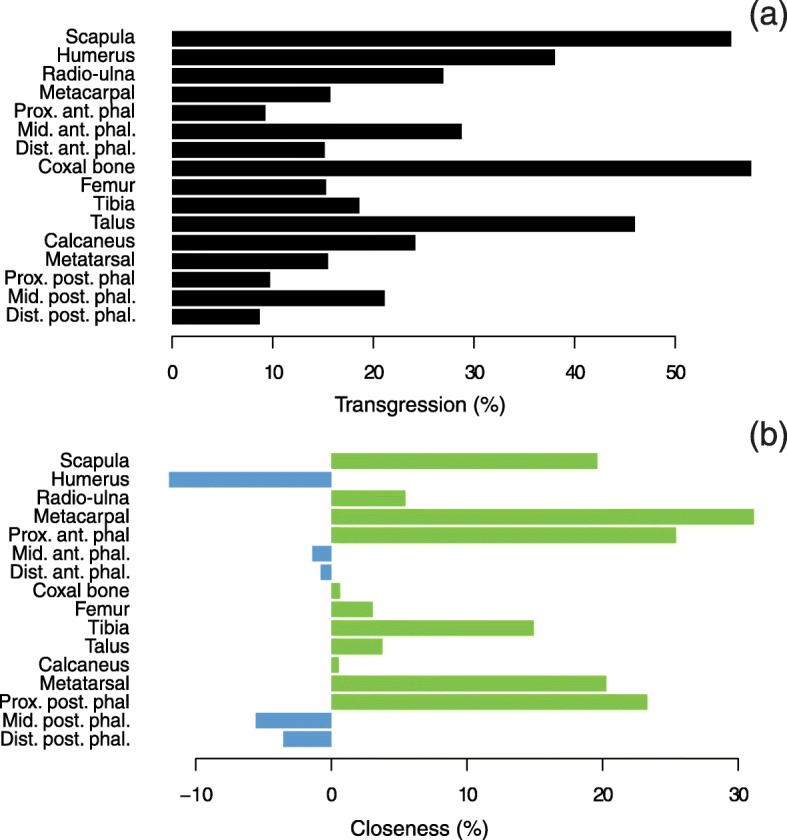
a Percentage of transgression of hybrids. b Percentage of closeness to parent species with positive values indicating greater closeness to donkey (in green) and negative ones to horse (in blue)
The results concerning the degree of dominance of the parental strains to hybrid morphology reveal a greater closeness to donkeys (Fig. 3b), on most of the bones, with the highest values (> 20%) found on the proximal autopodial bones (metapodial bones and proximal phalanges). On the contrary, a greater closeness to horses is observed for the humerus, and albeit to a lesser extent, middle and distal phalanges.
Shape integration patterns
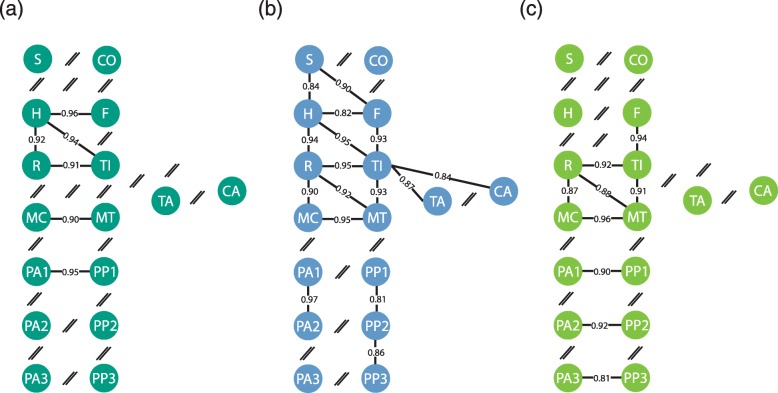
The covariation between the serial homologous bones is significant (p < 0.05) for most of the pairs in hybrids (Fig. 4). The absence of significant covariation between girdles (scapula and coxal bone) is also observed in both parent species, and that between middle and distal phalanges is shared with horses.
Fig. 4.
Graphical models of the rPLS coefficients obtained on the appendicular bones of hybrids (a), horses (b) and donkeys (c). The line thickness is proportional to the coefficient values (the boldest lines corresponding to the strongest intensity of covariation). The absence significant of covariation (non-significant PLS result) is represented by a double slash ‘//’. Abbreviations: S, scapula; H, humerus; R, radio-ulna; MC, metacarpal bone; PA1, proximal anterior phalanx; PA2, middle anterior phalanx; PA3, distal anterior phalanx; C, coxal bone; F, femur; T, tibia; MT, metatarsal bone; TA, talus; CA, calcaneus; PP1, proximal posterior phalanx; PP2, middle posterior phalanx; PP3, distal posterior phalanx
Concerning the intra-limb adjacent bones, only the covariation between the humerus and radio-ulna is significant in hybrids. In that respect, they differ from both parent species by the absence of significant covariation between the femur and tibia, and between the zeugopods and metapodials (radio-ulna/metacarpal bone, tibia/metatarsal bone).
Similarly, among the functional equivalent bones, only the covariation between the humerus and tibia is significant. Whereas the absence of significant covariation between the scapula and femur is also noticed in donkeys, the absence of significant covariation between the radio-ulna and metatarsal bone contrasts with both parent species.
When significant, the rPLS values in hybrids are globally high (rPLS ≥0.90) suggesting a strong degree of covariation between bones. However, intensity of integration in hybrids is globally lower than in horses, with significant differences in the z-scores for most of the pairs (see Additional file 6).
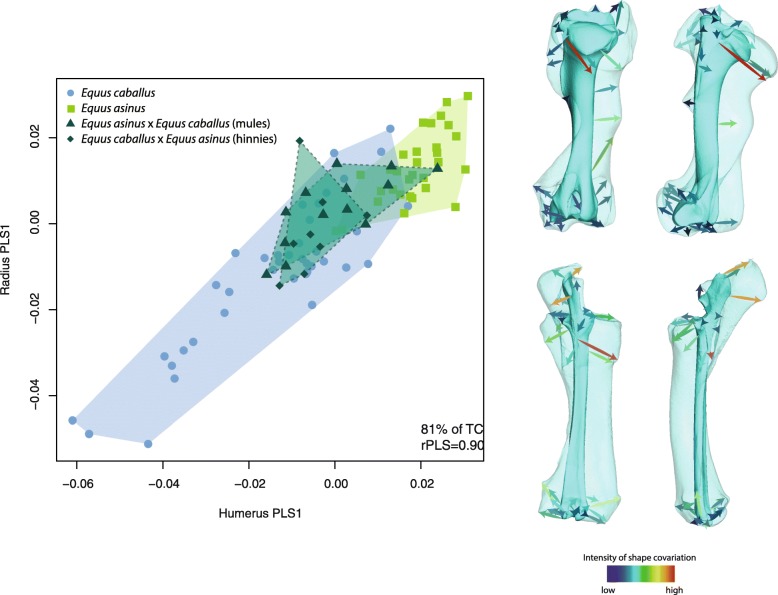
The first axis of covariation between the humerus and radio-ulna explain 90% of the total covariance. The distribution of the specimens along this axis follows a similar pattern than in the PCA with hybrids displaying an intermediate position between their parent species (Fig. 5). The morphological changes related to this axis are largely related to bone robusticity and curvature of the diaphysis (with horses displaying more robust and curved bones than donkeys). More precisely, some anatomical areas mainly contribute to the covariation: the proximal part of the caudal surface of the humerus (corresponding to the origin of the brachialis and triceps brachii muscles); the radial tuberosity on the medial side of the radius (corresponding to the insertion of the brachialis muscle) and the olecranon tuberosity on the ulna (corresponding to the insertion of the triceps brachii muscle).
Fig. 5.
Scatter plot of the first PLS axis describing shape covariation between the humerus and radio-ulna, with visualizations of the associated shape changes (in transparent: extreme negative; in opaque: extreme positive). The color of the arrows corresponds to the intensity of shape deformation along the PLS axis (red: high intensity; blue: low intensity). TC: total covariation; rPLS: PLS correlation coefficient
Discussion
Pattern of shape differentiation between the hybrids and their parent species
Hybridization has been previously reported as able to produce an increased shape variance from the first generation hybrids onwards [31]. In the absence of recombination between the parental genomes, this phenomenon has been related to decreased canalization in hybrids (i.e. the buffering of developmental processes against genetic and environmental variation in order to keep the phenotype constant). In our case, the absence of significant increased shape variance within the hybrid group in comparison with their parent species does not allow us to suggest that canalization is affected by hybridization in domestic equids. However, the impossibility to directly compare hybrids with their actual parents (or at least with a sub-sample comprised of the parental breeds used to produce the hybrids in our dataset) makes it impossible to discuss the potential impact of hybridization on shape variance in our sample. Further studies comparing shape variance between equid hybrids and their actual parents would be of interest to be able to shed light on the potential effect of hybridization on shape variance and canalization.
Although hardly discernible on the visualization of two first PCs, our results reveal transgression in hybrid shape. Indeed, the fact that shape differences between hybrids and both their parent species is significant for all the bones suggests that hybridization in domestic equids is able to produce a globally original morphology. The assessment of transgression in hybrid shape reveals that they do not display a strictly intermediate position between the parental strains with a percentage of transgression of over 40% of the between-parents distance on some bones. This confirms the idea that transgression may occur as soon as the first generation in hybrids [31, 32], in spite of the absence of genetic recombination expected to result in an intermediate morphology [26, 49]. In our dataset, we observe dominance effects with a greater closeness of hybrids to donkeys for most of the bones. This refutes the simple additive genetic model of strictly intermediate shape between parental strains for hybrids.
Size and allometry
A common effect of hybridization is the phenomenon of heterosis (or hybrid vigor) which corresponds to the improved quality of some biological and physiological characteristics of a hybrid organism, over those of its parents, generally providing a fitness advantage. Heterotic effects were noticed in hybrids from numerous taxa and may notably be expressed by an increase in size [29, 31, 47, 50, 51].
In our case, the fact that hybrid bones are significantly larger than those of donkeys and not significantly different from those of horses suggests that they exceed the size intermediacy between parent species, expected under the additive model. However, reliably characterizing such a heterotic effect on hybrid bone size is difficult for the same reasons than mentioned above: it would require a comparison of hybrids from our sample to parental groups constituted of similar breeds. Our current sample is indeed very disparate due to the great diversity in size of the modern parental species, especially horses (from Shetland ponies to draft breeds in the present study). A larger sample of hybrids specimens would also be of interest in order to be able to analyze mules separately from hinnies. Indeed, hinnies contribute to lower the mean size in the hybrid group whereas their smaller size is probably more related to the influence of physiological mechanisms (especially the mother size) rather than genetic ones [18].
Although we are unable to confidently confirm heterotic effect in size in our domestic equid sample, this phenomenon should not be neglected as a potential factor for transgression in shape. This is why allometry was examined in order to assess its possible contribution to the transgressive effect on shape, in cases of significant differences in bone size between the hybrids and their parent species (all the bones in donkeys, distal phalanges in horses). However, the low percentage of the shape variance related to size in most of the bones, coupled with the absence of homogeneity in the allometric slopes among species, suggest that transgression in hybrid shape is not driven by size differences (and hence potential heterotic effects on size).
The only exceptions are the anterior distal phalanges and the talus, in which a high percentage of shape variance is related to size, with hybrids and parent species sharing a common allometric trend. This consistency in the intergroup allometry suggests that their differentiation in shape could be partly due to allometric effects and size differences, probably revealing their role in guiding evolutionary changes in the distal parts.
Consequences of hybridization on development
In order to determine the factors that possibly contribute to explain these shape differences, we investigated morphological integration between the bones. In hybrids, covariation between serial-homologous bones is significant in most cases, contrasting with intra-limb adjacent bones. This reveals that the integration pattern in hybrid limbs is largely shaped by development and suggests that hybridization, although resulting from the combination of two different genomes, does not disrupt the developmental processes associated to the relationships between fore- and hind limbs. It should also be mentioned that some pairs of serial-homologous bones significantly covary in hybrids, even when this is not the case in both parent species. This confirms the strong impact of development in shaping covariation in hybrids, but also its underlying variation. Indeed, the significant covariation between the humerus and femur inherited from horses possibly contributes to driving the singular closeness of the humerus to that of horses. Similarly, the significant covariation between the proximal phalanges observed in hybrids, also only noticeable in donkeys, may echo the especially strong closeness of these two elements to donkeys.
The absence of significant covariation between middle and between distal phalanges is shared with horses, but also with various other tetrapods [39, 40] and could be explained by the proximo-distal increase in variability along the limb [39]. Similarly, the absence of significant covariation between girdles is also noticeable in parent species [52] and possibly results from the singular developmental pathway of the scapula [53, 54]. On another note, this relative morphological independence of the girdles from the rest of the skeleton in horses and donkeys echoes the high degree of transgression and the relative high shape variance noticed for these bones in hybrids. Indeed, the high degree of modularity of the parental scapula and the coxal bone could have contributed to facilitate shape variation in hybrids by increasing evolvability [55]. A similar finding applies to the talus which quite lowly covaries with adjacent elements in horses and donkeys, and is among the most transgressive bones in hybrids.
Consequence of hybridization on function
The absence of significant covariation between most of the intra-limb adjacent bones and functionally equivalent bones in hybrids reveals that functional factors do not contribute much to integration in the appendicular skeleton. This contrasts with horses and donkeys and suggests that hybridization may be accompanied by the relaxation of some processes producing phenotypic covariation between limb bones. Assuming that modularity would contribute to facilitate shape changes, loosening of covariation imputed to function could allow for a wider range of functional adaptations in hybrids, generally expressed by transgression and increased morphological variability [11, 31].
The only significant signal of integration between intra-limb adjacent bones concerns the humerus and radio-ulna, showing that the functional relationships between these two bones continues to generate shape covariation. More precisely, the interactions related to muscle action seem to underlie this covariation. Indeed, the PLS analysis reveals that the main areas of covariation correspond to the origin and insertion areas of the major muscles linking the humerus and radio-ulna, including the brachialis and triceps brachii muscles. The changes in diaphysis curvature could also be partly related to functional interactions, especially to the bending strains imposed by locomotion and muscular forces [56, 57]. The singular occurrence of significant covariation between the main forelimb bones involved in the muscular system probably suggests their functional importance in hybrid locomotion. In most tetrapods, the forelimb is mainly involved in support and braking [58] whereas the hind limb is optimized for generating propulsion [59]. In that respect, our result could appear as surprising considering that the intensity of morphological integration is higher in the hind limb of several taxa due to its major role in locomotion [40]. This was also observed on domestic equids [52] and could contribute to explain the lower degree of transgression noticed in the hind limb compared to the forelimb in hybrids. Indeed, the stronger intensity of integration between the femur and tibia in parent species could have participated to constraint transgression in hybrids.
The singular covariation between the humerus and radio-ulna in hybrids reminds the pattern of dominance noticed for the humerus, which contrasts from most of the bones by displaying greater closeness to the parental horse morphology. Knowing that functional interactions have been shown as contributing to bone shape in this anatomical area, the greater closeness to horses could be partly related to function, especially muscle characteristics. This is consistent with the fact that force and power are definitively the main features selected in horses and likely inherited by hybrids in their production [60]. Moreover, the hypothesis that special functional mechanisms underlie and drive the shape variation of the humerus is supported by the singular significant covariation with its functional equivalent, even absent in donkeys.
Conclusions
This study sought to assess the impact of hybridization on the morphology of the limb bones in domestic equids. Our results reveal that, for several bones, hybrids do not strictly display an intermediate position between their parent species, horses and donkeys, unlike what might be expected for first generation hybrids. Indeed, transgressive effects were brought to light, contributing to the global shape differentiation between hybrids and parent species. Our results show that the developmental mechanisms producing covariation are not disrupted by hybridization, contrary to functional ones. This would suggest that, surprisingly, the widely recognized vigor of these hybrids does not rest on a strongly integrated system which would drive bone shape variation as a result of functional interactions. Indeed, modularity might, on the contrary, contribute to facilitate shape changes, potentially as a way to increase overall fitness. The significant morphological covariation observed between the humerus and radio-ulna is an exception. It appears to be mostly related to muscle interactions and probably reveals the particular importance of this anatomical area in support and braking.
Additional studies comparing hybrids with their actual parents are needed to demonstrate the potential occurrence of phenomena generally associated to hybridization and prone to play a role in producing hybrid vigor including heterotic effects in size. Measuring the amount of fluctuating asymmetry as a marker of developmental (in) stability could also be a way to detect hybrid vigor as it is assumed to be correlated to overall fitness [51, 61].
More generally, this study contributes to show the importance of investigating the impact of hybridization on bone shape variation, but also on the processes which underlie it to gain insight into how developmental and functional mechanisms are affected. Our study also illustrates the interest of examining the appendicular skeleton which is prone to document the effect of hybridization on the locomotor system. This aspect, which is only rarely considered, can contribute to enrich our vision about the functional expression of hybrid vigor.
Methods
Acquisition of data
A 3D geometric morphometric approach was applied to the 16 main limb bones of the equid skeleton (scapula, humerus, radio-ulna, metacarpal bone, coxal bone, femur, tibia, calcaneus, talus, metatarsal bone, proximal, middle, and distal anterior and posterior phalanges). The 3D coordinates of anatomical landmarks were recorded according to the protocol of Hanot et al. [48] using a Microscribe 3D digitizer. Due to the fragmented nature of some bones, some landmarks were removed in the analyses (see Additional file 2).
For each bone, a generalized Procrustes Analysis (GPA) was performed on the landmark data in order to remove non-shape variation due to differences in position, scale, and orientation of the configurations [62].
Shape analyses
A Principal Component Analysis (PCA) was performed on the procrustes residuals of each bone to reduce the dimensionality of the multivariate datasets [63–66]. The first Principal Components (PCs) were then plotted in order to observe the distribution of the data in shape space. The PCAs were performed using the R package “Rmorph” [67, 68].
Shape differences between species (horses, donkeys and hybrids) were tested for each bone using Multivariate Analyses of Variance (MANOVA) with pairwise comparisons and Bonferroni corrections for multiple testing. They were performed on the Principal Components (PCs) explaining 90% of the total variance using the the “RVAideMemoire” library [69].
A two-way MANOVA was performed on the shape data to test the potential interaction between species differentiation and sexual dimorphism (on bones displaying a significant difference in shape between males and females). However, due to the small number of geldings in the sample, their potential impact on the shape variation was not taken into account.
The Procrustes variance of each bone was computed to assess the morphological disparity within each group using the “geomorph” library [70]. It was completed by pairwise comparisons among groups with Bonferroni corrections for multiple testing.
Size and allometry
Size differences between species were tested for each bone using Analyses of Variance (ANOVA) associated to pairwise post-hoc T-tests, using the “lsr” library [71], with Bonferroni corrections for multiple testing. Variations in size according to species were figured using box plots of the log10-transformed centroid sizes.
Allometry was assessed using multivariate regressions of shape variables on the log10-transformed centroid sizes. The homogeneity of allometric slopes among groups was then tested using the “geomorph” library [70].
Shape transgression and dominance
Closeness and transgression were quantified according to the method proposed by Renaud et al. (2012). First, for each bone, the group mean of each species was computed and the Euclidean distances between them measured using the “Morpho” library [72].
The degree of transgression was assessed by computing the percentage of the summed distance between the hybrids and each parent species on the distance between parent species themselves: (dDHy+dHoHy-dDHo)*100/ dDHo (with “d” for Euclidean distance, “Hy” for hybrids, “D” for donkeys and “Ho” for horses). Therefore, the higher this percentage, the stronger the transgression, considering that the absence of transgression (i.e. the strictly intermediate position of the hybrids between the parent species) should correspond to the equal distance between parent species (dDHo) and between hybrids and each parent species (dDHy+dHoHy).
The degree of dominance was assessed by comparing the average distance between the hybrids and their parent species to the distance between the hybrids and donkeys, as a percentage of the average distance between the hybrids and their parent species: ((dDHy+dCHy)/2-dDHy)*100/((dDHy+dCHy)/2). Hence, positive values indicate that hybrids display a greater closeness to donkeys and negative values to horses.
Shape integration patterns
The shape covariation between bones was explored for all the within-limb adjacent and serially homologous bones. We also investigated the covariation between the functionally equivalent bones from fore- and hind limb [44, 52, 73], defined according to the reorganization of the skeleton in therian mammals [74, 75].
In order to assess the intensity of shape co-variation between bones, Partial Least Squares coefficients (rPLS) were computed on the Procrustes shape variables. The Two blocks-PLS analysis extracts the eigenvectors and eigenvalues from blocks of variables and examines the covariance between them [76, 77]. A significant covariation is obtained when the observed rPLS is higher than those of a random distribution of values from permuted blocks. High values for rPLS correspond to strong morphological integration and vice versa. Bonferroni corrections were conducted to adjust the p-values from each set of tests including a same bone.
In addition, the z-scores (effect size) of rPLS values were computed in order to enable their comparison between datasets [78]. This test estimates the standard deviate of rPLS values obtained from different datasets displaying different expected values under the null hypothesis of no integration (due to variations in the number of variables and sample size). The difference in the effect-sizes was then assessed using two-sample tests with Bonferroni corrections for multiple testing. These analyses were computed using the “geomorph” library [70].
In some cases, in order to better investigate the shape covariation, the first PLS axes were plotted using the “Rmorph” library [67] and the associated shape changes were visualized. Visualizations of the extreme shapes associated with the PLS axes were produced using a 3D photogrammetric model (from the bones of the modern specimen: CV9 - ONIRIS-Nantes AC, with photographs taken using a Canon EOS 700D and 3D reconstructions computed on the software Agisoft PhotoScan;© 2014 Agisoft LLC, 27 Gzhatskaya st., St. Petersburg, Russia). The landmark coordinates of the 3D model were obtained from the “IDAV Landmark Editor” [79] and the shape deformation along the axes were finally visualized thanks to a Thin Plate Spline (TPS) deformation of the consensus surface, using the “Morpho” library [72].
For all the analyses previously described, test results were considered as significant when p-values (p) were below 0.05.
Supplementary information
Additional file 1. List of the specimens included in the analyses (table)
Additional file 2. List of the anatomical landmarks from the protocol of Hanot et al. 2017a which are not retained in the analyses due to the poor preservation of some bones (table).
Additional file 3. Scatter plot of the two first PCs of the PCA performed on the shape data of the coxal bone of females and males (figure); Percentage of transgression and closeness to parent species of hybrids (table).
Additional file 4. Scatter plot of the two first PCs of the PCA performed on the shape data (figure).
Additional file 5 P-values and coefficient of determination of the multivariate regressions of shape variables on the log10-transformed centroid sizes (table).
Additional file 6. Graphical models of z-scores (effect size) of rPLS values obtained on the appendicular bones of hybrids, horses and donkeys (figure).
Acknowledgments
We thank the morphometry platform from the MNHN (UMS 2700) and the movement analysis platform from the UMR CNRS/MNHN 7179 (Paris, France) who lent the digitizing devices. We also thank the researchers, curators and collection technicians who allowed access to the specimens: Luc Vives, Joséphine Lesur, Christine Lefèvre, Aurélie Verguin and Céline Bens (MNHN-Paris); Benoît Clavel, Jean-Hervé Yvinec and Gaëtan Jouanin (CRAVO-Compiègne); Christian Bussy (Clinique Vétérinaire du Grand Renaud-Saint Saturnin); Manuel Comte, Aurélia Borvon (ONIRIS-Nantes); Benoît Mellier (MSN-Angers); Philippe Migaud (Vetpole-Pays Mellois); Wim Van Neer, Georges Lenglet, Sébastien Bruaux and Terry Walschaerts (IRSNB-Bruxelles); Michael Hiermeier (ZSM-Munich); Henriette Obermaier (SAPM-Munich); Renate Schafberg (MLU/ZNS/H-Halle/Saale); Erich Pucher and Konstantina Saliari (NHM-Wien). Finally, we would like to express our thanks to the three anonymous reviewers for their constructive comments on the manuscript which helped us to improve the quality of this work.
Abbreviations
- ANOVA
Analysis of Variance
- C
Coxal bone
- CA
Calcaneus
- D
Donkey
- d
Euclidean distance
- F
Femur
- F1
First generation
- GPA
Generalized Procrustes Analysis
- H
Humerus
- Hi
Hinny
- Ho
Horse
- Hy
Hybrid
- M
Mule
- MANOVA
Multivariate Analysis of Variance
- MC
Metacarpal bone
- MT
Metatarsal bone
- p
P-value
- PA
Anterior phalanx
- PC
Principal Component
- PCA
Principal Component Analysis
- PLS
Partial Least Squares
- PP
Posterior phalanx
- R
Radio-ulna
- rPLS
PLS correlation coefficient
- S
Scapula
- T
Tibia
- TA
Talus
- TC
Total Covariation
- TPS
Thin Plate Spline
Authors’ contributions
All authors contributed to conception of the study. PH carried out the acquisition of data, performed the statistical analyses, and drafted the manuscript with input from AH and RC, CG contributes to collecting data. All authors revised and approved the manuscript.
Funding
P.H. is funded by a postdoctoral fellowship granted by the FYSSEN foundation. This work was partially supported by the “ATM blanche”/MNHN (Muséum national d’Histoire naturelle, Paris, France) and the UMR CNRS/MNHN 7209.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12862-019-1520-2.
References
- 1.Arnold ML, Bulger MR, Burke JM, Hempel AL, Williams JH. Natural hybridization: how Low can you go and still be important? Ecology. 1999;80:371–381. doi: 10.1890/0012-9658(1999)080[0371:NHHLCY]2.0.CO;2. [DOI] [Google Scholar]
- 2.Vamosi SM, Hatfield T, Schluter D. A test of ecological selection against young-of-the-year hybrids of sympatric sticklebacks. J Fish Biol. 2000;57:109–121. doi: 10.1111/j.1095-8649.2000.tb00779.x. [DOI] [Google Scholar]
- 3.Gow JL, Peichel CL, Taylor EB. Ecological selection against hybrids in natural populations of sympatric threespine sticklebacks. J Evol Biol. 2007;20:2173–2180. doi: 10.1111/j.1420-9101.2007.01427.x. [DOI] [PubMed] [Google Scholar]
- 4.Burke JM, Carney SE, Arnold ML. Hybrid fitness in the Louisiana irises: analysis of parental and F1 performance. Evolution. 1998;52:37–43. doi: 10.1111/j.1558-5646.1998.tb05136.x. [DOI] [PubMed] [Google Scholar]
- 5.Johansen-Morris AD, Latta RG. Fitness consequences of hybridization between ecotypes of Avena Barbata: hybrid breakdown, hybrid vigor, and Transgressive segregation. Evolution. 2006;60:1585–1595. doi: 10.1111/j.0014-3820.2006.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 6.Arnold ML, Martin NH. Hybrid fitness across time and habitats. Trends Ecol Evol. 2010;25:530–536. doi: 10.1016/j.tree.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annu Rev Ecol Syst. 1987;18:237–268. doi: 10.1146/annurev.es.18.110187.001321. [DOI] [Google Scholar]
- 8.Birchler JA, Yao H, Chudalayandi S. Unraveling the genetic basis of hybrid vigor. Proc Natl Acad Sci. 2006;103:12957–12958. doi: 10.1073/pnas.0605627103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, McArthur ED, Freeman DC. Narrow hybrid zone between two subspecies of big sagebrush (ARTEMISIA TRIDENTATA: Asteraceae). IX. Elemental uptake and niche separation. Am J Bot. 1999;86:1099–1107. doi: 10.2307/2656972. [DOI] [PubMed] [Google Scholar]
- 10.Welch ME, Rieseberg LH. Habitat divergence between a homoploid hybrid sunflower species, Helianthus paradoxus (Asteraceae), and its progenitors. Am J Bot. 2002;89:472–478. doi: 10.3732/ajb.89.3.472. [DOI] [PubMed] [Google Scholar]
- 11.Stelkens RB, Brockhurst MA, Hurst GDD, Miller EL, Greig D. The effect of hybrid transgression on environmental tolerance in experimental yeast crosses. J Evol Biol. 2014;27:2507–2519. doi: 10.1111/jeb.12494. [DOI] [PubMed] [Google Scholar]
- 12.Knox RE, Clarke FR, Clarke JM, Fox SL, DePauw RM, Singh AK. Enhancing the identification of genetic loci and transgressive segregants for preharvest sprouting resistance in a durum wheat population. Euphytica. 2012;186:193–206. doi: 10.1007/s10681-011-0557-0. [DOI] [Google Scholar]
- 13.Shivaprasad PV, Dunn RM, Santos BA, Bassett A, Baulcombe DC. Extraordinary transgressive phenotypes of hybrid tomato are influenced by epigenetics and small silencing RNAs. EMBO J. 2012;31:257–266. doi: 10.1038/emboj.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manwell C, Baker CMA. Molecular biology and the origin of species: heterosis, protein polymorphism and animal breeding. London: Sidgwick & Jackson; 1970. [Google Scholar]
- 15.Mingroni MA. The secular rise in IQ: giving heterosis a closer look. Intelligence. 2004;32:65–83. doi: 10.1016/S0160-2896(03)00058-8. [DOI] [Google Scholar]
- 16.Leighton AC. The mule as a cultural invention. Technol Cult. 1967;8:45–52. doi: 10.2307/3101524. [DOI] [Google Scholar]
- 17.Proops L, Burden F, Osthaus B. Mule cognition: a case of hybrid vigour? Anim Cogn. 2009;12:75–84. doi: 10.1007/s10071-008-0172-1. [DOI] [PubMed] [Google Scholar]
- 18.Castle WE. Size inheritance. Am Nat. 1941;75:488–498. doi: 10.1086/280988. [DOI] [Google Scholar]
- 19.Benirschke K, Low RJ, Sullivan MM, Carter RM. Chromosome study of an alleged fertile Mare mule. J Hered. 1964;55:31–38. doi: 10.1093/oxfordjournals.jhered.a107283. [DOI] [PubMed] [Google Scholar]
- 20.Steiner CC, Ryder OA. Characterization of Prdm9 in Equids and sterility in mules. PLoS One. 2013;8:e61746. doi: 10.1371/journal.pone.0061746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallet J. Hybrid speciation. Nature. 2007;446:279–283. doi: 10.1038/nature05706. [DOI] [PubMed] [Google Scholar]
- 22.Abbott R, Albach D, Ansell S, Arntzen JW, Baird SJE, Bierne N, et al. Hybridization and speciation. J Evol Biol. 2013;26:229–246. doi: 10.1111/j.1420-9101.2012.02599.x. [DOI] [PubMed] [Google Scholar]
- 23.Lamichhaney S, Han F, Webster MT, Andersson L, Grant BR, Grant PR. Rapid hybrid speciation in Darwin’s finches. Science. 2018;359:224–228. doi: 10.1126/science.aao4593. [DOI] [PubMed] [Google Scholar]
- 24.Savriama Y, Valtonen M, Kammonen JI, Rastas P, Smolander O-P, Lyyski A, et al. Bracketing phenogenotypic limits of mammalian hybridization. R Soc Open Sci. 2018;5:180903. doi: 10.1098/rsos.180903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanot P, Bochaton C. New osteological criteria for the identification of domestic horses, donkeys and their hybrids in archaeological contexts. J Archaeol Sci. 2018;94:12–20. doi: 10.1016/j.jas.2018.03.012. [DOI] [Google Scholar]
- 26.Grant PR, Grant BR. Phenotypic and genetic effects of hybridization in Darwin’s finches. Evolution. 1994;48:297–316. doi: 10.1111/j.1558-5646.1994.tb01313.x. [DOI] [PubMed] [Google Scholar]
- 27.Albertson RC, Kocher TD. Genetic architecture sets limits on transgressive segregation in hybrid cichlid fishes. Evolution. 2005;59:686–690. doi: 10.1111/j.0014-3820.2005.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 28.Bell MA, Travis MP. Hybridization, transgressive segregation, genetic covariation, and adaptive radiation. Trends Ecol Evol. 2005;20:358–361. doi: 10.1016/j.tree.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Thorpe RS, Leamy L. Morphometric studies in inbred and hybrid House mice (Mus sp.): Multivariate analysis of size and shape. J Zool. 1983;199:421–432. doi: 10.1111/j.1469-7998.1983.tb05097.x. [DOI] [Google Scholar]
- 30.Nolte AW, Sheets HD. Shape based assignment tests suggest transgressive phenotypes in natural sculpin hybrids (Teleostei, Scorpaeniformes, Cottidae) Front Zool. 2005;2:11. doi: 10.1186/1742-9994-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renaud S, Alibert P, Auffray J-C. Mandible shape in hybrid mice. Naturwissenschaften. 2009;96:1043–1050. doi: 10.1007/s00114-009-0563-4. [DOI] [PubMed] [Google Scholar]
- 32.Renaud S, Alibert P, Auffray J-C. Modularity as a source of new morphological variation in the mandible of hybrid mice. BMC Evol Biol. 2012;12:141. doi: 10.1186/1471-2148-12-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheverud JM. Phenotypic, genetic, and environmental morphological integration in the cranium. Evolution. 1982;36:499–516. doi: 10.1111/j.1558-5646.1982.tb05070.x. [DOI] [PubMed] [Google Scholar]
- 34.Wagner GP, Altenberg L. Perspective: complex adaptations and the evolution of Evolvability. Evolution. 1996;50:967–976. doi: 10.1111/j.1558-5646.1996.tb02339.x. [DOI] [PubMed] [Google Scholar]
- 35.Olson EC, Miller RL. A mathematical model applied to a study of the evolution of species. Evolution. 1951;5:325–338. doi: 10.1111/j.1558-5646.1951.tb02790.x. [DOI] [Google Scholar]
- 36.Olson EC, Miller RL. Morphological Integration. Chicago: University of Chicago Press; 1958.
- 37.Van Valen L. The Study of Morphological Integration. Evolution. 1965;19:347–349. doi: 10.2307/2406444. [DOI] [Google Scholar]
- 38.Hall BK. Homology and embryonic development. In: Hecht MK, Macintyre RJ, Clegg MT, editors. Evolutionary Biology: Springer US; 1995. p. 1–37. 10.1007/978-1-4615-1847-1_1.
- 39.Hallgrímsson B, Willmore K, Hall BK. Canalization, developmental stability, and morphological integration in primate limbs. Am J Phys Anthropol. 2002;119:131–158. doi: 10.1002/ajpa.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young NM, Hallgrímsson B. Serial homology and the evolution of mammalian limb covariation structure. Evolution. 2005;59:2691–2704. doi: 10.1111/j.0014-3820.2005.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 41.Lawler RR. Morphological integration and natural selection in the postcranium of wild verreaux’s sifaka (Propithecus verreauxi verreauxi) Am J Phys Anthropol. 2008;136:204–213. doi: 10.1002/ajpa.20795. [DOI] [PubMed] [Google Scholar]
- 42.Rolian C. Integration and Evolvability in primate hands and feet. Evol Biol. 2009;36:100–117. doi: 10.1007/s11692-009-9049-8. [DOI] [Google Scholar]
- 43.Goswami A, Smaers JB, Soligo C, Polly PD. The macroevolutionary consequences of phenotypic integration: from development to deep time. Phil Trans R Soc B. 2014;369:20130254. doi: 10.1098/rstb.2013.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martín-Serra A, Figueirido B, Pérez-Claros JA, Palmqvist P. Patterns of morphological integration in the appendicular skeleton of mammalian carnivores. Evolution. 2015;69:321–340. doi: 10.1111/evo.12566. [DOI] [PubMed] [Google Scholar]
- 45.Botton-Divet L, Houssaye A, Herrel A, Fabre A-C, Cornette R. Swimmers, Diggers, Climbers and More, a Study of Integration Across the Mustelids’ Locomotor Apparatus (Carnivora: Mustelidae). Evol Biol. 2018;45:182–195.
- 46.Goodwin HT. Supernumerary teeth in Pleistocene, recent, and hybrid individuals of the Spermophilus richardsonii Complex (Sciuridae) J Mammal. 1998;79:1161–1169. doi: 10.2307/1383007. [DOI] [Google Scholar]
- 47.Ackermann RR, Rogers J, Cheverud JM. Identifying the morphological signatures of hybridization in primate and human evolution. J Hum Evol. 2006;51:632–645. doi: 10.1016/j.jhevol.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Hanot P, Guintard C, Lepetz S, Cornette R. Identifying domestic horses, donkeys and hybrids from archaeological deposits: a 3D morphological investigation on skeletons. J Archaeol Sci. 2017;78:88–98. doi: 10.1016/j.jas.2016.12.002. [DOI] [Google Scholar]
- 49.Ryan PG. Morphological heritability in a hybrid bunting complex: Nesospiza at Inaccessible Island. Condor Ornithol Appl. 2001;103:429–438. [Google Scholar]
- 50.Cheverud JM, Jacobs SC, Moore AJ. Genetic differences among subspecies of the saddle-back tamarin (Saguinus fuscicollis):evidence from hybrids. Am J Primatol. 1993;31:23–39. doi: 10.1002/ajp.1350310104. [DOI] [PubMed] [Google Scholar]
- 51.Alibert P, Fel-Clair F, Manolakou K, Britton-Davidian J, Auffray J-C. Developmental stability, fitness, and trait size in laboratory hybrids between european subspecies of the house mouse. Evolution. 1997;51:1284–1295. doi: 10.1111/j.1558-5646.1997.tb03975.x. [DOI] [PubMed] [Google Scholar]
- 52.Hanot P, Herrel A, Guintard C, Cornette R. Morphological integration in the appendicular skeleton of two domestic taxa: the horse and donkey. Proc R Soc B. 2017;284:20171241. doi: 10.1098/rspb.2017.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young N. Modularity and integration in the hominoid scapula. J Exp Zoolog B Mol Dev Evol. 2004;302B:226–240. doi: 10.1002/jez.b.21003. [DOI] [PubMed] [Google Scholar]
- 54.Chamero B, Buscalioni ÁD, Marugán-Lobón J. Pectoral girdle and forelimb variation in extant Crocodylia: the coracoid–humerus pair as an evolutionary module. Biol J Linn Soc. 2013;108:600–618. doi: 10.1111/j.1095-8312.2012.02037.x. [DOI] [Google Scholar]
- 55.Goswami A, Polly PD. The influence of modularity on cranial morphological disparity in Carnivora and Primates (Mammalia) PLoS One. 2010;5:e9517. doi: 10.1371/journal.pone.0009517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milne N. Curved bones: an adaptation to habitual loading. J Theor Biol. 2016;407:18–24. doi: 10.1016/j.jtbi.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 57.Hanot P, Herrel A, Guintard C, Cornette R. The impact of artificial selection on morphological integration in the appendicular skeleton of domestic horses. J Anat. 2018;232:657–673. doi: 10.1111/joa.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGuigan MP, Wilson AM. The effect of gait and digital flexor muscle activation on limb compliance in the forelimb of the horse Equus caballus. J Exp Biol. 2003;206:1325–1336. doi: 10.1242/jeb.00254. [DOI] [PubMed] [Google Scholar]
- 59.Dutto DJ, Hoyt DF, Clayton HM, Cogger EA, Wickler SJ. Moments and power generated by the horse (Equus caballus) hind limb during jumping. J Exp Biol. 2004;207:667–674. doi: 10.1242/jeb.00808. [DOI] [PubMed] [Google Scholar]
- 60.Raepsaet G. Land transport, part 2: riding, harnesses, and vehicles. In: The Oxford Handbook of Engineering and Technology in the Classical World. Oxford: Oxford University Press; 2012.
- 61.Alibert P, Renaud S, Dod B, Bonhomme F, Auffray JC. Fluctuating asymmetry in the Mus musculus hybrid zone: a heterotic effect in disrupted co-adapted genomes. Proc Biol Sci. 1994;258:53–59. doi: 10.1098/rspb.1994.0141. [DOI] [PubMed] [Google Scholar]
- 62.Rohlf FJ, Slice D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Biol. 1990;39:40–59. [Google Scholar]
- 63.Baylac M, Frieß M. Fourier descriptors, Procrustes superimposition, and data dimensionality: an example of cranial shape analysis in modern human populations. In: Slice DE, editor. Modern Morphometrics in physical anthropology. New York: Springer Science & Business Media; 2005. pp. 145–165. [Google Scholar]
- 64.Jolliffe IT. Principal component analysis. Second. New York: Springer-Verlag; 2002. [Google Scholar]
- 65.Krzanowski WJ. Principles of multivariate analysis: a User’s perspective. New York: Oxford University Press, Inc.; 1988. [Google Scholar]
- 66.Krzanowski WJ. Cross-validation in principal component analysis. Biometrics. 1987;43:575–584. doi: 10.2307/2531996. [DOI] [Google Scholar]
- 67.Baylac M. Rmorph : a R Geometric and Multivariate Morphometrics Library. 2014. [Google Scholar]
- 68.R Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for statistical Computing; 2015. [Google Scholar]
- 69.Hervé M. RVAideMemoire: testing and plotting procedures for biostatistics. R Package Version 09–69-3. 2018. [Google Scholar]
- 70.Adams DC, Otárola-Castillo E. Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol. 2013;4:393–399. doi: 10.1111/2041-210X.12035. [DOI] [Google Scholar]
- 71.Navarro D. lsr: Companion to “Learning Statistics with R.” R Package Version 05. 2015. [Google Scholar]
- 72.Schlager S. Morpho: calculations and Visualisations related to geometric Morphometrics. 2016. [Google Scholar]
- 73.Schmidt M, Fischer MS. Morphological integration in mammalian limb proportions: dissociation between function and development. Evolution. 2009;63:749–766. doi: 10.1111/j.1558-5646.2008.00583.x. [DOI] [PubMed] [Google Scholar]
- 74.Gasc J-P. Comparative aspects of gait, scaling and mechanics in mammals. Comp Biochem Physiol A Mol Integr Physiol. 2001;131:121–133. doi: 10.1016/S1095-6433(01)00457-3. [DOI] [PubMed] [Google Scholar]
- 75.Fischer MS, Schilling N, Schmidt M, Haarhaus D, Witte H. Basic limb kinematics of small therian mammals. J Exp Biol. 2002;205:1315–1338. doi: 10.1242/jeb.205.9.1315. [DOI] [PubMed] [Google Scholar]
- 76.Rohlf FJ, Corti M. Use of two-block partial least-squares to study Covariation in shape. Syst Biol. 2000;49:740–753. doi: 10.1080/106351500750049806. [DOI] [PubMed] [Google Scholar]
- 77.Bookstein FL, Gunz P, Mitterœcker P, Prossinger H, Schæfer K, Seidler H. Cranial integration in Homo: singular warps analysis of the midsagittal plane in ontogeny and evolution. J Hum Evol. 2003;44:167–187. doi: 10.1016/S0047-2484(02)00201-4. [DOI] [PubMed] [Google Scholar]
- 78.Adams DC, Collyer ML. On the comparison of the strength of morphological integration across morphometric datasets. Evolution. 2016;70:2623–2631. doi: 10.1111/evo.13045. [DOI] [PubMed] [Google Scholar]
- 79.Wiley DF, Amenta N, Alcantara DA, Ghosh D, Kil YJ, Delson E, et al. Evolutionary morphing. In: Proceedings of IEEE Visualization 2005. Minneapolis: IEEE; 2005. p. 431–8.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. List of the specimens included in the analyses (table)
Additional file 2. List of the anatomical landmarks from the protocol of Hanot et al. 2017a which are not retained in the analyses due to the poor preservation of some bones (table).
Additional file 3. Scatter plot of the two first PCs of the PCA performed on the shape data of the coxal bone of females and males (figure); Percentage of transgression and closeness to parent species of hybrids (table).
Additional file 4. Scatter plot of the two first PCs of the PCA performed on the shape data (figure).
Additional file 5 P-values and coefficient of determination of the multivariate regressions of shape variables on the log10-transformed centroid sizes (table).
Additional file 6. Graphical models of z-scores (effect size) of rPLS values obtained on the appendicular bones of hybrids, horses and donkeys (figure).
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on request.