Abstract
Neurotransmission is sustained by endocytosis and refilling of synaptic vesicles (SVs) locally within the presynapse. Until recently, a consensus formed that after exocytosis, SVs are recovered by either fusion pore closure (kiss-and-run) or clathrin-mediated endocytosis directly from the plasma membrane. However, recent data have revealed that SV formation is more complex than previously envisaged. For example, two additional recycling pathways have been discovered, ultrafast endocytosis and activity-dependent bulk endocytosis, in which SVs are regenerated from the internalized membrane and synaptic endosomes. Furthermore, these diverse modes of endocytosis appear to influence both the molecular composition and subsequent physiological role of individual SVs. In addition, previously unknown complexity in SV refilling and reclustering has been revealed. This review presents a modern view of the SV life cycle and discusses how neuronal subtype, physiological temperature, and individual activity patterns can recruit different endocytic modes to generate new SVs and sculpt subsequent presynaptic performance.
Introduction
During chemical neurotransmission, synaptic vesicles (SVs) fuse with the plasma membrane to release neurotransmitter. To support high rates of release, synapses require a constant supply of neurotransmitter-filled SVs. This supply is maintained primarily through membrane endocytosis, cargo sorting, and rapid refilling of newly formed SVs with neurotransmitter, which occur locally within nerve terminals. This entire process of reconstituting SVs after fusion is referred to as SV “recycling.” In contrast, de novo synthesis of SVs in the cell body and axonal transport to synapses is usually too slow to support the high demand for neurotransmitter release. This review will focus mainly on the recycling aspect of the SV cycle. For mechanisms of exocytosis, please refer to recent excellent reviews (Rizo and Xu, 2015; Chanaday and Kavalali, 2018a; Neher and Brose, 2018; Brunger et al., 2019; Dittman and Ryan, 2019).
Since the 1970s, the mechanisms by which SVs are recycled and trafficked at synapses have been intensely contested. On one side, using the frog neuromuscular junction, Heuser and Reese (1973) reported that SVs are regenerated locally by the formation of clathrin-coated vesicles at the periphery of active zones. Thus, they proposed that SV recycling occurs via clathrin-mediated endocytosis (CME) from the plasma membrane (Fig. 1). Around the same time, using similar experimental conditions, Ceccarelli et al. (1973) reported scant evidence of clathrin-coated vesicles at synapses, but instead reported clear, uncoated vesicles potentially being internalized at the active zone. They proposed that SVs can be recycled by the reversal of an exocytic fusion pore, a model that was later termed “kiss-and-run” (Fig. 1) (Fesce et al., 1994). Thus ensued a 40-year debate about how SVs are recycled and the underlying mechanisms.
Figure 1.
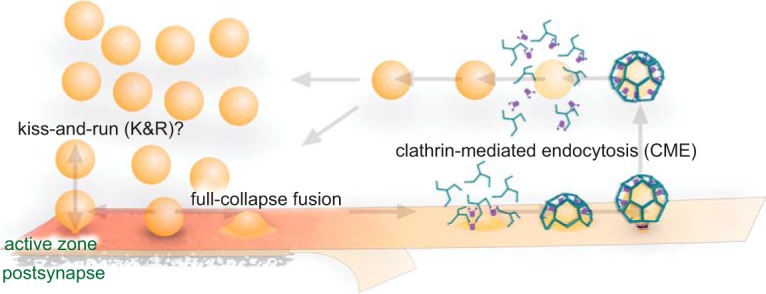
Classical view of the SV cycle. Action potentials trigger fusion of SVs at the active zone. After formation of the fusion pore, resulting in neurotransmitter release, two options are possible: the pore can reclose via kiss-and-run (K&R) or it can expand irreversibly, leading to full collapse. Compensating for full-collapse fusion, specific SV proteins are recruited by adaptor proteins at the periactive zone, triggering CME. Dynamin mediates vesicle scission, after which SVs are uncoated and refilled with neurotransmitters before being returned to the vesicle cluster.
In the intervening decades, these models were further tested as new molecular and imaging tools became available. However, instead of resolving the issue, two other models for SV recycling have emerged: activity-dependent bulk endocytosis (ADBE) and ultrafast endocytosis. It is possible that all four mechanisms coexist in nerve terminals, and are used differently depending on activity levels or synapse type (Gan and Watanabe, 2018). Adding to the complexity is that SVs are functionally heterogeneous, defined by distinct molecular compositions (Crawford and Kavalali, 2015). Thus, different recycling mechanisms may help to sort cargoes during SV reformation, ensuring that the proper molecular identity is maintained (Morgan et al., 2013). Additionally, recent data have revealed mechanistic insights into how newly endocytosed SVs are refilled with neurotransmitter and subsequently reclustered (Farsi et al., 2018; Milovanovic et al., 2018). In this review, we discuss these recent developments and, in doing so, present a modern view of the SV cycle with a specific emphasis on SV recycling mechanisms.
SV heterogeneity and functional pools
After action potentials, SVs fuse and release neurotransmitter either in a time-locked or delayed manner. The time-locked, synchronous phase of release transmits fast and reliable signals, while delayed asynchronous release influences network parameters, including efficacy of neurotransmission, synchronicity and plasticity (Otsu et al., 2004; Iremonger and Bains, 2016; Luo and Südhof, 2017). In addition, SVs can fuse spontaneously in the absence of action potentials, potentially affecting synapse formation and the strength of the connection (Chanaday and Kavalali, 2018a). Although not apparent from electron micrographs, increasing evidence suggests that visually identical SVs have different molecular compositions, and this heterogeneity may underlie functional organization of vesicle pools that differentially participate in the three phases of release (Chanaday and Kavalali, 2018a).
SVs are organized into four functional pools at presynapses: the readily releasable pool (RRP), recycling pool, reserve pool and resting pool. For synchronous neurotransmission, a subset of SVs are docked (physically in contact with the plasma membrane) and primed (ready-to-fuse upon calcium elevation) in a fusion-ready state at the active zone (Hammarlund et al., 2007; Südhof, 2013; Neher and Brose, 2018). Docked and primed SVs, located near the active zone, constitute the RRP that is immediately available for fusion upon the arrival of action potentials (Holderith et al., 2012). To sustain neurotransmitter release, the RRP must be constantly replenished with SVs (Guo et al., 2015) (Jonas, 2014). This replenishment can be accomplished by either rapid reuse of fused vesicles or recruitment of new SVs from the “reserve pool” during prolonged periods of activity. Collectively, all SVs that participate in activity-induced synaptic transmission comprise the recycling pool (i.e., RRP and a fraction of the reserve pool) (Denker and Rizzoli, 2010), which is estimated to be ∼50% of total SVs (Kim and Ryan, 2010), but may be as few as 1–5% (Denker et al., 2011). The remaining SVs may be referred to as the resting pool because they are reluctant to be mobilized during/after stimulation (Chanaday and Kavalali, 2018a).
Within the recycling pool, distinct molecular machineries determine whether vesicles fuse synchronously or asynchronously. Synchronous fusion is mediated by the canonical neuronal Soluble NSF Attachment Protein Receptor (SNARE) complex, which includes the SV protein synaptobrevin 2 (syb-2/VAMP2) (Jahn and Fasshauer, 2012; Rizo and Südhof, 2012) and the neuronal calcium-sensing proteins synaptotagmins 1, 2, and 9. Synaptotagmins clamp the SNARE complex in the absence of an action potential and trigger synchronous fusion in response to local calcium entry through voltage-gated calcium channels (Südhof, 2013). In contrast, asynchronous release is conferred by synaptotagmin 7 (Bacaj et al., 2013) or Doc2 (Yao et al., 2011) and by the noncanonical SNARE VAMP4 (Raingo et al., 2012). These molecular differences are likely maintained throughout the SV cycle. A recent report suggests that synaptotagmin 1 and 7 couple synchronous and asynchronous release to a fast (1–2 s) or slow (several seconds) mode of endocytosis, respectively (Li et al., 2017). Moreover, the asynchronous SNARE VAMP4 is required for ADBE after intense activity (Nicholson-Fish et al., 2015), during which asynchronous release becomes more prominent. Thus, current evidence suggests that synchronous and asynchronous release is maintained through different SV recycling pathways.
SVs can also spontaneously fuse and recycle in the absence of action potentials (Kavalali, 2015). Although it is debated whether spontaneous release draws from the recycling pool, resting pool, or its own pool (Sara et al., 2005; Fredj and Burrone, 2009), the presence of noncanonical SNAREs VAMP4, VAMP7, and Vti1a likely defines whether particular vesicles fuse spontaneously (Ramirez and Kavalali, 2012; Bal et al., 2013; Chanaday and Kavalali, 2018a). A number of calcium sensors have been proposed to trigger spontaneous neurotransmitter release, including the Doc2 family of proteins (Ramirez et al., 2017; Courtney et al., 2018). In addition to these molecular differences, endocytosis of spontaneously fused SVs occurs at a faster timescale (<1 s) and is partially calcium independent (Leitz and Kavalali, 2014), implicating a distinct mode of endocytosis. Thus, several modes of endocytosis may maintain the SV supply at presynaptic terminals to support distinct phases of neurotransmission.
Modes of SV recycling
At present, at least four modes of SV recycling have been identified, distinguished by their molecular mechanisms and speed: CME, kiss-and-run, ultrafast endocytosis, and ADBE (Fig. 2).
Figure 2.
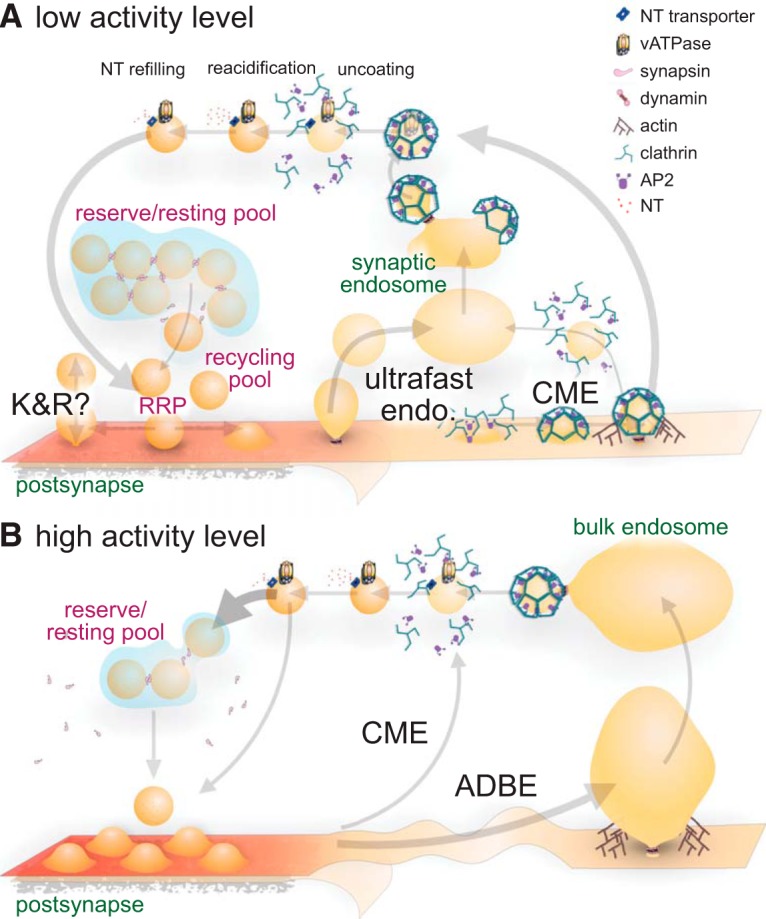
Modern view of the SV cycle. A, During low activity levels, SVs are recruited to the RRP from the reserve/resting pool and fuse at the active zone, after which they may be retrieved via one of several mechanisms: (1) the fusion pore may reclose by kiss-and-run, (2) ultrafast endocytosis at the periactive zone can retrieve endocytic vesicles that rapidly fuse with synaptic endosomes from which SVs regenerate in a clathrin-dependent manner, or (3) CME can generate SVs from the plasma membrane in certain circumstances, after which vesicle uncoating is necessary for the vATPase to acidify the lumen triggering concurrent neurotransmitter (NT) refilling by transporters. After refilling, SVs can be recruited back to the cluster, where they are segregated into functional pools. B, At high activity levels, many SVs are mobilized and exocytosed by full-collapse fusion. This activates ADBE, which retrieves large areas of membrane generating bulk endosomes from which SVs regenerate.
CME versus kiss-and-run
Over the last two decades, many studies have focused on addressing whether SVs are recycled via CME or kiss-and-run. The kinetics and molecular requirements distinguish these two modes of endocytosis: CME is relatively slow (10–30 s) and requires a distinct set of molecules (Fig. 2A) (Saheki and De Camilli, 2012; Milosevic, 2018). In contrast, kiss-and-run is fast (<1–2 s) and does not require clathrin-associated proteins. Thus, these features have been investigated extensively at model synapses ranging from those in invertebrates such as nematodes (Caenorhabditis elegans) (Nonet et al., 1999), fruit fly (Zhang et al., 1998; Heerssen et al., 2008), and squid (Morgan et al., 1999, 2000, 2001), to vertebrates such as lampreys (Shupliakov et al., 1997; Walsh et al., 2018) and rodents (Granseth et al., 2006; Mani et al., 2007). At squid synapses, disrupting the functions of core clathrin coat components, such as adaptor proteins (AP180, AP-2) or clathrin-uncoating proteins (Hsc70, auxilin) severely impaired neurotransmission, indicating an essential role for the clathrin pathway (Morgan et al., 1999, 2000, 2001). At mammalian nerve terminals, knock-down of clathrin-heavy chain suggested that almost all endocytosis is clathrin mediated (Granseth et al., 2006). A unified view from these studies is that SV recycling requires clathrin and clathrin-associated proteins, and, where measured, occurs slowly with a single kinetic component (Balaji and Ryan, 2007; but see Zhu et al., 2009). These studies led to the idea that CME predominates in these synapses. For more complete reviews on molecular mechanisms of CME, please refer to recent articles (Saheki and De Camilli, 2012; Milosevic, 2018).
Nonetheless, the essential role for CME in recycling of SVs from the plasma membrane and retrieval of SV cargos has been questioned. More recent data suggest that endocytosis can proceed after knockdown of clathrin heavy chain or its adaptor AP-2 (Kim and Ryan, 2009; Kononenko et al., 2014; Watanabe et al., 2014), pharmacological inhibition (Delvendahl et al., 2016), acute photo-inactivation (Heerssen et al., 2008; Kasprowicz et al., 2008) or using temperature-sensitive clathrin heavy chain mutants (Yu et al., 2018), though in some cases compensatory endocytosis was aberrant and insufficient for regenerating SVs or sustaining neurotransmission (Heerssen et al., 2008; Kasprowicz et al., 2008). This lack of an obligatory requirement for CME was most prevalent at physiological temperatures in mammalian neurons (Watanabe et al., 2014; Delvendahl et al., 2016; Soykan et al., 2017). However, in all cases, clathrin is necessary during the SV cycle either at the plasma membrane, as previously understood, or from intracellular endosomes, as described below.
Ultrafast endocytosis
Another mode for SV recycling called “ultrafast endocytosis” was recently identified (Fig. 2A). Ultrafast endocytosis can complete in as fast as 50 ms after exocytosis and continues stochastically only for seconds (Watanabe et al., 2013a,b; Delvendahl et al., 2016). This mode of SV recycling is predominant at physiological temperatures in both C. elegans neuromuscular junctions (NMJs, room temperature) and mouse hippocampal synapses (34−37°C). It occurs after brief neuronal activity, but may also operate during high-frequency stimulation (Watanabe et al., 2014; Soykan et al., 2017). In C. elegans NMJs and mouse central synapses, the lateral edges of an active zone mark sites of ultrafast endocytosis. Membrane at these sites rapidly invaginates to form a large endocytic vesicle (∼80 nm) without the requirement for clathrin. These endocytic vesicles are delivered immediately to synaptic endosomes from which SVs are regenerated via budding in a clathrin-dependent manner (Watanabe et al., 2014). Membrane flux through exocytosis and ultrafast endocytosis is approximately equal. During trains of stimuli, ultrafast endocytosis is triggered multiple times to compensate for excess membrane added through SV fusion. It is worth noting that a form of clathrin-independent fast endocytosis has been observed in retinal bipolar neurons (von Gersdorff and Matthews, 1994), and ultrafast endocytosis shares many features with this pathway.
Because ultrafast endocytosis was discovered only recently, its molecular mechanisms have not been explored as extensively as other modes of SV recycling. However, several studies suggest that ultrafast endocytosis shares many molecular players with other endocytic pathways including CME. For example, synaptojanin-1 and endophilin-A, two key players in CME (Verstreken et al., 2003; Milosevic et al., 2011), coordinately tubulate the invaginated membrane at its base, forming a narrow neck on the budding vesicle (Watanabe et al., 2018). The vesicle is then pinched off at the neck by the actions of a large GTPase, dynamin-1 (Watanabe et al., 2013a,b). Polymerized actin is also essential in ultrafast endocytosis (Watanabe et al., 2013a), as it is during clathrin-dependent and clathrin-independent endocytosis at synapses (Shupliakov et al., 2002; Soykan et al., 2017). Theoretical and computational modeling studies suggest that ultrafast endocytosis relies on proper maintenance of membrane tension (Shi and Baumgart, 2015), which may be influenced by actin. Interestingly, ultrafast endocytosis fails completely under conditions where membrane fluidity is reduced, for example by rapid cooling of cultured mouse neurons to room temperature (Watanabe et al., 2014). Further studies are required to elucidate the exact mechanism of ultrafast endocytosis.
Activity-dependent bulk endocytosis
In contrast to ultrafast endocytosis, longer bursts of intense activity trigger ADBE at invertebrate, amphibian, and mammalian synapses (Clayton et al., 2008; Gan and Watanabe, 2018) and in vivo (Körber et al., 2012). ADBE retrieves large areas of membrane within 1–2 s to form intracellular endosomes (average ∼150 nm) in a process that is clathrin-independent (Fig. 2B) (Clayton and Cousin, 2009; Kononenko and Haucke, 2015). This strict coupling of ADBE to neuronal activity is due to the transient activation of the calcium-dependent protein phosphatase calcineurin (Kokotos and Cousin, 2015). Recent studies have also highlighted a key role for the actin cytoskeleton in ADBE (Wu et al., 2016; Soykan et al., 2017). This suggests that a rapid, actin-dependent invagination drives formation of the bulk endosome, which may be coupled to neuronal activity by altered membrane tension during SV fusion events (Fig. 2B). Inhibition of ADBE results in a modest relief of short-term depression (Clayton et al., 2010; Smillie et al., 2013), potentially by increasing the efficiency of SV cargo capture at the periactive zone. However, ADBE inhibition results in a reduced capacity to sustain neurotransmitter release in the longer term (Nicholson-Fish et al., 2015). When one considers the scope for its bidirectional modulation (Smillie et al., 2013; Kokotos et al., 2018), this suggests that ADBE provides a plastic, scalable mechanism to alter neuronal output.
Typical SV proteins (cargoes) such as VAMP2, synaptophysin, and vesicular glutamate transporter (v-Glut) are retrieved by ADBE (Nicholson-Fish et al., 2015; Kokotos et al., 2018), though it is unclear whether this retrieval is direct or due to escape of excess cargo from saturated clustering mechanisms at the periactive zone. Some cargoes, such as VAMP4, are preferentially accumulated by ADBE, perhaps explaining why VAMP4 is also essential for this mode of endocytosis (Nicholson-Fish et al., 2015). Interestingly, the SV-associated calcium channel Flower, which is deposited into the plasma membrane during high activity, may provide calcium influx to trigger ADBE and thus facilitate the coupling of neuronal activity to ADBE (Yao et al., 2009, 2017). Therefore, specific vesicle proteins may play direct roles in ADBE rather than being passively retrieved.
After ADBE, subsequent SV budding from internalized membrane requires the efflux of previously accumulated extracellular calcium, which is driven by endosomal acidification (Cheung and Cousin, 2013). Cargo selection most likely occurs at this step, since both the classical plasma membrane adaptor AP-2 and endosomal AP-1/AP-3 are required for SV generation from bulk endosomes (Kononenko et al., 2014; Kokotos and Cousin, 2015). Because endophilin-dependent recruitment of synaptojanin-1 is determined by membrane curvature (Chang-Ileto et al., 2011; Milosevic et al., 2011), this hybrid requirement for adaptors during ADBE may arise from heterogeneity in bulk endosome size (range: 100–500 nm). With larger bulk endosomes, which have shallower membrane curvature, endophilin and synaptojanin-1 recruitment would be inefficient, resulting in stabilized PI(4,5)P2 and therefore enhanced AP-2-dependent cargo sorting, whereas smaller endosomes may use AP-1/AP-3. Consequently, the requirement of different adaptor proteins may result in SVs with varying molecular compositions, resulting in the functional heterogeneity discussed above (Silm et al., 2019).
Current view of SV recycling
Although decades of research implicate clathrin as an essential player in the regeneration of SVs, the location of these events may be dictated by stimulus intensity, temperature, and synapse type. In general, current data suggest that during lower activity levels and at temperatures significantly lower than physiological temperature most endocytic events are clathrin mediated, since ADBE is inactive and ultrafast endocytosis is highly temperature-sensitive (Fig. 2A). At near-physiological temperature, regardless of stimulation, nascent plasma membrane sites of clathrin-mediated budding may be relocated to rapidly forming endosomes, although exceptions do exist. For example, squid and lampreys, which live at cooler temperatures (4–25°C), may use CME exclusively for recycling SVs (Gad et al., 1998; Morgan et al., 2000). Thus, SV recycling might have evolved to adapt to changes in activity and environmental conditions.
The essential requirement for clathrin during SV reformation may underscore why mutations or alterations in the levels of several well characterized clathrin-associated proteins are linked to neurodegeneration. These include deficiency in membrane curvature sensing protein, endophilin-A, which is linked to age-dependent ataxia (Murdoch et al., 2016), as well as mutations in phosphoinositide phosphatase synaptojanin-1 and putative tyrosine-protein phosphatase auxilin, which are linked to inherited forms of Parkinson's disease or parkinsonism (Edvardson et al., 2012; Krebs et al., 2013). Similarly, a selective reduction of the clathrin adaptors AP180 and AP-2 has been reported in Alzheimer's disease (Yao and Coleman, 1998). Thus, there are numerous links between defects in the clathrin pathway and neurodegenerative diseases.
With several new endocytic models revealed, the debate on SV recycling mechanisms is far from being resolved (Wu et al., 2014). Under all conditions discussed above, additional roles for kiss-and-run cannot be ruled out. The presence of kiss-and-run is well established in non-neuronal secretory cells (Alés et al., 1999; Burgoyne et al., 2001). Although scarcer, several optical approaches also indicate its existence at mammalian central synapses (Stevens and Williams, 2000; Zhang et al., 2009; Chanaday and Kavalali, 2018b). Given the modulatory nature of SV cycling, it would be important to understand at what stimulation frequency and temperature kiss-and-run is prevalent and which molecules stabilize the rapidly expanding fusion pore. With the refinement of tools and approaches, a better understanding of these processes will likely arise in coming years.
Mechanisms of SV (re)acidification and (re)filling
After endocytosis and vesicle reformation, newly formed SVs must be refilled with neurotransmitter and made fusion-ready (Blakely and Edwards, 2012; Farsi et al., 2017). Regardless of the mechanism of vesicle reformation, each SV must be rapidly loaded with more than a thousand neurotransmitter molecules (Riveros et al., 1986; Burger et al., 1989). The key components that execute neurotransmitter filling are the vacuolar H+-ATPase (vATPase) and the vesicular neurotransmitter transporters. The evolutionarily conserved vATPase is a large multiprotein complex that consists of an integral V0 domain, which translocates protons across the membrane, and a peripheral V1 domain responsible for ATP hydrolysis (Stevens and Forgac, 1997; Toei et al., 2010). The vesicular neurotransmitter transporters determine neurotransmitter content (Grønborg et al., 2010). These two groups of proteins mediate distinct processes: the vATPase rapidly forms an electrochemical gradient (ΔμH+) across the membrane by pumping protons into the lumen of SVs with subsecond kinetics, whereas the transporters use this gradient to shuttle the neurotransmitter molecules into the SVs, although the exact loading mechanism differs depending on the charge of particular neurotransmitters (Blakely and Edwards, 2012). Nonetheless, under physiological conditions where ATP and neurotransmitter are abundant and readily available, these two processes likely occur in parallel.
Each SV isolated from mammalian brain contains many tens of copies of vesicular transporters, but only one or two copies of the vATPase (Takamori et al., 2006). The recycling of vATPases and neurotransmitter transporters must therefore be tightly coupled with SV recycling, and at least one copy of the vATPase must be sorted into each SV to allow subsequent neurotransmitter loading in the vesicle. In addition, recycled SVs should contain a proper set of transporters, particularly when more than one type of neurotransmitter transporters are available in the same neurons (i.e., vesicular monoamine and glutamate transporters). Sorting of transporters requires clathrin and multiple adaptor protein complexes (AP1, AP2, and AP3) (Onoa et al., 2010; Blakely and Edwards, 2012; Silm et al., 2019), again pointing to the essential roles of clathrin-mediated processes in SV recycling.
In addition to proper sorting of SV proteins, clathrin likely plays an essential role in determining the timing of vesicle acidification and thereby neurotransmitter loading. A recent study suggests that reacidification of SVs relies on removal of clathrin-coats from vesicles, due to steric hindrance of the vATPase by clathrin cages (Farsi et al., 2018). Upon uncoating, vesicles rapidly acidify, suggesting that the removal of clathrin-coats ensures that neurotransmitter is loaded as soon as SVs are reformed. Although partially filled SVs are fusion-competent, incompletely filled vesicles have a lower release probability (Rost et al., 2015). Thus, by ensuring proper loading of neurotransmitter into vesicles, fidelity of neurotransmission is maintained.
SV “maturation” and clustering
Finally, new SVs are captured into discrete SV clusters. During prolonged stimulation, vesicles are mobilized from these clusters to ensure continued neurotransmitter release. The primary components for vesicle clustering are the synapsins, which are highly abundant phosphoproteins that reversibly associate with SVs (De Camilli et al., 1983; Chi et al., 2001). Synapsins maintain the reserve pool via phosphorylation-dependent interactions with SVs and the actin cytoskeleton (Pieribone et al., 1995; Bloom et al., 2003; Gitler et al., 2008). Synapsins also functionally interact with α-synuclein (Atias et al., 2019), peripheral Rab3 proteins (Giovedì et al., 2004), and other Rab GTPases and their interactors (Pavlos and Jahn, 2011), to regulate SV clustering. Importantly, loss of function of synapsins is associated with a number of neurological and neuropsychiatric disorders, including autism, schizophrenia, and epilepsy (Garcia et al., 2004; Porton et al., 2011; Greco et al., 2013).
One critical aspect of vesicle clustering that has remained unclear is how all these proteins keep SVs clustered together while still allowing vesicle mobility. A recent study suggested that SV clusters represent an example of liquid condensates—distinct phase of liquid in aqueous environment, where lipid vesicles are captured by proteins of the interweaving matrix (Milovanovic and De Camilli, 2017). Indeed, synapsin was shown to organize vesicles in clusters in vitro by liquid–liquid phase separation, thereby suggesting that SV clustering at the presynaptic terminal can be explained at least in part by the phase separation principle (Milovanovic et al., 2018). In addition, some endocytic proteins, including amphiphysin, dynamin-1, and intersectin-1, have been found among the matrix components connecting SVs at resting state (Shupliakov et al., 2011), raising the possibility that the SV cluster may additionally provide a source for proteins involved in vesicle recycling. Upon stimulation, these endocytic proteins translocate to the periactive zone, thus coupling the processes of exocytosis and endocytosis (Evergren et al., 2004).
Conclusions
In summary, it is now recognized that the SV cycle is much more complex than previously thought. Given how important neurotransmission is to survival, in hindsight, it may not be so surprising that synapses harbor multiple modes of SV exocytosis and endocytosis to ensure their fidelity despite differences in activity levels and physiological temperatures and to accommodate different release modes or synapse types. In cold-blooded animals, for example, the modes of SV recycling may shift seasonally as the animals adapt to environmental changes in temperature. Emerging evidence also suggests that the different modes of vesicle recycling may supply SVs that are “tuned” (in molecular terms) to the function of the neuron. This might be especially important at synapses with phasic versus tonic activity or with different rates of spontaneous release, or at sensory synapses that require particularly fast forms of neurotransmission.
Given this new knowledge, it will become increasingly important to measure SV recycling under experimental conditions that best mimic the synapses' normal physiology or, in cases where this is not known, across different temperatures and stimulation intensities. Likewise, as we go forward in different model systems, it is essential to determine when and where the clathrin machinery acts during SV recycling. Such studies may reveal a molecular convergence between the different vesicle retrieval modes, or conversely highlight specific presynaptic adaptations driven by the variables listed above. Given the rapidly changing field, there are likely to be additional significant advances in the coming years that further illuminate the regulatory mechanisms of SV cycling and how they play together to ensure ongoing neurotransmission.
Footnotes
This work was supported by: Schram-Stiftung T287/25457 and Deutsche Forschungsgemeinschaft (Emmy Noether Young Investigator Award MI-1702/1 to I.M.); the Wellcome Trust (204954/Z/16/Z to M.A.C.); the National Science Foundation (1727260 to S.W.), the National Institutes of Health (NINDS DP2 NS111133 and R01 NS105810 to S.W.); the McKnight Foundation (S.W.); the Sloan Foundation (S.W.); and the National Institutes of Health (NINDS/NIA R01 NS078165 to J.R.M. and NIMH R01 MH066198 to Dr. Ege Kavalali, which supports N.L.C.). We thank Dragomir Milovanovic for helpful comments on this manuscript.
The authors declare no competing financial interests.
References
- Alés E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G (1999) High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol 1:40–44. 10.1038/9012 [DOI] [PubMed] [Google Scholar]
- Atias M, Tevet Y, Sun J, Stavsky A, Tal S, Kahn J, Roy S, Gitler D (2019) Synapsins regulate alpha-synuclein functions. Proc Natl Acad Sci U S A 116:11116–11118. 10.1073/pnas.1903054116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacaj T, Wu D, Yang X, Morishita W, Zhou P, Xu W, Malenka RC, Südhof TC (2013) Synaptotagmin-1 and synaptotagmin-7 trigger synchronous and asynchronous phases of neurotransmitter release. Neuron 80:947–959. 10.1016/j.neuron.2013.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji J, Ryan TA (2007) Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc Natl Acad Sci U S A 104:20576–20581. 10.1073/pnas.0707574105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal M, Leitz J, Reese AL, Ramirez DM, Durakoglugil M, Herz J, Monteggia LM, Kavalali ET (2013) Reelin mobilizes a VAMP7-dependent synaptic vesicle pool and selectively augments spontaneous neurotransmission. Neuron 80:934–946. 10.1016/j.neuron.2013.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely RD, Edwards RH (2012) Vesicular and plasma membrane transporters for neurotransmitters. Cold Spring Harb Perspect Biol 4:a005595. 10.1101/cshperspect.a005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom O, Evergren E, Tomilin N, Kjaerulff O, Löw P, Brodin L, Pieribone VA, Greengard P, Shupliakov O (2003) Colocalization of synapsin and actin during synaptic vesicle recycling. J Cell Biol 161:737–747. 10.1083/jcb.200212140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger AT, Choi UB, Lai Y, Leitz J, White KI, Zhou Q (2019) The pre-synaptic fusion machinery. Curr Opin Struct Biol 54:179–188. 10.1016/j.sbi.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger PM, Mehl E, Cameron PL, Maycox PR, Baumert M, Lottspeich F, De Camilli P, Jahn R (1989) Synaptic vesicles immunoisolated from rat cerebral cortex contain high levels of glutamate. Neuron 3:715–720. 10.1016/0896-6273(89)90240-7 [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Fisher RJ, Graham ME (2001) Regulation of kiss-and-run exocytosis. Trends Cell Biol 11:404–405. 10.1016/S0962-8924(01)02089-X [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP, Mauro A (1973) Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol 57:499–524. 10.1083/jcb.57.2.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanaday NL, Kavalali ET (2018a) Optical detection of three modes of endocytosis at hippocampal synapses. Elife 7:e36097. 10.7554/eLife.36097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanaday NL, Kavalali ET (2018b) Presynaptic origins of distinct modes of neurotransmitter release. Curr Opin Neurobiol 51:119–126. 10.1016/j.conb.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Ileto B, Frere SG, Chan RB, Voronov SV, Roux A, Di Paolo G (2011) Synaptojanin 1-mediated PI(4,5)P2 hydrolysis is modulated by membrane curvature and facilitates membrane fission. Dev Cell 20:206–218. 10.1016/j.devcel.2010.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung G, Cousin MA (2013) Synaptic vesicle generation from activity-dependent bulk endosomes requires calcium and calcineurin. J Neurosci 33:3370–3379. 10.1523/JNEUROSCI.4697-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA (2001) Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci 4:1187–1193. 10.1038/nn756 [DOI] [PubMed] [Google Scholar]
- Clayton EL, Cousin MA (2009) The molecular physiology of activity-dependent bulk endocytosis of synaptic vesicles. J Neurochem 111:901–914. 10.1111/j.1471-4159.2009.06384.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Evans GJ, Cousin MA (2008) Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation. J Neurosci 28:6627–6632. 10.1523/JNEUROSCI.1445-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Sue N, Smillie KJ, O'Leary T, Bache N, Cheung G, Cole AR, Wyllie DJ, Sutherland C, Robinson PJ, Cousin MA (2010) Dynamin I phosphorylation by GSK3 controls activity-dependent bulk endocytosis of synaptic vesicles. Nat Neurosci 13:845–851. 10.1038/nn.2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney NA, Briguglio JS, Bradberry MM, Greer C, Chapman ER (2018) Excitatory and inhibitory neurons utilize different Ca(2+) sensors and sources to regulate spontaneous release. Neuron 98:977–991.e5. 10.1016/j.neuron.2018.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Kavalali ET (2015) Molecular underpinnings of synaptic vesicle pool heterogeneity. Traffic 16:338–364. 10.1111/tra.12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Harris SM Jr, Huttner WB, Greengard P (1983) Synapsin I (Protein I), a nerve terminal-specific phosphoprotein. II. its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J Cell Biol 96:1355–1373. 10.1083/jcb.96.5.1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvendahl I, Vyleta NP, von Gersdorff H, Hallermann S (2016) Fast, temperature-sensitive and clathrin-independent endocytosis at central synapses. Neuron 90:492–498. 10.1016/j.neuron.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker A, Rizzoli SO (2010) Synaptic vesicle pools: an update. Front Synaptic Neurosci 2:135. 10.3389/fnsyn.2010.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker A, Bethani I, Kröhnert K, Körber C, Horstmann H, Wilhelm BG, Barysch SV, Kuner T, Neher E, Rizzoli SO (2011) A small pool of vesicles maintains synaptic activity in vivo. Proc Natl Acad Sci U S A 108:17177–17182. 10.1073/pnas.1112688108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Ryan TA (2019) The control of release probability at nerve terminals. Nat Rev Neurosci 20:177–186. 10.1038/s41583-018-0111-3 [DOI] [PubMed] [Google Scholar]
- Edvardson S, Cinnamon Y, Ta-Shma A, Shaag A, Yim YI, Zenvirt S, Jalas C, Lesage S, Brice A, Taraboulos A, Kaestner KH, Greene LE, Elpeleg O (2012) A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS One 7:e36458. 10.1371/journal.pone.0036458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evergren E, Marcucci M, Tomilin N, Löw P, Slepnev V, Andersson F, Gad H, Brodin L, De Camilli P, Shupliakov O (2004) Amphiphysin is a component of clathrin coats formed during synaptic vesicle recycling at the lamprey giant synapse. Traffic 5:514–528. 10.1111/j.1398-9219.2004.00198.x [DOI] [PubMed] [Google Scholar]
- Farsi Z, Jahn R, Woehler A (2017) Proton electrochemical gradient: driving and regulating neurotransmitter uptake. Bioessays. Advance online publication. Retrieved April 6, 2017. doi: 10.1002/bies.201600240. 10.1002/bies.201600240 [DOI] [PubMed] [Google Scholar]
- Farsi Z, Gowrisankaran S, Krunic M, Rammner B, Woehler A, Lafer EM, Mim C, Jahn R, Milosevic I (2018) Clathrin coat controls synaptic vesicle acidification by blocking vacuolar ATPase activity. Elife 7:e32569. 10.7554/eLife.32569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesce R, Grohovaz F, Valtorta F, Meldolesi J (1994) Neurotransmitter release: fusion or 'kiss-and-run'? Trends Cell Biol 4:1–4. 10.1016/0962-8924(94)90025-6 [DOI] [PubMed] [Google Scholar]
- Fredj NB, Burrone J (2009) A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci 12:751–758. 10.1038/nn.2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad H, Löw P, Zotova E, Brodin L, Shupliakov O (1998) Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron 21:607–616. 10.1016/S0896-6273(00)80570-X [DOI] [PubMed] [Google Scholar]
- Gan Q, Watanabe S (2018) Synaptic vesicle endocytosis in different model systems. Front Cell Neurosci 12:171. 10.3389/fncel.2018.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CC, Blair HJ, Seager M, Coulthard A, Tennant S, Buddles M, Curtis A, Goodship JA (2004) Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J Med Genet 41:183–186. 10.1136/jmg.2003.013680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovedì S, Vaccaro P, Valtorta F, Darchen F, Greengard P, Cesareni G, Benfenati F (2004) Synapsin is a novel Rab3 effector protein on small synaptic vesicles. I. Identification and characterization of the synapsin I-Rab3 interactions in vitro and in intact nerve terminals. J Biol Chem 279:43760–43768. 10.1074/jbc.M403293200 [DOI] [PubMed] [Google Scholar]
- Gitler D, Cheng Q, Greengard P, Augustine GJ (2008) Synapsin IIa controls the reserve pool of glutamatergic synaptic vesicles. J Neurosci 28:10835–10843. 10.1523/JNEUROSCI.0924-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granseth B, Odermatt B, Royle SJ, Lagnado L (2006) Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron 51:773–786. 10.1016/j.neuron.2006.08.029 [DOI] [PubMed] [Google Scholar]
- Greco B, Managò F, Tucci V, Kao HT, Valtorta F, Benfenati F (2013) Autism-related behavioral abnormalities in synapsin knockout mice. Behav Brain Res 251:65–74. 10.1016/j.bbr.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønborg M, Pavlos NJ, Brunk I, Chua JJ, Münster-Wandowski A, Riedel D, Ahnert-Hilger G, Urlaub H, Jahn R (2010) Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein. J Neurosci 30:2–12. 10.1523/JNEUROSCI.4074-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Ge JL, Hao M, Sun ZC, Wu XS, Zhu JB, Wang W, Yao PT, Lin W, Xue L (2015) A three-pool model dissecting readily releasable pool replenishment at the calyx of held. Sci Rep 5:9517. 10.1038/srep09517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Palfreyman MT, Watanabe S, Olsen S, Jorgensen EM (2007) Open syntaxin docks synaptic vesicles. PLoS Biol 5:e198. 10.1371/journal.pbio.0050198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerssen H, Fetter RD, Davis GW (2008) Clathrin dependence of synaptic-vesicle formation at the Drosophila neuromuscular junction. Curr Biol 18:401–409. 10.1016/j.cub.2008.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS (1973) Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol 57:315–344. 10.1083/jcb.57.2.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderith N, Lorincz A, Katona G, Rózsa B, Kulik A, Watanabe M, Nusser Z (2012) Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat Neurosci 15:988–997. 10.1038/nn.3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS (2016) Asynchronous presynaptic glutamate release enhances neuronal excitability during the post-spike refractory period. J Physiol 594:1005–1015. 10.1113/JP271485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Fasshauer D (2012) Molecular machines governing exocytosis of synaptic vesicles. Nature 490:201–207. 10.1038/nature11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas EA. (2014) Contributions of bcl-xL to acute and long term changes in bioenergetics during neuronal plasticity. Biochim Biophys Acta 1842:1168–1178. 10.1016/j.bbadis.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprowicz J, Kuenen S, Miskiewicz K, Habets RL, Smitz L, Verstreken P (2008) Inactivation of clathrin heavy chain inhibits synaptic recycling but allows bulk membrane uptake. J Cell Biol 182:1007–1016. 10.1083/jcb.200804162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET. (2015) The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci 16:5–16. 10.1038/nrn3875 [DOI] [PubMed] [Google Scholar]
- Kim SH, Ryan TA (2009) Synaptic vesicle recycling at CNS snapses without AP-2. J Neurosci 29:3865–3874. 10.1523/JNEUROSCI.5639-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ryan TA (2010) CDK5 serves as a major control point in neurotransmitter release. Neuron 67:797–809. 10.1016/j.neuron.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokotos AC, Cousin MA (2015) Synaptic vesicle generation from central nerve terminal endosomes. Traffic 16:229–240. 10.1111/tra.12235 [DOI] [PubMed] [Google Scholar]
- Kokotos AC, Peltier J, Davenport EC, Trost M, Cousin MA (2018) Activity-dependent bulk endocytosis proteome reveals a key presynaptic role for the monomeric GTPase Rab11. Proc Natl Acad Sci U S A 115:E10177–E10186. 10.1073/pnas.1809189115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko NL, Haucke V (2015) Molecular mechanisms of presynaptic membrane retrieval and synaptic vesicle reformation. Neuron 85:484–496. 10.1016/j.neuron.2014.12.016 [DOI] [PubMed] [Google Scholar]
- Kononenko NL, Puchkov D, Classen GA, Walter AM, Pechstein A, Sawade L, Kaempf N, Trimbuch T, Lorenz D, Rosenmund C, Maritzen T, Haucke V (2014) Clathrin/AP-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses. Neuron 82:981–988. 10.1016/j.neuron.2014.05.007 [DOI] [PubMed] [Google Scholar]
- Körber C, Horstmann H, Sätzler K, Kuner T (2012) Endocytic structures and synaptic vesicle recycling at a central synapse in awake rats. Traffic 13:1601–1611. 10.1111/tra.12007 [DOI] [PubMed] [Google Scholar]
- Krebs CE, Karkheiran S, Powell JC, Cao M, Makarov V, Darvish H, Di Paolo G, Walker RH, Shahidi GA, Buxbaum JD, De Camilli P, Yue Z, Paisán-Ruiz C (2013) The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive parkinsonism with generalized seizures. Hum Mutat 34:1200–1207. 10.1002/humu.22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitz J, Kavalali ET (2014) Fast retrieval and autonomous regulation of single spontaneously recycling synaptic vesicles. Elife 3:e03658. 10.7554/eLife.03658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Chanaday NL, Xu W, Kavalali ET (2017) Synaptotagmin-1- and synaptotagmin-7-dependent fusion mechanisms target synaptic vesicles to kinetically distinct endocytic pathways. Neuron 93:616–631.e3. 10.1016/j.neuron.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Südhof TC (2017) Synaptotagmin-7-mediated asynchronous release boosts high-fidelity synchronous transmission at a central synapse. Neuron 94:826–839.e3. 10.1016/j.neuron.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Mani M, Lee SY, Lucast L, Cremona O, Di Paolo G, De Camilli P, Ryan TA (2007) The dual phosphatase activity of synaptojanin1 is required for both efficient synaptic vesicle endocytosis and reavailability at nerve terminals. Neuron 56:1004–1018. 10.1016/j.neuron.2007.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic I. (2018) Revisiting the role of clathrin-mediated endoytosis in synaptic vesicle recycling. Front Cell Neurosci 12:27. 10.3389/fncel.2018.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic I, Giovedì S, Lou X, Raimondi A, Collesi C, Shen H, Paradise S, O'Toole E, Ferguson S, Cremona O, De Camilli P (2011) Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron 72:587–601. 10.1016/j.neuron.2011.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic D, De Camilli P (2017) Synaptic vesicle clusters at synapses: a distinct liquid phase? Neuron 93:995–1002. 10.1016/j.neuron.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic D, Wu Y, Bian X, De Camilli P (2018) A liquid phase of synapsin and lipid vesicles. Science 361:604–607. 10.1126/science.aat5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Zhao X, Womack M, Prasad K, Augustine GJ, Lafer EM (1999) A role for the clathrin assembly domain of AP180 in synaptic vesicle endocytosis. J Neurosci 19:10201–10212. 10.1523/JNEUROSCI.19-23-10201.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Prasad K, Hao W, Augustine GJ, Lafer EM (2000) A conserved clathrin assembly motif essential for synaptic vesicle endocytosis. J Neurosci 20:8667–8676. 10.1523/JNEUROSCI.20-23-08667.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Prasad K, Jin S, Augustine GJ, Lafer EM (2001) Uncoating of clathrin-coated vesicles in presynaptic terminals: roles for Hsc70 and auxilin. Neuron 32:289–300. 10.1016/S0896-6273(01)00467-6 [DOI] [PubMed] [Google Scholar]
- Morgan JR, Comstra HS, Cohen M, Faundez V (2013) Presynaptic membrane retrieval and endosome biology: defining molecularly heterogeneous synaptic vesicles. Cold Spring Harb Perspect Biol 5:a016915. 10.1101/cshperspect.a016915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JD, Rostosky CM, Gowrisankaran S, Arora AS, Soukup SF, Vidal R, Capece V, Freytag S, Fischer A, Verstreken P, Bonn S, Raimundo N, Milosevic I (2016) Endophilin-A deficiency induces the Foxo3a-Fbxo32 network in the brain and causes dysregulation of autophagy and the ubiquitin-proteasome system. Cell Rep 17:1071–1086. 10.1016/j.celrep.2016.09.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Brose N (2018) Dynamically primed synaptic vesicle states: key to understand synaptic short-term plasticity. Neuron 100:1283–1291. 10.1016/j.neuron.2018.11.024 [DOI] [PubMed] [Google Scholar]
- Nicholson-Fish JC, Kokotos AC, Gillingwater TH, Smillie KJ, Cousin MA (2015) VAMP4 is an essential cargo molecule for activity-dependent bulk endocytosis. Neuron 88:973–984. 10.1016/j.neuron.2015.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet ML, Holgado AM, Brewer F, Serpe CJ, Norbeck BA, Holleran J, Wei L, Hartwieg E, Jorgensen EM, Alfonso A (1999) UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Mol Biol Cell 10:2343–2360. 10.1091/mbc.10.7.2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoa B, Li H, Gagnon-Bartsch JA, Elias LA, Edwards RH (2010) Vesicular monoamine and glutamate transporters select distinct synaptic vesicle recycling pathways. J Neurosci 30:7917–7927. 10.1523/JNEUROSCI.5298-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu Y, Shahrezaei V, Li B, Raymond LA, Delaney KR, Murphy TH (2004) Competition between phasic and asynchronous release for recovered synaptic vesicles at developing hippocampal autaptic synapses. J Neurosci 24:420–433. 10.1523/JNEUROSCI.4452-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlos NJ, Jahn R (2011) Distinct yet overlapping roles of rab GTPases on synaptic vesicles. Small GTPases 2:77–81. 10.4161/sgtp.2.2.15201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik AJ, Greengard P (1995) Distinct pools of synaptic vesicles in neurotransmitter release. Nature 375:493–497. 10.1038/375493a0 [DOI] [PubMed] [Google Scholar]
- Porton B, Wetsel WC, Kao HT (2011) Synapsin III: role in neuronal plasticity and disease. Semin Cell Dev Biol 22:416–424. 10.1016/j.semcdb.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingo J, Khvotchev M, Liu P, Darios F, Li YC, Ramirez DM, Adachi M, Lemieux P, Toth K, Davletov B, Kavalali ET (2012) VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission. Nat Neurosci 15:738–745. 10.1038/nn.3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DM, Kavalali ET (2012) The role of non-canonical SNAREs in synaptic vesicle recycling. Cell Logist 2:20–27. 10.4161/cl.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DMO, Crawford DC, Chanaday NL, Trauterman B, Monteggia LM, Kavalali ET (2017) Loss of Doc2-dependent spontaneous neurotransmission augments glutamatergic synaptic strength. J Neurosci 37:6224–6230. 10.1523/JNEUROSCI.0418-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveros N, Fiedler J, Lagos N, Muñoz C, Orrego F (1986) Glutamate in rat brain cortex synaptic vesicles: influence of the vesicle isolation procedure. Brain Res 386:405–408. 10.1016/0006-8993(86)90181-2 [DOI] [PubMed] [Google Scholar]
- Rizo J, Südhof TC (2012) The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices–guilty as charged? Annu Rev Cell Dev Biol 28:279–308. 10.1146/annurev-cellbio-101011-155818 [DOI] [PubMed] [Google Scholar]
- Rizo J, Xu J (2015) The synaptic vesicle release machinery. Annu Rev Biophys 44:339–367. 10.1146/annurev-biophys-060414-034057 [DOI] [PubMed] [Google Scholar]
- Rost BR, Schneider F, Grauel MK, Wozny C, Bentz C, Blessing A, Rosenmund T, Jentsch TJ, Schmitz D, Hegemann P, Rosenmund C (2015) Optogenetic acidification of synaptic vesicles and lysosomes. Nat Neurosci 18:1845–1852. 10.1038/nn.4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki Y, De Camilli P (2012) Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol 4:a005645. 10.1101/cshperspect.a005645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deák F, Liu X, Kavalali ET (2005) An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron 45:563–573. 10.1016/j.neuron.2004.12.056 [DOI] [PubMed] [Google Scholar]
- Shi Z, Baumgart T (2015) Membrane tension and peripheral protein density mediate membrane shape transitions. Nat Commun 6:5974. 10.1038/ncomms6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Löw P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L (1997) Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science 276:259–263. 10.1126/science.276.5310.259 [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Bloom O, Gustafsson JS, Kjaerulff O, Löw P, Tomilin N, Pieribone VA, Greengard P, Brodin L (2002) Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc Natl Acad Sci U S A 99:14476–14481. 10.1073/pnas.212381799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Haucke V, Pechstein A (2011) How synapsin I may cluster synaptic vesicles. Semin Cell Dev Biol 22:393–399. 10.1016/j.semcdb.2011.07.006 [DOI] [PubMed] [Google Scholar]
- Silm K, Yang J, Marcott PF, Asensio CS, Eriksen J, Guthrie DA, Newman AH, Ford CP, Edwards RH (2019) Synaptic vesicle recycling pathway determines neurotransmitter content and release properties. Neuron 102:786–800.e5. 10.1016/j.neuron.2019.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie KJ, Pawson J, Perkins EM, Jackson M, Cousin MA (2013) Control of synaptic vesicle endocytosis by an extracellular signalling molecule. Nat Commun 4:2394. 10.1038/ncomms3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soykan T, Kaempf N, Sakaba T, Vollweiter D, Goerdeler F, Puchkov D, Kononenko NL, Haucke V (2017) Synaptic vesicle endocytosis occurs on multiple timescales and is mediated by formin-dependent actin assembly. Neuron 93:854–866.e4. 10.1016/j.neuron.2017.02.011 [DOI] [PubMed] [Google Scholar]
- Stevens CF, Williams JH (2000) “Kiss and run” exocytosis at hippocampal synapses. Proc Natl Acad Sci U S A 97:12828–12833. 10.1073/pnas.230438697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens TH, Forgac M (1997) Structure, function and regulation of the vacuolar (H+)-ATPase. Annu Rev Cell Dev Biol 13:779–808. 10.1146/annurev.cellbio.13.1.779 [DOI] [PubMed] [Google Scholar]
- Südhof TC. (2013) Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 80:675–690. 10.1016/j.neuron.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, et al. (2006) Molecular anatomy of a trafficking organelle. Cell 127:831–846. 10.1016/j.cell.2006.10.030 [DOI] [PubMed] [Google Scholar]
- Toei M, Saum R, Forgac M (2010) Regulation and isoform function of the V-ATPases. Biochemistry 49:4715–4723. 10.1021/bi100397s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Koh TW, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ (2003) Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron 40:733–748. 10.1016/S0896-6273(03)00644-5 [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G (1994) Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature 367:735–739. 10.1038/367735a0 [DOI] [PubMed] [Google Scholar]
- Walsh RB, Bloom OE, Morgan JR (2018) Acute manipulations of clathrin-mediated endocytosis at presynaptic nerve terminals. Methods Mol Biol 1847:65–82. 10.1007/978-1-4939-8719-1_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Rost BR, Camacho-Pérez M, Davis MW, Söhl-Kielczynski B, Rosenmund C, Jorgensen EM (2013a) Ultrafast endocytosis at mouse hippocampal synapses. Nature 504:242–247. 10.1038/nature12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Liu Q, Davis MW, Hollopeter G, Thomas N, Jorgensen NB, Jorgensen EM (2013b) Ultrafast endocytosis at Caenorhabditis elegans neuromuscular junctions. Elife 2:e00723. 10.7554/eLife.00723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Trimbuch T, Camacho-Pérez M, Rost BR, Brokowski B, Sohl-Kielczynski B, Felies A, Davis MW, Rosenmund C, Jorgensen EM (2014) Clathrin regenerates synaptic vesicles from endosomes. Nature 515:228–233. 10.1038/nature13846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Mamer LE, Raychaudhuri S, Luvsanjav D, Eisen J, Trimbuch T, Söhl-Kielczynski B, Fenske P, Milosevic I, Rosenmund C, Jorgensen EM (2018) Synaptojanin and endophilin mediate neck formation during ultrafast endocytosis. Neuron 98:1184–1197.e6. 10.1016/j.neuron.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Hamid E, Shin W, Chiang HC (2014) Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol 76:301–331. 10.1146/annurev-physiol-021113-170305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Lee SH, Sheng J, Zhang Z, Zhao WD, Wang D, Jin Y, Charnay P, Ervasti JM, Wu LG (2016) Actin is crucial for all kinetically distinguishable forms of endocytosis at synapses. Neuron 92:1020–1035. 10.1016/j.neuron.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CK, Lin YQ, Ly CV, Ohyama T, Haueter CM, Moiseenkova-Bell VY, Wensel TG, Bellen HJ (2009) A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell 138:947–960. 10.1016/j.cell.2009.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CK, Liu YT, Lee IC, Wang YT, Wu PY (2017) A Ca2+ channel differentially regulates Clathrin-mediated and activity-dependent bulk endocytosis. PLoS Biol 15:e2000931. 10.1371/journal.pbio.2000931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Gaffaney JD, Kwon SE, Chapman ER (2011) Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell 147:666–677. 10.1016/j.cell.2011.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PJ, Coleman PD (1998) Reduced O-glycosylated clathrin assembly protein AP180: implication for synaptic vesicle recycling dysfunction in Alzheimer's disease. Neurosci Lett 252:33–36. 10.1016/S0304-3940(98)00547-3 [DOI] [PubMed] [Google Scholar]
- Yu SC, Jánosi B, Liewald JF, Wabnig S, Gottschalk A (2018) Endophilin A and B join forces with clathrin to mediate synaptic vesicle recycling in Caenorhabditis elegans. Front Mol Neurosci 11:196. 10.3389/fnmol.2018.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Koh YH, Beckstead RB, Budnik V, Ganetzky B, Bellen HJ (1998) Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron 21:1465–1475. 10.1016/S0896-6273(00)80664-9 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li Y, Tsien RW (2009) The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science 323:1448–1453. 10.1126/science.1167373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Xu J, Heinemann SF (2009) Two pathways of synaptic vesicle retrieval revealed by single-vesicle imaging. Neuron 61:397–411. 10.1016/j.neuron.2008.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]


