Despite being potentially crucial for performance, recovery and rehabilitation, non-verbal cognitive functions have not been investigated comprehensively in patients with post-stroke aphasia. By administering a battery of tests of attention and executive function, Schumacher et al. identify six orthogonal non-verbal and language components that are associated with separable structural correlates.
Keywords: aphasia, executive functions, attention, principal components, univariate and multivariate brain-behaviour mapping
Abstract
There is growing awareness that aphasia following a stroke can include deficits in other cognitive functions and that these are predictive of certain aspects of language function, recovery and rehabilitation. However, data on attentional and executive (dys)functions in individuals with stroke aphasia are still scarce and the relationship to underlying lesions is rarely explored. Accordingly in this investigation, an extensive selection of standardized non-verbal neuropsychological tests was administered to 38 individuals with chronic post-stroke aphasia, in addition to detailed language testing and MRI. To establish the core components underlying the variable patients’ performance, behavioural data were explored with rotated principal component analyses, first separately for the non-verbal and language tests, then in a combined analysis including all tests. Three orthogonal components for the non-verbal tests were extracted, which were interpreted as shift-update, inhibit-generate and speed. Three components were also extracted for the language tests, representing phonology, semantics and speech quanta. Individual continuous scores on each component were then included in a voxel-based correlational methodology analysis, yielding significant clusters for all components. The shift-update component was associated with a posterior left temporo-occipital and bilateral medial parietal cluster, the inhibit-generate component was mainly associated with left frontal and bilateral medial frontal regions, and the speed component with several small right-sided fronto-parieto-occipital clusters. Two complementary multivariate brain-behaviour mapping methods were also used, which showed converging results. Together the results suggest that a range of brain regions are involved in attention and executive functioning, and that these non-language domains play a role in the abilities of patients with chronic aphasia. In conclusion, our findings confirm and extend our understanding of the multidimensionality of stroke aphasia, emphasize the importance of assessing non-verbal cognition in this patient group and provide directions for future research and clinical practice. We also briefly compare and discuss univariate and multivariate methods for brain-behaviour mapping.
Introduction
There is a growing understanding that a left hemispheric stroke leading to impairments in language processing—aphasia—often also affects other cognitive functions, such as attention or executive functions (Glosser and Goodglass, 1990; Helm-Estabrooks, 2002; Jefferies and Lambon Ralph, 2006; Murray, 2012; Villard and Kiran, 2017) and it has been shown that impairments in these cognitive functions play an important role in aphasia recovery and rehabilitation (Fillingham et al., 2005; van de Sandt-Koenderman et al., 2008; Lambon Ralph et al., 2010; Brownsett et al., 2014; El Hachioui et al., 2014; Geranmayeh et al., 2017; Simic et al., 2019). The occurrence and patterns of non-verbal cognitive dysfunctions in patients with aphasia, the relationship between non-verbal and language impairments, and their structural correlates have been examined separately in some studies. To date, however, no investigation has undertaken a detailed behavioural assessment of both verbal and non-verbal performance or combined this with structural imaging data.
A handful of previous behavioural studies have examined non-verbal cognition in patients with aphasia, but did so either with a narrow focus, for instance investigating the impact of domain-general executive dysfunctions on semantic cognition (Thompson et al., 2018), or on a rather general level with findings based on composite scores (Helm-Estabrooks, 2002), a few standardized tests per domain (Kauhanen et al., 2000; Fucetola et al., 2009; El Hachioui et al., 2014; Lee and Pyun, 2014; Marinelli et al., 2017; Wall et al., 2017) or experimental tasks (Villard and Kiran, 2015; Kuzmina and Weekes, 2017). This limited test selection stands in contrast to research efforts with healthy participants or other patient populations that have explored the nature of multiple components within attention and executive function (Mirsky et al., 1991; Miyake et al., 2000; Friedman and Miyake, 2017). One study including patients with aphasia used a broad range of attention assessments and indeed found that aspects of attention differed with respect to their predictive power regarding language function (Murray, 2012).
Another limitation of existing studies is that patient performance is often reported on a group level only (Glosser and Goodglass, 1990; Kauhanen et al., 2000; El Hachioui et al., 2014; Lee and Pyun, 2014; Naranjo et al., 2018) and information about the prevalence of impaired performance based on normative data is seldom available or incomplete. This information is, however, of clinical significance and relevant when performance in different aspects of cognitive functioning is to be compared.
Underlying patterns in impaired and preserved abilities of heterogeneous patient populations can be extracted using data reduction techniques, such as principal component analysis (PCA) (Kummerer et al., 2013; Butler et al., 2014; Mirman et al., 2015; Halai et al., 2017; Lacey et al., 2017). Applied to large, detailed datasets containing language measures and a handful of executive function assessments, a previous study of chronic post-stroke aphasia found three principal components (phonology, semantics, executive function) underlying participants’ performance (Butler et al., 2014), which was supplemented by a fourth speech quanta component (the quantity of speech produced in connected-speech tasks) in a subsequent study (Halai et al., 2017). One major advantage of data-driven approaches is that they can accommodate for the fact that multiple processes underlie performance in any given test (e.g. naming requires preserved visual perception, semantics, phonology and motor articulation) and no test is a pure measure of single cognitive/language processes. Indeed, sensibility regarding the linguistic demands of any test is particularly high within the field of aphasia. These concerns are usually expressed in the sense that impaired language functions may interfere with testing of other cognitive domains (Keil and Kaszniak, 2002), and more rarely the other way around (Heuer et al., 2017). Data-driven approaches offer a formal method to establish the mutual influences of language and non-verbal ability on test performance.
Based on studies with healthy controls and various neurological populations, a bilateral fronto-cingulo-parietal network is known to be involved in attention and executive function processes (Miller and Cohen, 2001; Duncan, 2010; Niendam et al., 2012; Petersen and Posner, 2012; Fedorenko et al., 2013; Power and Petersen, 2013) but little is known about the structural correlates of attentional and executive dysfunctions in patients with aphasia. Recent research combining data-driven decomposition of behavioural assessment with neuroimaging data, has revealed the structural correlates of behavioural performance in patients with aphasia (Kummerer et al., 2013; Butler et al., 2014; Mirman et al., 2015; Halai et al., 2017; Lacey et al., 2017). While extracting clear brain-behaviour relationships for various aspects of language, these studies struggled to find significant associations of tissue integrity with scores on executive function (but see Lacey et al., 2017), either because non-language assessment was not included (Kummerer et al., 2013; Mirman et al., 2015) or assessment coverage was too limited (Butler et al., 2014; Halai et al., 2017).
In addition to the form and analysis of patients’ behavioural assessment, the approach to mapping brain-behaviour relationships could also be critical. Univariate approaches, such as voxel-based lesion-symptom mapping (VLSM) (Bates et al., 2003) and voxel based correlational methodology (VBCM) (Tyler et al., 2005), are relatively easy to run and interpret. Recent debate has noted the potential shortcomings of univariate approaches (Karnath et al., 2018) including the inability to detect conditional voxel combinations (DeMarco and Turkeltaub, 2018) and mislocalization (Mah et al., 2014), which might be addressed by multivariate analyses (but see Sperber et al., 2019). The power of multivariate analyses, however, bring new interpretation challenges that are straightforward in univariate approaches: because all weights in multivariate models are conditional on each other, the interpretation or post hoc thresholding of individual weights becomes non-trivial (Haufe et al., 2014). Accordingly, making inferences about local brain-behaviour relationships based on multivariate models is, at best, complicated. One transparent way forward is for studies to begin to present both univariate and multivariate results. Therefore, in the current study we show the results for four different methodological approaches, which allows us to demonstrate some commonalities and differences.
To extend our understanding of stroke aphasia to potentially critical aspects of non-verbal cognitive function and their structural correlates, we administered a comprehensive battery of non-verbal tests of attention and executive function to a large and diverse group of individuals with chronic post-stroke aphasia. The key aims of the study were: (i) to assess the prevalence of attention and executive dysfunction in patients with post-stroke aphasia; (ii) to explore the underlying relationships between the tests of attention and executive function, as well as the link to the patients’ language profiles; and (iii) to map the structural correlates for these underlying attention, executive and language features by means of four different methodological approaches.
Materials and methods
Participants
Thirty-eight participants were recruited for the present study (11 female, 27 male; mean age 64 ± 11.9 years, range 45–88 years; see Supplementary Table 1 for more details). All participants had a single left hemispheric stroke (ischaemic or haemorrhagic) at least 1 year before assessment and imaging (see Fig. 1 for lesion overlap map) and had no additional significant neurological conditions and no contraindications for MRI. They were pre-morbidly right-handed native English speakers with normal or corrected-to-normal vision. All had been diagnosed with aphasia but no restrictions were applied regarding the type of aphasia or the severity. Five patients are identical to patients whose data were reported in Halai et al. (2017) and Butler et al. (2014). Informed consent was obtained from all participants prior to participation, in line with the Declaration of Helsinki and as approved by the local NHS ethics committee. MRI data from a healthy age and education matched control group (10 female, 12 male) was used as a reference to identify lesion/abnormal tissue for each patient (Seghier et al., 2008).
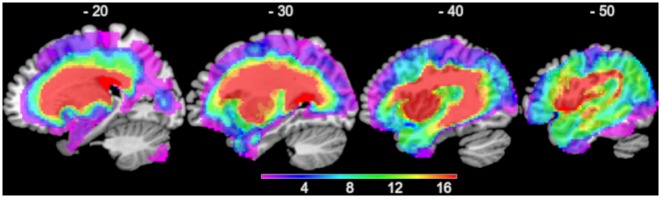
Figure 1.
Overlap of the 38 patients’ lesions.
Neuropsychological assessments
In addition to comprehensive language testing, described in more detail in Butler et al. (2014) and Halai et al. (2017), a broad range of standardized neuropsychological tests of attention and executive functions were administered. This included the subtests Alertness, GoNoGo, Divided Attention, and Distractibility from the Test of Attentional Performance (TAP Mobility version 1.3.1; Zimmermann and Fimm, 1995; www.psytest.net), a computerized test battery measuring reaction times and error rates in tests with varying attentional demands; the subtests Design Fluency and Trail Making (parts 2–4) from the Delis-Kaplan Executive Function System (D-KEFS; Delis et al., 2001), the former assessing non-verbal idea generation by requiring participants to draw as many different figures as possible (connecting dots with lines), and the latter assessing visuospatial attention, processing speed and flexibility by requiring participants to connect numbers (part 2), letters (part 3) or alternatingly both (part 4) in ascending order; a computerized version of the Tower of London (TOL-F by Schuhfried; Kaller et al., 2011), a visuospatial planning task; the Kramer test (Balzer et al., 2011), a categorization task requiring participants to find ways of sorting eight cards into two groups; the Raven’s Coloured Progressive Matrices (Raven, 1962), assessing reasoning abilities; and the Brixton test (Burgess and Shallice, 1997), assessing visuospatial rule detection. Test scores were compared to published norms; age- and/or education-corrected norms were considered if available. For the Raven Matrices, the norms for part B were taken from Smits et al. (1997). Following Brooks et al. (2011), performance was considered as at least mildly-to-moderately impaired if it was more than 1.5 standard deviations (SD) below the mean (i.e. a T-score < 35, a percentile rank < 6 or a scaled score of ≤ 5).
Data analysis
For a descriptive comparison of the impairments per patient and measure, and to account for missing data, percentages of impaired scores were calculated based on 16 measures from the 10 non-verbal tests and 14 measures from 12 language tests. The percentage of impaired scores per patient was taken as an indicator of the severity of their impairment and subsequently used in correlation analyses. Based on the raw test scores, three PCAs (correlation-based) were performed (using IBM SPSS 22.0) to elucidate the data’s underlying structure. The first PCA comprised just the non-verbal tests of attention and executive function. In the second PCA, only the language measures were included, which also provided a replication of previous results (Butler et al., 2014; Halai et al., 2017). Lastly, the third PCA comprised the combination of all measures included in the two other PCAs. To facilitate interpretation, it was ensured that a higher score would indicate better performance for all measures. To this end, reaction time measures were inverted, and accuracy rates were computed. Because of missing values and to include the same sample in all analyses, data of 32 of 38 patients were entered in the PCAs. TAP Distractibility and the letter and switching versions of the Trail Making Test were not included in order to not decrease the sample size further. Importantly, analyses including these measures showed that they were highly correlated with measures of the GoNoGo test or the number version of the Trail Making Test, respectively. To reduce the number of variables entered in the analysis, some comparable language measures were combined (Boston naming and Cambridge naming, immediate and delayed repetition of words and non-words, spoken and written word-picture matching, word and non-word minimal pairs). All components with eigenvalues ≥ 1 were extracted and then varimax rotated, yielding orthogonal and interpretable components. Two control analyses were performed to assess the stability and predictability of the PCA results. First, means and 95% confidence intervals for the component loadings were computed by leaving one case out each time. Second, the similarity between the observed data and those predicted was determined using a leave one case out method (by projecting the left-out case into the component space using the coefficient matrix). Correlations were computed to explore the relationship between component scores and the severity of the impairment in the neuropsychological tests as well as with patient characteristics such as lesion volume, age, and years of education.
Neuroimaging data acquisition and analysis
High resolution structural T1-weighted MRI scans were acquired on a 3.0 T Philips Achieva scanner (Philips Healthcare) using an 8-element SENSE head coil. A T1-weighted inversion recovery sequence with 3D acquisition was used with the following parameters: repetition time = 9.0 ms, echo time = 3.93 ms, flip angle = 8°, 150 contiguous slices, slice thickness = 1 mm, acquired voxel size 1.0 × 1.0 × 1.0 mm, matrix size 256 × 256, field of view = 256 mm, inversion time = 1150 ms, SENSE acceleration factor 2.5, total scan acquisition time = 575 s.
Structural MRI scans were preprocessed with Statistical Parametric Mapping software (SPM8: Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/). The images were normalized into standard Montreal Neurological Institute (MNI) space using a modified unified segmentation-normalization procedure optimized for focal lesioned brains (Seghier et al., 2008). Data from all participants with stroke aphasia and all healthy controls were entered into the segmentation-normalization. Images were then smoothed with an 8 mm full-width at half-maximum (FWHM) Gaussian kernel and used in the lesion analyses described below. An age and education matched healthy control group was used to determine the extent of abnormality per voxel. This was achieved using a fuzzy clustering fixed prototypes (FCP) approach, which measures the similarity between a voxel in the patient data with the mean of the same voxel in the control data (note: this method does not discriminate what caused the abnormality, but simply reflects how deviant the signal in the patient scan is from a healthy group). One can apply a threshold to the FCP to determine membership to abnormal/normal voxel. The default parameters were used apart from the lesion definition ‘U-threshold’, which was set to 0.5 to create a binary lesion image. We modified the U-threshold from 0.3 to 0.5 after comparing the results obtained from a sample of patients to what would be nominated as lesioned tissue by an expert neurologist. The images generated for each patient were visually inspected and manually corrected if necessary and were then used to create the lesion overlap map in Fig. 1.
The smoothed FCP images (% abnormality) were used to determine the brain regions where abnormality correlated with PCA component scores using a voxel-based correlational methodology (VBCM) (Tyler et al., 2005), a variant of voxel-lesion symptom mapping (Bates et al., 2003), in which both the behaviour and signal intensity measures are treated as continuous variables (conducted in SPM12). For the structural correlate analysis, we assume a negative correlation between abnormality and behavioural component score (i.e. greater abnormality leads to poorer performance). The participants’ component scores from the combined PCA, were entered simultaneously into a VBCM analysis. The resulting clusters thus account for the unique variance of a component. In additional analyses, lesion volume (calculated from the lesion identified by the automated lesion identification method; Seghier et al., 2008), age, education, and time post-stroke were entered as covariates. Unless noted otherwise, we applied the threshold at voxel-level P < 0.001 and family-wise error corrected (FWEc) cluster-level P < 0.05.
To supplement the univariate analysis, we conducted multivariate analyses in two ways. First, we used the support-vector regression lesion symptom mapping (SVR-LSM) toolbox recently updated by DeMarco and Turkeltaub (2018), which is based on Zhang et al. (2014). In this framework, we loaded the lesion binary images as the features and created a separate model for each component score. The following settings were used: MATLAB SVM implementation, hyper-parameter optimization (Bayes optimization with default settings) and lesion threshold = 3 (∼10% of sample). The resulting beta weights were evaluated by permutation testing (n = 10 000, voxel-wise P < 0.005 and cluster-wise P < 0.05), but note that the model performance (predicted versus observed scores) is not evaluated in this approach. We ran two models per component, with and without correction for lesion volume (‘regress on both’). Second, we used the pattern recognition of neuroimaging toolbox (PRoNTo V2.1) (http://www.mlnl.cs.ucl.ac.uk/pronto/) (Schrouff et al., 2013) as an alternative method because (i) it formally evaluates model predictions; and (ii) it does not truncate beta weights post hoc. For this toolkit, we entered the FCP % abnormality images as a continuous measure and followed the pipeline through in two pathways: (i) using the whole brain as input (similar to the VBCM); and (ii) restricted to lesion territory (n > 3) (similar to VLSM/SVR-LSM). Given the simplicity of the toolkit, we ran models using four regression machine implementations: (i) kernel ridge regression (KRR; Hastie et al., 2009); (ii) relevance vector regression (RVR; Tipping, 2001); (iii) Gaussian processes regression (GPR; Rasmussen and Williams, 2006); and (iv) multi-kernel regression (MKR; Bach et al., 2004; Rakotomamonjy et al., 2008). PRoNTo relies on kernel methods to overcome the high dimensionality problem in neuroimaging (using n × n pair-wise similarity matrix) and features were mean centred. The default parameters were used for all machines and where necessary hyper-parameter optimization was achieved using nested leave-one-out cross validation (default grid search). A leave-one-out cross-validation scheme was used to determine model performance. For model inference, we report P-values for correlation and mean square error (MSE) following a permutation test of the observed scores (n = 1000) with a P < 0.05 alpha threshold. As with the SVR-LSM, we ran each component model with and without lesion volume as a covariate.
The anatomical labels for the clusters were determined using the Harvard-Oxford atlas for grey matter and on the John Hopkins white matter atlas for white matter tracts. Furthermore, comparisons to existing findings were made by either overlapping the respective maps, if available, or by checking (in MRIcron) whether published peak coordinates overlapped with the clusters from the VBCM.
Data availability
Behavioural data are available in the Supplementary material. Further data are potentially available by request to M.A.L-R.
Results
Neuropsychological profiles
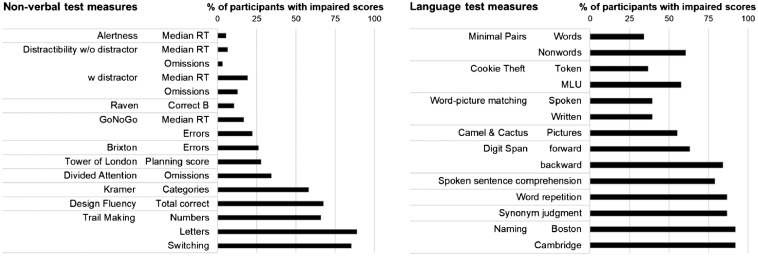
The first aim of this study was to assess the prevalence of impairments in attention and executive functions in patients with post-stroke aphasia. Patients’ performance was thus compared to available norm data to identify the number of impaired scores per patient and test. All participants scored below normal range in at least one measure of the 10 tests of attention and executive function, but no participant was impaired in all of these tests (mean percentage of impaired scores per patient 36.7 ± 20.8%, range 6.3–90.9%). Fifteen patients were impaired in at least half of the administered non-verbal tests. In comparison to the non-verbal test performance, all participants scored below normal range in at least three measures of the 12 language tests, 30 patients were impaired in at least half of the administered language tests, and five participants were impaired in all of these tests (mean percentage of impaired scores per patient 65.0 ± 22.4%, range 21.4–100%). Details on impaired performance in the non-verbal and language tests are depicted in Fig. 2, while Fig. 3 shows the patients’ overall impairment in the non-verbal versus language tests (as percentage of impaired scores in the respective tests). Individual patients’ scores are available in Supplementary Tables 2 and 3, while Supplementary Fig. 2 gives details about impaired performance on the different principal components.
Figure 2.
Percentage of participants with impaired performance on each measure of the non-verbal tests (left) and language tests (right).
Figure 3.
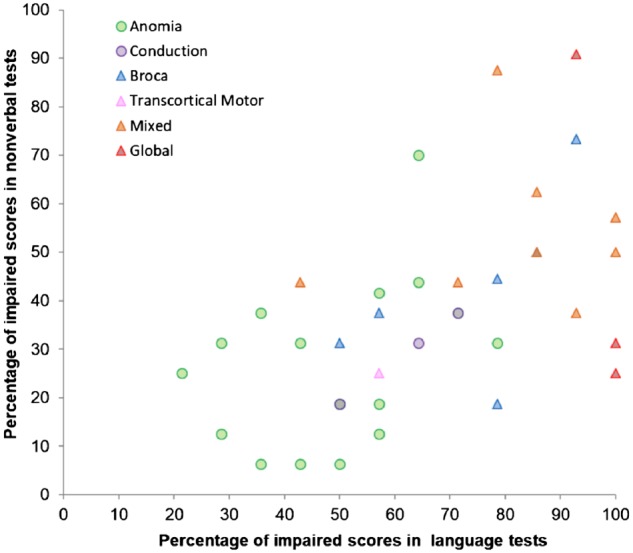
Patients’ overall impairment in the non-verbal versus language tests. The percentages of impaired scores correlated significantly (rs = 0.591, P < 0.01, n = 38, also if patient characteristics were accounted for by means of partial correlations). Symbols and colours denote an individual’s aphasia type based on the BDAE (triangles for non-fluent, circles for fluent patients, for colours see top left legend). More saturated or differently coloured symbols denote two patients in the same spot.
The Alertness test and the Distractibility without distractor condition were the only two non-verbal tests where the percentage of impaired scores was around or below 5% of the sample. These tests measure more basic attention functions and it has previously been reported that these aspects of attention are more commonly impaired in right-hemispheric stroke patients (Sturm et al., 1997). The tests with the highest percentages of impaired scores were the Trail Making Test [numbers impaired in 25 patients (65.8%), letters in 32 patients (88.9%), and switching in 29 patients (85.3%)], the Design Fluency Test (25 patients, 67.6%) and the Kramer Test (21 patients, 58.3%). We split the sample into two groups of ‘cognitive’ severity based on a median split of overall impairment in the non-verbal tests (see Supplementary Table 2 for details). Comparison of the two groups revealed that only the more cognitively-severe patients had impaired scores in the Tower of London and TAP Divided Attention tests. As such, the test of divided attention might be especially clinically useful as a predictor of impaired cognition in aphasic populations. In contrast, both groups showed a similar and high degree of impairment in two other tests, the Kramer and the letter version of the Trail Making Test. The high percentage of impaired performance in the Trail Making Test is particularly important considering the widespread use of this test with aphasic patients. Thus, impaired performance in the switching condition of the trail making test need not necessarily stem from difficulties in switching but from reduced automaticity of accessing the letters in order (and, to a lesser extent, numbers), which is a prerequisite for task completion.
Separate and combined principal component analyses of non-verbal and language tests
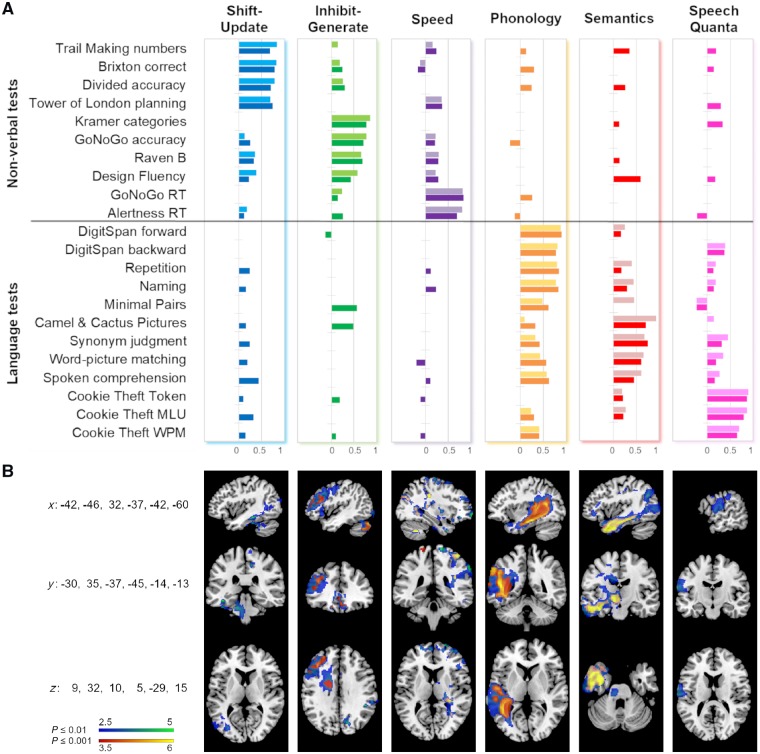
The second aim was to explore the underlying relationships between the tests of attention and executive function, as well as linking these to the patients’ language profiles. We computed separate PCAs for the non-verbal and verbal tests, as well as a combined PCA including all tests. The PCA including only the non-verbal tests of attention and executive functions yielded three orthogonal components accounting for 68.5% of the variance [Kaiser-Meyer-Olkin (KMO) = 0.704]. Based on the tests loading highest on each component (Fig. 4A), the first component (accounting for 28.1% of the variance) was interpreted as ‘shift-update’ as the tests loading highest are relatively demanding with respect to flexible (visuo-spatial) processing and working memory. Interestingly, the first component contains tests that are traditionally regarded as tests of executive function (Tower of London, Brixton) as well as tests that are more associated with attention (Divided attention and Trails numbers), which underlines the link between the two domains that is also reflected in the term ‘executive attention’ (Kane and Engle, 2002; Petersen and Posner, 2012). The second component (23.2%) was interpreted as ‘inhibit-generate’ as it included tests like the Kramer sorting test (requiring idea generation as well as inhibition of salient aspects of the stimuli) as well as simple response inhibition tasks like the GoNoGo test. The third component (17.2%) was interpreted as ‘speed’ as it contained the reaction time measures of both basic attention tasks.
Figure 4.
Component loadings and structural correlates associated with each component. (A) The darker coloured bars (from left to right: blue, green, purple, orange, red, pink) represent the loadings on the six components from the combined PCA. The lighter coloured bars represent the loadings on the three components in the separate non-verbal-only PCA (first three columns) and the language-only PCA (last three columns). Loadings < 0.1 are not depicted. MLU = mean length of utterance; WPM = words per minute. (B) Structural correlates associated with each component from the combined PCA. Clusters shown in blue-green were obtained by applying a voxel-level threshold of P ≤ 0.01, clusters in red-yellow correspond to a voxel-level threshold of P ≤ 0.001. A family-wise error correction of P ≤ 0.05 was applied to all clusters. The respective coordinates in MNI-space are indicated on the left side. Figures are in neurological convention (left is left).
The separate analysis of the language tests yielded three orthogonal components accounting for 78.3% of the variance (KMO = 0.718). The components can be interpreted as ‘phonology’ (accounting for 31.5% of the variance), ‘semantics’ (24.2%), and ‘speech quanta’ (22.6%), directly replicating previous research (Butler et al., 2014; Halai et al., 2017). The fact that the patient sample of this study largely consists of patients not included in previous reports shows the stability of these results. Moreover, other groups report similar patterns (Mirman et al., 2015; Lacey et al., 2017).
The third PCA—combining the non-verbal and language tests—yielded six orthogonal components accounting for 78.6% of the variance (KMO = 0.661). Figure 4A shows that the components from the two separate analyses remained relatively stable (also evidenced by high correlations between the separate and combined component scores; Table 1 and Supplementary Fig. 2). Their order and percentage of explained variance was as follows: phonology (21.6%), shift-update (13.4%), inhibit-generate (12.2%), speech quanta (11.7%), semantics (11.5%), speed (8.2%). Notably, apart from the phonology component which explained the highest amount of variance, the other language and non-verbal components are weighted similarly in terms of explained variance.
Table 1.
Spearman correlations within and between severity of non-verbal and language impairment, component scores, and patient characteristics
| Severity | Non-verbal PCA | Patient characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-verbal | Verbal | S-U | I-G | Speed | Lesion | Age | Education | Time post-stroke | |
| Severity | |||||||||
| Verbal | 0.535* | – | −0.521* | −0.105 | 0.150 | – | – | – | – |
| Non-verbal | – | – | −0.676 * | −0.283 | −0.110 | – | – | – | – |
| Verbal PCA | – | – | |||||||
| Phonology | −0.216 | −0.719 * | 0.261 | −0.294 | 0.025 | −0.208 | −0.126 | −0.209 | 0.131 |
| Semantics | −0.316 | −0.383* | 0.421* | 0.373* | 0.087 | −0.396* | −0.433* | 0.288 | −0.115 |
| Speech Quanta | −0.362* | −0.427* | 0.443* | 0.097 | −0.283 | −0.504* | 0.068 | 0.190 | −0.240 |
| Combined PCA | |||||||||
| Phonology | −0.164 | −0.744 * | 0.216 | −0.245 | −0.062 | −0.238 | −0.121 | −0.213 | 0.126 |
| S-U | −0.530* | −0.259 | 0.871* | −0.063 | −0.018 | −0.308 | −0.445* | 0.143 | −0.393* |
| I-G | −0.235 | −0.173 | −0.109 | 0.905* | −0.184 | 0.050 | −0.436* | 0.312 | −0.208 |
| Speech Quanta | −0.325 | −0.349 | 0.214 | 0.178 | −0.195 | −0.376* | 0.053 | 0.120 | −0.142 |
| Semantics | −0.139 | −0.118 | 0.194 | 0.201 | −0.010 | −0.370* | −0.014 | 0.247 | 0.061 |
| Speed | −0.172 | 0.103 | −0.122 | 0.037 | 0.902* | 0.177 | −0.305 | −0.003 | 0.294 |
| Patient characteristics | |||||||||
| Time post-stroke | 0.196 | 0.151 | −0.381* | −0.187 | 0.240 | 0.389* | 0.094 | −0.123 | – |
| Education | −0.279 | −0.061 | 0.254 | 0.494* | −0.174 | −0.132 | −0.321 | – | – |
| Age | 0.323 | 0.332 | −0.441* | −0.455* | −0.254 | 0.251 | – | – | – |
| Lesion | 0.353* | 0.555* | −0.518* | −0.146 | 0.156 | – | – | – | – |
S-U = shift-update; I-G = inhibit-generate.
*P < 0.05 two-tailed; bold = significant after Bonferroni correction (P < 0.0004); n = 32.
The stability analyses for all three PCAs revealed that all test loadings had very tight 95% confidence intervals. The most unstable tests were Design Fluency in the non-verbal PCA (mean loading = 0.58 ± 0.02), Camel and Cactus in the verbal PCA (0.86 ± 0.08), and Kramer in the combined PCA (0.75 ± 0.05). We also found generally high correlations between the predicted left-out cases and observed scores for the non-verbal (r = 0.83), verbal (r = 0.88) and combined (r = 0.88) PCAs.
Whilst the combined PCA preserves the nature of the six principal behavioural components, it is notable that many individual language tasks load across verbal and non-verbal components, reflecting the fact that many language activities and the tasks used to assess them require generalized attention and executive skills (e.g. comparing verbal stimuli, deciding between responses, etc.). This is true for both semantic tests (aligning with the fact that semantic cognition requires both access to semantic representation but also executively-related processes (Jefferies and Lambon Ralph, 2006; Thompson et al., 2018) and for phonological tests with demands on working memory (sentence comprehension) or abstract reasoning and problem-solving (minimal pairs).
Relationship between impairment, component scores, and patient characteristics
Previous research documents both the presence (Fucetola et al., 2009; Baldo et al., 2015) and absence (Helm-Estabrooks, 2002) of a significant correlation between non-verbal and verbal impairment. We found a moderate but significant relationship between simple indices of non-verbal and language impairment (in terms of percentage of impaired non-verbal/language test scores per patient), as shown in Fig. 3 and Table 1. This finding seems to relate primarily to the non-verbal shift-update component that correlates with both indices of severity. Beyond this, there is considerable variation, which results from the fact that even when combined into one PCA there are statistically-orthogonal components for the language and non-verbal test scores; they would collapse into a shared PCA component if performance in non-verbal and language tests was a reflection of simple severity alone.
Regarding patient characteristics, also shown in Table 1, non-verbal as well as verbal severity correlated significantly with lesion volume, but neither correlated with age, education or time post-stroke. More specifically, lesion volume correlated with the separate non-verbal shift-update component and with the semantic and speech quanta components of both PCAs. Age correlated with the non-verbal components apart from speed, and with the semantic component from the separate verbal PCA. Education only correlated significantly with the inhibit-generate component from the separate non-verbal PCA, and time post-stroke correlated moderately with the shift-update components.
Notably, the first non-verbal and language components, shift-update and phonology, were still significantly correlated with the severity of the non-verbal and language impairment, respectively, when age, education, time post-stroke and lesion volume were accounted for by means of partial correlation (separate shift-update component and non-verbal impairment r = − 0.629; separate/combined phonology component and language impairment r = −0.814/r = −0.851; all P < 0.0004).
Structural correlates
The third aim was to map the structural correlates for the underlying attention, executive and language features. We simultaneously entered all component scores obtained in the combined PCA and performed a VBCM with tissue abnormality, which yielded significant clusters for all components (though shift-update and speech quanta were present at a lower voxel-level threshold of 0.01, FWEc at cluster-level P < 0.05). The clusters are depicted in Figs 4B and 5, and details are listed in Table 2.
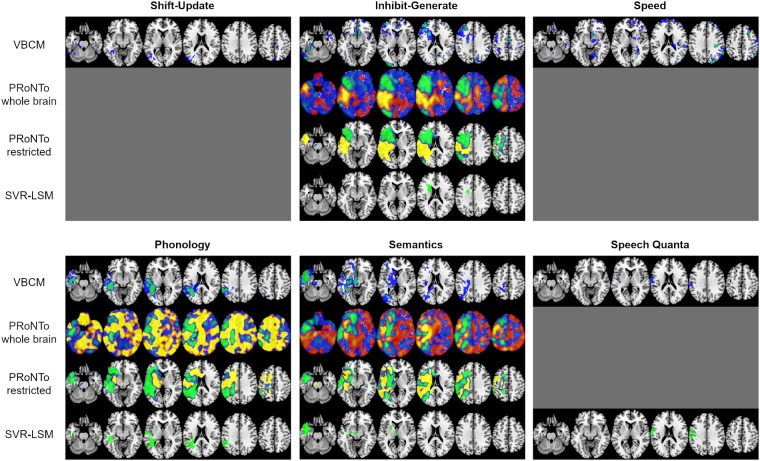
Figure 5.
Comparison of brain-behaviour mapping results based on the four different methodological approaches. The significant VBCM clusters are shown in blue (voxel-level threshold 0.01) and green (voxel-level threshold 0.001), a family-wise error correction of P ≤ 0.05 was applied to all clusters, and images are thresholded at the respective minimum t-value. The PRoNTo results depict the weights for the winning model if significant (see text), either including the whole brain space or restricting it to lesion territory (n > 3). They are thresholded from −0.005 to −0.0001 (green-blue) and 0.0001 to 0.005 (red-yellow). The negative weights are considered as more meaningful in this approach. The SVR-LSM images show voxels with significant beta weights after permutation testing (n = 10 000, voxel-wise P < 0.005 and cluster-wise P < 0.05). MNI coordinates of slices, from left to right, are z = −25, −10, 5, 20, 35, 50 and they are in neurological convention (left is left). A grey surface indicates that no significant results were found for the respective component and methodological approach.
Table 2.
Clusters and peaks associated with the non-verbal and language components
| Component | Extent | Location | Left/right | Z | x | y | z |
|---|---|---|---|---|---|---|---|
| Shift-Update | 2032 | Temporal fusiform cortex posterior | Left | 4.29 | −40 | −32 | −16 |
| Temporal fusiform cortex posterior | Left | 3.69 | −38 | −34 | −30 | ||
| Inferior longitudinal fasciculus | Left | 3.58 | −42 | −36 | −14 | ||
| Temporal fusiform cortex posterior | Left | 3.32 | −42 | −30 | −28 | ||
| Inferior temporal gyrus temporo-occipital | Left | 3.27 | −60 | −56 | −22 | ||
| Occipital fusiform gyrus | Left | 3.22 | −26 | −64 | −16 | ||
| Lateral occipital cortex superior | Left | 3.22 | −56 | −72 | 20 | ||
| Inferior temporal gyrus temporo-occipital | Left | 3.19 | −56 | −50 | −22 | ||
| 990 | Left Precuneous cortex | Left | 4.04 | −2 | −62 | 66 | |
| Postcentral gyrus | Right | 3.78 | 10 | −36 | 72 | ||
| Precentral gyrus | Right | 3.76 | 10 | −32 | 50 | ||
| Corticospinal tract | Right | 3.64 | 16 | −34 | 54 | ||
| Superior parietal lobule | Right | 3.57 | 10 | −48 | 72 | ||
| Inhibit-Generate | 1270 | Frontal pole | Left | 5.00 | −20 | 56 | 12 |
| Frontal pole | Left | 3.99 | −28 | 50 | 16 | ||
| Middle frontal gyrus | Left | 3.94 | −38 | 28 | 32 | ||
| Frontal pole | Left | 3.91 | −28 | 42 | 36 | ||
| Frontal pole | Left | 3.63 | −38 | 52 | 0 | ||
| Middle frontal gyrus | Left | 3.60 | −44 | 24 | 24 | ||
| Inferior frontal gyrus pars triangularis | Left | 3.50 | −40 | 32 | 18 | ||
| Middle frontal gyrus | Left | 3.45 | −52 | 18 | 30 | ||
| 530 | Subcallosal cortex | Right | 5.06 | 6 | 26 | −14 | |
| Accumbens | Right | 4.95 | 8 | 16 | −6 | ||
| Cingulate gyrus anterior | Right | 4.01 | 2 | 36 | 2 | ||
| Accumbens | Left | 3.88 | −8 | 12 | −8 | ||
| Subcallosal cortex | Left | 3.84 | −12 | 28 | −16 | ||
| 447 | Occipital pole | Left | 4.27 | −24 | −96 | 16 | |
| Occipital pole | Left | 4.21 | −22 | −94 | 10 | ||
| Lateral occipital cortex inferior | Left | 3.75 | −42 | −88 | −10 | ||
| 414 | Supplementary motor cortex | Left | 3.55 | −16 | −10 | 34 | |
| Anterior thalamic radiation | Left | 3.44 | −20 | 20 | 18 | ||
| Superior longitudinal fasciculus | Left | 3.34 | −22 | −4 | 30 | ||
| 337 | Supplementary motor cortex | Right | 4.50 | 6 | −12 | 46 | |
| Cingulate gyrus posterior | Right | 4.42 | 4 | −22 | 42 | ||
| Speed | 369 | Lateral occipital cortex superior | Right | 4.51 | 26 | −86 | 34 |
| Occipital pole | Right | 4.50 | 22 | −90 | 32 | ||
| 355 | Angular gyrus | Right | 4.53 | 62 | −54 | 38 | |
| Lateral occipital cortex superior | Right | 4.12 | 54 | −62 | 28 | ||
| Phonology | 5688 | Inferior longitudinal fasciculus | Left | 5.99 | −42 | −30 | −16 |
| Inferior longitudinal fasciculus | Left | 5.70 | −42 | −34 | −14 | ||
| Temporal fusiform cortex posterior | Left | 5.58 | −40 | −24 | −18 | ||
| Inferior temporal gyrus posterior | Left | 5.11 | −50 | −18 | −24 | ||
| Inferior temporal gyrus temporo-occipital | Left | 4.93 | −48 | −46 | 12 | ||
| Supramarginal gyrus posterior | Left | 4.61 | −60 | −48 | 34 | ||
| Angular gyrus | Left | 4.56 | −40 | −54 | 14 | ||
| Middle temporal gyrus temporo-occipital | Left | 4.47 | −42 | −54 | 8 | ||
| Planum temporale | Left | 4.37 | −36 | −32 | 14 | ||
| Semantics | 4994 | Temporal fusiform cortex posterior | Left | 5.48 | −40 | −30 | −16 |
| Inferior temporal gyrus posterior | Left | 5.15 | −52 | −16 | −24 | ||
| Parahippocampal gyrus anterior | Left | 5.12 | −34 | −6 | −26 | ||
| Thalamus | Left | 5.12 | −10 | −22 | −4 | ||
| Temporal Pole | Left | 5.04 | −52 | 10 | −36 | ||
| Hippocampus | Left | 5.02 | −34 | −10 | −24 | ||
| Anterior thalamic radiation | Left | 4.90 | −10 | −18 | −8 | ||
| Anterior thalamic radiation | Left | 4.82 | −8 | −18 | −12 | ||
| Inferior longitudinal fasciculus | Left | 4.71 | −40 | −36 | −14 | ||
| Speech Quanta | 1010 | Postcentral gyrus | Left | 3.24 | −66 | −16 | 16 |
| Postcentral gyrus | Left | 2.80 | −56 | −12 | 28 | ||
| Supramarginal gyrus anterior | Left | 2.63 | −62 | −28 | 36 | ||
| Postcentral gyrus | Left | 2.51 | −50 | −24 | 38 | ||
| Postcentral gyrus | Left | 2.50 | −44 | −24 | 44 | ||
| Precentral gyrus | Left | 2.37 | −60 | 0 | 38 |
Only clusters with cluster-level FWEc P ≤ 0.001 are shown in the table.
From the non-verbal components, shift-update was uniquely correlated with left lateral temporo-occipital regions (encompassing parts of the medial and inferior temporal gyrus, fusiform cortex as well as the lateral occipital cortex and extending to parahippocampal regions and brain stem), in addition to bilateral mainly parietal midline regions (postcentral gyrus, precuneus, superior parietal lobule). The inhibit-generate component was uniquely correlated with left lateral (middle and inferior frontal gyrus) and subcortical frontal regions (anterior thalamic radiation) as well as medial frontal regions bilaterally (subcallosal cortex, (para)cingulate gyrus, supplementary motor cortex), in addition to several smaller clusters in occipital and parietal regions. The speed component was also associated with several small, mainly right-sided parieto-occipital and frontal clusters.
The clusters associated with the three language components resembled the clusters reported in previous studies by our group (Butler et al., 2014; Halai et al., 2017). The phonology cluster was uniquely correlated with left temporo-parietal regions encompassing parts of the inferior, middle, and superior temporal gyri as well as supramarginal and angular gyrus. The semantics component was associated with a cluster of left cortical (anterior temporal lobe, extending inferiorly into occipital lobe) and subcortical (thalamus) regions. The speech quanta cluster was in the dorsal fronto-parietal cortex and included parts of the pre- and postcentral gyrus. When lesion volume was included as a covariate, inhibit-generate, speed, and phonology remained significant. Semantics was only significant at a less strict threshold; this applied as well to the shift-update component and is shown in Supplementary Fig. 3. The effects of including other patient characteristics such as age, education, and time post-stroke in the VBCM are also shown and discussed in the Supplementary material.
The multivariate analyses yielded similar results, as shown in Fig. 5. The SVR-LSM produced significant clusters for inhibit-generate, phonology, semantics and speech quanta. The evaluation of the best model within PRoNTo revealed significant brain-behaviour relationships for inhibit-generate (KRR model cross-validation r = 0.357, MSE = 0.854, P = 0.022), phonology (MKR model cross-validation r = 0.379, MSE = 1.008, P = 0.042), and semantics (KRR model cross-validation r = 0.750, MSE = 0.431, P < 0. 001) when using the whole brain. The results were the same when using the restricted lesion territory: inhibit-generate (KRR model cross-validation r = 0.400, MSE = 0.816, P = 0.019), phonology (GPR model cross-validation r = 0.359, MSE = 0.860, P = 0.013), and semantics (KRR model cross-validation r = 0.712, MSE = 0.478, P < 0.001). When lesion volume was added as a covariate, the SVR-LSM produced significant clusters for inhibit-generate and phonology only, while the PRoNTo toolkit found significant models for inhibit-generate and semantics (for both whole brain and restricted lesion territory), as detailed in Supplementary material.
As can be seen in Fig. 5, the VBCM and SVR-LSM results were strikingly similar. For inhibit-generate, VBCM yielded bigger and more distributed clusters but there was an overlap with the significant SVR-LSM result in left frontal subcortical regions. For phonology, the SVR-LSM and VBCM clusters were nearly identical, with the former extending slightly more into the superior parietal cortex, and the latter extending more anteriorly in the temporal lobe. Likewise, the VBCM and SVR-LSM results for the semantics component overlapped largely, with the former being slightly bigger and extending further posteriorly in the ventral temporal lobe. Finally, the main difference regarding the speech quanta results was that the SVR-LSM cluster extended slightly more dorsally and anteriorly. Furthermore, the unthresholded beta maps from PRoNTo showed some correspondence to both VBCM and SVR-LSM in terms of the negative beta weights. Apart from a small set of voxels in the medial temporal lobe that was part of the VBCM semantics cluster, all voxels identified in the VBCM and SVR-LSM analyses were within regions that were given a (strong) negative weight in the PRoNTo models. In contrast to SVR-LSM, the PRoNTo beta maps show the weights of the entire input space after confirming the model significantly maps to behaviour.
Discussion
Even though there is growing awareness of the importance of attentional and executive (dys)functions in aphasia, to date the occurrence and patterns of such impairments, the relationship between non-verbal and language functions, as well as their structural correlates have not been studied in detail in the same sample of patients. This study extended our understanding of the multidimensionality of chronic post-stroke aphasia and found that: (i) a considerable number of patients showed impaired performance in tests of attention and executive function; (ii) the variance underlying non-verbal and language test performance was best captured by three orthogonal components each; and (iii) both univariate and multivariate mapping approaches revealed brain-behaviour relationships in line with previous studies based on other methodologies and populations.
Given that our sample consisted of patients diagnosed with aphasia, unsurprisingly the incidence of language impairments was high and performance in language tests was overall worse than in non-verbal tests. However, patients’ performance in tests of attention and executive function was also considerably impaired, as none of the patients performed within normal range in all tests and nearly 50% of the patients showed deficits in at least half of the administered tests. While language impairments might be the most salient consequences of a left hemispheric stroke, our more thorough and systematic investigation replicates earlier observations of co-occurring deficits in other cognitive domains (Helm-Estabrooks, 2002; Murray, 2012; Marinelli et al., 2017; Ramsey et al., 2017); a pattern that is important for clinical management and response to rehabilitation.
Our comprehensive battery of non-verbal tests allowed us to identify three separable components of attention and executive function (shift-update, inhibit-generate, and speed), which mirror explorations in healthy participants (Petersen and Posner, 2012; Friedman and Miyake, 2017). This contrasts with current studies in aphasia and clinical practice that either fail to assess non-verbal functions at all, or if they do then only a few (screening) measures are used. Whilst there are clear co-occurrences and simple raw correlations between measures, there is little evidence that everything collapses to one simple severity-based metric. This is in line with a recent study by Marinelli et al. (2017), reporting that only a quarter of their severely aphasic patients was also severely impaired in non-verbal cognition, as well as classical findings showing that language and non-language performance in aphasia have low correlations, and that aphasia cannot be reduced to simple cognitive severity (Basso et al., 1973; Helm-Estabrooks, 2002; Fucetola et al., 2009).
It is important to note that performance on the various components is independent, suggesting that patients have variable combinations of verbal and non-verbal deficits. The common co-occurrence is relevant for three main reasons: (i) many language assessments also load on attention and executive functions; (ii) some aspects of language function require interactions between components (e.g. controlled semantic processing: Jefferies and Lambon Ralph, 2006); (iii) response to therapy and recovery has been shown to relate not only to language severity but also to more domain-general functions (Lambon Ralph et al., 2010; Geranmayeh et al., 2017; Conroy et al., 2018). Our findings thus imply that the three identified non-verbal cognitive components need to be assessed separately in future studies and in clinical practice, as they might have different implications for function and recovery. Likewise, interventions should be considered in this patient population that (i) specifically aim at improving domain-general cognitive deficits (Geranmayeh et al., 2017); (ii) integrate therapy of attentional or executive dysfunctions into speech-language remediation (Mayer et al., 2017); and (iii) adopt a multidisciplinary team approach.
Using univariate and multivariate brain-behaviour mapping approaches we identified separable structural correlates for all three non-verbal components, in addition to replicating previous findings regarding the structural correlates of the three verbal components. The clusters of all three non-verbal components overlapped to some degree with the multi-demand network (Duncan, 2010; Fedorenko et al., 2013). In addition, the shift-update cluster overlapped with the dorsal attention and control network, while the inhibit-generate cluster overlapped with the ventral attention and control network (Yeo et al., 2011). More specifically, the correlates of shift-update fit well with task-based functional imaging studies that report activations in lateral temporo-occipital areas for demanding visuo-spatial tasks (Fedorenko et al., 2013; Humphreys and Lambon Ralph, 2017) or when location and feature information must be combined (Simpson et al., 2011); both processes are inherent to shift-update. The findings for the inhibit-generate component are also in line with previous research. Although more extensive, this network of areas overlaps with the regions found in a previous study of aphasia (Lacey et al., 2017) and those identified in a meta-analysis of functional imaging studies on executive functions (Niendam et al., 2012).
From a methodological point of view, it is important to note the complementary differences between the interpretation of univariate and multivariate analyses (Hebart and Baker, 2018). In general, with univariate analyses, the beta values assigned to voxels are relatively transparent (i.e. their sign and strength indicates meaningful relationships with behaviour) and thus inferences about local function are easier to make (although inference using cluster-level thresholds can only show that there is signal somewhere in the cluster; Woo et al., 2014). However, univariate methods are limited by practical (i.e. multiple comparison correction, interactions between multiple variables that are typically not orthogonal) and theoretical concerns (i.e. assumption of voxel independence, mislocalization of effects; Mah et al., 2014; DeMarco and Turkeltaub, 2018; Karnath et al., 2018). In contrast, multivariate methods can be used for encoding or decoding (Naselaris et al., 2011; Hebart and Baker, 2018) and have different goals (i.e. to predict data from experimental conditions or to map brain status to behavioural performance and make formal predictions, respectively). These models can have problems with interpretability as feature weights become non-transparent (Haufe et al., 2014; Hebart and Baker, 2018), although encoding can assist with this challenge to some degree (such as partial least squares and canonical correlation analysis). By definition, in multivariate analyses all voxel/feature weights are non-independent and thus the importance of these weights is not easy to interpret. Furthermore, analysis steps that select a subsample of weights automatically mean that the overall multivariate model has been changed and one would need to test (i) whether the contribution of a voxel to the model is greater than chance; or (ii) whether the contribution of a voxel to the model is stable across different samples (e.g. via bootstrapping; Kuceyeski et al., 2016). Given these differences between the methods, it is striking that the multivariate models (both SVR-LSM and PRoNTo) produced beta maps that strongly correspond to the VBCM results. We assume this follows the fact that stroke tends to generate binary tissue status (intact versus infarcted) and this will dominate the predictions of behavioural variation in all models (and are the most likely features to be selected in any form of weight truncation such as that used in SVR-LSM). There are some potential avenues to help improve interpretations of both univariate and multivariate methods in the future. First, a recent study showed that it may be possible to compute a correction for the mislocalization caused by anatomical bias (Sperber and Karnath, 2017). Second, Haufe et al. (2014) and Naselaris et al. (2011) propose ways in which a decoding model can be transformed into an encoding model, which potentially leads to interpretable weights. Third, alternative sparse algorithms (such as LASSO, elastic net or recursive feature selection) have the benefit of introducing a penalty for complexity and therefore provide a solution with the smallest number of features (though the challenge of interpreting the resultant weights still holds). Finally, we note that multivariate decoding methodologies typically require a large dataset, as data are partitioned into training/test sets for cross validation. This can be practically challenging, as not only do we require neuroimaging data but also a large neuropsychological test battery to determine the underlying principal components. In a recent simulation study (Sperber et al., 2019), it was suggested that ∼100 subjects are required to have stable/reproducible beta parameter mapping, whereas for prediction of clinical outcomes the number peaked at 40 and was relatively stable from this point up to 100 cases. In the current study we obtained 32 cases (similar to Lacey et al., 2017) and so future work will require replication based on larger groups sizes.
Overall, the structural correlates align with areas of different cognitive functions in healthy participants. The variable combinations of verbal and non-verbal deficits observed across post-stroke aphasia (see above) presumably reflect differential encroachment of each person’s lesion on the various regions implicated for each non-verbal and verbal component and/or their connections. This would imply that interventions should target different brain regions depending on which component needs to be ameliorated to improve performance. Options to be explored include neurostimulation, for instance by targeting medial frontal areas (Sliwinska et al., 2017) or pharmacology (Berthier et al., 2011). It also has implications for building accurate prediction models (Price et al., 2010; Hope et al., 2013, 2018; Yourganov et al., 2015, 2016; Pustina et al., 2017; Thye and Mirman, 2018). First, it may be that predictions of language performance might be improved if the predictors include non-verbal cognitive abilities alongside patient characteristics. Second, it may be possible to improve prediction models of both verbal and non-verbal abilities by using these updated PCA-derived structural correlates (cf.Halai et al., 2018).
In conclusion, this study was able to demonstrate that functionally distinct aspects of attention and executive skills are commonly impaired in patients with post-stroke aphasia. The assessments successfully used here could be adopted in clinical assessment to guide management and choices over clinical pathways. Furthermore, future investigations can explore which specific aspects of attention and executive function are crucial for effective therapy and good rehabilitation outcomes, and how these features of non-verbal abilities can be supported or boosted through novel interventions.
Supplementary Material
Acknowledgements
We would like to thank the patients and their carers for contributing to this research project. Special thanks go also to Blanca De Dios Perez for collecting some of the data used in the analysis.
Glossary
Abbreviations
- PCA
principal component analysis
- PRoNTo
pattern recognition of neuroimaging toolbox
- SVR-LSM
support-vector regression lesion symptom mapping
- VBCM
voxel-based correlational methodology
Funding
This research was supported by the Swiss National Science Foundation (Grant P2BEP3_168670 to R.S.), by the Rosetrees Trust (Grant A1699 to A.D.H. and M.A.L-R.), by the Medical Research Council (Grant MR/R023883/1 to M.A.L-R.), and by the European Research Council (Grant GAP: 670428 to M.A.L-R.).
Competing interests
The authors report no competing interests.
References
- Bach FR, Lanckriet GR, Jordan MI. Multiple kernel learning, conic duality, and the SMO algorithm. In: Proceedings of the Twenty-First International Conference on Machine Learning. ACM; 2004. p. 6. [Google Scholar]
- Baldo JV, Paulraj SR, Curran BC, Dronkers NF. Impaired reasoning and problem-solving in individuals with language impairment due to aphasia or language delay. Front Psychol 2015; 6: 1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzer C, Berger JM, Caprez G, Gonser A, Gutbrod K, Keller M. Materialien und Normwerte fuer die neuropsychologische Diagnostik (MNND). Rheinfelden, Switzerland: Verlag Normdaten; 2011. [Google Scholar]
- Basso A, De Renzi E, Faglioni P, Scotti G, Spinnler H. Neuropsychological evidence for the existence of cerebral areas critical to the performance of intelligence tasks. Brain 1973; 96: 715–28. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT et al. Voxel-based lesion–symptom mapping. Nat Neurosci 2003; 6: 448. [DOI] [PubMed] [Google Scholar]
- Berthier ML, Pulvermuller F, Davila G, Casares NG, Gutierrez A. Drug therapy of post-stroke aphasia: a review of current evidence. Neuropsychol Rev 2011; 21: 302–17. [DOI] [PubMed] [Google Scholar]
- Brooks BL, Sherman EMS, Iverson GL, Slick DJ, Strauss E. Psychometric foundations for the interpretation of neuropsychological test results. In: Schoenberg MR, Scott JG, editors. Little Black Book of Neuropsychology: A Syndrome-Based Approach. New York: Springer; 2011. p. 893–922. [Google Scholar]
- Brownsett SLE, Warren JE, Geranmayeh F, Woodhead Z, Leech R, Wise RJS. Cognitive control and its impact on recovery from aphasic stroke. Brain 2014; 137: 242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P, Shallice T. The Hayling and Brixton Tests. Bury St Edmunds, England: Thames Valley Test Co Ltd; 1997. [Google Scholar]
- Butler RA, Lambon Ralph MA, Woollams AM. Capturing multidimensionality in stroke aphasia: mapping principal behavioural components to neural structures. Brain 2014; 137: 3248–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy P, Drosopoulou CS, Humphreys GF, Halai AD, Lambon Ralph MA. Time for a quick word? The striking benefits of training speed and accuracy of word retrieval in post-stroke aphasia. Brain 2018; 141: 1815–27. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System: Examiners Manual. San Antonio: Psychological Corporation; 2001. [Google Scholar]
- DeMarco AT, Turkeltaub PE. A multivariate lesion symptom mapping toolbox and examination of lesion-volume biases and correction methods in lesion-symptom mapping. Human Brain Mapping 2018; 39: 4169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 2010; 14: 172–9. [DOI] [PubMed] [Google Scholar]
- El Hachioui H, Visch-Brink EG, Lingsma HF, van de Sandt-Koenderman MWME, Dippel DWJ, Koudstaal PJ et al. Nonlinguistic cognitive impairment in poststroke aphasia: a prospective study. Neurorehabil Neural Repair 2014; 28: 273–81. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci USA 2013; 110: 16616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingham JK, Sage K, Lambon Ralph MA. Treatment of anomia using errorless versus errorful learning: are frontal executive skills and feedback important? Int J Lang Commun Disord 2005; 40: 505–23. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. Unity and diversity of executive functions: individual differences as a window on cognitive structure. Cortex 2017; 86: 186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucetola R, Connor LT, Strube MJ, Corbetta M. Unravelling nonverbal cognitive performance in acquired aphasia. Aphasiology 2009; 23: 1418–26. [Google Scholar]
- Geranmayeh F, Chau T, Wise RJS, Leech R, Hampshire A. Domain-general subregions of the medial prefrontal cortex contribute to recovery of language after stroke. Brain 2017; 140: 1947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glosser G, Goodglass H. Disorders in executive control functions among aphasic and other brain-damaged patients. J Clin Exp Neuropsychol 1990; 12: 485–501. [DOI] [PubMed] [Google Scholar]
- Halai AD, Woollams AM, Lambon Ralph MA. Using principal component analysis to capture individual differences within a unified neuropsychological model of chronic post-stroke aphasia: revealing the unique neural correlates of speech fluency, phonology and semantics. Cortex 2017; 86: 275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halai AD, Woollams AM, Lambon Ralph MA. Predicting the pattern and severity of chronic post-stroke language deficits from functionally-partitioned structural lesions. Neuroimage-Clinical 2018; 19: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: prediction, inference and data mining. New York: Springer; 2009. [Google Scholar]
- Haufe S, Meinecke F, Görgen K, Dähne S, Haynes J-D, Blankertz B et al. On the interpretation of weight vectors of linear models in multivariate neuroimaging. NeuroImage 2014; 87: 96–110. [DOI] [PubMed] [Google Scholar]
- Hebart MN, Baker CI. Deconstructing multivariate decoding for the study of brain function. Neuroimage 2018; 180: 4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm-Estabrooks N. Cognition and aphasia: a discussion and a study. J Commun Disord 2002; 35: 171–86. [DOI] [PubMed] [Google Scholar]
- Heuer S, Ivanova MV, Hallowell B. More than the verbal stimulus matters: visual attention in language assessment for people with aphasia using multiple-choice image displays. J Speech Lang Hear Res 2017; 60: 1348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope TMH, Leff AP, Price CJ. Predicting language outcomes after stroke: is structural disconnection a useful predictor? Neuroimage-Clin 2018; 19: 22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope TMH, Seghier ML, Leff AP, Price CJ. Predicting outcome and recovery after stroke with lesions extracted from MRI images. Neuroimage-Clin 2013; 2: 424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, Lambon Ralph MA. Mapping domain-selective and counterpointed domain-general higher cognitive functions in the lateral parietal cortex: evidence from fMRI comparisons of difficulty-varying semantic versus visuo-spatial tasks, and functional connectivity analyses. Cerebral Cortex 2017; 27: 4199–212. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain 2006; 129: 2132–47. [DOI] [PubMed] [Google Scholar]
- Kaller CP, Unterrainer JM, Kaiser S, Weisbrod M, Aschenbrenner S. Tower of London - Freiburg Version. Moedling, Austria: Schuhfried; 2011. [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev 2002; 9: 637–71. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Sperber C, Rorden C. Mapping human brain lesions and their functional consequences. Neuroimage 2018; 165: 180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauhanen ML, Korpelainen JT, Hiltunen P, Maatta R, Mononen H, Brusin E et al. Aphasia, depression, and non-verbal cognitive impairment in ischaemic stroke. Cerebrovascular Diseases 2000; 10: 455–61. [DOI] [PubMed] [Google Scholar]
- Keil K, Kaszniak AW. Examining executive function in individuals with brain injury: a review. Aphasiology 2002; 16: 305–35. [Google Scholar]
- Kuceyeski A, Navi BB, Kamel H, Raj A, Relkin N, Toglia J et al. Structural connectome disruption at baseline predicts 6-months post-stroke outcome. Human Brain Mapp 2016; 37: 2587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Kloppel S et al. Damage to ventral and dorsal language pathways in acute aphasia. Brain 2013; 136: 619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmina E, Weekes BS. Role of cognitive control in language deficits in different types of aphasia. Aphasiology 2017; 31: 765–92. [Google Scholar]
- Lacey EH, Skipper-Kallal LM, Xing SH, Fama ME, Turkeltaub PE. Mapping common aphasia assessments to underlying cognitive processes and their neural substrates. Neurorehabil Neural Repair 2017; 31: 442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Snell C, Fillingham JK, Conroy P, Sage K. Predicting the outcome of anomia therapy for people with aphasia post CVA: both language and cognitive status are key predictors. Neuropsychol Rehabil 2010; 20: 289–305. [DOI] [PubMed] [Google Scholar]
- Lee B, Pyun SB. Characteristics of cognitive impairment in patients with post-stroke aphasia. Ann Rehabil Med 2014; 38: 759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah Y-H, Husain M, Rees G, Nachev P. Human brain lesion-deficit inference remapped. Brain 2014; 137: 2522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli CV, Spaccavento S, Craca A, Marangolo P, Angelelli P. Different cognitive profiles of patients with severe aphasia. Behav Neurol 2017: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JF, Mitchinson SI, Murray LL. Addressing concomitant executive dysfunction and aphasia: previous approaches and the new brain budget protocol. Aphasiology 2017; 31: 837–60. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Ann Rev Neurosci 2001; 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Mirman D, Chen Q, Zhang YS, Wang Z, Faseyitan OK, Coslett HB et al. Neural organization of spoken language revealed by lesion-symptom mapping. Nat Commun 2015; 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, Kellam SG. Analysis of the elements of attention: a neuropsychological approach. Neuropsychol Rev 1991; 2: 109–45. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol 2000; 41: 49–100. [DOI] [PubMed] [Google Scholar]
- Murray LL. Attention and other cognitive deficits in aphasia: Presence and relation to language and communication measures. Am J Speech-Lang Pathol 2012; 21: S51–64. [DOI] [PubMed] [Google Scholar]
- Naranjo NP, Grande DD, Alted CG. Individual variability in attention and language performance in aphasia: a study using Conner’s Continuous Performance Test. Aphasiology 2018; 32: 436–58. [Google Scholar]
- Naselaris T, Kay KN, Nishimoto S, Gallant JL. Encoding and decoding in fMRI. Neuroimage 2011; 56: 400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 2012; 12: 241–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. In: Hyman SE, editor. Annual Review of Neuroscience, Vol 35 Palo Alto: Annual Reviews; 2012. p. 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Petersen SE. Control-related systems in the human brain. Curr Opin Neurobiol 2013; 23: 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Seghier ML, Leff AP. Predicting language outcome and recovery after stroke: the PLORAS system. Nat Rev Neurol 2010; 6: 202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustina D, Coslett HB, Ungar L, Faseyitan OK, Medaglia JD, Avants B et al. Enhanced estimations of post-stroke aphasia severity using stacked multimodal predictions. Hum Brain Mapp 2017; 38: 5603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakotomamonjy A, Bach F, Canu S, Grandvalet Y. Simplemkl. J Mach Learn Res 2008; 9: 2491–521. [Google Scholar]
- Ramsey LE, Siegel JS, Lang CE, Strube M, Shulman GL, Corbetta M. Behavioural clusters and predictors of performance during recovery from stroke. Nat Hum Behav 2017; 1: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen CE, Williams CK. Gaussian processes for machine learning. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- Raven JC. Coloured Progressive Matrices, Sets A, AB, B. London, England: H. K. Lewis; 1962. [Google Scholar]
- Schrouff J, Rosa MJ, Rondina JM, Marquand AF, Chu C, Ashburner J et al. PRoNTo: Pattern Recognition for Neuroimaging Toolbox. Neuroinformatics 2013; 11: 319–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Ramlackhansingh A, Crinion J, Leff AP, Price CJ. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. Neuroimage 2008; 41: 1253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic T, Rochon E, Greco E, Martino R. Baseline executive control ability and its relationship to language therapy improvements in post-stroke aphasia: a systematic review. Neuropsychol Rehabil 2019; 29: 395–439. [DOI] [PubMed] [Google Scholar]
- Simpson GV, Weber DL, Dale CL, Pantazis D, Bressler SL, Leahy RM et al. Dynamic activation of frontal, parietal, and sensory regions underlying anticipatory visual spatial attention. J Neurosci 2011; 31: 13880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinska MW, Violante IR, Wise RJS, Leech R, Devlin JT, Geranmayeh F et al. Stimulating multiple-demand cortex enhances vocabulary learning. J Neurosci 2017; 37: 7606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits CHM, Smit JH, vandenHeuvel N, Jonker C. Norms for an abbreviated Raven’s Coloured Progressive Matrices in an older sample. J Clin Psychol 1997; 53: 687–97. [DOI] [PubMed] [Google Scholar]
- Sperber C, Karnath HO. Impact of correction factors in human brain lesion-behavior inference. Hum Brain Mapp 2017; 38: 1692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber C, Wiesen D, Karnath H-O. An empirical evaluation of multivariate lesion behaviour mapping using support vector regression. Hum Brain Mapp 2019; 40: 1381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm W, Willmes K, Orgass B, Hartje W. Do specific attention deficits need specific training? Neuropsychol Rehabil 1997; 7: 81–103. [Google Scholar]
- Thompson HE, Almaghyuli A, Noonan KA, Barak O, Lambon Ralph MA, Jefferies E. The contribution of executive control to semantic cognition: convergent evidence from semantic aphasia and executive dysfunction. J Neuropsychol 2018; 12: 312–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thye M, Mirman D. Relative contributions of lesion location and lesion size to predictions of varied language deficits in post-stroke aphasia. Neuroimage-Clin 2018; 20: 1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping ME. Spare Bayesian learning and the relevance vector machine. J Mach Learn Res 2001; 1: 211–44. [Google Scholar]
- Tyler LK, Marslen-Wilson W, Stamatakis EA. Dissociating neuro-cognitive component processes: voxel-based correlational methodology. Neuropsychologia 2005; 43: 771–8. [DOI] [PubMed] [Google Scholar]
- van de Sandt-Koenderman WME, van Harskamp F, Duivenvoorden HJ, Remerie SC, van der Voort-Klees YA, Wielaert SM et al. MAAS (Multi-axial Aphasia System): realistic goal setting in aphasia rehabilitation. Int J Rehabil Res 2008; 31: 314–20. [DOI] [PubMed] [Google Scholar]
- Villard S, Kiran S. Between-session intra-individual variability in sustained, selective, and integrational non-linguistic attention in aphasia. Neuropsychologia 2015; 66: 204–12. [DOI] [PubMed] [Google Scholar]
- Villard S, Kiran S. To what extent does attention underlie language in aphasia? Aphasiology 2017; 31: 1226–45. [Google Scholar]
- Wall KJ, Cumming TB, Copland DA. Determining the association between language and cognitive Tests in Poststroke aphasia. Front Neurol 2017; 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage 2014; 91: 412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106: 1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourganov G, Fridriksson J, Rorden C, Gleichgerrcht E, Bonilha L. Multivariate Connectome-based symptom mapping in post-stroke patients: networks supporting language and speech. J Neurosci 2016; 36: 6668–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourganov G, Smith KG, Fridriksson J, Rorden C. Predicting aphasia type from brain damage measured with structural MRI. Cortex 2015; 73: 203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kimberg DY, Coslett HB, Schwartz MF, Wang Z. Multivariate lesion-symptom mapping using support vector regression. Hum Brain Mapp 2014; 35: 5861–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Fimm B. Test for attentional performance (TAP). Herzogenrath, Germany: PsyTest; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Behavioural data are available in the Supplementary material. Further data are potentially available by request to M.A.L-R.