The diverse roles of monocytes in SLE and RA
Keywords: autoimmune disease, B-cell activation, FcγRIIB, osteoclastogenesis
Abstract
AbstractMonocytes are evolutionally conserved innate immune cells that play essential roles for the protection of the host against pathogens and also produce several inflammatory cytokines. Thus, the aberrant functioning of monocytes may affect not only host defense but also the development of inflammatory diseases. Monocytes are a heterogeneous population with phenotypical and functional differences. Most recent studies have shown that monocytes are divided into three subsets, namely classical, intermediate and non-classical subsets, both in humans and mice. Accumulating evidence showed that monocyte activation is associated with the disease progression in autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). However, it remains to be determined how monocytes contribute to the disease process and which subset is involved. In this review, we discuss the pathogenic role of monocyte subsets in SLE and RA on the basis of current studies by ourselves and others to shed light on the suitability of monocyte-targeted therapies in these diseases.
Introduction
Monocytes originate from hematopoietic precursor cells in bone marrow in a CD115-dependent manner [CD115 is also known as the colony-stimulating factor-1 receptor (CSF1R) or c-fms] (1). A common progenitor that is committed to the monocyte lineage was recently identified in both humans and mice (2, 3). Bloodstream monocytes are recruited into peripheral tissues during both homeostasis and inflammation and differentiate into macrophages and dendritic cells (DCs) in response to the local milieu of cytokines and microbial products (1, 4). Monocytes and macrophages can perform multiple functions including phagocytosis, antigen presentation and cytokine production.
Aberrations of monocyte/macrophage phenotype and function are increasingly being recognized in murine lupus as well as in patients with systemic lupus erythematosus (SLE). A defective phagocytic function may underlie the pathogenesis of SLE since mice lacking molecules associated with apoptotic cell clearance develop SLE-like disease (5). Other studies, however, show an active role of monocytes in accelerating inflammation and injury in kidney glomerular lesions (6–8).
There has also been a focus on the involvement of monocytes in the pathogenesis of rheumatoid arthritis (RA). Monocytes/macrophages accumulate in arthritic synovial-joint tissues and produce large amounts of inflammatory cytokines (9). Furthermore, monocytes have the potential to differentiate into osteoclast precursors, which are an essential cell type for osteoclastogenesis (10, 11).
Recent studies have identified three types of monocyte subsets with different phenotypes and functions (12, 13). Thus, a knowledge of this broadening field is required to thoroughly understand the pathological role of monocyte subsets in SLE and RA.
Monocyte heterogeneity
Monocytes are a heterogeneous population, and each sub-population differently mediates host defense and inflammation (1, 4). They were initially divided into two sub-populations (1, 11, 14) and more recently into three sub-populations according to the differences in cell surface markers and functions (Table 1) (4, 12, 13). According to CD14 [Lipopolysaccharide (LPS) co-receptor] and CD16 (FcγRIII) expression levels, human monocytes are divided into the following three subsets: the CD14++CD16– classical, CD14++CD16+ intermediate and CD14lowCD16++ non-classical (4, 12). A subdivision into three subsets is also reported for mouse monocytes (Table 1) (13). However, murine monocytes are usually examined by dividing into two subsets namely Gr-1+ (Ly6C+) classical and Gr-1– (Ly6C-) non-classical subsets in contemporary studies, because it is difficult to discriminate an intermediate subset (4).
Table 1.
Monocyte subsets in humans and mice
| Subset | Markers | Chemokine receptors |
|---|---|---|
| Human | ||
| Classical | CD14++CD16– | CCR2highCX3CR1low |
| Intermediate | CD14++CD16+ | CCR2lowCX3CR1high |
| Non-classical | CD14lowCD16++ | CCR2lowCX3CR1high |
| Mouse | ||
| Classical | Ly6C+ (Gr-1+) | CCR2+CX3CR1low |
| Intermediate | Ly6Cint | CCR2intCX3CR1int |
| Non-classical | Ly6C– (Gr-1–) | CCR2–CX3CR1high |
The murine classical and non-classical monocyte subsets are CCR2+CX3CR1low and CCR2–CX3CR1high, and the human classical and non-classical monocyte subsets are likewise CCR2highCX3CR1low and CCR2lowCX3CR1high respectively (Table 1), indicating that murine and human monocyte subsets are functionally similar. Thus, studies conducted in mice are suitable for understanding the role of human monocytes, although we should be cautious because differences between species have been reported in gene expression profiles of the corresponding subsets (15). For instance, whereas human classical and non-classical monocyte subsets are CD16– and CD16++, respectively, both subsets in mice are CD16+, although the expression level is higher on the non-classical subset compared with the classical subset (15).
The classical monocytes migrate into inflammatory sites through interactions between CCR2 and its ligand MCP-1 expressed in inflamed sites, and is known as the ‘inflammatory’ subset (11). Most of the ‘inflammatory’ monocytes differentiate into macrophages and DCs in inflamed tissues and protect the host against infection. The non-classical monocytes show CX3CR1-dependent recruitment to resting tissues. They patrol blood vessels to survey endothelial cells and surrounding tissues for damage and are known as the ‘resident’ or ‘patrolling’ subset (16). Although classical monocytes are known to be the major players for host protection from pathogens, accumulating results in the past decade indicate the important roles of non-classical or intermediate monocyte subsets in the development of SLE and RA. Thus, the validated phenotypic and functional characterization of monocyte subsets should be essential to clarify the pathogenic roles in these diseases.
Monocyte subsets arise from a common precursor (2, 3), but show the different phenotypes and functions as mentioned above. Although the ontogenic relationship of these subsets was under debate for a time, it has been reported that Gr-1+ classical monocytes mature in the circulation and are the precursors for Gr-1– non-classical monocytes (13, 17–19). Stimulation with TLR7 or TLR9 can induce the maturation of a fraction of Gr-1high monocytes towards Gr-1low monocytes, indicating that the Gr-1– subset is in a more mature and active stage compared with the Gr-1+ subset (18). Consistently, the Gr-1– subset, but not the Gr-1+ subset, expresses the ‘activating’ IgG Fc receptor FcγRIV (i.e. ligation of this receptor activates the cell on which it is expressed, as does ligation of FcγRIII/CD16) (18). As in the case of murine monocytes, fate-mapping studies have shown that human classical monocytes differentiate sequentially into the intermediate subset and then into the non-classical subset (20).
The contribution of monocyte subsets in autoimmune diseases
The contribution of monocytes to the development of disease has long been studied in atherosclerosis (21). Atherosclerosis is an inflammatory vascular disease characterized by the formation of an atherosclerotic plaque that consists of a well-defined structure of lipids, calcified regions and foam cells (i.e. lipid-rich macrophages). Macrophages account for the majority of the cellular component in this lesion and they differentiate from circulating monocytes. Intriguingly, a raised incidence of accelerated atherosclerosis was reported in patients with SLE and RA (22, 23), indicating the possible contribution of monocyte dysfunction in the disease process of SLA and RA.
Genetic studies also suggest the possible role of monocytes in the pathogenesis of SLE and RA. Both SLE and RA are the genetically determined autoimmune diseases, and genome-wide association studies (GWAS) have identified >100 risk loci that are robustly associated with SLE and RA (24, 25). Among them, the most critical alleles are in the class II major histocompatibility complex (MHC) locus both in SLE and RA. Thus, MHC class II-mediated antigen presentation by DCs, T-cell activation and subsequent B-cell activation are supposed to be essential processes in the breakdown of self-tolerance in these diseases, and studies on the pathology have long been focused on the adaptive immune system in studies especially on SLE. However, since many GWAS loci reside outside of protein-coding regions, it is difficult to identify the cell types where these GWAS loci function. To clarify the involved cell types, expression quantitative trait loci (eQTLs) experiments are performed. These results have implicated aberrant regulation of not only adaptive but also innate immune cells including monocytes in the pathogenesis of SLE and RA (26, 27).
Recent studies have identified three types of monocyte subsets with different phenotypes and functions. The remainder of this review focuses on the pathogenic role of monocyte subsets in SLE and RA, especially in murine models, to understand whether they are suitable therapeutic targets in human diseases.
Systemic lupus erythematosus
SLE is a chronic autoimmune disease characterized by the production of anti-nuclear auto-antibodies and immune complex (IC)-mediated tissue inflammation such as lupus nephritis, a major cause of death of SLE patients. Multiple susceptibility genes determine the disease occurrence. Like human SLE, the haplotype of the H-2 locus, the murine MHC, strongly affects the disease severity in murine lupus (28, 29). The gene for the inhibitory IgG Fc receptor, FcγRIIB, is also an additional susceptibility locus for SLE both in humans and mice (30–32). There are three types of polymorphisms in the murine Fcgr2b gene, and lupus-prone strains, such as NZB, BXSB and MRL, all share an autoimmune-type deletion polymorphism in the Fcgr2b promoter region (30). This polymorphism causes down-regulation of FcγRIIB expression particularly on activated B cells, which results in increased IgG antibody production (33, 34). The signaling lymphocytic activation molecule (SLAM)-family genes located downstream of Fcgr2b are also polymorphic. SLAM-family members are known to be critical players in interactions between B cells and T cells during the germinal center reaction (35). In mice, there are two haplotypes of SLAM-family genes, and haplotype 2 associates with defective B-cell tolerance and the development of autoimmune disease (36). An association of a specific SLAM haplotype with human SLE has also been reported (37).
In addition to the aberrant activation of the adaptive immune system, the importance of the innate immune system is now emerging for the pathogenesis of SLE since convincing susceptibility genes are implicated in not only T-/B-cell signaling but also Toll-like receptors (TLRs) and type 1 interferon signaling (38). Innate immune cells express TLRs that recognize not only foreign nucleic acids, originating from intruding viruses and bacteria, but also self-derived nucleic acids from apoptotic cells in the host body and thus may contribute to the autoimmune responses against nuclear antigens. Accumulating evidence shows that among several TLRs, TLR7—a receptor for single-stranded RNA—plays an essential role in the development of SLE. The Yaa (Y chromosome-linked autoimmune acceleration) locus, a duplication of the TLR7 gene due to a translocation of the TLR7-containing region of the X-chromosome to the Y chromosome (39, 40), induces spontaneous lupus nephritis in male BXSB mice. Furthermore, TLR7-transgenic mice develop spontaneous lupus-like autoimmunity (41). TLR7 is an endosomal sensor and is mainly expressed on B cells and DCs. TLR7 over-expression on B cells renders RNA-reactive B cells hyper-active to produce anti-RNA auto-antibodies via TLR7 stimulation by RNA-containing antigens taken up through RNA-specific B-cell antigen receptors (42). Besides, TLR7 over-expression on CD11c+ DCs contributes to severe lupus nephritis because of the enhanced ability of these DCs to produce chemokines, resulting in increased recruitment of inflammatory monocytes into kidney lesions (43).
Monocytosis is one of the unique features of SLE-prone mice. Monocytes constitute ~4% of blood leukocytes in healthy mice, whereas the frequency of monocytes is >50% in aged BXSB male mice, and this age-associated monocytosis predominantly consists of the Gr-1– non-classical monocyte subset (44). A previous association study has shown that there is a remarkable correlation between monocytosis and serum levels of auto-antibodies in Yaa-locus associated lupus mice (45), suggesting a possible role for monocytes in B-cell activation. Monocytosis is dependent on FcRγ, which is a common component shared by some activating Fc receptors, since FcRγ-deficient BXSB mice do not develop monocytosis (44).
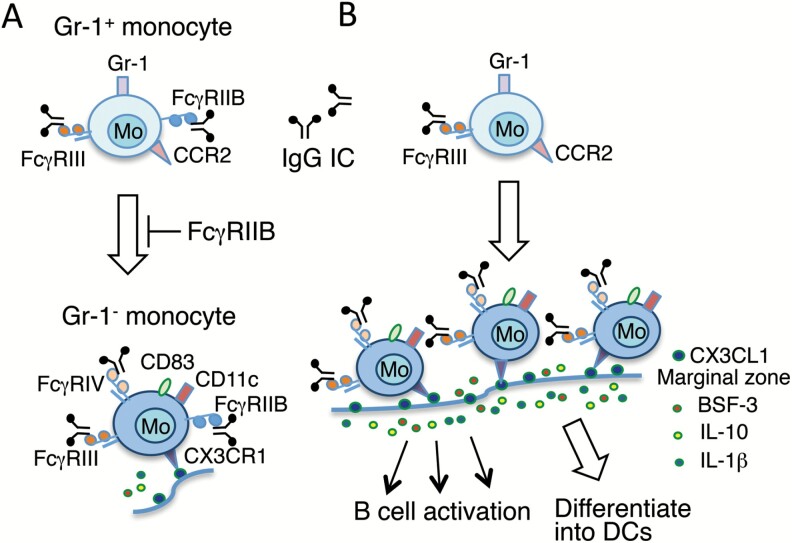
Whereas the Yaa locus in the normal C57BL/6 (B6) background induces neither monocytosis nor lupus nephritis, in mice deficient for the inhibitory IgG Fc receptor FcγRIIB, the Yaa locus induces both monocytosis and lupus nephritis (46, 47). Although FcγRIIB is the major negative regulator of B cells, FcγRIIB is also expressed on a wide variety of myeloid lineage cells (48). To study the cell type-specific role of FcγRIIB in lupus-prone B6.FcγRIIB–/–.Yaa mice, we established three strains of FcγRIIB-deficient B6.Yaa mice: B-cell-specific deficiency, myeloid cell-specific deficiency and CD11c+ DC-specific deficiency. The B-cell-specific and myeloid cell-specific FcγRIIB-deficient mice developed milder lupus than B6.FcγRIIB–/–.Yaa mice, whereas surprisingly DC-specific deficient mice stayed disease free (47). These findings indicate that FcγRIIB deficiency on not only B cells but also myeloid cells except DCs synergistically contributes to spontaneously occurring lupus nephritis in B6.FcγRIIB–/–.Yaa mice. The above three lupus-prone strains developed monocytosis. Intriguingly, in B6.FcγRIIB–/–.Yaa mice and myeloid cell-specific FcγRIIB-deficient B6.Yaa mice, monocytosis predominantly consisted of Gr-1– monocytes, whereas in B-cell-specific FcγRIIB-deficient B6.Yaa mice, monocytosis mostly consisted of Gr-1+ monocytes. These observations suggest that the lack of FcγRIIB expression on monocytes likely accelerates the FcRγ-mediated monocyte differentiation process from the Gr-1+ subset into the Gr-1- subset (Fig. 1).
Fig. 1.
A model for the accelerated maturation of Gr-1+ monocytes into active Gr-1– monocytes in lupus-prone B6.FcγRIIB–/–.Yaa mice. (A) In non-autoimmune B6.Yaa mice, monocytosis is not observed, and the differentiation process from Gr-1+ monocytes to Gr-1– monocytes is suppressed because of the negative signal from FcγRIIB. (B) In B6.FcγRIIB–/–.Yaa mice, because of the lack of FcγRIIB expression on monocytes, monocytosis occurs through the activation signals from IgG IC-stimulated FcγRIII (CD16) and the differentiation process from Gr-1+ monocytes to Gr-1– monocytes is accelerated. These increased Gr-1– monocytes with high expression of CX3CR1 can be recruited into the splenic marginal zone that is positive for CX3CL1, and activate B cells through their higher potential to produce BSF-3, IL-10 and IL-1β. Gr-1– monocytes are long-lived and may be committed to differentiate into DCs in the spleen since they are positive for DC markers such as CD11c, CD83 and Adamdec1 (47).
The frequency of Gr-1– monocytes was associated with the frequencies of activated B cells and plasma cells, suggesting a possible contribution from Gr-1– monocytes in B-cell activation/differentiation (47). Tanscriptome analysis of sorted monocyte subsets obtained from B6.FcγRIIB–/–. Yaa and cell-type-specific FcγRIIB-deficient B6.Yaa mice showed that, compared with Gr-1+ monocytes, Gr-1– monocytes have higher expression levels of several immunologically interesting genes as shown in Table 2. The critical point to note is that, while there is no difference in the expression level of interferon α (IFNα), CLCF1 [also known as B-cell stimulating factor-3 (BSF-3)], IL-1β, B-cell activating factor (BAFF) and IL-10—all of which have the potential to activate B cells—were up-regulated predominantly in Gr-1– monocytes. Moreover, anti-apoptotic Bcl2 and Bcl6 and DC markers, such as CD11c, Adamdec1 and CD83, were markedly up-regulated in Gr-1– monocytes, suggesting that Gr-1– monocytes are long-lived and committed to differentiate into DCs (47). Since the splenic marginal zone around B-cell follicles is CX3CL1+ (Fig. 2), CX3CR1+ Gr-1– non-classical monocytes are most likely recruited into the marginal zone, where they activate B cells and subsequently may differentiate into DCs in the splenic white pulp (Fig. 1). This notion is consistent with the result reported in an adoptive transfer experiment, showing that both classical and non-classical monocyte subsets can differentiate into DCs in inflamed and non-inflamed tissues, respectively (14).
Table 2.
Comparison of the expression levels of genes encoding immunologically interesting molecules between Gr-1+ and Gr-1– monocyte subsets
| Gene | Gr-1+ monocytes | Gr-1– monocytes | P value |
|---|---|---|---|
| Bcl2 | 4.084 ± 1.077 | 36.325 ± 2.756 | 0.0000232324 |
| Fcgr4 | 45.473 ± 11.789 | 465.176 ± 38.713 | 0.0000343138 |
| Bcl6 | 27.972 ± 2.818 | 66.939 ± 4.189 | 0.000166234 |
| Clcf1 (BSF-3) | 0.768 ± 0.385 | 8.752 ± 1.049 | 0.000348923 |
| Itgax (CD11c) | 2.066 ± 0.690 | 43.465 ± 7.543 | 0.00156422 |
| Adamdec1 | 0.258 ± 0.947 | 18.910 ± 3.492 | 0.00218494 |
| Il1b | 89.141 ± 32.658 | 456.647 ± 66.449 | 0.00260974 |
| Tnfsf13B (BAFF) | 4.437 ± 1.273 | 7.950 ± 1.022 | 0.00487835 |
| Cd83 | 1.766 ± 0.364 | 32.984 ± 7.656 | 0.00655325 |
| Il10 | 0.632 ± 0.370 | 9.052 ± 2.046 | 0.0088057 |
RNAs were extracted from sorted Gr-1+ monocyte subsets (n = 4) and Gr-1– monocyte subsets (n = 5) obtained from 8-month-old, randomly selected B6.FcγRIIB–/–.Yaa mice and cell-type-specific FcγRIIB-deficient B6.Yaa mice (47). The gene expression levels (mean ± SE) are shown as FPKM (fragments per kilo base of exon per million reads).
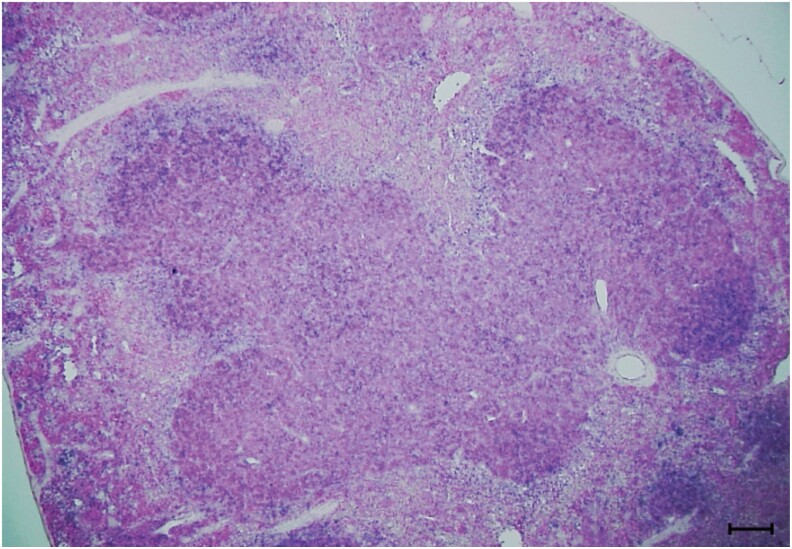
Fig. 2.
In situ hybridization for CX3CL1 expression in the marginal zone around B-cell follicles in the spleen from 6-mo-old B6 mice. The antisense probe used is the mouse CX3CL1 (NM_009142.3, sequence position 1151–1951) and positive areas are shown by the blue color. The bar in the picture represents 100 µm.
Experimental lupus induced by 2,6,10,14-tetramethyl-pentadecane (commonly known as pristane) is the experimental mouse model of IFNα-induced SLE. The injection of pristane into the peritoneal cavity of BALB/c mice induces the accumulation of Gr-1+ classical monocytes in the peritoneal cavity, which produce high amounts of IFNα and promote DC maturation, T-cell survival, B-cell maturation into plasma cells, and auto-antibody production (49). While plasmacytoid DCs are known to be the specialized cells to produce type 1 interferons (50), Gr-1+ monocytes intriguingly produce IFNα through TLR7 signals in this model (51). Thus, spontaneous and induced lupus models both demonstrate the importance of monocytes and TLR7 signals, but the involved monocyte subsets and TLR7-mediated pathway differ in each model.
The possible contribution of TLR7 signaling has been reported in human SLE (52). The association of monocyte subsets with the disease severity has also been studied. Contributing subsets vary among studies (53–58); however, several reports show a contribution of non-classical monocytes in the disease (53, 55–57). Biesen et al. (53) have reported that serum levels of anti-dsDNA antibodies highly correlate with the percentage of sialoadhesin+ CD14lowCD16++ non-classical monocytes in circulation. Furthermore, Cros et al. (59) have shown that non-classical monocytes secrete high amounts of IL-1β in a TLR signaling-dependent manner. Also, the contributions of non-classical monocytes to the antigen presentation and the activation of T cells and B cells have been reported in SLE patients (56, 57). These findings are consistent with those in Yaa-associated lupus models. As SLE is a heterogeneous autoimmune disease, in which different combinations of multiple susceptibility genes and a variety of environmental factors may result in the development of SLE via different mechanisms. Thus, further studies are needed to clarify the role of monocyte subsets for disease development in each type of SLE.
Defective clearance of ICs and apoptotic cells by macrophages is detected in some patients with SLE (60). However, accumulating observations suggest that aberrant activation, but not defective function, of monocytes may be a more appropriate concept and plays a dynamic role in the initiation and progression of disease both in human SLE and murine lupus (61). Current non-specific immunosuppressive treatments for SLE sometimes cause serious side effects. Moreover, the clinical trials of biotherapies targeting IFNα receptor or activated lymphocytes did not meet the successful end-point (62). Monocyte targeting may provide an alternative treatment approach (63). A pilot study for monocyte and neutrophil depletion was associated with clinical improvement in patients with SLE (64), and the therapeutic effects of the inhibition of monocyte activation, differentiation and migration were reported in murine lupus models (65–67). Strategies to manipulate FcγR function to overcome IC-mediated autoimmune diseases are additional but challenging options (68); for example, the treatment of lupus nephritis with a soluble decoy FcγR was applied in lupus-prone NZB/NZW F1 mice (69). These trials are summarized in Table 3. Further therapeutic approaches will be helpful to design suitable strategies without side effects.
Table 3.
Monocyte-targeting therapeutic approaches for SLE and RA in humans and mice
| Ref. | |
|---|---|
| Human SLE | |
| Removal by cytapheresis | (64) |
| Murine lupus models | |
| Migration inhibition by anti-MCP-1 gene therapy in MRL/lpr mice | (65) |
| Migration inhibition by CX3CL1 antagonist in MRL/lpr mice | (66) |
| CSF1R signal inhibition by CSF1R inhibitor in MRL/lpr mice | (67) |
| Activation inhibition by soluble FcγR in NZB/NZW F1 mice | (69) |
| Human RA | |
| Treatment with anti-RANKL antibody | (70, 71) |
| Treatment with anti-CX3CL1 antibody | (72) |
| Murine arthritis models | |
| Migration inhibition and depletion by anti-CCR2 antibody in CIA model | (73) |
| CSF1R signal inhibition by anti-CSF1R antibody and by CSF1R inhibitor in CIA, CAIA, K/BxN serum transfer models |
(74) |
| CSF1R signal inhibition by anti-CSF1R antibody in CIA and K/BxN serum transfer models |
(75) |
| Migration inhibition by anti-CX3CL1 antibody in CIA model | (76) |
| Migration inhibition and depletion by anti-CD11b antibody in KO1 mice | (77) |
Rheumatoid arthritis
RA is characterized by marked synovial hyperplasia in multiple synovial joints associated with pannus formation, which contains a massive infiltration of cytokine-producing inflammatory cells, proliferating fibroblasts and increased numbers of mature osteoclasts. The generation and activation of osteoclasts in inflamed joint tissues are essential for the progressive destruction of cartilage and bone. Osteoclasts are multinucleated giant cells positive for tartrate-resistant acid phosphatase (TRAP) and cathepsin K and they resorb bone matrix. These cells differentiate from osteoclast precursors, which originate from monocytes in the bone marrow and peripheral blood (10, 11). The process of osteoclastogenesis is controlled by the interaction of receptor activator of NF-κB (RANK) expressed on osteoclast precursors with its ligand RANKL expressed on synovial fibroblasts, osteoblasts and Th17 cells (78). RANKL expression on these cells is up-regulated by inflammatory cytokines such as TNFα, IL-1, IL-6 and IL-17 (78).
RA is also a complex autoimmune disease, and multiple susceptibility genes and environmental factors are involved in the disease susceptibility. The most important risk factor is the class II MHC locus, as in the case of SLE. Intriguingly, it has been suggested that RA-associated class II alleles present citrullinated peptides efficiently to T cells, and subsequently activate B cells to produce anti-cyclic citrullinated peptide (CCP) antibodies (79), suggesting the essential role of the adaptive immune system in the disease progression since anti-CCP antibodies provide the diagnostic marker for RA (80, 81). Auto-antibody production and the resultant IC formation are suggested to be involved in the disease process. Several studies have shown that vascular endothelial cells increase vascular permeability, adhesion molecule expression and inflammatory cytokine production after the deposition of circulating ICs (82–84). Recently, it has been shown that IgG ICs sensitize monocytes for inflammatory hyperactivity in RA patients (85).
Monocytes/macrophages are the major producer of inflammatory cytokines in arthritic lesions and some of these cytokines promote the polarization of CD4+ T cells to Th1 cells and Th17 cells, which are considered to be critical mediators of RA (86). Intriguingly, monocytes include precursors of osteoclasts, the specialized cell type for osteoclastogenesis (10, 11). Thus, monocytes are an essential cell type in both increased inflammation and bone destruction. The binding of IgG ICs to the activating Fcγ receptors is essential for inflammatory myelomonocytic cell activation to produce inflammatory cytokines. Moreover, it has been shown that the cross-linking of FcγRIV on osteoclasts by ICs is critical for osteoclast development in inflammatory arthritis (87). The activating signal through the FcRγ is counterbalanced by the inhibitory signal mediated by FcγRIIB. Thus, the lack of FcγRIIB may augment IC-mediated inflammation and bone loss. We previously found that a subline of FcγRIIB-deficient strains (designated as KO1) spontaneously developed severe arthritis closely resembling human RA (88). This strain was established by backcrossing of the initially constructed FcγRIIB–/– mice on a hybrid (129 × B6) background into a B6 background and was carrying a 129-derived autoimmune-susceptible SLAM haplotype 2 locus in the vicinity of the Fcgr2b gene (88). FcγRIIB–/– mice on a pure B6 background did not develop arthritis, suggesting that the combined effect of FcγRIIB-deficiency and SLAM haplotype 2 is responsible for the development of arthritis. An association of gene polymorphisms of FCGR2B and the SLAM family with RA was also reported in studies of humans (89–91).
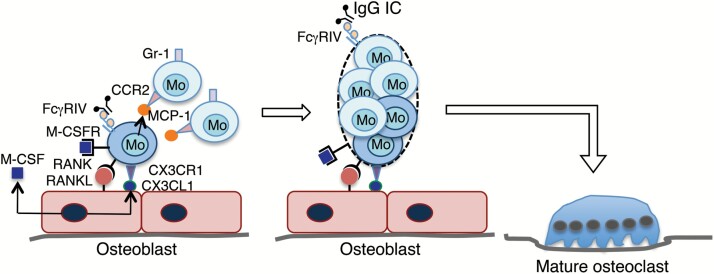
It has been shown that the mouse Gr-1+ classical monocyte subset, but not the Gr-1– non-classical subset, can differentiate into osteoclasts in vitro when stimulated with M-CSF and RANKL (87). However, when cultured in vitro together with osteoblasts, the interaction between CX3CR1 expressed on osteoclast precursors and CX3CL1 constitutively expressed on osteoblasts is essential for the osteoblast-induced osteoclast differentiation, indicating that the Gr-1–CX3CR1high non-classical monocyte subset is responsible for osteoclastogenesis in these culture conditions (92). CX3CL1 exists as a soluble form and a membrane-bound form, and mediates migration and adhesion as well (93). Thus, CX3CL1+ osteoblasts attract CX3CR1high non-classical monocytes and induce firm adhesion by membrane-bound CX3CL1 (Fig. 3). These adherent CX3CR1high monocytes proliferate in response to M-CSF secreted by osteoblasts (94) and are activated by RANKL expressed on osteoblasts to produce MCP-1, which in turn attracts CCR2+ classical monocytes (95). Intriguingly, the interaction between CCR2 and its ligand MCP-1 was shown to be essential for cell fusion to form multinucleated giant cells including mature osteoclasts (96). Thus, secreted MCP-1 promotes fusion of CCR2+ classical monocytes with the RANKL-stimulated CX3CR1high non-classical monocytes, resulting in the formation of multinucleated mature osteoclasts (Fig. 3). This process may be accelerated by large amounts of MCP-1 produced by activated inflammatory cells, resulting in the augmented bone loss in inflamed joint tissues.
Fig. 3.
A model for the generation of multinucleated mature osteoclasts from osteoclast precursor monocytes in inflammatory arthritis. Gr-1–CX3CR1+ osteoclast precursor monocytes adhere to CX3CL1+ osteoblasts and are stimulated by secreted M-CSF and membrane-bound RANKL. Activated Gr-1–CX3CR1+ monocytes secrete MCP-1, which attracts Gr-1+CCR2+ monocytes and promotes fusion of these Gr-1+CCR2+ monocytes with the RANKL-stimulated Gr-1–CX3CR1+ monocytes, resulting in the formation of multinucleated mature osteoclasts. The cross-linking of FcγRIV on osteoclasts by IgG ICs is critical for osteoclast development in inflammatory arthritis (87).
Previous studies showed that classical monocytes differentiate into macrophages in inflamed tissues (11); however, the more recent study indicates that non-classical monocytes give rise to inflammatory macrophages and are crucial for the initiation of joint inflammation in K/BxN serum transfer arthritis model (97). Recently, it has also been reported that non-classical monocytes are pivotal cells for osteoclast differentiation in the same arthritis model (98). These findings suggest that non-classical monocytes have the potential to differentiate into both macrophages and mature osteoclasts in inflammatory arthritis.
Like lupus-prone B6.FcγRIIB–/–.Yaa mice, arthritis-prone KO1 mice also developed monocytosis that predominantly consists of the Gr-1– subset. The introduction of TNFα deficiency into KO1 mice suppressed both monocytosis and arthritis, suggesting an essential role of TNFα for monocyte generation and also an association of monocytosis with the development of RA (99). When KO1 mice were treated with an anti-CD11b monoclonal antibody (5C6), the development of arthritis was markedly suppressed with much less inflammatory cell infiltration and less osteoclast generation in joint tissues (77) (Table 3). This suppression is due to the 5C6-mediated blockade of the recruitment of peripheral circulating CD11b+ inflammatory cells and osteoclast precursors into the arthritic lesions (100). The 5C6 treatment also suppressed monocytosis probably because of the 5C6-mediated cytotoxic effect. Furthermore, in 5C6-treated KO1 mice, serum levels of auto-antibodies such as rheumatoid factor and anti-CCP antibodies were significantly suppressed compared to the non-treated KO1 mice. It has been shown that a large amount of auto-antibody is secreted by age-associated B cells (ABCs), which uniquely express CD11c and also CD11b in mice (101, 102). Thus, it is possible that the suppression of auto-antibody levels is due to the decreased incidence of ABCs by the 5C6 treatment. Real-time PCR studies revealed that the 5C6 treatment suppressed the expression levels of B-cell activation/differentiation-related cytokines such as BAFF, IL-1β, BSF-3, IL-6 and IL-10 in spleen and peripheral leukocytes (77), consistent with the lower level of auto-antibodies in 5C6-treated KO1 mice. The decreased expression of these cytokines may be due to the reduced frequency of monocytes in 5C6-treated mice since these cytokines are preferentially secreted by Gr-1− monocytes as shown in Table 2, although the possible contribution of cytokine-producing CD11b+ other cell types including ABCs is not denied.
The expression level of CD64 (FcγRI; a high affinity activating receptor) is known to be elevated in monocytes in RA patients suggesting that monocytes from RA patients are in a more differentiated and active stage compared with those from the healthy individuals (103). Moreover, the association of monocyte subsets with the development of arthritis has been examined in patients with RA. A previous study showed increased frequencies of CD14+CD16+ monocytes in peripheral blood (104) and more recent studies have shown that the frequency of the CD14++CD16+ intermediate subset is increased in peripheral blood (105–107) and in synovial fluid (108, 109). It has been shown that the frequency of intermediate monocytes in the periphery is significantly associated with the disease severity (106, 107) and that intermediate monocytes are the predominant subset in the differentiation into inflammatory macrophages in arthritic joints (110). Also, it has been suggested that while classical monocytes are the main source of osteoclasts in physiology, osteoclasts generated from intermediate monocytes are responsible for the increased bone resorption in arthritic joints (111, 112).
Biotherapies targeting several inflammatory cytokines as well as those targeting T cells or B cells have been considered in RA (113, 114). TNFα is the master inflammatory element, and anti-TNFα biotherapy is effective in many patients; however, a proportion of patients remains resistant (115). These disadvantaged patients need alternatives to protect them from destructive arthritis, and the blocking of migration, activation, differentiation and function of osteoclast precursor monocytes is the most promising approach as suggested (116, 117). Table 3 summarizes several of these approaches ongoing in human RA and murine arthritis models such as collagen-induced arthritis (CIA), collagen antibody-induced arthritis (CAIA) and K/BxN serum transfer arthritis models, as well as KO1 mice (70–77).
Conclusions and future directions
SLE and RA are both IC-mediated autoimmune diseases, and IgG ICs activate the monocyte lineage through stimulating signals from FcRγ. These activation signals accelerate the monocyte differentiation process from the classical monocyte subset to the intermediate subset and the subsequent non-classical subset. In SLE and RA, more differentiated monocyte subsets play an essential role for the disease progression. An association of the frequency of the non-classical monocyte subset with the production of auto-antibodies is reported in SLE patients and lupus mouse models. Intriguingly, murine non-classical monocytes have a high potential to produce B-cell-stimulating cytokines, suggesting a pathological role for non-classical monocytes in auto-antibody production. Furthermore, an antigen presentation capacity of non-classical monocytes is reported in SLE patients. In murine arthritis model, non-classical monocytes differentiate into both inflammatory macrophages and osteoclasts in arthritic joints. In RA patients, the intermediate monocyte frequency is associated with the disease severity, suggesting the essential role of these monocytes in both inflammation and osteoclastogenesis. These findings suggest that monocyte-targeting therapies are promising alternative therapeutic approaches for patients who are resistant to the immunosuppressive treatments or the biotherapies that are widely used at present. Our additional, broadening knowledge could be beneficial to bring about these approaches.
Funding
The work related to this review was supported in part by a Grant-in-Aid for Scientific Research (C) (15K08432 to SH and 26460493 to QL) from the Ministry of Education, Culture, Sports, Science and Technology Japan and a Grant-in-Aid for Japan Society of the Promotion of Science (JSPS) Long-Term Fellows (FY2017 to JSV).
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Auffray, C., Sieweke, M. H. and Geissmann, F. 2009. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27:669. [DOI] [PubMed] [Google Scholar]
- 2. Hettinger, J.,, Richards, D. M.,, Hansson, J., et al. 2013. Origin of monocytes and macrophages in a committed progenitor. Nat. Immunol. 14:821. [DOI] [PubMed] [Google Scholar]
- 3. Kawamura, S.,, Onai, N.,, Miya, F., et al. 2017. Identification of a human clonogenic progenitor with strict monocyte differentiation potential: a counterpart of mouse cMoPs. Immunity 46:835.e4. [DOI] [PubMed] [Google Scholar]
- 4. Shi, C. and Pamer, E. G. 2011. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martinez, J.,, Cunha, L. D.,, Park, S., et al. 2016. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature 533:115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Wada, T.,, Furuichi, K.,, Segawa-Takaeda, C., et al. 1999. MIP-1alpha and MCP-1 contribute to crescents and interstitial lesions in human crescentic glomerulonephritis. Kidney Int. 56:995. [DOI] [PubMed] [Google Scholar]
- 7. Bergtold, A., Gavhane, A., D’Agati, V., Madaio, M. and Clynes, R. 2006. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J. Immunol. 177:7287. [DOI] [PubMed] [Google Scholar]
- 8. Nakatani, K.,, Yoshimoto, S.,, Iwano, M., et al. 2010. Fractalkine expression and CD16+ monocyte accumulation in glomerular lesions: association with their severity and diversity in lupus models. Am. J. Physiol. Renal Physiol. 299:F207. [DOI] [PubMed] [Google Scholar]
- 9. McInnes, I. B. and Schett, G. 2011. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365:2205. [DOI] [PubMed] [Google Scholar]
- 10. Boyle, W. J., Simonet, W. S. and Lacey, D. L. 2003. Osteoclast differentiation and activation. Nature 423:337. [DOI] [PubMed] [Google Scholar]
- 11. Gordon, S. and Taylor, P. R. 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5:953. [DOI] [PubMed] [Google Scholar]
- 12. Ziegler-Heitbrock, L.,, Ancuta, P.,, Crowe, S., et al. 2010. Nomenclature of monocytes and dendritic cells in blood. Blood 116:e74. [DOI] [PubMed] [Google Scholar]
- 13. Mildner, A.,, Schönheit, J.,, Giladi, A., et al. 2017. Genomic characterization of murine monocytes reveals C/EBPβ transcription factor dependence of Ly6C- cells. Immunity 46:849.e7. [DOI] [PubMed] [Google Scholar]
- 14. Geissmann, F., Jung, S. and Littman, D. R. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71. [DOI] [PubMed] [Google Scholar]
- 15. Ingersoll, M. A.,, Spanbroek, R.,, Lottaz, C., et al. 2010. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 115:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Auffray, C.,, Fogg, D.,, Garfa, M., et al. 2007. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317:666. [DOI] [PubMed] [Google Scholar]
- 17. Sunderkötter, C.,, Nikolic, T.,, Dillon, M. J., et al. 2004. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172:4410. [DOI] [PubMed] [Google Scholar]
- 18. Santiago-Raber, M. L., Baudino, L., Alvarez, M., van Rooijen, N., Nimmerjahn, F. and Izui, S. 2011. TLR7/9-mediated monocytosis and maturation of Gr-1(hi) inflammatory monocytes towards Gr-1(lo) resting monocytes implicated in murine lupus. J. Autoimmun. 37:171. [DOI] [PubMed] [Google Scholar]
- 19. Yona, S.,, Kim, K. W.,, Wolf, Y., et al. 2013. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel, A. A.,, Zhang, Y.,, Fullerton, J. N., et al. 2017. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 214:1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woollard, K. J. and Geissmann, F. 2010. Monocytes in atherosclerosis: subsets and functions. Nat. Rev. Cardiol. 7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mikołajczyk, T. P.,, Osmenda, G.,, Batko, B., et al. 2016. Heterogeneity of peripheral blood monocytes, endothelial dysfunction and subclinical atherosclerosis in patients with systemic lupus erythematosus. Lupus 25:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winchester, R.,, Giles, J. T.,, Nativ, S., et al. 2016. Association of elevations of specific T cell and monocyte subpopulations in rheumatoid arthritis with subclinical coronary artery atherosclerosis. Arthritis Rheumatol. 68:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Langefeld, C. D.,, Ainsworth, H. C.,, Cunninghame Graham, D. S., et al. 2017. Transancestral mapping and genetic load in systemic lupus erythematosus. Nat. Commun. 8:16021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Okada, Y.,, Wu, D.,, Trynka, G., et al. ; RACI Consortium; GARNET Consortium . 2014. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bentham, J.,, Morris, D. L.,, Graham, D. S. C., et al. 2015. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 47:1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walsh, A. M.,, Whitaker, J. W.,, Huang, C. C., et al. 2016. Integrative genomic deconvolution of rheumatoid arthritis GWAS loci into gene and cell type associations. Genome Biol. 17:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Izui, S., Ibnou-Zekri, N., Fossati-Jimack, L. and Iwamoto, M. 2000. Lessons from BXSB and related mouse models. Int. Rev. Immunol. 19:447. [DOI] [PubMed] [Google Scholar]
- 29. Zhang, D.,, Fujio, K.,, Jiang, Y., et al. 2004. Dissection of the role of MHC class II A and E genes in autoimmune susceptibility in murine lupus models with intragenic recombination. Proc. Natl Acad. Sci. USA 101:13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang, Y.,, Hirose, S.,, Abe, M., et al. 2000. Polymorphisms in IgG Fc receptor IIB regulatory regions associated with autoimmune susceptibility. Immunogenetics 51:429. [DOI] [PubMed] [Google Scholar]
- 31. Pritchard, N. R., Cutler, A. J., Uribe, S., Chadban, S. J., Morley, B. J. and Smith, K. G. 2000. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcgammaRII. Curr. Biol. 10:227. [DOI] [PubMed] [Google Scholar]
- 32. Willcocks, L. C.,, Carr, E. J.,, Niederer, H. A., et al. 2010. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc. Natl Acad. Sci. USA 107:7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang, Y.,, Hirose, S.,, Sanokawa-Akakura, R., et al. 1999. Genetically determined aberrant down-regulation of FcgammaRIIB1 in germinal center B cells associated with hyper-IgG and IgG autoantibodies in murine systemic lupus erythematosus. Int. Immunol. 11:1685. [DOI] [PubMed] [Google Scholar]
- 34. Xiu, Y.,, Nakamura, K.,, Abe, M., et al. 2002. Transcriptional regulation of Fcgr2b gene by polymorphic promoter region and its contribution to humoral immune responses. J. Immunol. 169:4340. [DOI] [PubMed] [Google Scholar]
- 35. Chan, A. Y., Westcott, J. M., Mooney, J. M., Wakeland, E. K. and Schatzie, J. D. 2006. The role of SAP and the SLAM family in autoimmunity. Curr. Opin. Immunol. 3:813. [DOI] [PubMed] [Google Scholar]
- 36. Wang, A., Batteux, F. and Wakeland, E. K. 2010. The role of SLAM/CD2 polymorphisms in systemic autoimmunity. Curr. Opin. Immunol. 22:706. [DOI] [PubMed] [Google Scholar]
- 37. Ota, Y.,, Kawaguchi, Y.,, Takagi, K., et al. 2010. Single nucleotide polymorphisms of CD244 gene predispose to renal and neuropsychiatric manifestations with systemic lupus erythematosus. Mod. Rheumatol. 20:427. [DOI] [PubMed] [Google Scholar]
- 38. Delgado-Vega, A., Sánchez, E., Löfgren, S., Castillejo-López, C. and Alarcón-Riquelme, M. E. 2010. Recent findings on genetics of systemic autoimmune diseases. Curr. Opin. Immunol. 22:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Subramanian, S.,, Tus, K.,, Li, Q. Z., et al. 2006. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl Acad. Sci. USA 103:9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pisitkun, P., Deane, J. A., Difilippantonio, M. J., Tarasenko, T., Satterthwaite, A. B. and Bolland, S. 2006. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312:1669. [DOI] [PubMed] [Google Scholar]
- 41. Deane, J. A.,, Pisitkun, P.,, Barrett, R. S., et al. 2007. Control of Toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rothstein, A. M. 2006. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Celhar, T.,, Hopkins, R.,, Thornhill, S. I., et al. 2015. RNA sensing by conventional dendritic cells is central to the development of lupus nephritis. Proc. Natl Acad. Sci. USA 112:E6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Santiago-Raber, M. L.,, Amano, H.,, Amano, E., et al. 2009. Fcgamma receptor-dependent expansion of a hyperactive monocyte subset in lupus-prone mice. Arthritis Rheum. 60:2408. [DOI] [PubMed] [Google Scholar]
- 45. Kikuchi, S.,, Santiago-Raber, M. L.,, Amano, H., et al. 2006. Contribution of NZB autoimmunity 2 to Y-linked autoimmune acceleration-induced monocytosis in association with murine systemic lupus. J. Immunol. 176:3240. [DOI] [PubMed] [Google Scholar]
- 46. Boross, P.,, Arandhara, V. L.,, Martin-Ramirez, J., et al. 2011. The inhibiting Fc receptor for IgG, FcγRIIB, is a modifier of autoimmune susceptibility. J. Immunol. 187:1304. [DOI] [PubMed] [Google Scholar]
- 47. Lin, Q.,, Ohtsuji, M.,, Amano, H., et al. 2018. FcγRIIb on B cells and myeloid cells modulates B cell activation and autoantibody responses via different but synergistic pathways in lupus-prone Yaa mice. J. Immunol. 201:3199. [DOI] [PubMed] [Google Scholar]
- 48. Ravetch, J. V. and Bolland, S. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275. [DOI] [PubMed] [Google Scholar]
- 49. Lee, P. Y.,, Weinstein, J. S.,, Nacionales, D. C., et al. 2008. A novel type I IFN-producing cell subset in murine lupus. J. Immunol. 180:5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Swiecki, M. and Colonna, M. 2015. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 15:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bossaller, L.,, Christ, A.,, Pelka, K., et al. 2016. TLR9 deficiency leads to accelerated renal disease and myeloid lineage abnormalities in pristane-induced murine lupus. J. Immunol. 197:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shen, N.,, Fu, Q.,, Deng, Y., et al. 2010. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc. Natl Acad. Sci. USA 107:15838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Biesen, R.,, Demir, C.,, Barkhudarova, F., et al. 2008. Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus. Arthritis Rheum. 58:1136. [DOI] [PubMed] [Google Scholar]
- 54. Li, Y., Lee, P. Y. and Reeves, W. H. 2010. Monocyte and macrophage abnormalities in systemic lupus erythematosus. Arch. Immunol. Ther. Exp. (Warsz) 58:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jiang, W., Zhang, L., Lang, R., Li, Z. and Gilkeson, G. 2014. Sex differences in monocyte activation in systemic lupus erythematosus (SLE). PLoS One 9:e114589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mukherjee, R., Kanti Barman, P., Kumar Thatoi, P., Tripathy, R., Kumar Das, B. and Ravindran, B. 2015. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci. Rep. 5:13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhu, H.,, Hu, F.,, Sun, X., et al. 2016. CD16+ monocyte subset was enriched and functionally exacerbated in driving T-cell activation and B-cell response in systemic lupus erythematosus. Front. Immunol. 7:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu, Z.,, Zhang, S.,, Zhao, L., et al. 2017. Upregulation of CD16- monocyte subsets in systemic lupus erythematous patients. Clin. Rheumatol. 36:2281. [DOI] [PubMed] [Google Scholar]
- 59. Cros, J.,, Cagnard, N.,, Woollard, K., et al. 2010. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mahajan, A., Herrmann, M. and Muñoz, L. E. 2016. Clearance deficiency and cell death pathways: a model for the pathogenesis of SLE. Front. Immunol. 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Katsiari, C. G., Liossis, S. N. and Sfikakis, P. P. 2010. The pathophysiologic role of monocytes and macrophages in systemic lupus erythematosus: a reappraisal. Semin. Arthritis Rheum. 39:491. [DOI] [PubMed] [Google Scholar]
- 62. Dolgin, E. 2019. Lupus in crisis: as failures pile up, clinicians call for new tools. Nat. Biotechnol. 37:7. [DOI] [PubMed] [Google Scholar]
- 63. Chalmers, S. A., Chitu, V., Ramanujam, M. and Putterman, C. 2015. Therapeutic targeting of macrophages in lupus nephritis. Discov. Med. 20:43. [PubMed] [Google Scholar]
- 64. Soerensen, H.,, Schneidewind-Mueller, J. M.,, Lange, D., et al. 2006. Pilot clinical study of Adacolumn cytapheresis in patients with systemic lupus erythematosus. Rheumatol. Int. 26:409. [DOI] [PubMed] [Google Scholar]
- 65. Shimizu, S.,, Nakashima, H.,, Masutani, K., et al. 2004. Anti-monocyte chemoattractant protein-1 gene therapy attenuates nephritis in MRL/lpr mice. Rheumatology (Oxford) 43:1121. [DOI] [PubMed] [Google Scholar]
- 66. Inoue, A.,, Hasegawa, H.,, Kohno, M., et al. 2005. Antagonist of fractalkine (CX3CL1) delays the initiation and ameliorates the progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum. 52:1522. [DOI] [PubMed] [Google Scholar]
- 67. Chalmers, S. A., Wen, J., Shum, J., Doerner, J., Herlitz, L. and Putterman, C. 2017. CSF-1R inhibition attenuates renal and neuropsychiatric disease in murine lupus. Clin. Immunol. 185:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hogarth, P. M. and Pietersz, G. A. 2012. Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat. Rev. Drug Discov. 11:311. [DOI] [PubMed] [Google Scholar]
- 69. Werwitzke, S.,, Trick, D.,, Sondermann, P., et al. 2008. Treatment of lupus-prone NZB/NZW F1 mice with recombinant soluble Fc gamma receptor II (CD32). Ann. Rheum. Dis. 67:154. [DOI] [PubMed] [Google Scholar]
- 70. Deodhar, A.,, Dore, R. K.,, Mandel, D., et al. 2010. Denosumab-mediated increase in hand bone mineral density associated with decreased progression of bone erosion in rheumatoid arthritis patients. Arthritis Care Res. (Hoboken) 62:569. [DOI] [PubMed] [Google Scholar]
- 71. Tanaka, S., Tanaka, Y., Ishiguro, N., Yamanaka, H., and Takeuchi, T. 2018. RANKL: a therapeutic target for bone destruction in rheumatoid arthritis. Mod. Rheumatol. 28:9. [DOI] [PubMed] [Google Scholar]
- 72. Tanaka, Y.,, Takeuchi, T.,, Umehara, H., et al. 2018. Safety, pharmacokinetics, and efficacy of E6011, an antifractalkine monoclonal antibody, in a first-in-patient phase 1/2 study on rheumatoid arthritis. Mod. Rheumatol. 28:58. [DOI] [PubMed] [Google Scholar]
- 73. Brühl, H.,, Cihak, J.,, Plachý, J., et al. 2007. Targeting of Gr-1+,CCR2+ monocytes in collagen-induced arthritis. Arthritis Rheum. 56:2975. [DOI] [PubMed] [Google Scholar]
- 74. Paniagua, R. T.,, Chang, A.,, Mariano, M. M., et al. 2010. c-Fms-mediated differentiation and priming of monocyte lineage cells play a central role in autoimmune arthritis. Arthritis Res. Ther. 12:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Toh, M. L.,, Bonnefoy, J. Y.,, Accart, N., et al. 2014. Bone- and cartilage-protective effects of a monoclonal antibody against colony-stimulating factor 1 receptor in experimental arthritis. Arthritis Rheumatol. 66:2989. [DOI] [PubMed] [Google Scholar]
- 76. Hoshino-Negishi, K.,, Ohkuro, M.,, Nakatani, T., et al. 2019. Role of anti-fractalkine antibody in suppression of joint destruction by inhibiting migration of osteoclast precursors to the synovium in experimental arthritis. Arthritis Rheumatol. 71:222. [DOI] [PubMed] [Google Scholar]
- 77. Ohtsuji, M.,, Lin, Q.,, Okazaki, H., et al. 2018. Anti-CD11b antibody treatment suppresses the osteoclast generation, inflammatory cell infiltration, and autoantibody production in arthritis-prone FcγRIIB-deficient mice. Arthritis Res. Ther. 20:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Takayanagi, H. 2012. New developments in osteoimmunology. Nat. Rev. Rheumatol. 8:684. [DOI] [PubMed] [Google Scholar]
- 79. Firestein, G. S. and McInnes, I. B. 2017. Immunopathogenesis of rheumatoid arthritis. Immunity 46:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wegner, N.,, Lundberg, K.,, Kinloch, A., et al. 2010. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol. Rev. 233:34. [DOI] [PubMed] [Google Scholar]
- 81. Bax, M., Huizinga, T. W. and Toes, R. E. 2014. The pathogenic potential of autoreactive antibodies in rheumatoid arthritis. Semin. Immunopathol. 36:313. [DOI] [PubMed] [Google Scholar]
- 82. Binstadt, B. A.,, Patel, P. R.,, Alencar, H., et al. 2006. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat. Immunol. 7:284. [DOI] [PubMed] [Google Scholar]
- 83. Sun, W., Jiao, Y., Cui, B., Gao, X., Xia, Y. and Zhao, Y. 2013. Immune complexes activate human endothelium involving the cell-signaling HMGB1-RAGE axis in the pathogenesis of lupus vasculitis. Lab. Invest. 93:626. [DOI] [PubMed] [Google Scholar]
- 84. Atehortúa, L.,, Rojas, M.,, Vásquez, G., et al. 2019. Endothelial activation and injury by microparticles in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res. Ther. 21:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhong, Q., Gong, F. Y., Gong, Z., Hua, S. H., Zeng, K. Q. and Gao, X. M. 2018. IgG immunocomplexes sensitize human monocytes for inflammatory hyperactivity via transcriptomic and epigenetic reprogramming in rheumatoid arthritis. J. Immunol. 200:3913. [DOI] [PubMed] [Google Scholar]
- 86. Roberts, C. A., Dickinson, A. K. and Taams, L. S. 2015. The interplay between monocytes/macrophages and CD4(+) T cell subsets in rheumatoid arthritis. Front. Immunol. 6:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Seeling, M.,, Hillenhoff, U.,, David, J. P., et al. 2013. Inflammatory monocytes and Fcγ receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proc. Natl Acad. Sci. USA 110:10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sato-Hayashizaki, A.,, Ohtsuji, M.,, Lin, Q., et al. 2011. Presumptive role of 129 strain-derived Sle16 locus in rheumatoid arthritis in a new mouse model with Fcγ receptor type IIb-deficient C57BL/6 genetic background. Arthritis Rheum. 63:2930. [DOI] [PubMed] [Google Scholar]
- 89. Chen, J. Y.,, Wang, C. M.,, Ma, C. C., et al. 2008. A transmembrane polymorphism in FcgammaRIIb (FCGR2B) is associated with the production of anti-cyclic citrullinated peptide autoantibodies in Taiwanese RA. Genes Immun. 9:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Radstake, T. R.,, Franke, B.,, Wenink, M. H., et al. 2006. The functional variant of the inhibitory Fcgamma receptor IIb (CD32B) is associated with the rate of radiologic joint damage and dendritic cell function in rheumatoid arthritis. Arthritis Rheum. 54:3828. [DOI] [PubMed] [Google Scholar]
- 91. Suzuki, A.,, Yamada, R.,, Kochi, Y., et al. 2008. Functional SNPs in CD244 increase the risk of rheumatoid arthritis in a Japanese population. Nat. Genet. 40:1224. [DOI] [PubMed] [Google Scholar]
- 92. Koizumi, K.,, Saitoh, Y.,, Minami, T., et al. 2009. Role of CX3CL1/fractalkine in osteoclast differentiation and bone resorption. J. Immunol. 183:7825. [DOI] [PubMed] [Google Scholar]
- 93. Bazan, J. F.,, Bacon, K. B.,, Hardiman, G., et al. 1997. A new class of membrane-bound chemokine with a CX3C motif. Nature 385:640. [DOI] [PubMed] [Google Scholar]
- 94. Matsuo, K. and Irie, N. 2008. Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 473:201. [DOI] [PubMed] [Google Scholar]
- 95. Kim, M. S., Day, C. J. and Morrison, N. A. 2005. MCP-1 is induced by receptor activator of nuclear factor-{kappa}B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J. Biol. Chem. 280:16163. [DOI] [PubMed] [Google Scholar]
- 96. Khan, U. A., Hashimi, S. M., Bakr, M. M., Forwood, M. R. and Morrison, N. A. 2016. CCL2 and CCR2 are essential for the formation of osteoclasts and foreign body giant cells. J. Cell. Biochem. 117:382. [DOI] [PubMed] [Google Scholar]
- 97. Misharin, A. V.,, Cuda, C. M.,, Saber, R., et al. 2014. Nonclassical Ly6C(-) monocytes drive the development of inflammatory arthritis in mice. Cell Rep. 9:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Puchner, A.,, Saferding, V.,, Bonelli, M., et al. 2018. Non-classical monocytes as mediators of tissue destruction in arthritis. Ann. Rheum. Dis. 77:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Okazaki, H.,, Lin, Q.,, Nishikawa, K., et al. 2014. TNFα but not IL-17 is critical in the pathogenesis of rheumatoid arthritis spontaneously occurring in a unique FcγRIIB-deficient mouse model. Mod. Rheumatol. 24:931. [DOI] [PubMed] [Google Scholar]
- 100. Rosen, H. and Gordon, S. 1987. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J. Exp. Med. 166:1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Naradikian, M. S., Hao, Y. and Cancro, M. P. 2016. Age-associated B cells: key mediators of both protective and autoreactive humoral responses. Immunol. Rev. 269:118. [DOI] [PubMed] [Google Scholar]
- 102. Rubtsov, A. V.,, Rubtsova, K.,, Fischer, A., et al. 2011. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c⁺ B-cell population is important for the development of autoimmunity. Blood 118:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Luo, Q.,, Xiao, P.,, Li, X., et al. 2018. Overexpression of CD64 on CD14++CD16- and CD14++CD16+ monocytes of rheumatoid arthritis patients correlates with disease activity. Exp. Ther. Med. 16:2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kawanaka, N.,, Yamamura, M.,, Aita, T., et al. 2002. CD14+, CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 46:2578. [DOI] [PubMed] [Google Scholar]
- 105. Rossol, M., Kraus, S., Pierer, M., Baerwald, C. and Wagner, U. 2012. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 64:671. [DOI] [PubMed] [Google Scholar]
- 106. Tsukamoto, M., Suzuki, K., Seta, N. and Takeuchi, T. 2018. Increased circulating CD14brightCD16+ intermediate monocytes are regulated by TNF-α and IL-6 axis in accordance with disease activity in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 36:540. [PubMed] [Google Scholar]
- 107. Tsukamoto, M., Seta, N., Yoshimoto, K., Suzuki, K., Yamaoka, K. and Takeuchi, T. 2017. CD14brightCD16+ intermediate monocytes are induced by interleukin-10 and positively correlate with disease activity in rheumatoid arthritis. Arthritis Res. Ther. 19:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yoon, B. R.,, Yoo, S. J.,, Choi, Y. h., et al. 2014. Functional phenotype of synovial monocytes modulating inflammatory T-cell responses in rheumatoid arthritis (RA). PLoS One 9:e109775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Smiljanovic, B.,, Radzikowska, A.,, Kuca-Warnawin, E., et al. 2018. Monocyte alterations in rheumatoid arthritis are dominated by preterm release from bone marrow and prominent triggering in the joint. Ann. Rheum. Dis. 77:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rana, A. K., Li, Y., Dang, Q. and Yang, F. 2018. Monocytes in rheumatoid arthritis: circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int. Immunopharmacol. 65:348. [DOI] [PubMed] [Google Scholar]
- 111. Komano, Y., Nanki, T., Hayashida, K., Taniguchi, K. and Miyasaka, N. 2006. Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res. Ther. 8:R152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sprangers, S., Schoenmaker, T., Cao, Y., Everts, V. and de Vries, T. J. 2016. Different blood-borne human osteoclast precursors respond in distinct ways to IL-17A. J. Cell. Physiol. 231:1249. [DOI] [PubMed] [Google Scholar]
- 113. Kopf, M., Bachmann, M. F. and Marsland, B. J. 2010. Averting inflammation by targeting the cytokine environment. Nat. Rev. Drug Discov. 9:703. [DOI] [PubMed] [Google Scholar]
- 114. Buch, M. H. and Emery, P. 2011. New therapies in the management of rheumatoid arthritis. Curr. Opin. Rheumatol. 23:245. [DOI] [PubMed] [Google Scholar]
- 115. Monaco, C., Nanchahal, J., Taylor, P. and Feldmann, M. 2015. Anti-TNF therapy: past, present and future. Int. Immunol. 27:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kikuta, J. and Ishii, M. 2013. Osteoclast migration, differentiation and function: novel therapeutic targets for rheumatic diseases. Rheumatology (Oxford) 52:226. [DOI] [PubMed] [Google Scholar]
- 117. Davignon, J. L.,, Hayder, M.,, Baron, M., et al. 2013. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 52:590. [DOI] [PubMed] [Google Scholar]