Abstract
BACKGROUND
To compare in individuals with type 1 diabetes the prediction of incident coronary artery disease (CAD) by components of resting blood pressure—systolic, diastolic, pulse pressure, and mean arterial pressure.
METHODS
In 605 participants without known CAD at baseline and followed sequentially for 25 years, we used Cox modeling built for each blood pressure component associated with incident CAD, overall and stratified by age (<35 and ≥35 years) or hemoglobin A1c (HbA1c) (<9% and ≥9%).
RESULTS
Baseline mean age and diabetes duration were 27 and 19 years, respectively. We observed an early asymptote and then fall in diastolic blood pressure in their late 30s and early 40s in this group of type 1 diabetes individuals, followed by an early rise of pulse pressure. Adjusted hazard ratios (HR) (95% con) for CAD associated with 1 SD pressure increase were 1.35 (1.17, 1.56) for systolic pressure; 1.30 (1.12, 1.51) for diastolic pressure; 1.20 (1.03, 1.39) for pulse pressure; and 1.35 (1.17, 1.56) for mean arterial pressure. Pulse pressure emerged as a strong predictor of CAD at age ≥ 35 years (HR: 1.49 [1.15, 1.94]) and for HbA1c ≥ 9% (HR: 1.32 [1.01, 1.72]).
CONCLUSIONS
Individuals with type 1 diabetes may manifest early vascular aging by an early decline in diastolic blood pressure and rise in pulse pressure, the latter parameter becoming a comparable to systolic blood pressure in predictor incident CAD in those aged over 35 years and those with poor glycemic control.
Keywords: arterial stiffness, blood pressure, coronary artery disease, hypertension, pulse pressure, type 1 diabetes
Individuals with type 1 diabetes carry substantially increased cardiovascular risk.1–3 Blood pressure is a major modifiable risk factor of cardiovascular disease4,5 that displays a series of distinct changes with increasing age.6 The predictive utilities of different blood pressure components, i.e., systolic (SBP), diastolic (DBP), pulse (PP), and mean arterial pressure (MAP), are altered by aging. SBP has been recommended as the primary measure reflecting cardiovascular risk in current hypertension guidelines.7 DBP and MAP may carry further value for risk prediction at younger ages. PP is an independent determinant of adverse cardiovascular outcomes especially in older people but appeared less informative than the other blood pressure measures in the general population.4 However, the degree to which similar relationships exist in people with type 1 diabetes remains unclear, which is particularly important given that an “accelerated vascular aging” has been suggested in this high-risk population.1–3
Because hyperglycemia and insulin resistance are associated with greater stiffening of arteries and premature vascular aging, PP may be more informative for risk prediction in the diabetes population.8,9 However, very few studies have tested the relative importance of PP compared with other blood pressure measures in diabetes, and previous data were exclusively from the older aged type 2 diabetes population.10,11 High blood pressure can affect type 1 diabetes individuals as early as in their childhood.12 Compared with the nondiabetes population, individuals with type 1 diabetes experience an elevated SBP at all ages and an earlier decline in DBP, resulting in a premature increase in PP.13 However, the discriminatory abilities of different blood pressure components for cardiovascular risk have not been established in the type 1 diabetes population. In this study, therefore, we assessed the comparative predictive utilities of different blood pressure components for coronary artery disease (CAD) in type 1 diabetes. In addition, we examined the effect modification of age and glycemic exposure on the association of blood pressure with CAD outcomes. This study was conducted using 25-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, a cohort of well-characterized childhood-onset type 1 diabetes individuals.
METHODS
Study population
Participants were from the Pittsburgh EDC Study, which has previously been described in detail.14 In brief, this is a prospective longitudinal cohort study of childhood-onset (<17 years of age) type 1 diabetes, diagnosed between 1950 and 1980 at Children’s Hospital of Pittsburgh. There were 658 eligible participants who were initially examined between 1986 and 1988. Subsequent clinical assessments, including resting blood pressure measurements, took place biennially for 10 years, with further examinations at the 18- and 25-year follow-up visits. Importantly, the EDC cohort has been shown to be epidemiologically representative of the type 1 diabetes population in Allegheny County, Pennsylvania.15 There were 605 participants who were free from CAD at the study entry and these were selected for the present analysis.
Ascertainment of cardiovascular outcomes
Cardiovascular disease status was evaluated biennially from the baseline visit. CAD was defined as EDC physician-diagnosed angina; myocardial infarction confirmed by Q-waves on an electrocardiogram (Minnesota codes 1.1 or 1.2) or hospital records; angiographic stenosis ≥ 50%; revascularization; or ischemic electrocardiograph changes (Minnesota codes 1.3, 4.1–4.3, 5.1–5.3, and 7.1).
Measurement of blood pressure
Blood pressure was measured by a random-zero sphygmomanometer for the initial 10 years of the study and subsequently by an aneroid device. At each clinic visit, blood pressure was measured 3 times by trained and certified research staff, after the participant had been peacefully sitting for 5 minutes in a quiet room, according to the Hypertension Detection and Follow-up Program protocol.16 PP was defined as the difference between SBP and DBP, and MAP was calculated as the sum of one third of the SBP and two thirds of the DBP.
Measurement of covariates
Demographic and medical history information was obtained through biennial questionnaires beginning at study initiation. Participants self-reported all medication use via questionnaires. Antihypertensive medication use was identified using the Anatomical Therapeutic Chemical Classification System/Defined Daily Dose (ATC/DDD) index. An ever smoker was defined as someone who had smoked at least 100 cigarettes in a lifetime. Body mass index was calculated as weight in kilograms divided by the square of height in meters. HbA1 was obtained by ion-exchange chromatography (Isolab, Akron, OH) for the first 18 months, and the subsequent 10 years by automated high-performance liquid chromatography (Diamat, Bio-Rad, Hercules, CA). Results from the 2 methods were highly correlated (r = 0.95). Hemoglobin A1c (HbA1c) was subsequently obtained using the DCA 2000 analyzer (Bayar, Taneytown, NY) for assessments beyond the first 10 years. The DCA and Diamat assays were also highly correlated (r = 0.95). Before being used in the analysis, all glycosylated hemoglobin values were converted to DCCT-aligned HbA1c using regression equations derived from duplicate assays.17 Total cholesterol was determined enzymatically.18 High-density lipoprotein (HDL) cholesterol was obtained enzymatically with a precipitation technique (heparin and manganese chloride) using a modified version of the Lipid Research Clinics method.19 Non-HDL cholesterol was estimated from total cholesterol minus HDL cholesterol. Urinary albumin was measured by immunonephelometry.20
Statistical analysis
Baseline characteristics of the study population were examined; categorical variables were presented as a percentage (number), and continuous variables as a mean (SD) or median (first and third quantiles), as appropriate.
Cox proportional hazard models were constructed to estimate the associations of each baseline blood pressure measure with incident CAD, adjusting for sex, age, age at diabetes onset, HbA1c, and antihypertensive use. In all of the Cox models, time was measured as time since the study entry (i.e., time 0 = date of study entry). The adjusted hazards ratios (HR) with 95% confidence intervals (CIs) were presented. To evaluate whether the predictive abilities of blood pressure measures vary at different ages or glycemic levels, stratified analysis was conducted by age < 35 and ≥ 35 years and by HbA1c < 9% and ≥ 9%, respectively. The age cutoff of 35 years is based on previous findings that PP showed a steep rise from the 30s to 40s in type 1 diabetes13; the HbA1c cutoff of 9% was close to the median of baseline HbA1c in this cohort. In addition to stratified analysis, the interaction effect of different age and HbA1c groups on the association of blood pressure with outcome events were tested and then plotted (model-based interaction plot).21 The predictive ability of each baseline blood pressure measure was also assessed using the C-statistic method by Uno et al.22; all pairwise comparisons were tested.
The least absolute selection and shrinkage operator (LASSO)-penalized Cox regression23 was also employed for variable selection, allowing for all 4 blood pressure measures (SBP, DBP, PP, and MAP) and a wide range of potential baseline risk factors (age, sex, age at diabetes onset, ever smoker, body mass index, pulse rate, HbA1c, urinary albumin excretion rate, HDL and non-HDL cholesterol, white blood cell count, and antihypertensive use). The optimal penalty parameter was determined using the 10-fold cross-validation method. The considerations of using LASSO regression were as follows: (i) penalized regression handles multicollinearity between predictors by imposing a penalty factor in the estimation of coefficients24,25 and (ii) LASSO, as one of the most widely used penalized models, is applicable for both low- and high-dimensional data and has been shown to be superior to the stepwise technique.26
The assessment of improvement in model fit of the combination of blood pressure variables (SBP and DBP, MAP and PP, or SBP and PP) vs. a single blood pressure variable was conducted using the likelihood-ratio χ 2 test. The combined MAP and PP was tested as the MAP and PP reflects 2 distinct blood pressure characteristics, the steady and pulsatile components, receptively.27 PP has been suggested to be more informative for cardiovascular risk prediction in the diabetes population,8,9 and SBP has been recommended as the primary blood pressure measure in current guidelines.7 Thus, we indented to test whether risk prediction might be improved with the combination of SBP and PP.
A 2-sided P < 0.05 was considered significant. The analyses were performed with SAS v 9.4 (SAS Institute, Cary, NC) and R version 3.5.1 (R Core Team, Vienna, Austria).
RESULTS
Baseline characteristics are shown in Table 1. Among 605 eligible participants without known CAD at baseline, mean age and diabetes duration were 27 and 19 years, respectively. During the 25 years of follow-up, 219 (36.2%) incident CAD cases were identified. Among the 219 CAD cases that were identified in this study, the proportions of myocardial infarction (fatal or nonfatal), coronary revascularization, physician-diagnosed angina, and electrocardiograph-determined ischemia were 37.0%, 18.7%, 29.2%, and 15.1%, respectively.
Table 1.
Baseline characteristics of the study participants (n = 605)
| Variablesa | Data |
|---|---|
| Age, years | 27.2 (7.7) |
| Age at diabetes onset, years | 8.2 (4.1) |
| Diabetes duration, years | 19.0 (7.4) |
| Female, % (n) | 49.8 (301) |
| BP, mm Hg | |
| SBP | 112.9 (14.7) |
| DBP | 72.5 (10.8) |
| PP | 40.4 (10.3) |
| MAP | 85.9 (11.3) |
| Antihypertensive medication use, % (n) | 12.9 (78) |
| Hypertension, % (n) | 14.4 (87) |
| Pulse rate, beats/min | 78 (10) |
| HbA1c, % | 8.8 (1.5) |
| Ever smoker, % (n) | 37.2 (225) |
| BMI, kg/m2 | 23.5 (3.2) |
| High WHRb, % (n) | 4.8 (29) |
| AER, μg/min | 14.4 (7.2, 101.7) |
| Raised albuminuria, % (n) | 44.4 (269) |
| Cholesterol, mg/dL | |
| Total | 189.6 (41.0) |
| Non-HDL | 135.6 (41.0) |
| HDL | |
| Male | 49.5 (9.8) |
| Female | 58.4 (12.9) |
Abbreviations: AER, urinary albumin excretion rate; BMI, body mass index; BP = Blood pressure; CAD, coronary artery disease; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; IQR, interquartile range; MAP, mean artery pressure; PP, pulse pressure; SBP, systolic blood pressure; WHR, waist–hip ratio.
aCategorical variables were presented as percentage (number) and continuous variables as mean (SD) or median (IQR).
bHigh WHR defined as >1 if men or >0.85 if women.
The Pearson correlation coefficients of different baseline blood pressure measures were 0.72 (SBP vs. DBP), 0.68 (SBP vs. PP), 0.90 (SBP vs. MAP), −0.02 (DBP vs. PP), 0.95 (DBP vs. MAP), and 0.28 (PP vs. MAP).
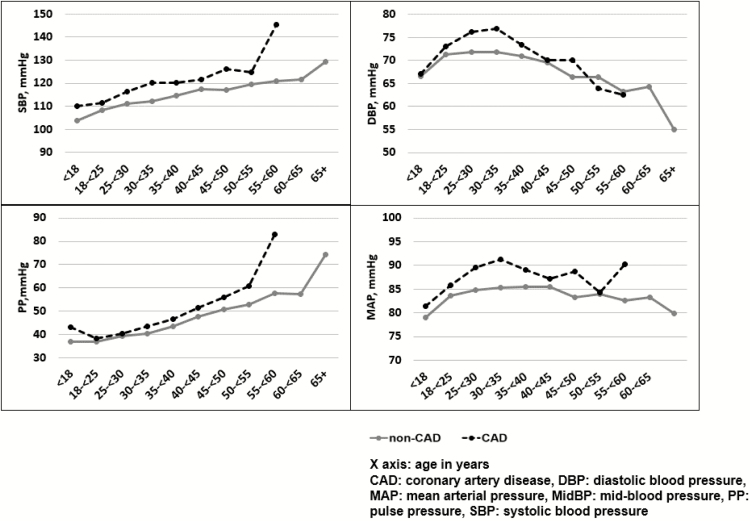
Age-specific means of blood pressures were calculated among CAD cases and noncases, comprising all blood pressure values from the baseline to the last available measure, prior to the event occurrence for cases or at the end of the follow-up for noncases (Figure 1). CAD cases experienced a higher SBP and a higher PP than noncases across all age groups; the differences of blood pressure between cases and noncases became greater with older age. The DBP increased until late 30s and early 40s and started falling thereafter; the CAD cases showed a decline in DBP 10 years earlier than seen in noncases.
Figure 1.
Age-specific means of blood pressure between coronary artery disease cases and noncases.
Controlling for sex, age, age at diabetes onset, HbA1c levels, and antihypertensive medication use, the HRs (95% CI) associated with one increment in SD for CAD risk were 1.35 (1.17, 1.56) for SBP; 1.30 (1.12, 1.51) for DBP; 1.20 (1.03, 1.39) for PP; and 1.35 (1.17, 1.56) for MAP (Table 2). In an analysis stratified by baseline age < vs. ≥ 35 years, PP was not significantly associated with CAD at age < 35 years (HR [95% CI]: 1.07 [0.88, 1.29], P = 0.500), but PP became the strongest blood pressure predictor in those aged 35 years or older (HR [95% CI]: 1.49 [1.15, 1.94], P = 0.003). In a stratified analysis by baseline HbA1c < vs. ≥9%, the HR of PP associated with CAD was statistically significant, and the effect size was similar to that of other blood pressure measures (HR [95% CI]: 1.32 [1.01, 1.72], P = 0.043) in those with worse glycemic control; however, the association was less powerful and nonstatistically significant (HR [95% CI]: 1.13 [0.94, 1.37], P = 0.202) in those with better glycemic control.
Table 2.
Hazard ratios and C statistics for incident CAD of different baseline blood pressure measures, adjusted for gender, age, age of diabetes onset, HbA1c, and antihypertensive medication use
| BP measure | Cox model | C-statistics | |
|---|---|---|---|
| HR (95% CI) per SD | P value | C-statistic (95% CI) | |
| Overall | n = 605, events = 219 | ||
| SBP | 1.35 (1.17, 1.56) | <0.001 | 0.789 (0.752, 0.827)* |
| DBP | 1.30 (1.12, 1.51) | <0.001 | 0.784 (0.746, 0.822)* |
| PP | 1.20 (1.03, 1.39) | 0.021 | 0.770 (0.729, 0.810) |
| MAP | 1.35 (1.17, 1.56) | <0.001 | 0.789 (0.747, 0.831)* |
| Stratified by age | |||
| Baseline age < 35 years | |||
| n = 500, events = 150 | |||
| SBP | 1.31 (1.10, 1.57) | 0.003 | 0.760 (0.705, 0.815) |
| DBP | 1.33 (1.12, 1.59) | 0.001 | 0.773 (0.719, 0.827)* |
| PP | 1.07 (0.88, 1.29) | 0.500 | 0.748 (0.691, 0.805) |
| MAP | 1.35 (1.14, 1.61) | <0.001 | 0.770 (0.713, 0.826)* |
| Baseline age ≥ 35 years | |||
| n = 105, events = 69 | |||
| SBP | 1.38 (1.06, 1.79) | 0.018 | 0.656 (0.567, 0.764) |
| DBP | 1.08 (0.78, 1.49) | 0.640 | 0.640 (0.551, 0.729) |
| PP | 1.49 (1.15, 1.94) | 0.003 | 0.658 (0.562, 0.753) |
| MAP | 1.24 (0.92, 1.68) | 0.156 | 0.648 (0.545, 0.751) |
| Stratified by HbA1c | |||
| Baseline HbA1c < 9% | |||
| n = 380, events = 141 | |||
| SBP | 1.35 (1.12, 1.62) | 0.001 | 0.751 (0.696, 0.806) |
| DBP | 1.37 (1.13, 1.66) | 0.001 | 0.768 (0.715, 0.822)* |
| PP | 1.13 (0.94, 1.37) | 0.202 | 0.731 (0.678, 0.784) |
| MAP | 1.39 (1.16, 1.68) | <0.001 | 0.774 (0.710, 0.838)* |
| Baseline HbA1c ≥ 9% | |||
| n = 225, events = 78 | |||
| SBP | 1.29 (1.02, 1.63) | 0.018 | 0.840 (0.780, 0.900) |
| DBP | 1.20 (0.95, 1.52) | 0.135 | 0.836 (0.773, 0.898) |
| PP | 1.32 (1.01, 1.72) | 0.043 | 0.838 (0.786, 0.890) |
| MAP | 1.26 (1.00, 1.60) | 0.050 | 0.839 (0.792, 0.887) |
*P < 0.05 compared with PP. P > 0.05 for the rest of pairwise comparisons between different blood pressure measures (SBP, DBP, PP, and MAP). Abbreviations: BP, blood pressure; CAD, coronary artery disease; CI, confidence interval; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HR, hazard ratio; MAP, mean artery pressure; PP, pulse pressure; SBP, systolic blood pressure.
The effect modification was significant for HbA1c (HR: 1.41, P = 0.023) and was marginally significant for age (HR: 1.29, P = 0.093) on the association of PP with CAD outcomes (Supplementary Figure S1). No significant interaction effect was found for the other 4 blood pressure measures (SBP, DBP, and MAP) in this analysis (data not shown).
Consistently, although the C-statistic value was significantly lower for PP compared with the other 4 blood pressure indices in the entire cohort, PP performed similarly to that of other indices in those with higher HbA1c levels (≥9%) or at an older age (≥35 years) (Table 2).
Table 3 shows the baseline risk factor selection for the prediction of CAD using a LASSO–Cox model for overall participants as well as subgroups of different age and glycemic levels. Allowing for a wide range of potential risk factors as well as 4 blood pressure measures (SBP, DBP, PP, and MAP), SBP was retained in the model after LASSO selection in the entire cohort, and the 2 subcohorts with HbA1c < 9% and ≥ 9%. Consistent with the conventional Cox models, PP exerted an important role in CAD risk prediction for those over 35 years of age and in those with an HbA1c of over 9%. As expected, MAP, compared with other blood pressure measures, showed a stronger effect in the younger group.
Table 3.
Baseline risk factors selection for the prediction of CAD using LASSO–Cox regression model
| Risk factors | β coefficient | ||||
|---|---|---|---|---|---|
| Overall | Subset age < 35 years | Subset age ≥ 35 years | Subset HbA1c < 9% | Subset HbA1c ≥ 9% | |
| SBP | 0.168** | 0.033 | 0.081 | ||
| DBP | |||||
| PP | 0.247* | 0.128 | |||
| MAP | 0.153 | 0.147 | |||
| Age | 0.717** | 0.526** | 0.588** | 0.801** | |
| Age of diabetes onset | −0.192** | −0.177** | −0.172 | −0.207** | −0.151 |
| Female | |||||
| Ever smoking | 0.137 | 0.073 | 0.219* | 0.075 | 0.192 |
| BMI | 0.024 | 0.108 | |||
| Pulse rate | |||||
| HbA1c | |||||
| Urinary AER | 0.197 | 0.191* | 0.075 | 0.212* | 0.155 |
| HDL cholesterol | −0.134* | −0.388** | −0.085 | −0.125 | |
| Non-HDL cholesterol | 0.208** | 0.230** | 0.214* | 0.167 | |
| WBC | 0.090 | 0.082 | 0.030 | 0.066 | 0.094 |
| Antihypertensive use | 0.098 | 0.078 | 0.107 | 0.042 | 0.179 |
Inference with fixed lambda (optimal lambda based on 10-fold cross-validation). Abbreviations: AER, urinary albumin excretion rate; BMI, body mass index; CAD, coronary artery disease; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LASSO, least absolute shrinkage and selection operator; MAP, mean artery pressure; PP, pulse pressure; SBP, systolic blood pressure; WBC, white blood cell counts.
*P < 0.05, **P <0.01.
A model with combined SBP and DBP or combined SBP and PP was not remarkably superior to a model with a single SBP for CAD risk prediction. A model with combined MAP and PP was not remarkably superior to a model with a single MBP (Supplementary Table S1).
The current analysis included all CAD events (n = 219). Of these CAD events, 122 (56%) were hard CAD events (revascularization, myocardial infarction, and CAD death), and the rest 97 (44%) were more “soft” CAD end points (new onset of angina and electrocardiograph-identified ischemic heart disease). We also conducted analyses for hard CAD events only. Being consistent with the analysis for the overall CAD outcomes, PP was a significant predictor of hard CAD outcome events in those with older age ≥ 35 years and in those with higher HbA1c levels ≥ 9% (Supplementary Table S2).
DISCUSSION
This study presents a comprehensive comparison of CAD risk prediction among 4 blood pressure measures in a cohort with long duration type 1 diabetes. We observed an early asymptote and then fall in DBP between ages 30 and 39, followed by an early rise of PP. This early fall in DBP and early rise in PP occurred approximately 20 years earlier in these type 1 diabetes individuals than that seen in the general population.6 In this study, significant positive associations were observed between each blood pressure measure (SBP, DBP, PP, and MAP) and incident CAD in the entire study cohort. PP appears to be inferior to the other 4 blood pressure measures but becomes comparable in those aged 35 years or older and in those with worse glycemic control.
Stiffening of the arterial wall as a sign of vascular aging is thought to be a complex process involving collagen overproduction and accumulation, elastin fiber degradation, vascular smooth muscle cell proliferation, and vascular calcification.28 Increased cardiovascular risk in diabetes is at least partially explained by glycation-induced accelerated vascular aging, such as advanced glycated end products (AGE), cross-linking, and decreased turnover of collagen on the arterial wall, leading to a decline of artery elasticity.29,30 An increased PP results from age-related stiffening of the large arteries,31 leading to increasing interest in the role of PP in contributing to cardiovascular risk, particularly in diabetes.9 According to the present study, type 1 diabetes individuals have an increased SBP across all ages compared with the nondiabetes population and also have a sharp rise of PP as early as the third and fourth decade of life. We found a weaker, but significant association of PP with incident CAD in the entire cohort, in line with the results of other type 1 diabetes studies, such as the EURODIAB study32 and the FinnDiane study.33 Our observations extend previous findings by showing that PP is a powerful determinant for CAD risk particularly in those 35 years or older and/or with poorer glycemic control. These findings strongly support the hypothesis that vascular aging is accelerated by glycation-mediated changes in this high-risk population.
The 2017 American Diabetes Association position paper “Diabetes and Hypertension” 34 states that a higher PP (>60 mm Hg, systolic hypertension in association with low DBP) in older people with diabetes may result in an increased risk of adverse cardiovascular outcomes. Our findings suggest that, in type 1 diabetes individuals, PP may start playing an important role from an early age, i.e., late 30s, in predicting cardiovascular disease. An increased PP may not only be a marker of cardiovascular risk but also reflect a diseased state of stiffened arteries. Thus, studies focusing on premature vascular aging in type 1 diabetes may open a window for us to better understand the pathogenesis of general vascular aging that is typically occurred at older ages in the general population.
This EDC Study is a well-characterized type 1 diabetes cohort with an extended follow-up duration. Different blood pressure measures for CAD risk prediction were examined, along with age and HbA1c stratified analyses. Advanced statistical methods (e.g., LASSO regression) were employed to further confirm the study findings. The present study has characterized age-related blood pressure changes from youth throughout middle age in individuals with childhood-onset type 1 diabetes: DBP had an earlier decline since late 30s, leading to an earlier rise of PP. In addition, our findings suggest an accelerated vascular aging process in type 1 diabetes, which was shown to be linked to the increased cardiovascular risk at younger age in this patient population.
Several limitations of the current work should also be noted. Our sample consisted primarily of white type 1 diabetes individuals and therefore may not be representative of other ethnic or racial groups. The cohort was relatively young at study entry, which allowed us to observe a long disease course of type 1 diabetes. However, some types of outcome events, such as stroke and cause-specific mortality, were relatively rare even at the 25-year follow-up, which have limited our ability to fully evaluate different classes of cardiovascular outcomes.
In a group of individuals with childhood-onset type 1 diabetes with over 25 years follow-up, we observed that an early fall of DBP beginning from late 30s, leads to an early rise of PP. Although PP is less effective for risk prediction in CAD in the entire cohort, its prognostic significance may improve and become comparable to SBP in those aged 35 years or more or those under poor glycemic control, reflecting an early onset of glycation-induced vascular stiffening in type 1 diabetes. PP may be necessary to incorporate into the clinical evaluation in type 1 diabetes, especially in those over 35 years old and/or in poor glycemic control.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (grant R01-DK-034818) and the Rossi Memorial Fund. S.J.A. is supported by the National Institutes of Health (grant P30-MH-090333). The authors thank the staff and the participants of the EDC Study for their contributions. Preliminary results were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 21–24 June 2018.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Bernardi L, Gordin D, Bordino M, Rosengård-Bärlund M, Sandelin A, Forsblom C, Groop PH. Oxygen-induced impairment in arterial function is corrected by slow breathing in patients with type 1 diabetes. Sci Rep 2017; 7:6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gordin D, Rönnback M, Forsblom C, Heikkilä O, Saraheimo M, Groop PH. Acute hyperglycaemia rapidly increases arterial stiffness in young patients with type 1 diabetes. Diabetologia 2007; 50:1808–1814. [DOI] [PubMed] [Google Scholar]
- 3. Nunley KA, Ryan CM, Orchard TJ, Aizenstein HJ, Jennings JR, Ryan J, Zgibor JC, Boudreau RM, Costacou T, Maynard JD, Miller RG, Rosano C. White matter hyperintensities in middle-aged adults with childhood-onset type 1 diabetes. Neurology 2015; 84:2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 5. Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC, Jacobs DR Jr, Liu K, Lloyd-Jones D. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA 2014; 311:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Franklin SS, Gustin W 4th, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997; 96:308–315. [DOI] [PubMed] [Google Scholar]
- 7. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 8. Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 2008; 118:968–976. [DOI] [PubMed] [Google Scholar]
- 9. Kodama S, Horikawa C, Fujihara K, Yoshizawa S, Yachi Y, Tanaka S, Ohara N, Matsunaga S, Yamada T, Hanyu O, Sone H. Meta-analysis of the quantitative relation between pulse pressure and mean arterial pressure and cardiovascular risk in patients with diabetes mellitus. Am J Cardiol 2014; 113:1058–1065. [DOI] [PubMed] [Google Scholar]
- 10. Schram MT, Kostense PJ, Van Dijk RA, Dekker JM, Nijpels G, Bouter LM, Heine RJ, Stehouwer CD. Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. J Hypertens 2002; 20:1743–1751. [DOI] [PubMed] [Google Scholar]
- 11. Kengne AP, Czernichow S, Huxley R, Grobbee D, Woodward M, Neal B, Zoungas S, Cooper M, Glasziou P, Hamet P, Harrap SB, Mancia G, Poulter N, Williams B, Chalmers J; ADVANCE Collaborative Group . Blood pressure variables and cardiovascular risk: new findings from ADVANCE. Hypertension 2009; 54:399–404. [DOI] [PubMed] [Google Scholar]
- 12. Schwab KO, Doerfer J, Krebs A, Krebs K, Schorb E, Hallermann K, Superti-Furga A, Zieger B, März W, Schmidt-Trucksäss A, Winkler K. Early atherosclerosis in childhood type 1 diabetes: role of raised systolic blood pressure in the absence of dyslipidaemia. Eur J Pediatr 2007; 166:541–548. [DOI] [PubMed] [Google Scholar]
- 13. Rönnback M, Fagerudd J, Forsblom C, Pettersson-Fernholm K, Reunanen A, Groop PH; Finnish Diabetic Nephropathy (FinnDiane) Study Group . Altered age-related blood pressure pattern in type 1 diabetes. Circulation 2004; 110:1076–1082. [DOI] [PubMed] [Google Scholar]
- 14. Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes 1990; 39:1116–1124. [DOI] [PubMed] [Google Scholar]
- 15. Wagener DK, Sacks JM, LaPorte RE, Macgregor JM. The Pittsburgh study of insulin-dependent diabetes mellitus. Risk for diabetes among relatives of IDDM. Diabetes 1982; 31:136–144. [DOI] [PubMed] [Google Scholar]
- 16. Borhani N, Kass E, Langford H, Payne G, Remington R, Stamler J. The hypertension detection and follow-up program: hypertension detection and follow-up program cooperative group. Prev Med (Baltim) 1976; 5:207–215. [DOI] [PubMed] [Google Scholar]
- 17. Prince CT, Becker DJ, Costacou T, Miller RG, Orchard TJ. Changes in glycaemic control and risk of coronary artery disease in type 1 diabetes mellitus: findings from the Pittsburgh Epidemiology of Diabetes Complications Study (EDC). Diabetologia 2007; 50:2280–2288. [DOI] [PubMed] [Google Scholar]
- 18. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974; 20:470–475. [PubMed] [Google Scholar]
- 19. Warnick GR, Albers JJ. Heparin–Mn2+ quantitation of high-density-lipoprotein cholesterol: an ultrafiltration procedure for lipemic samples. Clin Chem 1978; 24:900–904. [PubMed] [Google Scholar]
- 20. Ellis D, Coonrod BA, Dorman JS, Kelsey SF, Becker DJ, Avner ED, Orchard TJ. Choice of urine sample predictive of microalbuminuria in patients with insulin-dependent diabetes mellitus. Am J Kidney Dis 1989; 13:321–328. [DOI] [PubMed] [Google Scholar]
- 21. Harrell FEJ. rms: regression modeling strategies. R package version 5.1-2. https://cran.r-project.org/web/packages/rms/rms.pdf. Accessed April 24, 2019. [Google Scholar]
- 22. Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 2011; 30:1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goeman JJ. L1 Penalized Estimation in the Cox Proportional Hazards Model. Biometrical J. 2009; 52(1):70–84. doi:10.1002/bimj.200900028 [DOI] [PubMed] [Google Scholar]
- 24.Oyeyemi GM, Ogunjobi EO, Folorunsho AI. On Performance of Shrinkage Methods – A Monte Carlo Study. Int J Stat Appl. 2015; 5:72–76. doi:10.5923/j.statistics.20150502.04
- 25. Shahraki HR, Salehi A, Zare N. Survival prognostic factors of male breast cancer in Southern Iran: a LASSO-Cox regression approach. Asian Pac J Cancer Prev 2015; 16:6773–6777. [DOI] [PubMed] [Google Scholar]
- 26. Ojeda FM, Müller C, Börnigen D, Trégouët DA, Schillert A, Heinig M, Zeller T, Schnabel RB. Comparison of Cox model methods in a low-dimensional setting with few events. Genom Proteom Bioinf 2016; 14:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Darne B, Girerd X, Safar M, Cambien F, Guize L. Pulsatile versus steady component of blood pressure: a cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension 1989; 13:392–400. [DOI] [PubMed] [Google Scholar]
- 28. Guo J, Fujiyoshi A, Willcox B, Choo J, Vishnu A, Hisamatsu T, Ahuja V, Takashima N, Barinas-Mitchell E, Kadota A, Evans RW, Miura K, Edmundowicz D, Masaki K, Shin C, Kuller LH, Ueshima H, Sekikawa A; ERA JUMP Study Group . Increased aortic calcification is associated with arterial stiffness progression in multiethnic middle-aged men. Hypertension 2017; 69:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation – a mini-review. Gerontology 2012; 58:227–237. [DOI] [PubMed] [Google Scholar]
- 30. Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia 2008; 51:527–539. [DOI] [PubMed] [Google Scholar]
- 31. Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: emerging concepts. Hypertension 2010; 55:9–14. [DOI] [PubMed] [Google Scholar]
- 32. Schram MT, Chaturvedi N, Fuller JH, Stehouwer CD; EURODIAB Prospective Complications Study Group . Pulse pressure is associated with age and cardiovascular disease in type 1 diabetes: the Eurodiab Prospective Complications Study. J Hypertens 2003; 21:2035–2044. [DOI] [PubMed] [Google Scholar]
- 33. Gordin D, Wadén J, Forsblom C, Thorn L, Rosengård-Bärlund M, Tolonen N, Saraheimo M, Harjutsalo V, Groop PH; FinnDiane Study Group . Pulse pressure predicts incident cardiovascular disease but not diabetic nephropathy in patients with type 1 diabetes (The FinnDiane Study). Diabetes Care 2011; 34:886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P, Rossing P, Zoungas S, Bakris G. Diabetes and hypertension: a position statement by the American diabetes association. Diabetes Care 2017; 40:1273–1284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.