A novel mechanism for Treg-mediated control of immune responses
Keywords: heterogeneity, lymphopenia, proliferation, regulation model, T-APC interaction
Abstract
The immune system in tolerance maintains cell diversity without responding to self-antigens. Foxp3-expressing CD25+CD4+ regulatory T cells (Tregs) inhibit T-cell activation through various molecular mechanisms. However, several key questions are still not resolved, including how Tregs control the immune response on the basis of their self-skewed T-cell receptor repertoire and how Tregs avoid impeding relevant immunity against pathogens. Here, we show that Tregs promote the proliferation of conventional T cells in the presence of excessive co-stimulation when murine T cells are stimulated in vitro with allogeneic antigen-presenting cells (APCs). Antigen-specific Tregs increase the number of cells interacting with dendritic cells (DCs) by increasing the number of viable DCs and the expression of adhesion molecules on DCs. Theoretical simulations and mathematical models representing the dynamics of T-APC interaction and T-cell numbers in a lymph node indicate that Tregs reduce the dissociation probability of T cells from APCs and increase the new association. These functions contribute to tolerance by enhancing the interaction of low-affinity T cells with APCs. Supporting the theoretical analyses, we found that reducing the T-cell numbers in mice increases the ratio of specific T cells among CD4+ T cells after immunization and effectively induces autoimmune diabetes in non obese diabetes mice. Thus, as a critical function, antigen-specific Tregs stabilize the immune state, irrespective of it being tolerant or responsive, by augmenting T-APC interaction. We propose a novel regulation model in which stable tolerance with large heterogeneous populations proceeds to a specific immune response through a transient state with few populations.
Introduction
The immune system is capable of maintaining tolerance toward self but providing immunity against foreign antigens. During tolerance, the proliferation of T cells with reactive T-cell receptors (TCRs) against self-antigens is inhibited despite the lifelong exposure. The broad diversity of TCRs is maintained to preserve T cells specific to foreign antigens. In immunity, infection by pathogens promptly induces selective expansion of T cells with reactive TCRs. Elucidating the mechanisms that regulate tolerance and immunity is a primary goal of immunology research.
Foxp3-expressing CD25+CD4+ regulatory T cells (Tregs), accounting for ~10% of CD4+ T cells, constitute a distinct sub-population with high self-reactivity and suppressive function that plays a crucial role in maintaining self-tolerance (1, 2). CTLA-4 (CD152) on Tregs inhibits T-cell activation by interrupting co-stimulatory CD28 signaling from the ligands CD80 and CD86 on antigen-presenting cells (APCs) (3). Tregs secrete immune suppressive cytokines, such as transforming growth factor-β (TGF-β) and Interleukin-10 (IL-10), and absorb IL-2 through the high-affinity IL-2 receptor CD25, thus depriving conventional T cells (Tconvs) of IL-2 (4). In addition to these well-characterized mechanisms, many other molecules, including those involved in chemotaxis, cell adhesion and signal transduction, are reported to contribute to suppression of T-cell proliferation (2, 5–9) and even more molecules are preferentially expressed on Tregs without clear significance to suppression (10, 11). How self-reactive Tregs maintain robust tolerance is still an open and crucial question since the key molecules on Tregs could be targets of immune checkpoint inhibitors.
The role of Tregs in immunity against infection seems to be inconsistent depending on the condition (12). The TCR repertoire of Tregs overlaps with that of Tconvs, indicating that both Tregs and Tconvs are potentially activated in either state with or without infection (13, 14). A volume of reports on infection models have shown that the depletion of Tregs can enhance the titer of pathogens (15–19). These are paradoxical findings, as infection activates Tregs and the Tregs are able to inhibit proliferation of or cytokine production by effector T cells. The conflicting effects of Tregs make it more difficult to understand how Tregs control the balance between tolerance and response.
In many biological systems, dynamic behaviors with bistable states are not strictly controlled by specific signaling, but can be tuned under stochastic fluctuation or noise (20, 21). Substantial degrees of fluctuations are observed in gene expression and the movements of a motor molecule, and these fluctuations are essential during transition between states (22–25). The immune system might also be dependent on stochastic fluctuation in various processes. Preceding theoretical studies on Treg-mediated suppression have revealed the crucial role of global factors (e.g. competition for IL-2 and CD28 ligands) in controlling the proliferation of Tconvs (26–32), further suggesting the importance of regulating stochastic noise. In this study, we show that Tregs are able to enhance proliferation of effector T cells under strong stimulatory conditions and that Tregs augment the interaction between T cells and APCs. Further, theoretical analyses show that a large degree of total T-APC interaction is crucial for stable tolerance mediated by self-reactive Tregs. These experimental and theoretical studies reveal that Tregs inhibit transition from the current immune state by increasing the degree of total T-APC interaction.
Methods
Theoretical analysis
All mathematical calculations were performed using Wolfram Mathematica 10 (Wolfram Research, Champaign, IL, USA). Details of the simulation parameters are described in Supplementary Text 1.
Mice
DEREG mice with green fluorescent protein (GFP) under the control of a BAC transgenic Foxp3 promoter (33), DO11.10 transgenic mice with a TCR specific to ovalbumin (OVA) peptide and congenic Thy1.1 (CD90.1) mice were bred on the BALB/c background in our specific pathogen-free facility. Wild-type BALB/c, C57BL/6 and non obese diabetes (NOD) mice were purchased from CLEA (Tokyo, Japan). Age-matched 4- to 8-week-old co-housed female mice were used for in vivo experiments. All experiments were conducted according to the institutional guidelines for animal welfare under approvals by the Animal Care Committees at Osaka University and at the Research Institute, Nozaki Tokushukai Hospital.
Cell preparation for culture and flow cytometry
Cell suspensions from the lymph nodes or the spleens of 6- to 12-week-old mice were stained with specific antibodies (listed in the ‘Reagents’ section) and sorted using a FACS Aria III (BD Biosciences, San Jose, CA, USA) with a typical final purity of >97%. Where required, CD8+ cells and APCs were sorted using magnetic MACS Separation Beads (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were cultured in RPMI 1640 medium with 10% fetal bovine serum, 100 U ml−1 penicillin and 100 µg ml−1 streptomycin. For flow cytometry, cells were stained with specific antibodies for 30 min on ice after blocking Fc receptors with anti-CD16/CD32. Propidium iodide (PI) solution (Dojindo Laboratories, Kumamoto, Japan) was added at 0.1 µg ml−1 to exclude dead cells. Stained cells were examined using a MACS Quant flow cytometer (Miltenyi Biotec). Live cells were identified as PI− cells with appropriate intensities on FSC and SSC, and further gating was performed as described in the figure legends using FlowJo software (FlowJo LLC, Ashland, OR, USA).
Proliferation and suppression assays
Responder T cells (Tresps; 1 × 105), labeled with 1 µM carboxyfluorescein succinimidyl ester (CFSE; Dojindo Laboratories), were stimulated with allogeneic CD11c+ dendritic cells (DCs) (2 × 104) in the presence or absence of 1 × 105 of either Tregs or Tconvs in 96-well round-bottom plates for 5 days. GFP+CD4+CD8− Tregs and CD45RBhigh GFP−CD4+CD8− Tconvs were sorted from DEREG mice. CD8+ Tresps and CD11c+ DCs were sorted from wild-type BALB/c and C57BL/6 mice, respectively. CD25−CD4+ Tresps from Thy1.1+ congenic BALB/c mice, CD25−CD4+ Tconvs and CD25+CD4+ Tregs from Thy1.2 BALB/c mice and CD11c+ DCs from C57BL/6 mice were sorted for the CD4+ Tresp proliferation assay. In the indicated cases, 5 µg ml−1 anti-CD28, 5 µg ml−1 anti-CD40, 1 µg ml−1 anti-CD3 antibodies or 50 ng ml−1 IL-2 (PeproTech, Rocky Hill, NJ, USA) were added to the culture. To compare APC types, CD11c+, CD11b+ or CD19+ cells were sorted from C57BL/6 splenocytes using FACS. Whole splenocytes were irradiated with 15 Gy by a gamma irradiator Gammacell 40 (Nordion, Ontario, Canada) before using as APCs. CFSE dilution and the number of Tresps were determined using flow cytometry.
For the proliferation assay with antigen-specific T cells, 5 × 103 DO11.10+ T cells and 5 × 104 BALB/c T cells were mixed for each Tresp, Tconv and Treg population. The Tresps, with or without the same number of Tconvs or Tregs, were stimulated with CD11c+ DCs from BALB/c mice for 5 days in the presence of 1 µM ovalbumin peptide (OVA323–339; MBL, Nagoya, Japan), 50 ng ml−1 IL-2 and 5 µg ml−1 anti-CD28. For Tresps, CD25−CD4+ T cells from Thy1.1+ DO11.10+ mice were mixed with Thy1.2+CD25−CD4+ T cells from wild-type Thy1.2+ BALB/c mice at a 1:10 ratio. For Tregs and Tconvs, Thy1.2+ DO11.10+ and wild-type Thy1.2+ T cells were mixed at a 1:10 ratio. The number of Thy1.1+CD4+ Tresps was determined using flow cytometry.
Assays for cell aggregates
To form cell aggregates, CD25+CD4+ Tregs or CD25−CD4+ Tconvs from DO11.10 mice (2 × 104 cells), CD25−CD4+ T cells (1.8 × 105 cells) and splenic CD11c+ DCs (1 × 104 cells) were cultured in the presence of 1 µM OVA peptide in 96-well round-bottom plates for 1 day. Anti-CD16/CD32 was added at 0.5 µg ml−1 to block interactions with Fc receptors. Cell aggregates were transferred using a micropipette to a glass slide and stained with 5 µM DRAQ5 (BioStatus, Shepshed, UK) to label nuclei and fluorescein isothiocyanate (FITC)-conjugated anti-major histocompatibility complex II (MHC) (2G9) to label DCs. These were then observed by fluorescence microscopy (BIOREVO; Keyence, Osaka, Japan). The number of cells in each colony with at least one DC and at least three cells was counted. To take a picture without DRAQ5, DO11.10+ T cells were stained with 1 µM eFluor 670 (eBioscience, Santa Clara, CA, USA) to label them before co-culturing.
To analyze DCs, MACS-sorted CD11c+ DCs (5 × 104 cells) and CD25−CD4+ Tconvs (2.5 × 105 cells) from wild-type BALB/c mice and KJ1.26+CD45RBhigh CD25−CD4+ Tconvs or KJ1.26+CD45RBlowCD25+CD4+ Tregs (5 × 104 cells) from DO11.10 mice were co-cultured in the presence of 1 µM OVA peptide for 3 days. The KJ1.26 antibody clone recognizes the DO11.10 TCR and facilitates identification of TCR-positive cells in DO11.10 TCR transgenic mice. DCs expressing CD11c and MHC class II were analyzed using flow cytometry.
Immunization
BALB/c mice were injected intravenously with 5 × 105 KJ1.26+CD45RBhigh CD25−CD4+ Tconvs. One-week post-injection, the mice were intravenously injected with 100 µg anti-Thy1.2 or control rat IgG, and on the next day they were immunized with 100 µg OVA protein emulsified in incomplete Freund adjuvant (IFA; Sigma-Aldrich, St Louis, MO, USA) by injection into the right footpads. Five days after immunization, the right (OVA+) and left (OVA−) popliteal lymph nodes were stained with anti-CD4, anti-CD44 and KJ1.26 antibodies and analyzed using flow cytometry.
Diabetes model
Female NOD mice were intravenously injected with 100 µg anti-Thy1.2 or control rat IgG at 4 weeks of age. Urine was tested for glucose weekly using test strips (Uropaper III; Eiken Chemical, Tokyo, Japan). Mice were sacrificed at 3 months of age and their pancreata were fixed with 10% formaldehyde and stained with hematoxylin and eosin (HE) by GenoStaff, Tokyo, Japan. The histological severity of insulitis was semi-quantitatively scored as 0 (intact islets), 1 (mild insulitis with limited cellular infiltration), 2 (moderate insulitis with cellular infiltration and mild destruction of islets), 3 (severe insulitis with extensive cellular infiltration and destruction of islets) or 4 (severe destruction of islets).
Reagents
For flow cytometric analysis and sorting, the following antibodies were purchased from BD Biosciences: anti-CD4 (RM4-5), anti-CD8a (53-6.7), anti-CD11c (HL3), anti-CD25 (PC61), anti-I-A/I-E (MHC class II, 2G9), anti-DO11.10 TCR (KJ1.26), anti-CD16/CD32 (2.4G2, for blocking Fc receptors) and anti-Thy1.1 (CD90.1, OX-7). The following antibodies were purchased from eBioscience: anti-CD80 (16-10A1), anti-CD86 (GL1), anti-LFA-1 (CD11a, M17/4), anti-ICAM-1 (CD54, YN1/1.7.4), anti-CD44 (IM7) and anti-Thy1.2 (CD90.2, 53-2.1). Anti-CD45RB (C363-16A) was purchased from BioLegend (San Diego, CA, USA). For reagents used in culture, anti-CD28 (37.51) and anti-CD3 (2C11) antibodies were purchased from BD Biosciences, and anti-CD40 (1C10) from eBioscience. For in vivo injections, anti-Thy1.2 (CD90.2, 30-H12) was purchased from BD Biosciences and also purified from ascites with hybridoma cells. Control rat IgG was purchased from Molecular Innovations (Novi, MI, USA).
Results
Tregs augment proliferation of T cells under strong stimulatory conditions
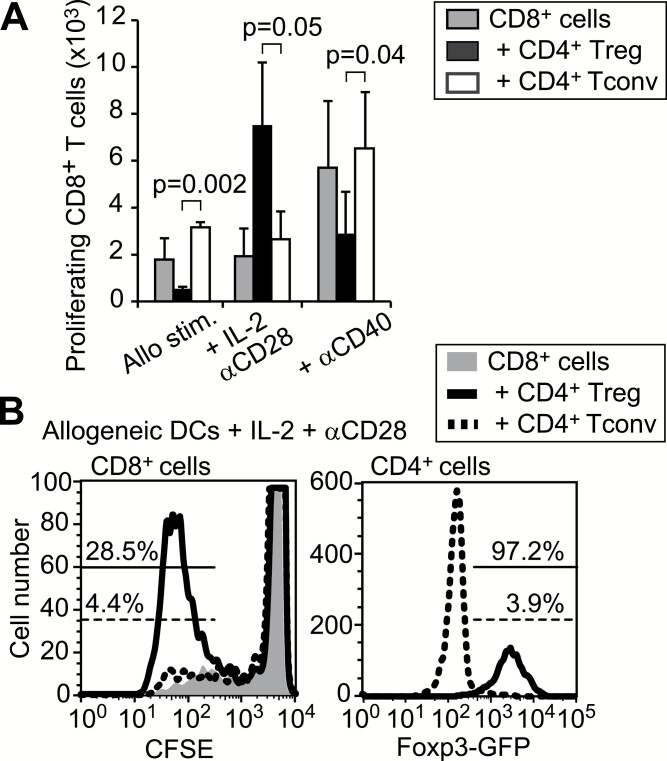
To assess the function of Tregs in infection or under strong stimulatory conditions, CFSE-labeled CD8+ Tresps from BALB/c mice were stimulated in vitro with allogeneic DCs from C57BL/6 mice, exogenous IL-2 and agonistic anti-CD28, either in the presence or absence of Foxp3+ Tregs isolated from Foxp3-reporter mice on the BALB/c background (33). We found that Tregs, rather than Foxp3−CD4+ Tconvs, increased the proliferation of Tresps in the presence of IL-2 and anti-CD28, although the same Tregs inhibited proliferation in the absence of the exogenous co-stimulation (Fig. 1). Conversely, agonistic anti-CD40 that can directly activate DCs maintained some inhibitory activity of Tregs against CD8+ Tresp proliferation (Fig. 1A). Notably, Tregs that enhanced proliferation of CD8+ Tresps expressed Foxp3 after 5 days of culture (Fig. 1B).
Fig. 1.
Augmented CD8+ T-cell proliferation by Tregs. (A) CFSE-labeled CD8 Tresps with or without the same number of Tregs or CD4+ Tconvs were stimulated with allogenic DCs for 5 days under the indicated conditions. The mean ± SD of the numbers of proliferating CD8+ T cells with more than two divisions are shown from four independent experiments. Paired t-tests were used for statistical analyses. (B) CFSE dilutions in CD8+ Tresps and Foxp3-GFP expression in CD4+CD8− T cells after co-culture with allogenic DCs, exogenous IL-2 and agonistic anti-CD28.
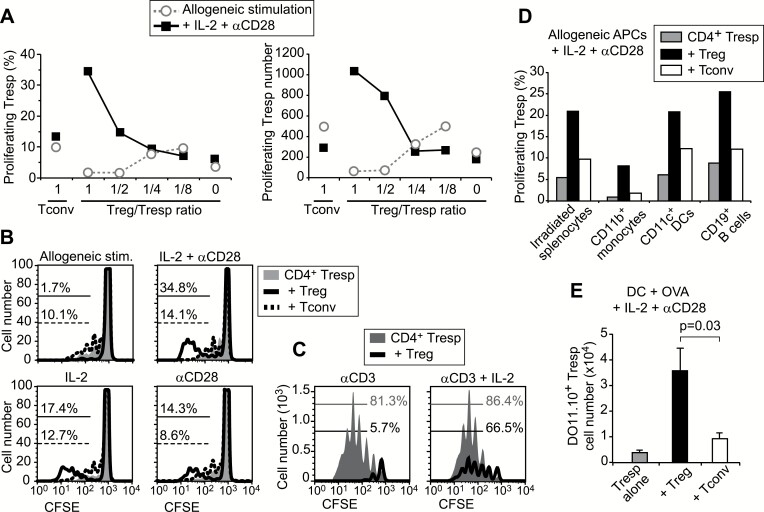
The immune-activating effect of Tregs under excessive co-stimulation was further examined using CD25−CD4+ Tresps (Fig. 2). The proportion and the number of proliferating CD4+ Tresps was reduced by Tregs in the presence of allogeneic DCs alone, but proliferation in the presence of IL-2, anti-CD28 and allogeneic DCs was increased by Tregs in a dose-dependent manner (Fig. 2A). Either IL-2 or anti-CD28 also showed augmentation of Tresp proliferation by Tregs as the combination of IL-2 and anti-CD28 (Fig. 2B). However, the activating effect of Tregs was not observed in the presence of anti-CD3 (Fig. 2C). When we used irradiated splenocytes, CD11b+ splenic monocytes or CD19+ B cells as allogeneic APCs, Treg-mediated augmentation of Tresp proliferation was observed in the context of all APCs (Fig. 2D). Finally, we examined the proliferation of antigen-specific T cells with transgenic TCRs. Instead of allogeneic stimulation, DO11.10 T cells expressing a specific TCR against OVA peptide were mixed with wild-type BALB/c T cells at a 1:10 ratio and stimulated with 1 µM OVA peptide and syngeneic DCs for 5 days in the presence of IL-2 and anti-CD28 (Fig. 2E). The number of antigen-specific DO11.10+CD4+ Tresps, which were distinguished by Thy1.1+ expression from other Thy1.2+ populations, was significantly higher in the co-culture with Tregs that included DO11.10+ Tregs. These results indicate that the immune response under strong stimulatory conditions is augmented in the presence of Tregs.
Fig. 2.
Enhanced proliferation of CD4+ Tresps in the presence of Tregs and excessive co-stimulation. (A) CFSE-labeled CD25−CD4+ Tresps from Thy1.1 mice were stimulated with allogeneic CD11c+ DCs in the presence of the same number of CD25−CD4+ Tconvs, or graded amounts of CD25+CD4+ Tregs from Thy1.2 BALB/c mice. The proportion and number of Thy1.1+ T cells with more than two divisions are shown. (B) CD25−CD4+ Tresp proliferation with allogenic DCs in the presence of the same number of Tregs or Tconvs. (C) Anti-CD3 was added to co-cultures to stimulate all T cells. (D) Proliferation of Tresps on different types of allogeneic APCs. Representative data from at least three independent experiments are shown in A–D. (E) Proliferation of OVA antigen-specific T cells. For Tresp, Treg and Tconv, CD4+ T-cell fractions from DO11.10 and wild-type mice were mixed at a 1:10 ratio. The means and SD of the number of DO11.10+Thy1.1+ Tresps are presented from three independent experiments. A paired t-test was used to assess statistical significance.
Tregs increase T-cell interaction with APCs
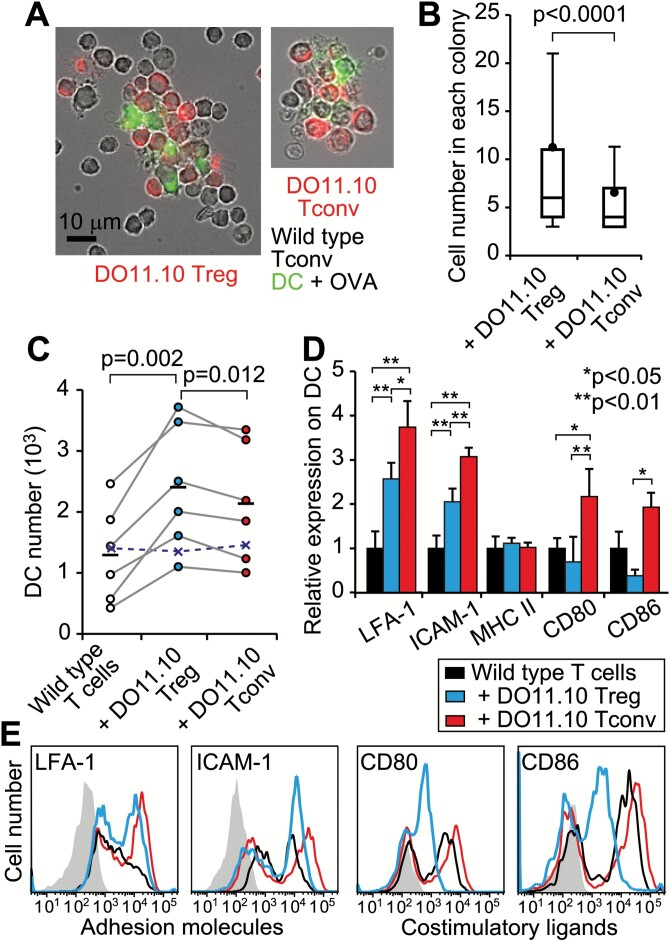
To explore mechanisms of the augmented Tresp proliferation by Tregs, we observed T cells interacting with antigen-presenting DCs under a fluorescence microscope. CD25+CD4+ Tregs or CD25−CD4+ Tconvs from DO11.10 mice were co-cultured with OVA peptide, DCs and polyclonal Tconvs from wild-type mice for 1 day. Each colony contained several DCs and a number of polyclonal T cells, in addition to many antigen-specific T cells (Fig. 3A). Counting the number of nuclei in each colony revealed higher cell numbers in the presence of antigen-specific Tregs than antigen-specific Tconvs (Fig. 3B). Next, we assessed the mechanism of how a colony with specific Tregs contains a large number of cells. The number of viable DCs increased in the presence of antigen-specific Tregs (Fig. 3C). Additionally, adhesion molecules, such as LFA-1 (CD11a) and ICAM-1 (CD54), were expressed at high levels by DCs cultured with antigen-specific T cells, whereas co-stimulatory ligands, such as CD80 and CD86, were expressed at low levels by DCs cultured with specific Tregs (Fig. 3D and E). These results indicate that DCs, in the presence of antigen-specific Tregs, engage many T cells with limited co-stimulation. These findings can explain how Tregs augment Tresp proliferation in the presence of exogenous IL-2 and anti-CD28.
Fig. 3.
Increase in T-DC interaction induced by Tregs. (A) Representative in vitro colonies are shown with eFluor 670-labeled DO11.10 T cells, wild-type Tconvs and FITC-anti MHC II+ DCs. (B) The numbers of cells in each colony were counted after nuclear staining. Box and whisker plots indicate 10, 25, 50, 75 and 90th percentiles, in addition to the mean (data are a summary of 328 colonies with Tregs and 298 colonies with Tconvs) from 10 cultures each. A Mann–Whitney U-test was used to assess statistical significance. (C–E) OVA-pulsed CD11c+ DCs were co-cultured with wild-type Tconvs ± DO11.10 Tregs or Tconvs. (C) The number of DCs in each experiment (circles) is shown with the means (bars). Paired t-tests were used to assess statistical significance. Crosses indicate the mean number of DCs without OVA. (D) The expression of adhesion molecules (LFA-1 and ICAM-1), MHC class II and co-stimulatory ligands (CD80 and CD86) on DCs. Mean ± SD from five independent experiments are shown after normalizing to DCs without DO11.10+ T cells. Analysis of variance (ANOVA) tests with Tukey–Kramer post hoc analysis were used to assess statistical significance. (E) Representative histograms. The shaded lines indicate the IgG-stained control.
Simulation of Treg-mediated suppression reveals crucial factors for tolerance
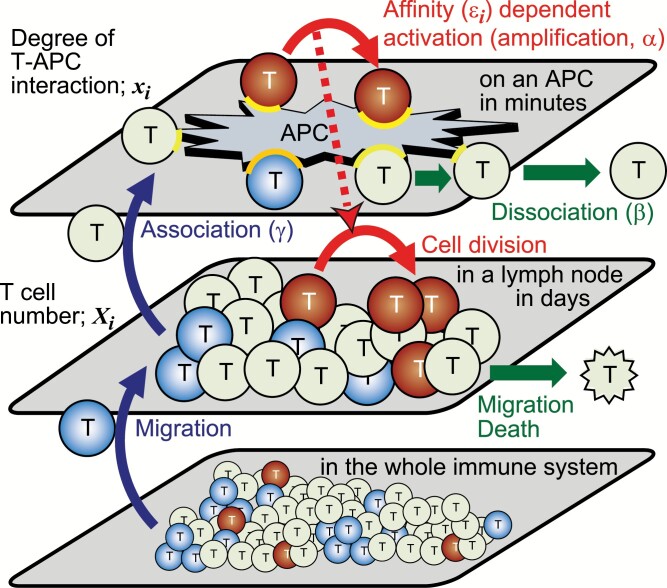
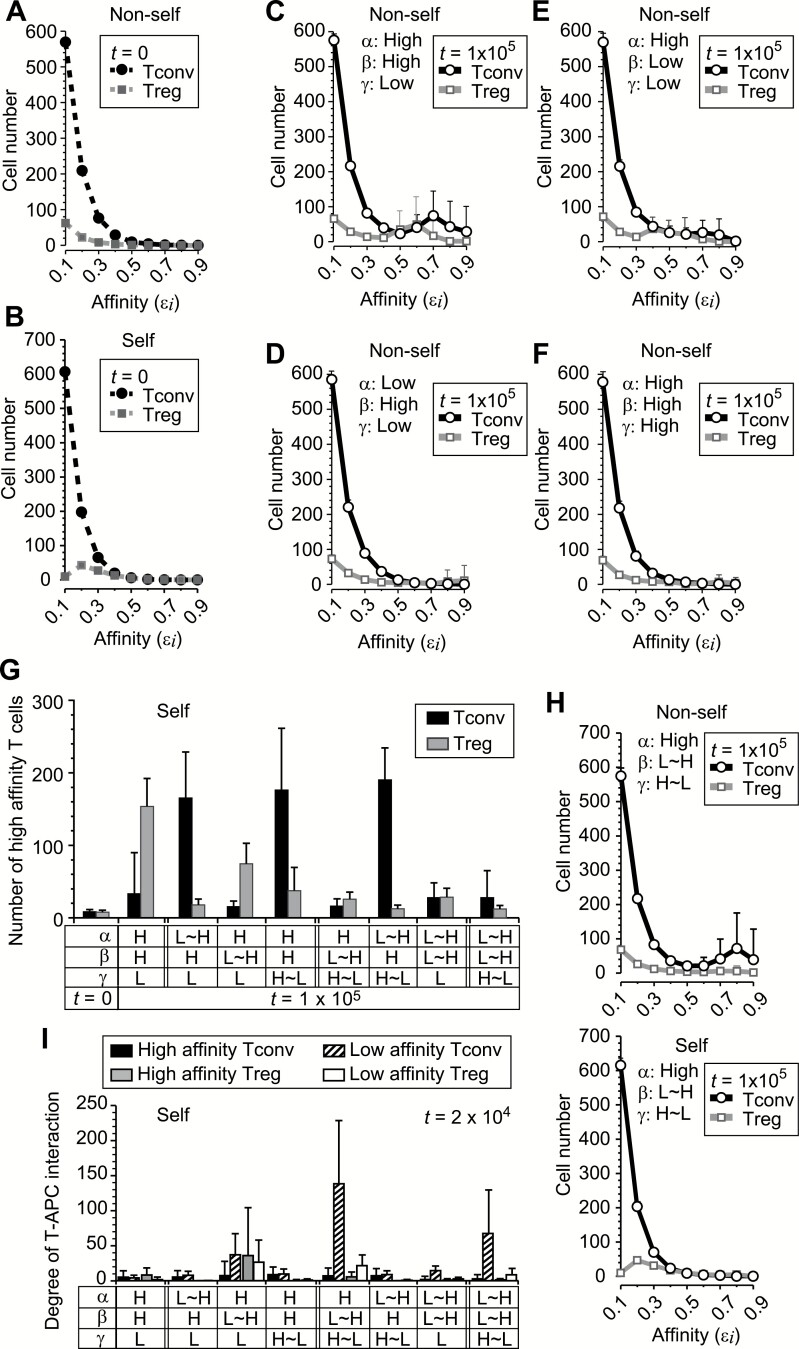
To understand why Tregs increase T-APC interaction, we attempted to unveil crucial factors for T-cell proliferation and for Treg suppression using theoretical analysis. We simulated the dynamics of T-cell numbers in a lymph node, using a Monte Carlo simulation model (Supplementary Text 1). Here, we assumed that the number of T cells in a lymph node changes throughout cell division, death or migration from the lymph node, and migration into the lymph node from the whole immune system (the lower layers in Fig. 4). T cells in this model are composed of Tconvs and Tregs with nine different affinities to APCs (defined in Steps 1 and 2 of Supplementary Text 1). We also introduced variables representing the degree of T-APC interaction (denoted xi) between each APC and each type of T cells (the top layer of Fig. 4). The cell division is assumed to depend on the product of this degree of T-APC interaction (xi) and the TCR affinity (denoted εi). The degree of T-APC interaction increases with new association of the same type of T cells in the lymph node onto the APC (parameter γ), and decreases by dissociation (β). It also increases due to affinity-dependent activation of APC-bound T cells. The per-unit time rate constant of this T-cell affinity-dependent increase is denoted as α, and named amplification rate (the top layer of Fig. 4). A high ratio of Tregs to Tconvs on an APC is assumed to reduce the probability of T-cell division through the reduction of an inflammatory cytokine like IL-2. T cells migrating to the lymph node were randomly sampled from the T-cell populations in the whole immune system, where the fractions with a particular affinity are set to be different depending on self or non-self condition (the bottom layer of Fig. 4). In the non-self condition, we assumed that Tregs and Tconvs in the whole immune system have similar affinity distribution as shown in T-cell population in the lymph node at t = 0 (Fig. 5A). In the self condition, we assumed that positive selection for self-reactive Tregs and negative selections against self-reactive Tregs and Tconvs confer Tregs with higher affinities than those of Tconvs, as shown in Fig. 5B; and Supplementary Figure 1. While the T-cell fractions in the whole immune system are fixed throughout each simulation, the number of cells in each T-cell population in the lymph node and the degree of each T-APC interaction change through six stochastic processes in the lymph node and on APCs (Fig. 4). The T-cell numbers in the lymph node were evaluated after repeating the stochastic processes 105 times (Supplementary Text 1).
Fig. 4.
Scheme of the theoretical model. The top layer shows the change in the degree of T-APC interaction on an APC. The degrees of interaction (yellow arcs) are increased by TCR affinity-dependent activation of APC-bound T cells (amplification, α) and by new association of T cells in a lymph node onto the APC (γ), and decreased by dissociation of interacting molecules (β). The middle layer shows the change in T-cell number in a lymph node. In some models, the numbers increase by cell division that is dependent on the activation on APCs and by migration from the whole immune system and decrease by migration or death. The color of T cells indicates the T-cell population expressing a particular TCR.
Fig. 5.
Identification of parameters controlled by self-reactive Tregs in simulation. The T-cell numbers in a lymph node were simulated as described in Supplementary Text 1. (A and B) The TCR affinity (εi) distribution of Tconvs and Tregs at the initial time point (t = 0) in the non-self (A) and self condition (B). The mean initial T-cell numbers in 15 × 4 simulations used in (C–F) are shown. See also Supplementary Figure 1. (C–F) The numbers of Tconvs and Tregs at t = 105 in a condition where the probabilities of TCR affinity-dependent amplification of the degree of T-APC interaction (α), dissociation from APC (β) and new association to APC (γ) were fixed at high or low values that have 10-fold difference. The average numbers of T cells from 15 simulations are shown. (G) Proliferation of high-affinity T cells in self condition. The parameters α, β and γ were fixed at high or low values (indicated by H or L) or changed depending on the Treg/Tconv ratio on each APC (indicated by ~). The mean numbers of T cells with high affinity (εi ≥ 0.5) at t = 105 are shown with SD from 50 simulations. (H) T-cell numbers are shown with mean + SD of 50 simulations where high Treg/Tconv ratio on each APC induces low cell division, low β and high γ. (I) Degree of T-APC interaction of the indicated T-cell populations (high affinity: εi ≥ 0.5, low affinity: εi ≤ 0.4). The averaged values at t = 2 × 104 in conditions where Treg/Tconv ratios on APCs do or do not change α, β and γ are shown with SD from 10 APCs × 15 simulations.
We first performed the simulation where Tregs were assumed to decrease the cell-division probability through the cytokine concentration without directly affecting T-APC interaction (Fig. 5C–F). The efficient proliferation of high-affinity T cells occurred in a parameter set where amplification probability was high (High α), dissociation probability was high (High β) and association probability was low (Low γ). However, under these conditions, high-affinity T cells, especially self-reactive Tregs, proliferated also in the self condition, which indicated the failure of stable tolerance (the second pair from the left in Fig. 5G).
Next, we further assumed that Tregs might affect one of the T-APC interaction processes by decreasing α or β, or increasing γ if the Treg/Tconv ratio on an APC was higher than a threshold (the third to fifth pairs from the left in Fig. 5G). We found that the regulation of β by Tregs, but not α or γ, effectively suppressed proliferation of high-affinity Tconvs in the self condition. Additional regulation of α or γ by Tregs, which resulted in decreasing the α/γ ratio, was further required to inhibit proliferation of high-affinity Tregs (Fig. 5G). When Tregs were set to decrease the dissociation probability β, and increase the association probability γ, the expansion of high-affinity T cells in the self condition was stably inhibited with the lowest deviation (F-test < 0.0003 when comparing with any others in Fig. 5G), preserving reactivity in the non-self condition (Fig. 5H; Supplementary Figure 2). In the self tolerant condition, APCs interacted with a large number of T cells, especially with low-affinity Tconvs (Fig. 5I). These results indicate the importance of a large degree of total T-APC interaction for stable tolerance, which might be achieved with the augmented survival and adhesion molecule expression of DCs with Tregs as indicated in the co-culture experiments.
Mathematical modeling reveals that low dissociation stabilizes T-cell fraction dynamics
Next, we developed a mathematical model that represents T-cell interaction with an APC to further clarify how low dissociation and high association contribute to stable tolerance. Similar to our simulation model in the previous section, we modeled the degree of T-cell interaction between an APC and T cells assuming that the interaction degree increases with affinity-dependent activation of APC-bound T cells (amplification, parameter α) and with new association of T cells (γ), and decreases by dissociation (β) (the top layer of Fig. 4).
| (e1) |
The equation (e1) is the time evolution of the degree of total T-APC interaction xtot. The symbol α represents the maximal increase in the degree of T-APC interaction per unit time in the immunological synapse. The symbol 〈ε〉 is the efficiency of the amplification at a given time point, ranging from 0 to 1. It is equivalent to the average affinity between TCRs and antigens. The symbol β is the dissociation rate of T-APC interaction per unit time. The symbol γ represents new associations formed between the APC and T cells in a T-cell pool or a lymph node per unit time. Notably, the regulation by Tregs or the effect of stochastic noise were not represented explicitly in this mathematical model.
To assess the interaction of T cells with the APC with various affinities, we next constructed a model of the degree of T-APC interaction between an APC and each type of T cell with a particular TCR or the affinity εi, as the underlying model of equation (e1).
| (e2) |
The appearance of xi and in the first term of the equation (e2) indicates a positive feedback and competition among T-cell populations, respectively. The ratio accounts for the available resources at a junction or synapse between the APC and the i-th T-cell population. Even though there is a positive feedback, an unlimited exponential increase does not occur due to the competition. Upon new association of a T cell, the T cell is randomly sampled from the T-cell pool (lymph node) and the parameter pi is the fraction of the i-th T-cell population in the T-cell pool, i.e..
The equations (e2) can be separated into two parts, (e1) and (e3).
| (e3) |
The equation (e1) is obtained by summing up the equations (e2). The variable xtot is the degree of total T-APC interaction and is given by . The symbol 〈ε〉 is redefined as the average affinity, given by . On the other hand, the equations (e3) represent how the fraction or occupancy of the i-th T-cell population on the APC changes under the time evolution. The first term of the right-hand side of equations (e3) represents the advantage of T cells with high affinities, while the second represents the disadvantage of rare T cells in the T-cell pool. Following the equations (e1 and e3), the degree of total T-APC interaction and the fractions approach an equilibrium which satisfies the following equations.
| (e4) |
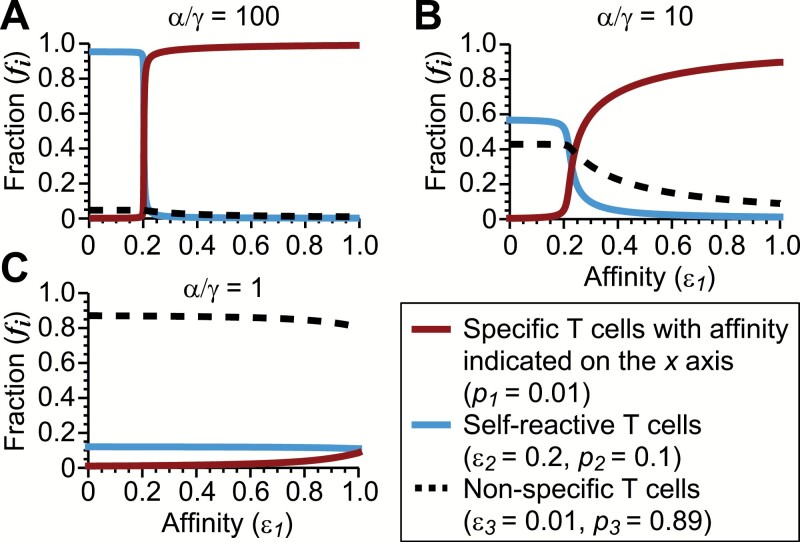
The interacting fractions at the equilibrium largely depend on the relative importance of amplification and new association. If the amplification of existing T-APC interaction with positive feedback is the major contributor to the increase in the degree of T-APC interaction, namely, (where Δɛ is the difference between the highest affinity and second-highest affinity among the T-cell populations), then in the first term of the right-hand side of equation (e3) should be nearly 0 at the equilibrium. T cells with the highest affinity (i = h) increase the fraction fh due to , approaching (Supplementary Text 2). Other lower affinity T cells are excluded from the APC; . On the other hand, if the new association of T cells is more important for the increase in the degree of T-APC interaction, , interacting fractions on the APC become similar to the fractions in the T-cell pool regardless of the affinities; . To illustrate these results, we considered only three T-cell populations with a particular given affinity (p1 = 0.01, rare specific T cells), intermediate affinity (ε2 = 0.2, p2 = 0.1, self-reactive T cells) or low affinity (ε3 = 0.01, p3 = 0.89, non-specific T cells), and calculated the cell fractions on an APC at the equilibrium (Fig. 6).
Fig. 6.
Calculation of T-cell fractions on an APC on the basis of the mathematical model. (A–C) T-cell fractions on an APC at the equilibrium were calculated in three different α/γ conditions in the presence of three T-cell populations in the T-cell pool. The affinity of the first population (ε1) is indicated on the x-axis.
On the basis of this simplified mathematical model, we have attempted to resolve the question of why the regulation of dissociation and association by Tregs was crucial in the simulation in the previous section where T cells were assumed to divide depending on the T-APC interaction (Supplementary Text 3). In conditions where , the dominating T cells with highest affinity on APCs can be activated selectively, even if the population is rare in the T-cell pool or the affinity is not very high (Supplementary Figure 3). When α ≪ γ, activation of rare T cells with high affinity is inhibited by the small degree of T-APC interaction. Importantly, however, the Treg fraction on APCs would be kept equivalent to the fraction in the T-cell pool (lymph node) regardless of self and non-self conditions (Fig. 6C), making it difficult to establish tolerance based on the high self-reactivity of Tregs. On the other hand, a low β condition increases the degree of total T-APC interaction, xtot, without altering the T-cell fractions on APCs at the equilibrium (e4). As a result, Treg ratio to xtot on each APC in self condition would be kept stably higher than a threshold even in the presence of a certain range of stochastic fluctuation. High xtot also negatively affects the dynamics of interacting fractions as indicated by equations (e3). Thus, although the concrete functions regulating the parameters β and γ are not assessed in the mathematical model, low dissociation and a high association/amplification ratio would be crucial for self-reactive Tregs to sustain robust tolerance avoiding possible rapid proliferation of high-affinity T cells.
T-cell sufficiency is crucial for stable tolerance in vivo
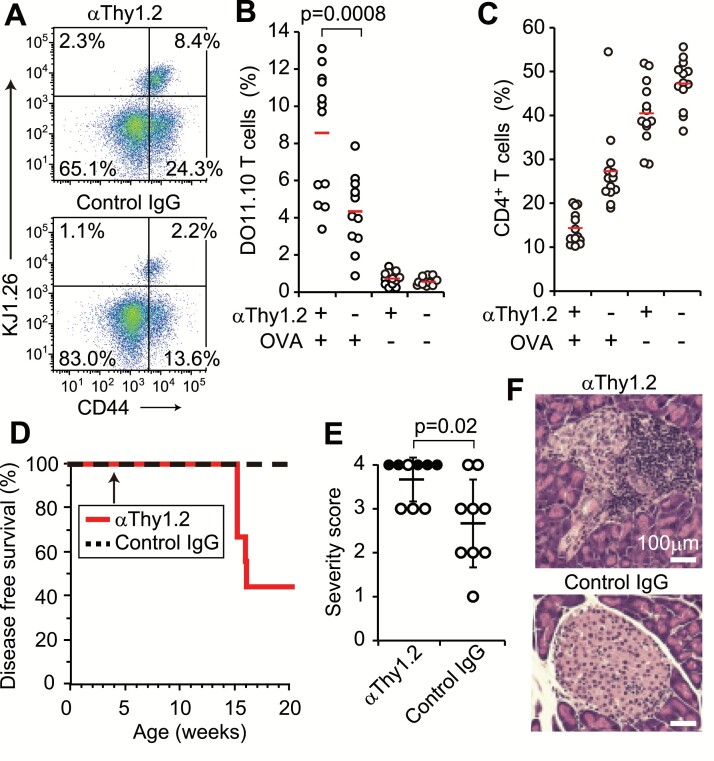
Finally, we attempted to manipulate immune responses in mice by targeting the T-cell number. To test this, we injected 100 µg of T cell-depleting antibody (anti-Thy1.2) into Thy1.2+ BALB/c mice pre-transferred with DO11.10 Tconvs (Thy1.2+). The treatment reduced the numbers of total and CD4+ T cells in a lymph node to approximately half (data not shown). When immunized with OVA protein, the T-cell reduction increased the ratio of KJ1.26+-specific T cells and CD44high memory and/or activated T cells among CD4+ T cells (Fig. 7A and B). This inversely correlated with the ratio of CD4+ T cells in the lymph node (Fig. 7C). Furthermore, after treating autoimmune type 1 diabetes-prone NOD mice with anti-Thy1.2 at 4 weeks of age, a majority of them developed diabetes with glycosuria until week 20, when no NOD mice with control IgG showed glycosuria (Fig. 7D). Histological examination confirmed severe damage to the pancreatic islets in diabetic mice (Fig. 7E and F). These results indicate that lymphopenia and Treg deficiency induce similar effects on the immune response, further suggesting that Treg-mediated tolerance requires a sufficient number of T cells.
Fig. 7.
Enhanced immune responses in vivo after T-cell reduction. (A) BALB/c mice pre-transferred with DO11.10+ Tconvs were immunized with OVA after injection with either anti-Thy1.2 or control IgG. CD44 and KJ1.26 expression on CD4+ T cells in the draining lymph nodes are shown. (B) KJ1.26+ T-cell ratios among CD4+ T cells, and (C) CD4+ T-cell ratios in draining and non-draining lymph nodes are shown. Thirteen mice in each group from four independent experiments. Red bars indicate the means. (D) The incidence of glycosuria in NOD mice injected with either anti-Thy1.2 or IgG at 4 weeks of age. Nine mice in each group from three independent experiments. (E) Histological scores for each mouse from three independent experiments (closed circles indicate glycosuria), and (F) representative HE staining of pancreatic islets. The brightness of the two images was adjusted in the same way. An unpaired t-test was used to assess the statistical significance in B and E.
Discussion
In this combined study of theoretical analyses and experimental examinations, we proposed a novel mechanism by which Tregs regulate robust self-tolerance. Theoretically, as crucial functions of Tregs, the augmentation of total T-APC interaction by inhibiting dissociation and increasing new association is critical in addition to reducing the probability of cell division. Results of in vivo and in vitro experiments support the theoretical analyses qualitatively. A large number of T cells interacting with DCs in colonies were observed in the presence of antigen-specific Tregs, whereas proliferation was inhibited by limiting co-stimulation. Accordingly, the combination of high exogenous co-stimulation and antigen-specific Tregs enhanced Tresp proliferation most effectively. Further, the reduction of whole T-cell number promoted the expansion of antigen-specific T cells and the development of autoimmune diabetes in diabetes-prone mice. Although the model contains many parameters and our experimental data are insufficient to prove the hypothesis quantitatively among other possible models, our results indicate the importance of T-cell numbers for tolerance.
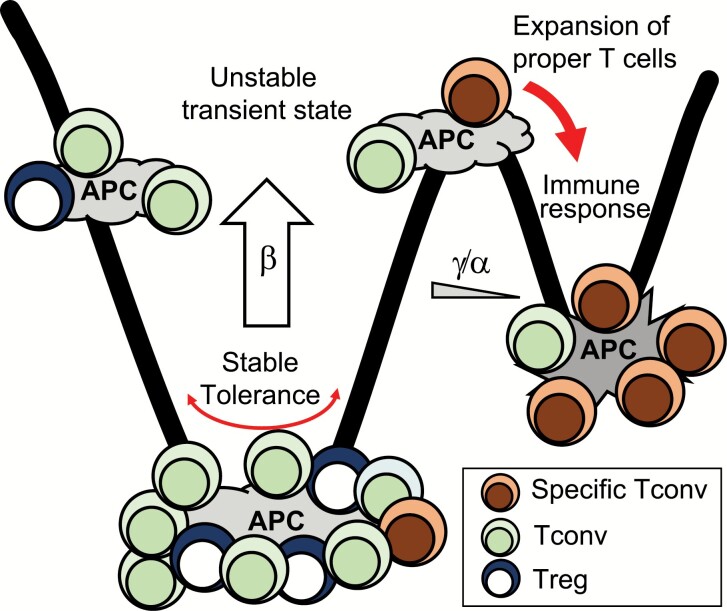
According to a theory in developmental biology, the differentiation of a stem cell might be a process of symmetry breaking on an epigenetic landscape (21, 34). In the immune system, transition from tolerance to immune response might also proceed through a transient unstable state of T-cell fractions on each APC (Fig. 8). In the tolerant state, which means the state where T cells do not proliferate, the amplification/association ratio in the contribution to T-APC interaction increase should be low enough to prevent high-affinity T cells from amplifying their interaction with APCs. Proliferation of high-affinity T cells is inhibited by their small fractions on APCs and the low cell-division probability. In the self condition, the higher affinity of Tregs causes the mean Treg/Tconv ratio on APCs to be higher than a threshold even at this low amplification/association ratio. Most importantly, the deviation and fluctuation of T-cell fractions on an APC (Treg/Tconv ratio or specific/non-specific T-cell ratio) are suppressed by a large degree of total T-APC interaction due to low dissociation. On the other hand, the immunity state should have a high amplification/association ratio and a high cell-division probability. The highest affinity T cells on each APC amplify their interaction. While high-affinity Tregs tend to amplify their interaction in the self condition, high-affinity Tconvs might amplify their interaction with a certain probability. Cell division further augments the amplification of high-affinity Tconvs. In a real immune response, higher affinity T cells would continuously replace T cells on APCs, and most relevant T cells would proliferate depending on the presented antigens and cytokines. A low degree of total T-APC interaction facilitates the change to concentrate high-affinity T cells. When high-affinity Tconvs are activated, the activated Tconvs would inhibit dissociation and sustain the proliferating state by increasing the expression of adhesion molecules on APCs (Fig. 3D and E). This regulation model indicates that tolerance requires stabilization of the state in addition to simple suppression. This might be a reason why inhibitory molecules are more effective targets than stimulatory molecules to treat cancers. The control of the number and heterogeneity of T-APC interaction would be an immune checkpoint.
Fig. 8.
A regulation model of the immune system. Tregs on APCs increase heterogeneous T cells interacting with APCs by reducing the dissociation probability (β) and by increasing the ratio of new association to amplification (γ/α). When infected, the lack of reactive Tregs reduces the number of T cells on APCs by increasing dissociation probability. The low number of T cells on APCs induces high deviation of the fraction. While APCs with Tregs return to the tolerant state, some APCs are dominated by Tconvs because of the stochasticity. On the APC dominated by Tconvs, specific Tconvs with high affinity amplify the interaction and preferentially proliferate inevitably.
The suppressive function of Tregs might not always inhibit relevant reactions of the immune system. In the presence of IL-2 or CD28 stimulations in vitro, Tregs augmented T-cell proliferation. While it is still not clear whether conditions similar to those under in vitro IL-2 or anti-CD28 stimulations exist in vivo, some mouse and human diseases have shown that Treg deficiency worsens the infection, suggesting an immune-activating function of Tregs in vivo (12, 35). CTLA-4 deficient Tregs might augment Tresp proliferation in vitro (3). Recent quantitative analysis of Treg suppression in vitro has surprisingly shown that an intermediate number of Tregs increase Tresp numbers by promoting their survival, possibly via the cytokines TGF-β and IL-10 (36). Stimulation of APCs by CD40 on T cells might also contribute to the prolonged survival of APCs (37). The activating effect of Tregs was not observed in the presence of agonistic anti-CD3 or anti-CD40 (38, 39), suggesting that interaction between APCs and a small number of specific T cells could be enhanced by Tregs. Consistently, Tregs during infection could allow pathogen persistence, augment antigen exposure for effector T cells and induce development of pathogen-specific memory CD8+ T cells (12, 15–19). Theoretically, low-affinity T cells are able to proliferate in conditions where rates of cell division and association are high (Supplementary Figure 3). The quality and function of the proliferating Tconvs in the presence of Tregs is an issue to be clarified in the future. Augmentation of the degree of total T-APC interaction, which is a key mechanism Tregs use to stabilize the tolerance, might be utilized to promote proliferation of effector T cells in some highly inflammatory conditions.
Lymphopenia is known to exaggerate immune responses, especially after transfer of specific T cells (40, 41). Lymphopenic proliferation and the resulting excessive immune responses are inhibited by Tregs (42). Further, lymphopenia itself might be also controlled by Tregs (43). Our results indicate that low T-cell number and low average affinity reduce the affinity threshold for T cells to increase (Supplementary Text 3). In addition to the generation of Tregs, positive selection of T cells in the thymus that induces a sufficient number of T cells with higher affinities may play a role in tolerance. This prediction is compatible with the immune deficiency-associated autoimmunity in humans and mice (44–46). All these findings support our conclusion that Tregs would elicit their suppressive effects through sufficient T-APC interaction and T-cell number.
A central feature of living systems, including evolution, developmental differentiation, neural network in the brain and the immune system, is the balance between robustness with heterogeneity and plasticity with selective response. Our mathematical equation representing T-APC interaction shows that selective expansion of the fittest is induced in some parameter conditions. In the opposite condition, rare ones with high fitness are inhibited in expansion. Importantly, the rate of decrease acts as a checkpoint and influences the dynamics of and fluctuations in the composition. A low rate of decrease results in a large total amount, stabilizing the current composition, whereas a high rate of decrease can induce a drastic change under the influence of stochastic fluctuation. Our theoretical analyses and their experimental validation reveal novel key functions of Tregs and also provide insights into understanding of various biological systems.
Funding
This work was supported by JSPS KAKENHI (Grant Number 15K08530), Immunology Frontier Research Center in Osaka University and Nozaki Tokushukai Hospital.
Supplementary Material
Acknowledgements
We thank T. Sparwasser for providing the DEREG mice; K. Matsuda, M. Fukami, M. Okuda and Y. Matsumoto for maintaining mice; H. Takagi, J. Wing and K. Okamoto for critical reading of the manuscript; and Editage for English language editing.
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Sakaguchi, S., Powrie, F. and Ransohoff, R. M. 2012. Re-establishing immunological self-tolerance in autoimmune disease. Nat. Med. 18:54. [DOI] [PubMed] [Google Scholar]
- 2. Li, M. O. and Rudensky, A. Y. 2016. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 16:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wing, K.,, Onishi, Y.,, Prieto-Martin, P., et al. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322:271. [DOI] [PubMed] [Google Scholar]
- 4. Pandiyan, P., Zheng, L., Ishihara, S., Reed, J. and Lenardo, M. J. 2007. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 8:1353. [DOI] [PubMed] [Google Scholar]
- 5. Vignali, D. A., Collison, L. W. and Workman, C. J. 2008. How regulatory T cells work. Nat. Rev. Immunol. 8:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Onishi, Y., Fehervari, Z., Yamaguchi, T. and Sakaguchi, S. 2008. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl Acad. Sci. USA 105:10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamaguchi, T., Kishi, A., Osaki, M.et al. 2013. Construction of self-recognizing regulatory T cells from conventional T cells by controlling CTLA-4 and IL-2 expression. Proc. Natl Acad. Sci. USA 110:E2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shevach, E. M. 2009. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30:636. [DOI] [PubMed] [Google Scholar]
- 9. Josefowicz, S. Z., Lu, L. F. and Rudensky, A. Y. 2012. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marson, A.,, Kretschmer, K.,, Frampton, G. M., et al. 2007. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature 445:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng, Y., Josefowicz, S. Z., Kas, A., Chu, T. T., Gavin, M. A. and Rudensky, A. Y. 2007. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 445:936. [DOI] [PubMed] [Google Scholar]
- 12. Belkaid, Y. and Tarbell, K. 2009. Regulatory T cells in the control of host-microorganism interactions (*). Annu. Rev. Immunol. 27:551. [DOI] [PubMed] [Google Scholar]
- 13. Pacholczyk, R., Kern, J., Singh, N., Iwashima, M., Kraj, P. and Ignatowicz, L. 2007. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity 27:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsieh, C. S., Zheng, Y., Liang, Y., Fontenot, J. D. and Rudensky, A. Y. 2006. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 7:401. [DOI] [PubMed] [Google Scholar]
- 15. Belkaid, Y., Piccirillo, C. A., Mendez, S., Shevach, E. M. and Sacks, D. L. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502. [DOI] [PubMed] [Google Scholar]
- 16. Lund, J. M., Hsing, L., Pham, T. T. and Rudensky, A. Y. 2008. Coordination of early protective immunity to viral infection by regulatory T cells. Science 320:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pace, L.,, Tempez, A.,, Arnold-Schrauf, C., et al. 2012. Regulatory T cells increase the avidity of primary CD8+ T cell responses and promote memory. Science 338:532. [DOI] [PubMed] [Google Scholar]
- 18. Kalia, V., Penny, L. A., Yuzefpolskiy, Y., Baumann, F. M. and Sarkar, S. 2015. Quiescence of memory CD8(+) T cells is mediated by regulatory T cells through inhibitory receptor CTLA-4. Immunity 42:1116. [DOI] [PubMed] [Google Scholar]
- 19. Laidlaw, B. J.,, Cui, W.,, Amezquita, R. A., et al. 2015. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat. Immunol. 16:871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eldar, A. and Elowitz, M. B. 2010. Functional roles for noise in genetic circuits. Nature 467:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang, S. 2016. Where to Go: breaking the symmetry in cell motility. PLoS Biol. 14:e1002463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Süel, G. M., Kulkarni, R. P., Dworkin, J., Garcia-Ojalvo, J. and Elowitz, M. B. 2007. Tunability and noise dependence in differentiation dynamics. Science 315:1716. [DOI] [PubMed] [Google Scholar]
- 23. Chang, H. H., Hemberg, M., Barahona, M., Ingber, D. E. and Huang, S. 2008. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature 453:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iwaki, M., Iwane, A. H., Shimokawa, T., Cooke, R. and Yanagida, T. 2009. Brownian search-and-catch mechanism for myosin-VI steps. Nat. Chem. Biol. 5:403. [DOI] [PubMed] [Google Scholar]
- 25. Shaffer, S. M.,, Dunagin, M. C.,, Torborg, S. R., et al. 2017. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 546:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scheffold, A., Hühn, J. and Höfer, T. 2005. Regulation of CD4+CD25+ regulatory T cell activity: it takes (IL-)two to tango. Eur. J. Immunol. 35:1336. [DOI] [PubMed] [Google Scholar]
- 27. Carneiro, J.,, Leon, K.,, Caramalho, I., et al. 2007. When three is not a crowd: a crossregulation model of the dynamics and repertoire selection of regulatory CD4+ T cells. Immunol. Rev. 216:48. [DOI] [PubMed] [Google Scholar]
- 28. Busse, D.,, de la Rosa, M.,, Hobiger, K., et al. 2010. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc. Natl Acad. Sci. USA 107:3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Almeida, A. R.,, Amado, I. F.,, Reynolds, J., et al. 2012. Quorum-sensing in CD4(+) T cell homeostasis: a hypothesis and a model. Front. Immunol. 3:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Butler, T. C., Kardar, M. and Chakraborty, A. K. 2013. Quorum sensing allows T cells to discriminate between self and nonself. Proc. Natl Acad. Sci. USA 110:11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Voisinne, G., Nixon, B. G., Melbinger, A., Gasteiger, G., Vergassola, M. and Altan-Bonnet, G. 2015. T cells integrate local and global cues to discriminate between structurally similar antigens. Cell Rep. 11:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Furusawa, C. and Yamaguchi, T. 2016. Robust and accurate discrimination of self/non-self antigen presentations by regulatory T cell suppression. PLoS One 11:e0163134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lahl, K.,, Loddenkemper, C.,, Drouin, C., et al. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Waddington, C. H. 1957. The strategy of the genes, George Allen & Unwin, London, UK. [Google Scholar]
- 35. Wildin, R. S., Smyk-Pearson, S. and Filipovich, A. H. 2002. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J. Med. Genet. 39:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dowling, M. R., Kan, A., Heinzel, S., Marchingo, J. M., Hodgkin, P. D. and Hawkins, E. D. 2018. Regulatory T cells suppress effector T cell proliferation by limiting division destiny. Front. Immunol. 9:2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharma, M. D.,, Hou, D. Y.,, Baban, B., et al. 2010. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity 33:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takahashi, T.,, Kuniyasu, Y.,, Toda, M., et al. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969. [DOI] [PubMed] [Google Scholar]
- 39. Thornton, A. M. and Shevach, E. M. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gleeson, P. A., Toh, B. H. and van Driel, I. R. 1996. Organ-specific autoimmunity induced by lymphopenia. Immunol. Rev. 149:97. [DOI] [PubMed] [Google Scholar]
- 41. Singh, N. J. and Schwartz, R. H. 2006. The lymphopenic mouse in immunology: from patron to pariah. Immunity 25:851. [DOI] [PubMed] [Google Scholar]
- 42. Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. and Toda, M. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151. [PubMed] [Google Scholar]
- 43. Moltedo, B., Hemmers, S. and Rudensky, A. Y. 2014. Regulatory T cell ablation causes acute T cell lymphopenia. PLoS One 9:e86762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. King, C., Ilic, A., Koelsch, K. and Sarvetnick, N. 2004. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 117:265. [DOI] [PubMed] [Google Scholar]
- 45. Sakaguchi, N.,, Takahashi, T.,, Hata, H., et al. 2003. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426:454. [DOI] [PubMed] [Google Scholar]
- 46. Holst, J.,, Wang, H.,, Eder, K. D., et al. 2008. Scalable signaling mediated by T cell antigen receptor-CD3 ITAMs ensures effective negative selection and prevents autoimmunity. Nat. Immunol. 9:658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.