Abstract
Objective
We aimed to determine the reproducibility of TSH testing in pediatric patients referred to pediatric endocrinologists and to identify the threshold TSH levels that would predict the presence of antithyroid autoantibodies and inform decisions by pediatric endocrinologists to initiate or continue treatment with levothyroxine.
Study Design
We analyzed a retrospective case series of 325 children aged 1 to 18 years referred for hypothyroidism to the endocrinology clinic at a tertiary care children’s hospital. The receiver operating characteristic area under curve (AUC) determined the ability of the initial TSH level to predict pediatric endocrinologists’ treatment decisions, presence of thyroid autoantibodies, and reproducibility of elevated TSH on repeat testing.
Results
Of 325 patients, 191 were treated. The treated patients were more likely to have had a higher referral TSH, positive autoantibodies, and abnormal thyroid gland examination findings. An initial TSH of 5 had a specificity of only 14% for a repeat TSH of ≥5. An initial TSH level of 11 had a specificity of 90% for a repeat TSH of ≥11, with sensitivity of 90%. TSH was a relatively poor predictor (AUC, 0.711) of the presence of autoantibodies with optimal classification at TSH >8.8 mIU/L. It was better (AUC, 0.878) at predicting whether endocrinologists started or continued treatment with levothyroxine, with optimal classification at 8.2 mIU/L. TSH levels combined with antibody status and thyroid examination findings had the best ability to predict treatment (AUC, 0.930).
Conclusions
TSH levels slightly above the reference range should not prompt referral to pediatric endocrinologists unless another basis for clinical concern is present.
Keywords: autoantibodies, levothyroxine, receiver operating characteristic curve, subclinical hypothyroidism
Because reference ranges are 95% limits, elevated levels of TSH are frequently obtained (3.3% of a large normal pediatric population, with the distribution of TSH skewed to the right, resulting in more values above the reference range than below it) [1]. Most asymptomatic children with TSH values slightly above the reference range will have a normal TSH level on repeat testing [1]. Nevertheless, they are often referred by primary care practitioners, at least in our large American city, to pediatric endocrinologists when abnormal values are first detected.
Subclinical hypothyroidism has been defined as a TSH concentration consistently greater than the upper limit of the reference range associated with a normal T4 or free T4 (FT4) level. In the adult population, there appears to be no clinical benefit to treating subclinical hypothyroidism with levothyroxine [2]. Nevertheless, prescriptions of levothyroxine have increased markedly in recent years in the United States and the United Kingdom [3]. The prevalence of subclinical hypothyroidism in children is lower than in adults, progression to overt hypothyroidism is uncommon [1, 4], and children with subclinical hypothyroidism do not differ from healthy controls in linear growth, neurocognitive outcome, bone mineral density, blood pressure, or lipid profile (reviewed by Salerno et al. [5] and Monzani et al. [6]). As many as 12% of children with nonautoimmune elevations of TSH could carry variants in the TSH receptor [7], and these generally will not require treatment [8]. Treating children with subclinical hypothyroidism with levothyroxine has shown no clear benefit. Overtreatment is a possibility (and occurs in 20% of adults taking levothyroxine [9]). Although the pediatric data have been quite limited, there is some evidence that overtreatment of congenital hypothyroidism, at least during infancy, can have adverse cognitive effects [10].
Limited data are available to help primary care practitioners distinguish abnormal laboratory results that reflect actual thyroid disease requiring treatment from subclinical hypothyroidism requiring only observation or from transient abnormalities attributable to laboratory error, circadian variation [11], normal interindividual variation, and acute illness. Such information could help primary care practitioners limit referrals to pediatric endocrinologists for evaluation of abnormal thyroid function to those patients likely to require treatment.
Therefore, we sought to determine the reproducibility of TSH testing in pediatric patients referred to pediatric endocrinologists. We also aimed to identify the threshold TSH levels that would predict presence of antithyroid autoantibodies and inform decisions by pediatric endocrinologists at our institution to initiate or continue treatment with levothyroxine.
1. Methods
The institutional review board at UT Southwestern Medical Center approved the present study. A retrospective review of the medical records was performed on children aged 1 to 18 years with abnormal thyroid test results who had been referred to the Endocrinology Center at the Children’s Medical Center in Dallas, Texas.
The subjects were identified by the presence of abnormal thyroid function tests results or a diagnosis of acquired hypothyroidism or autoimmune thyroiditis (International Classification of Diseases, Ninth Revision, codes 794.5, 794.6, 244.x, or 245.2, respectively), who had been seen at the Children’s Medical Center’s endocrinology clinic.
The inclusion criteria were age 1 to 18 years at the initial laboratory test with a referral TSH level above the upper limit of the specific laboratory’s reference range. The exclusion criteria were a diagnosis of trisomy 21, care transferred from another institution or endocrinologist, referral for another endocrine disease, or if the patient was taking medications known to cause abnormal thyroid function tests (lithium and oxcarbazepine, one patient each).
A review of the medical records identified the demographic information, laboratory data, physical findings from the thyroid examination documented by the pediatric endocrinologist (for this analysis, graded as “normal” or “abnormal,” with the latter category including both goiter and abnormally firm consistency), and body mass index (BMI) z-score. The laboratory data included the referral and repeat thyroid function tests and antithyroglobulin and antithyroid peroxidase antibody levels.
We examined the medical records sequentially from 1 March 2012 through 28 June 2013. To ensure that no important changes had occurred in the referral patterns over time, we also included medical records randomly selected by the medical record number from patients first seen from 5 November 2008 through 28 February 2012. No differences were found between these groups in age, sex, referring TSH level, referring FT4, or the proportions of patients for whom treatment was continued or started (data not shown). Therefore, these groups were pooled for all further analyses. Of 527 medical records reviewed, 325 (175 in the 2012 to 2013 group) met the inclusion criteria for the final analysis.
Repeat TSH levels were available for 212 patients. We were unable to calculate the BMI for one patient owing to a lack of documented height. Missing data were not imputed.
A. Statistical Analysis
We performed a receiver operating characteristic (ROC) analysis to determine the threshold values for TSH with 95% sensitivity, 95% specificity, and optimal classification for three outcomes: a similarly elevated TSH level on repeat testing, the presence of autoantibodies, and the need for treatment as judged by the pediatric endocrinologists. The area under curve (AUC) was used as a measure of predictive power, with an AUC of 0.5 indicating that the test was no better than chance, and an AUC of 1.0 indicating a perfect test with 100% sensitivity (proportion of actual positives correctly identified) and 100% specificity (proportion of actual negatives correctly identified). The optimal classification level was that which maximized Youden’s J statistic (sensitivity + specificity − 1). ROC analysis was performed using a web-based calculator (available at: www.jrocfit.org). Other statistical analyses were conducted with Statview, version 5.0 (SAS Institute, Cary, NC). We used χ2 tests to assess the correlations between categorical variables. Continuous variables that were not normally distributed (in particular, TSH) were reported as medians and interquartile ranges, and intergroup differences for such variables were assessed nonparametrically (Mann-Whitney U tests). Normally distributed variables were presented as means and standard deviations, and differences were assessed using Student t tests. The combined ability of TSH measurement, thyroid examination findings, and antibody status to predict treatment decisions was assessed using multivariable logistic regression analysis. Statistical significance was assessed using Wald tests.
2. Results
Of the 325 patients included in the present study, 113 had been prescribed levothyroxine by the referring practitioner before their endocrinology appointment. Treatment was continued by the pediatric endocrinologist for 103 patients and initiated by the pediatric endocrinologist for 88 patients. In all, 191 patients were treated and 134 were not treated (Table 1).
Table 1.
Baseline Characteristics
| Characteristic | Continued Treatment (n = 103) | Started Treatment (n = 88) | Discontinued Treatment (n = 10) | Not Treated (n = 124) | P Valuea |
|---|---|---|---|---|---|
| Female sex, n (%) | 80 (78) | 65 (74) | 4 (40) | 64 (52) | <0.001 |
| Mean age, y (SD) | 11.9 (3.2) | 11.6 (3.2) | 13.4 (3.3) | 11.5 (3.3) | NS |
| Ethnicity, n (%) | |||||
| White | 30 (29) | 45 (51) | 3 (30) | 50 (40) | NS |
| Hispanic | 54 (52) | 33 (38) | 6 (60) | 54 (44) | |
| Black | 5 (5) | 2 (23) | 0 (0) | 9 (7) | |
| Other | 14 (14) | 8 (9) | 1 (10) | 11 (9) | |
| Median TSH, mIU/L (IQR) | 32.0 (13.6–123) | 9.7 (7.0–15.8) | 7.0 (5.7–8.0) | 6.3 (5.4–7.6) | <0.001 |
| Median FT4, ng/dL (IQR) | 0.78 (0.50–0.90) | 1.00 (0.82–1.16) | 1.58 (1.11–2.00) | 1.10 (1.00–1.20) | <0.001 |
| Abnormal thyroid examination results, n (%) | 52 (50) | 36 (38) | 1 (10) | 4 (3) | <0.001 |
| Positive antibodies, n (%) | 88 (85) | 70 (80) | 3 (30) | 28 (23) | <0.001 |
| Obese, n (%) | 26 (25) | 19 (22) | 3 (30) | 35 (28) | NS |
P values for treated patients (first 2 columns, n = 191) vs untreated patients (last 2 columns, n = 134).
The treated patients were more likely to have had a higher referral TSH level, lower FT4, positive autoantibodies (antithyroid peroxidase and/or antithyroglobulin), and an abnormal thyroid gland on physical examination. No differences were found in sex, ethnicity, or the percentage of obese patients (those with BMI z-scores, ≥2.0) between the two groups. No differences were found for any parameter between patients who had never been treated and those for whom treatment had been discontinued by the endocrinologist. In contrast, compared with the patients for whom the endocrinologist had started treatment, those patients for whom treatment had been started by the referring physician and continued by the endocrinologist were more likely (P = 0.02) to be of Hispanic ethnicity instead of white, and they had higher TSH and lower FT4 levels (P < 0.001 for both).
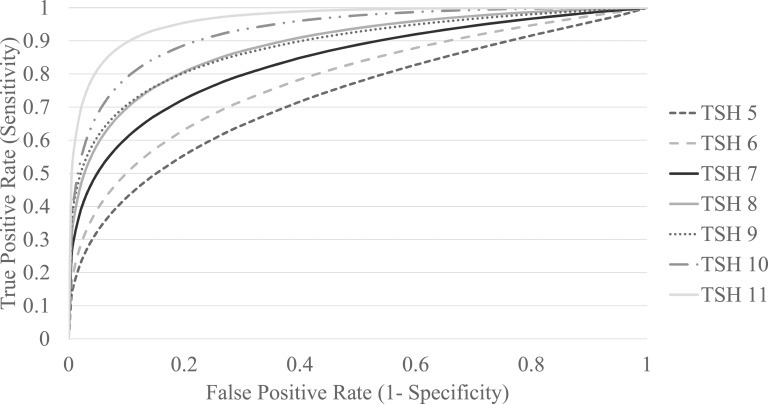
For the 212 patients in whom a repeat TSH level was available before starting treatment, we examined how well a given referral TSH level predicted a subsequent TSH of an equal or higher level. We constructed a series of seven ROC curves, each testing the ability of the referral TSH level to predict whether a repeat TSH level would be at or above a particular level T, where T was an integer between 5 and 11 (Fig. 1). The AUC steadily increased at higher values of T, indicating increased predictive power. Using the ROC curve for each value of T, we derived the sensitivity, specificity, and positive and negative predictive values for a referral TSH level of T to predict a repeat TSH level of ≥T (Table 2). Although an initial TSH of 5 had a specificity of only 14% for a repeat TSH of ≥5, an initial TSH level of 11 had a specificity of 90% for a repeat TSH of ≥11, with similar sensitivity.
Figure 1.
ROC curves for an initial TSH value to predict a repeat TSH value ≥T.
Table 2.
Ability of an Initial TSH Value of T to Predict a Repeat TSH Value of >T (n = 219)
| T (mIU/L) | Patients With Repeat TSH >T | ROC AUC | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|---|---|---|---|---|---|
| 5 | 89 | 0.718 | 94 | 15 | 43 | 79 |
| 6 | 61 | 0.781 | 87 | 35 | 34 | 88 |
| 7 | 46 | 0.836 | 83 | 58 | 35 | 93 |
| 8 | 40 | 0.885 | 90 | 80 | 50 | 97 |
| 9 | 35 | 0.882 | 86 | 83 | 48 | 97 |
| 10 | 31 | 0.928 | 90 | 88 | 55 | 98 |
| 11 | 22 | 0.963 | 91 | 88 | 47 | 99 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
The referral TSH level was a relatively poor predictor (AUC, 0.711) of presence of antithyroglobulin or antithyroid peroxidase antibodies with optimal classification at TSH >8.8 mIU/L (sensitivity, 63%; specificity, 80%; Table 3).
Table 3.
Ability of Referral TSH to Predict Presence of Thyroid Antibodies and Inform Treatment Decisions by Pediatric Endocrinologists
| ROC Outcome | ROC AUC | TSH, mIU/L | Sensitivity, % | Specificity, % | NPV, % | PPV, % |
|---|---|---|---|---|---|---|
| Positive antibodies | 0.711 | |||||
| 5.1 | 95 | 11 | 55 | 67 | ||
| 8.8a | 63 | 80 | 55 | 88 | ||
| 15.1 | 42 | 95 | 48 | 96 | ||
| Treatment | 0.878 | |||||
| 5.6 | 95 | 33 | 77 | 66 | ||
| 8.2a | 76 | 86 | 71 | 88 | ||
| 11.0 | 64 | 95 | 65 | 95 |
Optimal cutoff using Youden index (J).
TSH was better (AUC, 0.878) at predicting whether endocrinologists started or continued treatment with levothyroxine, with the optimal classification at 8.2 mIU/L (sensitivity, 76%; specificity, 85%; positive predictive value, 75%; negative predictive value, 86%). The result from a multivariable logistic regression analysis indicated that the TSH levels, thyroid examination findings, and antibody status were independent predictors of the treatment decision (Table 4). The model defined a probability: Pr(treatment) = 1/[1 + exp(−l)], where l = 1.854 [antibodies positive?] +2.18 [abnormal examination?] + 0.339 [TSH] − 4.238. A ROC curve assessing the performance of Pr(treatment) in predicting the decision to treat had an AUC of 0.930, indicating excellent predictive power. Thus, endocrinologists generally considered the combination of TSH level, the presence of autoantibodies, and abnormal thyroid examination results in deciding whether to treat each patient.
Table 4.
Ability of Referral TSH and Autoantibody Status To Predict Treatment Decisions by Pediatric Endocrinologists
| A. Multivariable Logistic Regression | |||||
|---|---|---|---|---|---|
| Variable | B | SE | P Value | Exp(B) | 95% CI, Exp(B) |
| Reference TSH | 0.339 | 0.07 | 0.000 | 1.404 | 1.225–1.609 |
| Positive antibodies | 1.854 | 0.367 | 0.000 | 6.383 | 3.11–13.101 |
| Abnormal examination results | 2.18 | 0.592 | 0.000 | 8.848 | 2.773–28.23 |
| Constant | −4.238 | 0.62 | 0.000 | 0.014 | |
| B. Operating Points for 95% Sensitivity, Optimal Classification, and 95% Specificity | |||||
| Pr(treatment) | Sensitivity, % | Specificity, % | NPV, % | PPV, % | |
| 0.18 | 95 | 59 | 89 | 78 | |
| 0.57a | 82 | 91 | 76 | 93 | |
| 0.70 | 77 | 95 | 73 | 96 | |
Abbreviations: B, regression coefficient; CI, confidence interval for Exp(B); Exp(B), eB, relative risk; Pr(treatment) = 1/(1 + exp(−l)), where l =1.854 [antibodies positive?] + 2.18 [abnormal examination?] + 0.339 [TSH] − 4.238; SE, standard error of regression coefficient.
Optimal classification.
3. Discussion
Pediatricians’ referral rates to subspecialists have varied widely [12, 13], suggesting a lack of uniformity in the referral criteria. Locale-specific referral guidelines might improve the appropriateness of referrals [14, 15]. Because <60% of referred patients in the present study were judged by pediatric endocrinologists to require treatment of hypothyroidism, we sought to develop evidence-based guidelines that could help primary care practitioners decide when to refer patients for further treatment. Reported data on the therapeutic benefits of treating subclinical hypothyroidism in children have been sparse. Therefore, we concentrated on finding TSH levels that were reproducible and predicted other findings of thyroid disease.
Many of the referred patients were obese. Although mildly elevated TSH levels are frequently observed in obese patients and will resolve with weight loss [16, 17], a recent consensus statement [18] has recommended against screening for hypothyroidism in patients with obesity unless they have a short stature relative to their genetic potential and decreased growth velocity in the setting of continued weight gain. Similarly, although constipation is a common finding in hypothyroidism, hypothyroidism has been identified in very few constipated children without other signs or symptoms of hypothyroidism [19].
Mild elevations of TSH (5 to 6 mIU/L) were unlikely to be reproduced with repeat testing, to be associated with positive autoantibodies, or to result in decisions by pediatric endocrinologists to treat the patients. As the TSH increased, the reproducibility on repeat testing improved. Based on the ROC AUC data presented, a referral TSH of 8 to 9 mIU/L (and even more so if ≥10 to 11 mIU/L) was more likely to be reproducible and to be associated with the presence of positive autoantibodies. Our findings are consistent with a previous study from Israel [1]. In their study, an initial TSH level >10 mIU/L was also more likely to remain elevated and to be associated with positive antibodies than was a TSH level of 5 to 10 mIU/L [1]. In the present study, we examined a referral population. In contrast, the Israeli study was population-based and had been conducted in a health care environment in which almost 12% of the children had had at least one TSH level determined [1]. Consequently, a much lower proportion of children in their study had been treated compared with the proportion in ours (0.4% vs 59% overall; 51% vs 94% of patients with an initial TSH level >10).
A. Guidelines for Primary Care Practitioners
Currently, no consensus statements or guidelines have been reported on subclinical hypothyroidism in children from the Pediatric Endocrine Society or the Endocrine Society. The American Thyroid Association has discussed this topic briefly in their most recent guideline on treating hypothyroidism, recommending against treatment except in special circumstances [20]. The European Thyroid Association guidelines in 2014 attempted to address this question, although the recommendations were brief and primarily offered guidance on the frequency of the laboratory evaluations [21]. Using the data we have presented and from previous studies [5], suggested referral guidelines for primary care practitioners are presented in the next paragraph. However, our data were derived from a single large subspecialty practice in a major American city; thus, the resulting guidelines could require modification for other venues with more limited access to subspecialists.
If a patient has signs or symptoms concerning for hypothyroidism (including goiter or other abnormalities on the thyroid examination, postnatal growth failure, fatigue, or constipation) and initial laboratory test results reveal a normal FT4 with a TSH above the reference range, the FT4 and TSH should be repeated by the primary care practitioner with the thyroid autoantibody (antithyroid peroxidase, antithyroglobulin) levels also determined. This agrees with previous recommendations [5].
Because trivial elevations of TSH above the reference range (∼5 mIU/L) are unlikely to be reproducible, they should not generally prompt referral unless another basis for clinical suspicion is present (e.g., low T4, goiter, positive antibodies). In contrast, if the initial TSH level is >10 to 11 mIU/L, the primary care physician should consider starting treatment with levothyroxine, especially if the FT4 is below the reference range, pending repeat laboratory test results, with referral to a pediatric endocrinologist if further evaluation and management by a subspecialist is desired. Although a TSH level of 11 was slightly more reproducible (Table 2), it would be reasonable to use a threshold TSH of 10 for considering treatment, consistent with previous recommendations [5].
If repeat test results reveals a normal FT4 with the TSH level above the reference range but <8 mIU/L, the follow-up protocol should be stratified according to the presence of antithyroglobulin and antithyroid peroxidase autoantibodies or goiter. If the antibodies are negative and the thyroid examination findings are normal, no further workup or repeat testing is needed. If one or both antibodies are positive, thyroid function testing should be repeated within 6 months, or sooner if the patient develops signs or symptoms of hypothyroidism. The evaluation and management of goiter and thyroid nodules were beyond the scope of our report (reviewed by Francis et al. [22]). However, the presence of either should prompt referral and further evaluation, including thyroid ultrasonography [23]. Finally, regardless of the presence of antibodies, if the FT4 level is low, treatment should be initiated with levothyroxine. If the repeat TSH level is >8 mIU/L, referral to a pediatric endocrinologist would be appropriate if further evaluation and management by a subspecialist were desired.
B. Study Limitations
The present study had potential limitations. By design, we examined a referral population, which could have been biased toward more severely affected patients. One of our outcomes—the decision by the pediatric endocrinologist to begin or continue treatment—was inherently subjective. Initiation of therapy before seeing the endocrinologist could have potentially influenced the decision for treatment to continue. However, our informal impression was that most referring pediatricians who prescribed levothyroxine to their patients had done so after a telephone consultation with an endocrinologist in our practice. This was supported by the findings that the children who started treatment by the referring pediatrician and continued therapy with the pediatric endocrinologist had higher TSH and lower FT4 levels than children whose therapy was initiated by the pediatric endocrinologist at the first visit. Despite the number of children who continued therapy, we found that 41% of the patients referred were not treated by the pediatric endocrinologists.
As an additional limitation, the TSH reference ranges in children have varied considerably in the reported data [24]. TSH levels vary the most in the first year of life. Beyond the neonatal period, the upper limit of normal for TSH decreases as age increases [25]. However, in the present study, >25% of children had had a referral TSH measured at a laboratory with a single reference range for children aged 1 to 19 years. The variability of the TSH level in this age range and the lack of pediatric normative data have made it difficult to clinically interpret a given TSH level using this single reference range. We did not study the difference in treatment decisions according to data from different laboratories nor differences based on age or pubertal stage.
Finally, the decision to continue or start therapy was determined by each individual endocrinologist, and no written standard of care was used. The present study was undertaken to provide data for such a guideline.
4. Conclusion
If concern exists regarding the presence of hypothyroidism and the initial thyroid function tests have revealed a normal FT4 with a TSH level above the reference range, repeat TSH and FT4 levels, a careful thyroid examination, and measurement of antithyroid autoantibody levels will assist the primary care practitioner in accurately triaging patients who meet the criteria for referral. This will decrease referrals of children who are unlikely to be treated by pediatric endocrinologists with levothyroxine.
Acknowledgments
Financial Support: P.C.W. is the Audry Newman Rapoport Distinguished Chair in Pediatric Endocrinology.
Presentation: A version of our study was presented as a poster presentation at the Endocrine Society Annual Meeting, Chicago, Illinois, 17-20 March 2018.
Additional Information
Current Affiliation: S.G.’s current affiliation is the Baylor College of Medicine, Houston, Texas 77030.
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Glossary
Abbreviations:
- AUC
area under the curve
- BMI
body mass index
- FT4
free T4
- ROC
receiver operating characteristic; T, integer between 5 and 11
References and Notes
- 1. Lazar L, Frumkin RB, Battat E, Lebenthal Y, Phillip M, Meyerovitch J. Natural history of thyroid function tests over 5 years in a large pediatric cohort. J Clin Endocrinol Metab. 2009;94(5):1678–1682. [DOI] [PubMed] [Google Scholar]
- 2. Feller M, Snel M, Moutzouri E, Bauer DC, de Montmollin M, Aujesky D, Ford I, Gussekloo J, Kearney PM, Mooijaart S, Quinn T, Stott D, Westendorp R, Rodondi N, Dekkers OM. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320(13):1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodriguez-Gutierrez R, Maraka S, Ospina NS, Montori VM, Brito JP. Levothyroxine overuse: time for an about face? Lancet Diabetes Endocrinol. 2017;5(4):246–248. [DOI] [PubMed] [Google Scholar]
- 4. Wasniewska M, Salerno M, Cassio A, Corrias A, Aversa T, Zirilli G, Capalbo D, Bal M, Mussa A, De Luca F. Prospective evaluation of the natural course of idiopathic subclinical hypothyroidism in childhood and adolescence. Eur J Endocrinol. 2009;160(3):417–421. [DOI] [PubMed] [Google Scholar]
- 5. Salerno M, Capalbo D, Cerbone M, De Luca F. Subclinical hypothyroidism in childhood—current knowledge and open issues. Nat Rev Endocrinol. 2016;12(12):734–746. [DOI] [PubMed] [Google Scholar]
- 6. Monzani A, Prodam F, Rapa A, Moia S, Agarla V, Bellone S, Bona G. Endocrine disorders in childhood and adolescence: natural history of subclinical hypothyroidism in children and adolescents and potential effects of replacement therapy: a review. Eur J Endocrinol. 2012;168(1):R1–R11. [DOI] [PubMed] [Google Scholar]
- 7. Calebiro D, Gelmini G, Cordella D, Bonomi M, Winkler F, Biebermann H, de Marco A, Marelli F, Libri DV, Antonica F, Vigone MC, Cappa M, Mian C, Sartorio A, Beck-Peccoz P, Radetti G, Weber G, Persani L. Frequent TSH receptor genetic alterations with variable signaling impairment in a large series of children with nonautoimmune isolated hyperthyrotropinemia. J Clin Endocrinol Metab. 2012;97(1):E156–E160. [DOI] [PubMed] [Google Scholar]
- 8. Tenenbaum-Rakover Y, Almashanu S, Hess O, Admoni O, Hag-Dahood Mahameed A, Schwartz N, Allon-Shalev S, Bercovich D, Refetoff S. Long-term outcome of loss-of-function mutations in thyrotropin receptor gene. Thyroid. 2015;25(3):292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534. [DOI] [PubMed] [Google Scholar]
- 10. Bongers-Schokking JJ, Resing WCM, Oostdijk W, de Rijke YB, de Muinck Keizer-Schrama SMPF. Relation between early over- and undertreatment and behavioural problems in preadolescent children with congenital hypothyroidism. Horm Res Paediatr. 2018;90(4):247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rose SR. Improved diagnosis of mild hypothyroidism using time-of-day normal ranges for thyrotropin. J Pediatr. 2010;157(4):662–667, 667.e1. [DOI] [PubMed] [Google Scholar]
- 12. Vernacchio L, Muto JM, Young G, Risko W. Ambulatory subspecialty visits in a large pediatric primary care network. Health Serv Res. 2012;47(4):1755–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forrest CB, Glade GB, Baker AE, Bocian AB, Kang M, Starfield B. The pediatric primary-specialty care interface: how pediatricians refer children and adolescents to specialty care. Arch Pediatr Adolesc Med. 1999;153(7):705–714. [DOI] [PubMed] [Google Scholar]
- 14. Cornell E, Chandhok L, Rubin K. Implementation of referral guidelines at the interface between pediatric primary and subspecialty care. Healthc (Amst). 2015;3(2):74–79. [DOI] [PubMed] [Google Scholar]
- 15. Akbari A, Mayhew A, Al-Alawi MA, Grimshaw J, Winkens R, Glidewell E, Pritchard C, Thomas R, Fraser C. Interventions to improve outpatient referrals from primary care to secondary care. Cochrane Database Syst Rev. 2008; (4):CD005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baş VN, Aycan Z, Ağladıoğlu SY, Kendirci HN. Prevalence of hyperthyrotropinemia in obese children before and after weight loss. Eat Weight Disord. 2013;18(1):87–90. [DOI] [PubMed] [Google Scholar]
- 17. Reinehr T. Thyroid function in the nutritionally obese child and adolescent. Curr Opin Pediatr. 2011;23(4):415–420. [DOI] [PubMed] [Google Scholar]
- 18. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, Yanovski JA. Pediatric obesity—assessment, treatment, and prevention: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(3):709–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chogle A, Saps M. Yield and cost of performing screening tests for constipation in children. Can J Gastroenterol. 2013;27(12):e35–e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3(2):76–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S; American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kambalapalli M, Gupta A, Prasad UR, Francis GL. Ultrasound characteristics of the thyroid in children and adolescents with goiter: a single center experience. Thyroid. 2015;25(2):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Önsesveren I, Barjaktarovic M, Chaker L, de Rijke YB, Jaddoe VWV, van Santen HM, Visser TJ, Peeters RP, Korevaar TIM. Childhood thyroid function reference ranges and determinants: a literature overview and a prospective cohort study. Thyroid. 2017;27(11):1360–1369. [DOI] [PubMed] [Google Scholar]
- 25. Bailey D, Colantonio D, Kyriakopoulou L, Cohen AH, Chan MK, Armbruster D, Adeli K. Marked biological variance in endocrine and biochemical markers in childhood: establishment of pediatric reference intervals using healthy community children from the CALIPER cohort. Clin Chem. 2013;59(9):1393–1405. [DOI] [PubMed] [Google Scholar]