Abstract
A critical contributor to the health consequences of the obesity epidemic is dysregulated adipose tissue (AT) homeostasis. While white, brown, and beige AT function is altered in obesity-related disease, white AT is marked by progressive inflammation and adipocyte dysfunction and has been the focus of extensive “immunometabolism” research in the past decade. The exact triggering events initiating and sustaining AT inflammation are still under study, but it has been shown that reducing inflammation improves insulin action in AT. Scientific efforts seeking interventions to mitigate obesity-associated AT inflammation continue, and many groups are now determining how lean healthy AT homeostasis is maintained in order to leverage these mechanisms as therapeutic targets. Such studies have revealed that an elaborate network of immune cells, cytokines, and other cellular mediators coordinate AT function. Recent studies elucidated the involvement of the innate immune system in AT homeostasis (e.g., beiging and insulin sensitivity), including M2-like macrophages, eosinophils, innate lymphoid type 2 cells, and several others. In this review, we summarize the existing literature on innate type 2 inflammation in AT; additionally, we draw attention to areas of debate where seemingly conflicting data promises to yield more surprising and elegant biology as studies continue to dissect AT physiology.
Keywords: obesity, adipose tissue, innate immune system, macrophage, eosinophil, innate lymphoid type 2 cell, beiging, homeostasis
ROLE OF ADIPOSE TISSUE IN SYSTEMIC ENERGY HOMEOSTASIS
Due to the epidemic rise in obesity rates, there has been renewed interest in understanding and manipulating energy storage and energy expenditure, with the hope that these systems could be harnessed to promote weight loss. As of 2016, not a single state in the US had an obesity rate less than 20% (1). The most recent data report that ∼70% of the American population is overweight (BMI ≥25 kg/m2) and ∼35% is obese (BMI ≥30 kg/m2) (2, 3). The need to reduce obesity stems from the increased risk of comorbidities such as cardiovascular disease, type 2 diabetes, asthma, certain cancers, and various other diseases (4–6). A report compiled of 57 prospective analyses of nearly a million adults showed that obesity can decrease a person’s life span up to 10 years (7). Thus, a better understanding of adipose tissue (AT), the organ that expands the most in size during obesity, should provide solutions to this health concern.
When discussing AT, white AT (WAT) is most commonly considered; however, other types of AT, brown AT (BAT) and beige AT, also exist. Adipocytes in WAT and BAT are generated from unique progenitors. WAT stores excess energy in the form of triglycerides. BAT can store small amounts of triglycerides; however, its primary role is to burn fatty acids to generate heat. Beige fat has brown-like properties, but is transiently present in WAT depots (especially subcutaneous) upon β-adrenergic stimulation. Regulation of all three of these fat depots is critically important for lipid and energy homeostasis.
WAT consists of spatially distinct beds: visceral, omental, subcutaneous, perivascular, epicardial, and many others. These depots serve to cushion the organs they encompass, store excess lipids so as to protect other organs from ectopic lipotoxic lipid deposition, provide fatty acids as a fuel source locally and systemically, store toxins (8), and secrete adipokines with wide-ranging autocrine, paracrine, and endocrine functions. Recent work suggests that AT may even serve as a long-term reservoir for educated lymphocytes to respond to subsequent infections (9).
BAT is localized primarily in subscapular regions in rodents, and was only discovered in adult humans in the past decade (10, 11). BAT has a characteristic brown appearance grossly, and is easily distinguished from WAT. This distinctive color is due to the presence of concentrated mitochondria within each brown adipocyte, accompanied by high iron concentrations required for activation of the electron transport chain during β-oxidation. High expression of uncoupling protein 1 (UCP-1) causes this β-oxidation to result in generation of heat rather than ATP (thermogenesis), thus assisting to maintain core body temperatures upon cold-induced activation of the sympathetic nervous system (12).
Beige fat, primarily found intercalated within subcutaneous WAT, also has a characteristic darker appearance, UCP-1 expression, and fuel burning phenotype (12). Beiging, or browning as it is sometimes called, is favorable during obesity because it decreases weight and inflammation, while improving metabolic functions such as insulin sensitivity and glucose tolerance (13, 14). The presence of beige fat is most notably induced by both acute and prolonged cold exposure, and is increased in humans during the colder winter months (15). Caloric restriction was also shown to induce beiging in obese mice (16), but a recent study indicates that this mechanism may not be conserved in humans (17); or at the very least, that there is greater variability of beiging capacity among humans. Additionally, exercise has been shown in multiple studies to increase beige, or “brite” (brown-white), adipocyte characteristics (18–21). Aside from physiological stimuli, pharmacological stimulation with PPARγ agonists, such as rosiglitazone, or β-adrenergic stimulation with drugs, such as CL-316,243, can also stimulate beiging of WAT adipocytes to a thermogenic phenotype (22). Together WAT, BAT, and beige AT control systemic energy storage and expenditure.
One of the most surprising discoveries about AT was the accumulation of pro-inflammatory immune cells in WAT in obesity (23, 24). In the last few decades, it has come to light that the immune system is a key regulator of WAT homeostasis. Virtually all cells of the immune system have been observed in WAT and much of the literature (both primary and review articles) has focused on the pro-inflammatory cells of type 1 immune responses during the dysfunctional state of WAT in obesity (25–27). We will briefly highlight these core findings, but then explore in greater detail the less well-discussed type 2 immune interactions associated with lean healthy WAT that have come to the forefront of recent research in AT inflammation.
In addition to WAT, there is some evidence for immune cells contributing to BAT function. We showed that BAT contains macrophages, eosinophils, and B cells; however, based on both flow cytometry and histology, they are very scarce (28). In addition, we showed that none of these immune cells change with aging; yet during obesity, B cells increase and macrophages and eosinophils decrease in BAT. Other groups have also shown the presence of macrophages (29–31), eosinophils (31, 32), T cells (31), regulatory T cells (33), natural killer T (NKT) cells (31), and γδ T cells in BAT (31). In the most thorough study of BAT macrophages, Wolf et al. (34) demonstrated evidence for their role in tissue innervation and energy expenditure. Despite these studies, much work needs to be done to determine the extent to which immune cells contribute to BAT function.
A BRIEF SUMMARY OF PRO-INFLAMMATORY TYPE 1 INFLAMMATION IN AT
Macrophages were discovered to be central players in AT homeostasis in the last few decades. Localization of macrophages to WAT was reported as early as 1988, but the difference in lean versus obese macrophage populations was not appreciated until 2003 when Weisberg et al. (24) and Xu et al. (23) showed a more inflammatory profile of obese WAT macrophages. These studies showed that WAT macrophage accumulation, and their inflammatory activation, positively correlated with weight gain and adipocyte size. Furthermore, WAT macrophage accumulation was noted in genetic models of obesity and in diet-induced obesity. The increase in WAT macrophages during obesity was also seen in humans (24). The studies in mice showed that during obesity, WAT macrophage inflammatory gene expression preceded rising circulating insulin levels and that treatment with a known insulin-sensitizing agent (rosiglitazone) reduced macrophage markers (i.e., MAC-1, F4/80, CD68) in WAT (23). Mechanisms by which macrophages accumulate in WAT in the chronic setting of obesity include recruitment, retention (35), proliferation (36, 37), and reduced apoptotic turnover (38, 39). The reasons for their accrual are incompletely understood, but likely include hypoxia-induced adipocyte death and chemokine signaling. It should also be noted that acute pro-inflammatory signaling in WAT has been shown to be an adaptive response that enables proper storage of excess energy (40). This vast literature is nicely covered in a recent review written by McLaughlin et al. (41).
Macrophages are highly plastic and can switch between pro- and anti-inflammatory phenotypes depending on environmental cues, in what has been termed macrophage polarization (42). Classically activated M1-like macrophages are defined by their in vitro stimulation with lipopolysaccharide and/or interferon-γ (IFN-γ) and are subsequently more pro-inflammatory, expressing cytokines, such as TNF-α and interleukin (IL)-1β, and are associated with clearing bacterial pathogens. Alternatively activated M2-like macrophages are defined by in vitro stimulation with IL-4 and IL-13 and are subsequently more anti-inflammatory, expressing cytokines, such as transforming growth factor β (TGF-β) and IL-10, and are associated with wound healing and tissue homeostasis. Anti-inflammatory M2-like macrophages are the predominant cells in lean AT, while pro-inflammatory M1-like macrophages greatly outnumber M2s in the obese state (43). In addition, recent studies suggest that M1-like macrophages in AT have a slightly different phenotype than lipopolysaccharide-stimulated macrophages and, instead, reflect a state of metabolic activation (saturated fatty acids + glucose + insulin); and are referred to as metabolically active macrophages (44). This study highlights that the M1 and M2 terminology and the associated phenotypes and gene expression originally derived from in vitro polarization studies often do not correspond precisely with macrophages in vivo; thus it is becoming standard in the field to examine a variety of markers to determine the polarization state of any given population of macrophages.
Other immune cells, such as cytotoxic CD8 T cells (45, 46), CD4 type 1 helper T cells (47), natural killer (NK) cells (48, 49), and B cells (50, 51), contribute to an overall type 1 immune phenotype in obesity. Concomitantly, there is a reduction in the ratio of type 2 immune cells, such as M2-polarized macrophages (43), regulatory T cells (52), CD4 type 2 helper T cells (53), regulatory B cells (54), invariant NKT (iNKT) cells (55, 56), eosinophils (32), and innate lymphoid type 2 cells (ILC2s), to the pro-inflammatory cells (57). This has led investigators to begin interrogating a role for type 2 immunity in maintaining homeostasis of lean AT.
The majority of what is known about AT inflammation was discovered in mouse studies; as such, there is some uncertainty as to how well these findings apply to human AT. Supporting a conserved mechanism of AT inflammation in humans, Weisberg et al. (24) showed that the percent of AT macrophages correlated with adipocyte size to similar degrees in mouse and human subcutaneous fat. It is important to note that while AT macrophage number in humans also increases with obesity and/or insulin resistance, like mice, immunohistochemistry shows fewer total macrophages in human AT compared with what is typically seen in mouse AT (58, 59). However, genome-wide association studies identifying genes underlying obesity-associated metabolic disease, such as T2D, have strongly implicated genes relating to pancreatic β cells rather than inflammatory markers (60). While it is no surprise that genes of insulin-producing β cells would be identified in T2D, it has caused adipose biologists to question the role of obesity-associated AT inflammation in such metabolic diseases. Importantly, only ∼10% of T2D heritability is currently explained by genetic variants (60); thus, as remaining loci responsible for T2D heritability are discovered, it may be found that loci variation in tissues that contribute to T2D in less straightforward ways, such as AT and the immune system, are found to also contribute to susceptibility.
Despite the genome-wide association study argument against a role for AT or generalized inflammation in metabolic disease, the use of anti-inflammatory drugs in humans partially supports the case. There has been some progress made in understanding how drugs can modulate inflammation to improve obesity and/or its comorbidities, such as T2D and insulin resistance. The most commonly prescribed drug for T2D patients, metformin, improves insulin sensitivity and reduces hepatic glucose production, and has also been shown to reduce inflammatory markers (61, 62). Yet it is unclear whether the reduced inflammation was required for the metabolic benefits seen in humans, and furthermore, whether resolving AT inflammation in particular is necessary. Examining the role of AT in the effect of anti-diabetic drugs on patients, Di Gregorio et al. (58) found that a 10 week treatment with pioglitazone improved insulin sensitivity by 60% and reduced AT gene expression of macrophage markers, CD68 and MCP-1, and the number of AT CD68+ macrophages; the reduced inflammation was not seen in muscle. In the same study, the same duration of treatment with metformin did not recapitulate the results achieved with pioglitazone (58). These results suggest that reduced inflammation in AT may partially confer the metabolic benefits of some, but not all, anti-diabetic drugs. The CANTOS trial targeted IL-1β in an attempt to reduce cardiovascular disease. While myocardial infarction was reduced by 15%, secondary effects of IL-1β blockade on insulin sensitivity and secretion have not been as promising, showing some trends but not statistically significant improvements (63–65). This may be due to the targeting of a single inflammatory IL, whereas obesity increases a multitude of inflammatory markers. In contrast, the anti-inflammatory, salicylate (nonacetylated form of aspirin), was shown to reduce glycemia, HbA1c, and inflammatory markers in a 48 week trial (66). A recent study showed that the anti-inflammatory drug, amlexanox, previously used to treat allergic conditions, reduced HbA1c in obese and T2D patients (67). Furthermore, a subset of patients also had improved insulin sensitivity and reduced hepatic steatosis. While not all patients received every benefit, the best responders had higher subcutaneous AT inflammation at baseline before treatment (67). This study suggests that while not all patients will have AT inflammation at the root of their metabolic impairments, a subset that does have preexisting AT inflammiton can gain metabolic benefits from anti-inflammatory treatments. Nevertheless, more human studies of AT inflammation are needed to fully understand its contribution to obesity-associated diseases. Specifically regarding type 2 inflammation, at least a few groups are exploring its role in humans. For example, Lynch et al. (56) found that iNKT cells are reduced in both mouse and human obesity, and alleviating obesity restored AT iNKT cells. Furthermore, a study by Zhu et al. (68) showed that peripheral eosinophils in Chinese adults inversely correlated with T2D and insulin resistance, indicating a potential protective role of type 2 inflammation in metabolic disease, although this study did not specifically look at AT eosinophils.
COUNTERPOINT TO THE PRO-INFLAMMATORY AT MACROPHAGE: THE M2-LIKE MACROPHAGE AND ITS REGULATORY ROLES IN AT HOMEOSTASIS
As described above, the initial postulates surrounding a role for type 2 immune cells in WAT focused on M2-like polarized macrophages. As macrophages are the predominant immune cell type in both lean and obese WAT, this focus has been warranted. M2-like macrophages are traditionally associated with wound healing, but investigators have considered their anti-inflammatory phenotype to promote appropriate glucose and lipid control in WAT as well (43). The M2-like phenotype of WAT macrophages is sustained through transcriptional activators, such as PPARs, microRNAs (miRs), such as miR-330-5p (69), adipose-derived stem cells (70), and cytokines, such as IL-4 and IL-13 (32, 71–74). Due to their proximity, M2-like macrophages are thought to interact with adipocytes, and may play roles in apoptotic clearance, angiogenesis, WAT development, antigen presentation, and inflammatory resolution [reviewed in (26, 75)].
More recent studies have pointed to additional roles for M2-like macrophages in WAT homeostasis. For example, an emerging literature suggests that M2-like macrophages have an iron handling phenotype (76, 77). Interestingly, CD163, the haptoglobin-hemoglobin receptor, has long been used as a marker for M2-macrophages. In studies of human atherosclerosis, CD163+ macrophages were found in hemorrhagic plaques and were iron-enriched (78–80). Our laboratory has shown a similar population of highly M2-polarized macrophages in WAT that have a 2-fold increase in iron content and iron-related gene expression (81, 82). Because adipocyte iron-overload can lead to insulin resistance (83), proper iron handling by M2-like macrophages may account for part of their contribution to WAT homeostasis.
Exciting work by Ferrante and colleagues demonstrates a noninflammatory function of WAT macrophages in lipid trafficking (84). They show that, in obesity, AT macrophages upregulate a program of lysosome biogenesis that is linked to lipid content and catabolism. Furthermore, inhibiting lysosomal functions increased lipid accumulation in macrophages and reduced the lipolytic functions of adipocytes. In another recent study, Wilson et al. (85) demonstrate that deficiency of macrophage neuropili-1 results in impaired fatty acid uptake and oxidation, resulting in an exaggerated obese glucose-intolerant phenotype in mice. Overall, these data suggest a role for resident macrophages in WAT homeostasis by controlling lipid turnover.
Emerging evidence also suggests a homeostatic role for M2 macrophages via secretion of extracellular vesicles (EVs) that carry various cargoes, such as adipokines and miRNAs, which impact adipocytes and whole-body insulin action. Several recent studies have demonstrated that EVs secreted from WAT have systemic functions and contribute to the cross-talk between adipocytes and macrophages [(86, 87) and reviewed in (88)]. EVs derived from lean versus obese human WAT have at least 55 differentially expressed miRNAs (89). Although macrophage-derived exosomes have been studied most extensively in the context of tumor-associated macrophages; it is possible that changes in overall WAT exosome-miRNA profiles in lean compared with obese samples partially derive from M2 versus M1 macrophages in the AT. With regard to miRNA released from WAT macrophages, miR-155 is upregulated in M1-like macrophages and seems to control macrophage inflammatory phenotype (90). miR-330-5p is also inflammatory, and its inhibition promotes M2 polarization (69).
These are a few examples of alternative roles for macrophages in regulating WAT homeostasis, and future studies are sure to reveal additional functions for these regulatory cells, in particular with the various cargoes that exosomes from M2-like macrophages might deliver.
AN EMERGING PARADIGM OF ADIPOSE TYPE 2 INFLAMMATION: AN AXIS OF ILC2 CELLS, EOSINOPHILS, MACROPHAGES, AND WAT BEIGING
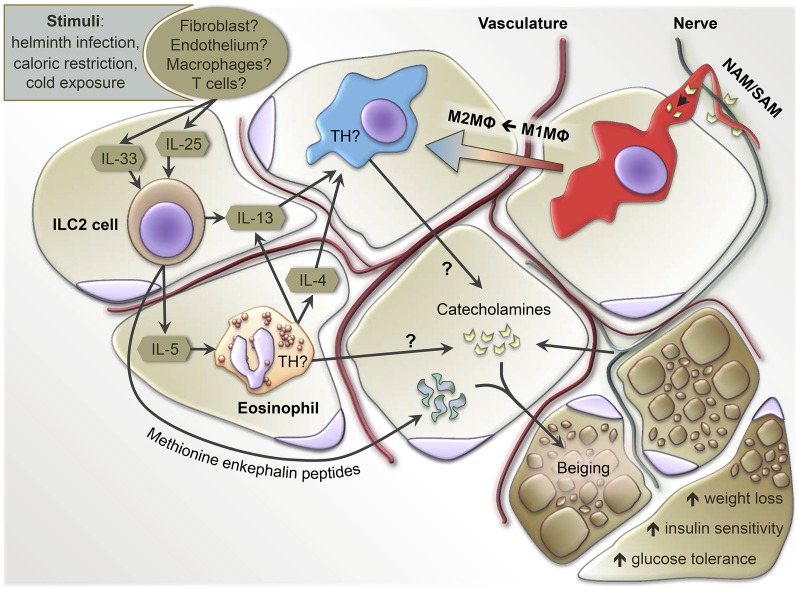
Due to the abundance of M2-like macrophages in lean functional AT, investigators have sought to understand what other type 2 immune cells may be present, and what their role is in sustaining an anti-inflammatory state. A variety of T cell subsets were discovered to influence macrophage inflammatory state and, subsequently, WAT function, which we will not discuss here as it is nicely summarized in a review by Winer and Winer (91). Another more recently identified mediator of WAT macrophage polarization and WAT function is the eosinophil, observed for the first time in AT in 2011 (32). Eosinophils are classically associated with parasitic infections and allergic diseases like asthma (92), but are more recently acknowledged for potential roles to regulate “local immunity and/or remodeling/repair in both health and disease” (Ref. 93; p.563). Initial studies in WAT showed eosinophils associated with lean healthy WAT, and that eosinophils promoted M2-like polarization of WAT macrophages (32, 94, 95). Wu et al. (32) were the first to show that WAT eosinophils decline with weight gain in association with increased AT M1-like macrophages and metabolic impairments. Interestingly, mouse models with systemically increased eosinophils were impervious to high-fat diet (HFD)-induced obesity and insulin resistance, while eosinophil-deficient mice were more vulnerable to the onset of insulin resistance (32). Molofsky et al. (94) further elucidated that WAT eosinophil numbers are maintained by IL-5, which is largely secreted by local ILC2s. Building upon these initial findings, Hams et al. (95) showed that body weight and glucose control are improved by ILC2 and NKT cells in a model of HFD-induced obesity, attributed to the accumulation of eosinophils and M2-like macrophages in visceral WAT. Aiding in maintaining an anti-inflammatory state of WAT, resident eosinophils were shown to express IL-4 and ILC2s to express IL-13 (32, 94). Published simultaneously, Rao et al. (73) and Qiu et al. (74) provided evidence that, upon cold-exposure, eosinophils could induce WAT beiging by polarizing M2-like macrophages. These combined studies suggested that ILC2s, eosinophils, and M2-like macrophages influence metabolic health through the regulation of AT inflammation, beiging capacity, and insulin resistance by facilitating a network of immune cell interactions (Fig. 1). In the simplest terms, AT M2-like macrophages, eosinophils, and ILC2s positively correlated with AT health.
Fig. 1.
Summary of known and potential innate type 2 immune cell interactions in the regulation of AT homeostasis. Stimuli, such as helminth infection, caloric restriction, or cold exposure, can induce the expression of IL-33/IL-25 to drive ILC2 numbers and activation. ILC2s secrete IL-5 to increase eosinophil numbers, which, in concert with one another, produce IL-13 and/or IL-4 to maintain M2-like macrophages (M2MΦ) or polarize M1-like macrophages (M1MΦ) into M2 macrophages. While innervating nerves are classically understood as the primary source of AT catecholamines, controversial data suggests that M2 macrophages and eosinophils may also supply catecholamines via expression of TH. Sympathetic NAMs/SAMs tightly align with AT nerves and can both uptake and catabolize nerve-derived catecholamines, reducing the available pool. Regardless of the source, catecholamines induce a thermogenic active state in adipocytes of WAT known as beiging. Alternatively, ILC2s have been shown to induce beiging via methionine enkephalin peptides, independent of eosinophils and M2 macrophages.
AN EVOLUTION OF THE ADIPOSE INNATE TYPE 2 INFLAMMATION PARADIGM: CAVEATS AND CONTROVERSIES
While the few reports mentioned above helped to formulate an initial idea of how a mechanism of innate type 2 inflammation may occur in WAT, a series of studies followed that have either supported, further refined, or, in some cases, refuted those initial findings. Given the recent discovery of these innate type 2 cellular mediators in WAT, the scientific rigor of consecutive studies by multiple groups has continued to shape this new emerging paradigm.
Macrophages in WAT type 2 inflammation: caveats and controversies
There has been a great deal of discussion within the immunometabolism field around work showing that IL-4/IL-13 secreted by WAT eosinophils induced macrophage expression of tyrosine hydroxylase (TH). In the first two publications on this topic, the authors suggested that macrophage TH was required for catecholamine production that resulted in beiging of subcutaneous WAT (74, 96). These findings were disputed in a multi-laboratory report providing strong evidence that WAT macrophages cannot express TH and, therefore, cannot produce catecholamines to beige WAT (97), which called into question the upstream role of eosinophils in beiging as well. Additional studies have provided further insight by revealing the presence of nerve-associated macrophages (NAMs)/sympathetic NAMs (SAMs) that line TH-producing sympathetic nerves within WAT (98, 99). It was found that the NAMs/SAMs could collect and catabolize catecholamines secreted by nerves, and in this way modulate the available pool of catecholamines that could otherwise act on proximal adipocytes (Fig. 1). While it is not yet known whether eosinophils interact with these NAMs/SAMs, it appears that these specialized macrophages indirectly adjust the catecholamine reserves responsible for beiging WAT. Another potential explanation that would require WAT macrophages for beiging, but still allow for WAT nerve-derived catecholamines, could be similar to what was discovered in BAT in the Wolf et al. (34) study, in which macrophages were required for proper BAT nerve innervation. Thus, without functional macrophages, it is possible that WAT nerve innervation would be impaired and catecholamines would not be produced under beiging stimuli.
Macrophages have the capacity to regulate beiging of WAT, yet the mechanism is still debatable because it is unknown which WAT cells exclusively express the TH required to produce catecholamines that enact beiging (Fig. 1). Fewer studies have attempted to measure TH directly in WAT macrophages, compared with whole WAT levels. Of those that have tested for WAT macrophage TH under a variety of conditions, four studies indicated detectable levels (70, 74, 96, 100), while four studies equally rigorously showed that WAT macrophages do not express TH (97–99, 101). Authors of one study supporting macrophage TH expression interpreted a positive TH result based on selective depletion of TH in myeloid cells with the LysMcre mouse (74); however, a subsequent publication has shown heavy neuron and nerve fiber expression of the lysozyme 2 (lyz2) promoter (102) and, thus, TH would likely also be removed from such cell types, potentially confounding the otherwise assumed selective manipulation of macrophage-derived versus nerve-derived TH. To add yet another layer of complexity, another WAT myeloid cell has been identified as a source of catecholamines: the eosinophil. Work by Withers et al. (103) provided evidence that eosinophils can induce the anti-contractile effect of perivascular AT (PVAT) on surrounding vasculature via catecholamines. They showed that vessels with PVAT from ΔdblGATA mice (Gata1 mutation mouse line) lacking eosinophils were more constricted upon stimulation than vessels with normal PVAT. However, reconstitution of purified eosinophils to ΔdblGATA PVAT vessels restored the relaxation effect. Furthermore, immunohistochemistry showed TH in eosinophils, and that preincubation of purified eosinophils with a TH inhibitor [α-methyl-p-tyrosine (AMPT)] impaired their relaxation effect on PVAT vessels. Lastly, they measured detectable levels of catecholamines (epinephrine, norepinephrine, and dopamine) produced by purified eosinophils in vitro. In light of the studies summarized above, this topic remains controversial and will need further study to delineate the exact source(s) of catecholamines required for beiging and other processes in WAT.
Eosinophils in WAT type 2 inflammation: caveats and controversies
The role of eosinophils in AT has now been examined in at least 30 different studies (14, 16, 31, 32, 72–74, 94, 95, 103–123), many of which are highlighted in Table 1. One of the most common readouts in examining the role of AT eosinophils has been whole-body glucose tolerance, tested in at least 21 of these works (14, 16, 32, 68, 73, 74, 94, 95, 103–106, 108, 110, 111, 113, 116, 117, 120, 122, 123). The first study to show improvements in glucose tolerance associated with increased WAT eosinophils used the IL-5 transgenic (IL-5tg) mouse (32). The primary caveat of this model is that this mouse line is known to be somewhat sickly, even at baseline, before any experimental manipulation. It is not too surprising then that IL-5tg mice did not gain as much weight as their wild-type counterparts when placed on a HFD (32). This is an issue because weight loss or a lack of weight gain, often regardless of mechanism or disease, can impart improved glucose tolerance and reduced AT inflammation (124, 125). Therefore, it is difficult to know whether the increased eosinophils in WAT were responsible for the reduced weight and improved glucose tolerance, or whether the weight of the mouse was influenced by the systemic increases in eosinophils that occur throughout the rest of the IL-5tg mouse, which then accounted for improved glucose homeostasis. Several studies have used alternative ways to increase WAT eosinophils, such as the parasitic infection model (14, 94, 104, 109, 111, 112). Yet again, the question must be asked of whether the weight loss simply occurred due to the mouse being energetically taxed while clearing an infection. One study used a honeybee extract, propolis, to increase WAT eosinophils and reported improved glucose tolerance (122). It must be acknowledged, however, that a parasitic infection most certainly modulates a number of other immune cells, and it is unknown how propolis influences other immune processes; for this reason, it is not possible to say that the increased eosinophils are definitively responsible for the improved glucose tolerance in such models, as they were nonspecific modulations of eosinophils. In our own studies, we found that despite specifically elevating numbers of WAT eosinophils in obese mice with either recombinant IL-5 (rIL-5) treatment or using CC motif chemokine receptor 2 (CCR2)-deficient mice, there was no improvement in glucose tolerance (72, 110, 123). Several studies have utilized IL-33 to increase ILC2s, eosinophils, and/or M2-like macrophages in WAT, often associated with weight loss and improved glucose control (31, 94, 106, 112, 115, 117, 119). However, one group noted that IL-33 administration resulted in diarrhea and reduced food intake (106), either of which could induce weight loss (and subsequent metabolic benefits) independent of modulating WAT immune cell populations. This is an example of a complication that all metabolic and weight studies attempt to avoid in order to conclusively assume that the improved metabolic readouts of the study are due to the proposed biological mechanism and not an unintended off-target induction of weight loss.
TABLE 1.
Comparative assessment of the role of eosinophils in at homeostasis and systemic metabolic health across multiple studies
| Metabolic Parameter | Experiment | Study Authors (Citation) | Definitive Role of Eosinophils | Rationale |
| Glucose tolerance | GTT | Wu et al., 2011 (32) | Yes | ΔdblGATA (eosinophil−/−) mice → impaired GTT on HFD |
| Wu et al., 2011 (32) | No | Sickly nature of IL-5tg mice may explain ameliorated weight gain and improved GTT | ||
| Molofsky et al., 2013 (94) | Yes | IL-5−/− (eosinophil−/−) mice → impaired GTT on HFD | ||
| Zhu et al., 2013 (68) | No | Eosinophils correlated with better GTT, but no causation | ||
| Hams et al., 2013 (95) | No | Glucose tolerance was not proportional to eosinophil number | ||
| Kitamura et al., 2013 (122) | No | Propolis → could target cells other than eosinophils | ||
| Berbudi et al., 2016 (111) | No | Litomosoides sigmodontis → also targets cells other than eosinophils | ||
| Bolus et al., 2017 (110) | No | ↑ Eosinophils via rIL-5 did not alter GTT | ||
| Lipid tolerance | LTT | Bolus et al., 2017 (110) | No | ↑ Eosinophils via rIL-5 did not alter LTT |
| Mixed-meal tolerance | MTT | Bolus et al., 2017 (110) | No | ↑ Eosinophils via rIL-5 did not alter MTT |
| Insulin sensitivity | AT insulin sensitivity: | Wu et al., 2011 (32) | Yes | ΔdblGATA mice → ↓ pAKT on HFD |
| pAKT signaling | Molofsky et al., 2013 (94) | Yes | IL-5−/− mice → impaired ITT on HFD | |
| ITT | Zhu et al., 2013 (68) | No | Eosinophils correlated with better HOMA-IR, but no causation shown | |
| HOMA-IR | Kitamura et al., 2013 (122) | No | Propolis → not specific to eosinophils | |
| Bolus et al., 2017 (110) | No | ↑ Eosinophils via rIL-5 did not alter pAKT | ||
| Weight gain | Fat mass | Wu et al., 2011 (32) | Yes | ΔdblGATA mice → ↑ fat mass on HFD |
| Body mass | Wu et al., 2011 (32) | No | Sickly nature of IL-5tg mice may explain ameliorated weight gain | |
| Molofsky et al., 2013 (94) | Yes | IL-5−/− mice → ↑ fat mass on HFD | ||
| Hams et al., 2013 (95) | No | Weight loss was not proportional to eosinophil # | ||
| Kitamura et al., 2013 (122) | No | Despite ↑ eosinophils → no Δ in weight | ||
| Satoh et al., 2013 (120) | No | Reduced weight in Trib1−/− mice correlates with low eosinophils → no causation | ||
| Berbudi et al., 2016 (111) | No | L. sigmodontis → not specific to eosinophils | ||
| Fabbiano et al., 2016 (16) | No | ↓ Weight from caloric restriction correlates with ↑ eosinophils → no causation | ||
| Bolus et al., 2017 (110) | No | ↑ Eosinophils via rIL-5 did not alter fat mass | ||
| Beiging | UCP-1 expression | Qiu et al., 2014 (74) | Yes | 4get-ΔdblGATA mice → reduced expression of AT UCP-1 during cold |
| Brestoff et al., 2015 (117) | No | ↑ UCP-1 expression not dependent on eosinophils → occurred with ILC2 alone | ||
| Rao et al., 2014 (73) | Yes | ΔdblGATA mice → ↓ UCP-1 when induced by Metrnl | ||
| Suarez-Zamorano et al., 2015 (116) | No | ↑ Eosinophils from microbiota depletion correlate with UCP-1 → no causation | ||
| Lee et al., 2015 (115) | No | ΔdblGATA mice still elicited beige progenitors upon IL-33 stimulation | ||
| ΔdblGATA mice have lower baseline beige progenitors | ||||
| Fabbiano et al., 2016 (16) | No | ↑ UCP-1 from caloric restriction correlates with ↑ eosinophils → but no causation | ||
| Ding et al., 2016 (31) | No | ↑ Eosinophils from rIL-33 correlate with UCP-1 → no causation | ||
| ILC2s were also ↑ with eosinophils → ILC2s can ↑ UCP-1 so it’s unclear which is responsible | ||||
| Bolus et al., 2017 (110) | No | ↑ Eosinophils via rIL-5 did not alter UCP-1 | ||
| Metabolic rate | EE | Molofsky et al., 2013 (94) | Yes | ΔdblGATA mice → ↓ EE on HFD |
| Bolus et al., 2017 (110) | No | ↑ Eosinophils via rIL-5 did not alter EE | ||
| Food intake/ locomotion | Mass eaten | Bolus et al., 2017 (110) | No | ↑ Eosinophils via rIL-5 did not alter food intake or movement |
| Line-breaks | ||||
| Inflammation | Type 2 cytokines | Wu et al., 2011 (32) | No | Sickly nature of IL-5tg mice may explain ameliorated weight gain → thus more M2 macrophages |
| M2-like macrophage polarization | Wu et al., 2011 (32) | No | IL-4/13−/− mouse model is not specific to eosinophils | |
| Molofsky et al., 2013 (94) | No | Rag2−/− × γC mice lack AT eosinophils and ILC2s → ILC2s can directly impact macrophages | ||
| Hams et al., 2013 (95) | No | M2 macrophages not proportional to eosinophil number | ||
| Kitamura et al., 2013 (122) | No | Propolis → not specific to eosinophils | ||
| M2 macrophages → some genes ↑ and some ↓ | ||||
| Satoh et al., 2013 (120) | No | Lack of M2 macrophages in Trib1−/− mice correlates with low eosinophils → but no causation | ||
| Bolus et al., 2015 (72) | No | ↑ Eosinophils in CCR2−/− mice correlate with M2 macrophages → no causation | ||
| Suarez-Zamorano et al., 2015 (116) | No | ↑ Eosinophils from microbiota depletion correlate with M2 macrophages → no causation | ||
| Berbudi et al., 2016 (111) | No | L. sigmodontis → also targets cells other than eosinophils | ||
| Fabbiano et al., 2016 (16) | No | ↑ Type 2 cytokines and M2 macrophages from caloric restriction correlates with ↑ eosinophils → but no causation | ||
| Ding et al., 2016 (31) | No | ↓ Eosinophils did not reduce M2 macrophages, and in some cases, ↑ M2 macrophages | ||
| Qin et al., 2017 (126) | No | Eosinophils preincubated with oxidized-LDL polarize M2 macrophages to M1 macrophages | ||
| Bolus et al., 2017 (110) | No | ↑ Eosinophils via rIL-5 did not alter M2 macrophages | ||
| Adipose architecture | Perivascular | Gouon-Evans, Rothenberg, and Pollard, 2000 (127) | Yes | Eotaxin−/− mice → poor mammary branch formation |
| Mammary gland | Heredia et al., 2013 (128) | Yes | ΔdblGATA mice → impaired FAP proliferation and muscle regeneration | |
| ΔdblGATA mice → impaired anti-contractile perivascular function | ||||
| FAP muscle regeneration | Withers et al., 2017 (103) | Yes | Reconstituting eosinophils restored anti-contractile perivascular function | |
| GTT, glucose tolerance test; LTT, lipid tolerance test; MTT, mixed-meal tolerance test; ITT, insulin tolerance test; HOMA-IR, homeostatic model assessment of insulin resistance; EE, energy expenditure; CCR2, CC motif chemokine receptor 2; Eotaxin−/−, eotaxin null mouse line; FAP, fibro/adipocyte progenitor; pAKT, phosphorylated adenosine triphosphate; Rag2−/−, recombination activating gene 2 null mouse line; Trib1−/−, tribbles pseudokinase 1 null mouse line. | ||||
In contrast, models of eosinophil deficiency do seem to definitely support a role for eosinophils in maintaining glucose tolerance. In ΔdblGATA and IL-5−/− transgenic mouse lines, which have a near complete absence of eosinophils, HFD feeding resulted in greater glucose intolerance compared with wild-type mice (32, 94, 111). Thus, while it is difficult to be confident that increasing WAT eosinophils over baseline improves glucose tolerance, depleting eosinophils negatively impacts glucose tolerance in all published studies to date.
In addition to testing glucose tolerance, studies have also yielded mixed results on the role of eosinophils in WAT beiging. Several reports have data suggesting that eosinophils may be required for the WAT beiging in their models (16, 31, 73, 74, 115). While Van den Berg et al. (109) corroborated data that helminth stimulation elicited eosinophilia in epididymal AT and subcutaneous AT, they saw no associated beiging in epididymal AT and a very minimal indication of beiging in subcutaneous AT. Likewise, our own laboratory has published data showing that while WAT eosinophils increased with 48 h cold exposure, fat pads with further increased eosinophils by rIL-5 treatment had no indication of increased beiging capacity (110). In the same study, we found that increased WAT eosinophils from rIL-5 treatment yielded no improvements in lipid and mixed-meal tolerance, insulin sensitivity, weight gain, metabolic rate, food intake and locomotion, or general inflammation (Table 1).
The literature suggests that a minimum number of eosinophils are necessary for WAT homeostasis, but increasing eosinophils is not always sufficient to impart metabolic improvements (e.g., weight loss, glucose tolerance, insulin sensitivity, WAT beiging). A major caveat remains that the effect of depleting eosinophils post normal development has never been tested. To our knowledge, all eosinophil depletion models studying metabolic deficits have been carried out in genetically modified mice that lacked eosinophils throughout all of development. Therefore, it is unknown whether the greater impact of eosinophil depletion is pre or post in utero development. This underscores a potentially important area of AT biology that has yet to be explored.
ILC2s in WAT type 2 inflammation: caveats and controversies
To date, at least 10 primary research articles have examined the role of ILC2s in WAT homeostasis (14, 16, 31, 94, 95, 112, 113, 115, 117, 119). The initial studies proposed that IL-33/IL-25 upregulates WAT ILC2s that then recruit eosinophils via IL-5, which are capable of increasing M2-like macrophages and ultimately yielding better WAT homeostasis (94, 95, 119). While data supporting a role of ILC2s in WAT homeostasis grows stronger, it appears that their downstream target, the eosinophil, may not be required for the beneficial effects. A publication by Brestoff et al. (117) showed that while IL-33 can stimulate ILC2s to induce WAT beiging, their model was independent of eosinophils. Instead, their evidence showed that ILC2s act directly on adipocytes via methionine-enkephalin peptides to increase UCP-1. Likewise, a study by Hams et al. (14) used an RNase glycoprotein extracted from the Helminth egg to elicit weight loss and improve glucose tolerance associated with increased ILC2s, eosinophils, and M2-like macrophages. However, upon administering a deactivated form of the RNase, weight loss and glucose tolerance improvements were abolished along with inhibition of increased ILC2s and M2-like macrophages, despite eosinophils remaining elevated (14). Thus, the elevated eosinophils in this model using a helminth extract were not necessary or sufficient to polarize macrophages and improve WAT homeostasis. In fact, the RNase was able to act directly on adipocytes and macrophages via the mannose receptor, CD206, to impart metabolic improvements (i.e., WAT beiging) (14).
As is evident in the studies discussed above, a complication of most WAT ILC2 studies conducted is the concurrent rise in WAT eosinophils. Therefore, any mechanism increasing ILC2s or their activity may appear to improve WAT health via eosinophils because eosinophils often also increase under such circumstances, but the increased eosinophils may not be required and may rather be a secondary effect of altering ILC2s. Future studies could determine under what conditions (e.g., exercise, cold exposure, caloric restriction, etc.) ILC2s and eosinophils are both required or whether one cell type is sufficient to impart metabolic benefits. One study has begun more specifically probing this elusive mechanism by performing a selective transfer of only ILC2s and inducing beiging independent of eosinophils, IL-4 receptor signaling, or the adaptive immune system (117). More technical approaches like this, that manipulate a single cell type at a time, will be required to fully elucidate the key cell types necessary for innate type 2 regulation of WAT homeostasis.
CONCLUDING REMARKS ON INNATE TYPE 2 INFLAMMATION IN AT
AT is now recognized as much more than a simple storage site for excess energy. Rather, it is now appreciated as an endocrine organ capable of secreting hormones and cytokines that regulate both local and systemic functions. Dysregulated AT homeostasis impairs the functional output of various other tissues, largely through ectopic lipid storage and excess inflammation, leading to systemic metabolic consequences and disease (e.g., cardiovascular disease, type 2 diabetes, insulin resistance, glucose intolerance, certain cancers, infertility, respiratory issues, hypertension, etc.).
Decades of research endeavors have revealed that broad and diverse immune cell populations reside in AT and are integral to homeostatic preservation. Obesity is perhaps the most well-studied dysfunctional state of AT; during obesity, an existing type 2 anti-inflammatory network of immune cells gives way to an accrual of pro-inflammatory immune cells, which generate a type 1 cytokine cocktail that disrupts AT homeostasis. Despite extensive research of the pro-inflammatory state of AT in obesity, there has been a significant knowledge gap left as to how type 2 immune cells contribute to AT function under healthy lean conditions.
Recent research has shown that type 2 immune cells in WAT have diverse roles ranging from generating appropriate angiogenesis and clearance of apoptotic cells to regulating local iron stores and inducing thermally active energy-burning adipocytes. These homeostatic properties of WAT are micro-managed in part by cells of both the innate immune system (e.g., M2 macrophages, eosinophils, ILC2s, and iNKT cells) and the adaptive immune system (e.g., regulatory T cells, CD4 type 2 helper T cells, and B cells). This review has primarily highlighted the contribution of a portion of the innate type 2 immune cells, due to an intense drive of late to understand the axis of M2-like macrophages, eosinophils, and ILC2s in WAT homeostasis. We have attempted to emphasize the commendable efforts of the pioneers in this field, while still acknowledging caveats where they exist. Further study will elucidate the full extent to which innate type 2 immune cells influence AT homeostasis; ultimately yielding a greater basic understanding of human physiology with the promise of revealing potential therapeutic targets to tackle the healthcare burdens of today’s world.
Footnotes
Abbreviations:
- AT
- adipose tissue
- BAT
- brown adipose tissue
- ΔdblGATA
- Gata1 mutation mouse line
- EV
- extracellular vesicle
- HFD
- high-fat diet
- IL
- interleukin
- IL-5tg
- interleukin 5 transgenic
- ILC2 cell
- innate lymphoid type 2 cell
- iNKT
- invariant natural killer T
- miR
- microRNA
- NAM
- nerve-associated macrophage
- NK
- natural killer
- NKT
- natural killer T
- PVAT
- perivascular adipose tissue
- rIL-5
- recombinant interleukin 5
- SAM
- sympathetic nerve-associated macrophage
- TH
- tyrosine hydroxylase
- UCP-1
- uncoupling protein 1
- WAT
- white adipose tissue
This work was supported by Health Services Research and Development Grant 5I01BX002195 (A.H.H.) and American Diabetes Association Innovative Basic Science Award 1-17-IBS-140. The authors declare no conflicts of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2016. Behavioral Risk Factor Surveillance System. Accessed October 1, 2017, at http://www.cdc.gov/obesity/data/prevalence-maps.html.
- 2.Flegal K. M., Carroll M. D., Kit B. K., and Ogden C. L.. 2012. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 307: 491–497. [DOI] [PubMed] [Google Scholar]
- 3.Ogden C. L., Carroll M. D., Fryar C. D., and Flegal K. M.. 2015. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 219: 1–8. [PubMed] [Google Scholar]
- 4.Shoelson S. E., Herrero L., and Naaz A.. 2007. Obesity, inflammation, and insulin resistance. Gastroenterology. 132: 2169–2180. [DOI] [PubMed] [Google Scholar]
- 5.Stone T. W., McPherson M., and Gail Darlington L.. 2018. Obesity and cancer: existing and new hypotheses for a causal connection. EBioMedicine. 30: 14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalid F., and Holguin F.. 2018. A review of obesity and asthma across the life span. J. Asthma. 8: 1–15. [DOI] [PubMed] [Google Scholar]
- 7.Prospective Studies Collaboration, Whitlock G., Lewington S., Sherliker P., Clarke R., Emberson J., Halsey J., Qizilbash N., Collins R., and Peto R.. 2009. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 373: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson E., Shoemaker R., Larian N., and Cassis L.. 2017. Adipose tissue as a site of toxin accumulation. Compr. Physiol. 7: 1085–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han S. J., Glatman Zaretsky A., Andrade-Oliveira V., Collins N., Dzutsev A., Shaik J., Morais da Fonseca D., Harrison O. J., Tamoutounour S., Byrd A. L., et al. 2017. White adipose tissue is a reservoir for memory T cells and promotes protective memory responses to infection. Immunity. 47: 1154–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., et al. 2009. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoneshiro T., Aita S., Matsushita M., Okamatsu-Ogura Y., Kameya T., Kawai Y., Miyagawa M., Tsujisaki M., and Saito M.. 2011. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring). 19: 1755–1760. [DOI] [PubMed] [Google Scholar]
- 12.Rui L. 2017. Brown and beige adipose tissues in health and disease. Compr. Physiol. 7: 1281–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harms M., and Seale P.. 2013. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19: 1252–1263. [DOI] [PubMed] [Google Scholar]
- 14.Hams E., Bermingham R., Wurlod F. A., Hogan A. E., O’Shea D., Preston R. J., Rodewald H. R., McKenzie A. N., and Fallon P. G.. 2016. The helminth T2 RNase omega1 promotes metabolic homeostasis in an IL-33- and group 2 innate lymphoid cell-dependent mechanism. FASEB J. 30: 824–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kern P. A., Finlin B. S., Zhu B., Rasouli N., McGehee R. E. Jr., Westgate P. M., and Dupont-Versteegden E. E.. 2014. The effects of temperature and seasons on subcutaneous white adipose tissue in humans: evidence for thermogenic gene induction. J. Clin. Endocrinol. Metab. 99: E2772–E2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabbiano S., Suarez-Zamorano N., Rigo D., Veyrat-Durebex C., Stevanovic Dokic A., Colin D. J., and Trajkovski M.. 2016. Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metab. 24: 434–446. [DOI] [PubMed] [Google Scholar]
- 17.Barquissau V., Leger B., Beuzelin D., Martins F., Amri E. Z., Pisani D. F., Saris W. H. M., Astrup A., Maoret J. J., Iacovoni J., et al. 2018. Caloric restriction and diet-induced weight loss do not induce browning of human subcutaneous white adipose tissue in women and men with obesity. Cell Reports. 22: 1079–1089. [DOI] [PubMed] [Google Scholar]
- 18.Stanford K. I., Middelbeek R. J., Townsend K. L., Lee M. Y., Takahashi H., So K., Hitchcox K. M., Markan K. R., Hellbach K., Hirshman M. F., et al. 2015. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 64: 2002–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutherland L. N., Bomhof M. R., Capozzi L. C., Basaraba S. A., and Wright D. C.. 2009. Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue. J. Physiol. 587: 1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trevellin E., Scorzeto M., Olivieri M., Granzotto M., Valerio A., Tedesco L., Fabris R., Serra R., Quarta M., Reggiani C., et al. 2014. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes. 63: 2800–2811. [DOI] [PubMed] [Google Scholar]
- 21.Boström P., Wu J., Jedrychowski M. P., Korde A., Ye L., Lo J. C., Rasbach K. A., Bostrom E. A., Choi J. H., Long J. Z., et al. 2012. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 481: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrovic N., Walden T. B., Shabalina I. G., Timmons J. A., Cannon B., and Nedergaard J.. 2010. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285: 7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., Sole J., Nichols A., Ross J. S., Tartaglia L. A., et al. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., and Ferrante A. W. Jr.. 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumeng C. N., and Saltiel A. R.. 2011. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121: 2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill A. A., Reid Bolus W., and Hasty A. H.. 2014. A decade of progress in adipose tissue macrophage biology. Immunol. Rev. 262: 134–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant R. W., and Dixit V. D.. 2015. Adipose tissue as an immunological organ. Obesity (Silver Spring). 23: 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson K. R., Flaherty D. K., and Hasty A. H.. 2017. Obesity alters B cell and macrophage populations in brown adipose tissue. Obesity (Silver Spring). 25: 1881–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., et al. 2006. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116: 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinh C. H., Szabo A., Yu Y., Camer D., Zhang Q., Wang H., and Huang X. F.. 2015. Bardoxolone methyl prevents fat deposition and inflammation in brown adipose tissue and enhances sympathetic activity in mice fed a high-fat diet. Nutrients. 7: 4705–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding X., Luo Y., Zhang X., Zheng H., Yang X., Yang X., and Liu M.. 2016. IL-33-driven ILC2/eosinophil axis in fat is induced by sympathetic tone and suppressed by obesity. J. Endocrinol. 231: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu D., Molofsky A. B., Liang H. E., Ricardo-Gonzalez R. R., Jouihan H. A., Bando J. K., Chawla A., and Locksley R. M.. 2011. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 332: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medrikova D., Sijmonsma T. P., Sowodniok K., Richards D. M., Delacher M., Sticht C., Gretz N., Schafmeier T., Feuerer M., and Herzig S.. 2015. Brown adipose tissue harbors a distinct sub-population of regulatory T cells. PLoS One. 10: e0118534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf Y., Boura-Halfon S., Cortese N., Haimon Z., Sar Shalom H., Kuperman Y., Kalchenko V., Brandis A., David E., Segal-Hayoun Y., et al. 2017. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol. 18: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramkhelawon B., Hennessy E. J., Menager M., Ray T. D., Sheedy F. J., Hutchison S., Wanschel A., Oldebeken S., Geoffrion M., Spiro W., et al. 2014. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat. Med. 20: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamarron B. F., Mergian T. A., Cho K. W., Martinez-Santibanez G., Luan D., Singer K., DelProposto J. L., Geletka L. M., Muir L. A., and Lumeng C. N.. 2017. Macrophage proliferation sustains adipose tissue inflammation in formerly obese mice. Diabetes. 66: 392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braune J., Weyer U., Hobusch C., Mauer J., Bruning J. C., Bechmann I., and Gericke M.. 2017. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. J. Immunol. 198: 2927–2934. [DOI] [PubMed] [Google Scholar]
- 38.Hill A. A., Anderson-Baucum E. K., Kennedy A. J., Webb C. D., Yull F. E., and Hasty A. H.. 2015. Activation of NF-kappaB drives the enhanced survival of adipose tissue macrophages in an obesogenic environment. Mol. Metab. 4: 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muir L. A., Kiridena S., Griffin C., DelProposto J. B., Geletka L., Martinez-Santibañez G., Zamarron B. F., Lucas H., Singer K., O’Rourke R. W., et al. 2018. Frontline Science: Rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages. J. Leukoc. Biol. 103: 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wernstedt Asterholm I., Tao C., Morley T. S., Wang Q. A., Delgado-Lopez F., Wang Z. V., and Scherer P. E.. 2014. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 20: 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLaughlin T., Ackerman S. E., Shen L., and Engleman E.. 2017. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Invest. 127: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray P. J. 2017. Macrophage polarization. Annu. Rev. Physiol. 79: 541–566. [DOI] [PubMed] [Google Scholar]
- 43.Lumeng C. N., Bodzin J. L., and Saltiel A. R.. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kratz M., Coats B. R., Hisert K. B., Hagman D., Mutskov V., Peris E., Schoenfelt K. Q., Kuzma J. N., Larson I., Billing P. S., et al. 2014. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 20: 614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S., et al. 2009. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15: 914–920. [DOI] [PubMed] [Google Scholar]
- 46.Rausch M. E., Weisberg S., Vardhana P., and Tortoriello D. V.. 2008. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. (Lond.). 32: 451–463. [DOI] [PubMed] [Google Scholar]
- 47.Kintscher U., Hartge M., Hess K., Foryst-Ludwig A., Clemenz M., Wabitsch M., Fischer-Posovszky P., Barth T. F., Dragun D., Skurk T., et al. 2008. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler. Thromb. Vasc. Biol. 28: 1304–1310. [DOI] [PubMed] [Google Scholar]
- 48.Caspar-Bauguil S., Cousin B., Galinier A., Segafredo C., Nibbelink M., Andre M., Casteilla L., and Penicaud L.. 2005. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 579: 3487–3492. [DOI] [PubMed] [Google Scholar]
- 49.Wensveen F. M., Jelencic V., Valentic S., Sestan M., Wensveen T. T., Theurich S., Glasner A., Mendrila D., Stimac D., Wunderlich F. T., et al. 2015. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat. Immunol. 16: 376–385. [DOI] [PubMed] [Google Scholar]
- 50.Winer D. A., Winer S., Shen L., Wadia P. P., Yantha J., Paltser G., Tsui H., Wu P., Davidson M. G., Alonso M. N., et al. 2011. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17: 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duffaut C., Galitzky J., Lafontan M., and Bouloumie A.. 2009. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem. Biophys. Res. Commun. 384: 482–485. [DOI] [PubMed] [Google Scholar]
- 52.Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A., Lee J., Goldfine A. B., Benoist C., Shoelson S., et al. 2009. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winer S., Chan Y., Paltser G., Truong D., Tsui H., Bahrami J., Dorfman R., Wang Y., Zielenski J., Mastronardi F., et al. 2009. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 15: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishimura S., Manabe I., Takaki S., Nagasaki M., Otsu M., Yamashita H., Sugita J., Yoshimura K., Eto K., Komuro I., et al. 2013. Adipose natural regulatory B cells negatively control adipose tissue inflammation. Cell Metab. 18: 759–766. [DOI] [PubMed] [Google Scholar]
- 55.Lynch L., O’Shea D., Winter D. C., Geoghegan J., Doherty D. G., and O’Farrelly C.. 2009. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur. J. Immunol. 39: 1893–1901. [DOI] [PubMed] [Google Scholar]
- 56.Lynch L., Nowak M., Varghese B., Clark J., Hogan A. E., Toxavidis V., Balk S. P., O’Shea D., O’Farrelly C., and Exley M. A.. 2012. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 37: 574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moro K., Yamada T., Tanabe M., Takeuchi T., Ikawa T., Kawamoto H., Furusawa J., Ohtani M., Fujii H., and Koyasu S.. 2010. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 463: 540–544. [DOI] [PubMed] [Google Scholar]
- 58.Di Gregorio G. B., Yao-Borengasser A., Rasouli N., Varma V., Lu T., Miles L. M., Ranganathan G., Peterson C. A., McGehee R. E., and Kern P. A.. 2005. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 54: 2305–2313. [DOI] [PubMed] [Google Scholar]
- 59.Spencer M., Yao-Borengasser A., Unal R., Rasouli N., Gurley C. M., Zhu B., Peterson C. A., and Kern P. A.. 2010. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am. J. Physiol. Endocrinol. Metab. 299: E1016–E1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Billings L. K., and Florez J. C.. 2010. The genetics of type 2 diabetes: what have we learned from GWAS? Ann. N. Y. Acad. Sci. 1212: 59–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo T., Nocon A., Fry J., Sherban A., Rui X., Jiang B., Xu X. J., Han J., Yan Y., Yang Q., et al. 2016. AMPK activation by metformin suppresses abnormal extracellular matrix remodeling in adipose tissue and ameliorates insulin resistance in obesity. Diabetes. 65: 2295–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evia-Viscarra M. L., Rodea-Montero E. R., Apolinar-Jimenez E., Munoz-Noriega N., Garcia-Morales L. M., Leanos-Perez C., Figueroa-Barron M., Sanchez-Fierros D., and Reyes-Garcia J. G.. 2012. The effects of metformin on inflammatory mediators in obese adolescents with insulin resistance: controlled randomized clinical trial. J. Pediatr. Endocrinol. Metab. 25: 41–49. [DOI] [PubMed] [Google Scholar]
- 63.Ridker P. M., Everett B. M., Thuren T., MacFadyen J. G., Chang W. H., Ballantyne C., Fonseca F., Nicolau J., Koenig W., Anker S. D., et al. 2017. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- 64.Rissanen A., Howard C. P., Botha J., Thuren T., and Global I.. 2012. Effect of anti-IL-1beta antibody (canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: results of a randomized, placebo-controlled trial. Diabetes Obes. Metab. 14: 1088–1096. [DOI] [PubMed] [Google Scholar]
- 65.Choudhury R. P., Birks J. S., Mani V., Biasiolli L., Robson M. D., L’Allier P. L., Gingras M. A., Alie N., McLaughlin M. A., Basson C. T., et al. 2016. Arterial effects of canakinumab in patients with atherosclerosis and type 2 diabetes or glucose intolerance. J. Am. Coll. Cardiol. 68: 1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldfine A. B., Fonseca V., Jablonski K. A., Chen Y. D., Tipton L., Staten M. A., and Shoelson S. E.. 2013. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann. Intern. Med. 159: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oral E. A., Reilly S. M., Gomez A. V., Meral R., Butz L., Ajluni N., Chenevert T. L., Korytnaya E., Neidert A. H., Hench R., et al. 2017. Inhibition of IKKε and TBK1 improves glucose control in a subset of patients with type 2 diabetes. Cell Metab. 26: 157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu L., Su T., Xu M., Xu Y., Li M., Wang T., Sun J., Zhang J., Xu B., Lu J., et al. 2013. Eosinophil inversely associates with type 2 diabetes and insulin resistance in Chinese adults. PLoS One. 8: e67613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun J., Huang Q., Li S., Meng F., Li X., and Gong X.. 2018. miR-330-5p/Tim-3 axis regulates macrophage M2 polarization and insulin resistance in diabetes mice. Mol. Immunol. 95: 107–113. [DOI] [PubMed] [Google Scholar]
- 70.Zhao H., Shang Q., Pan Z., Bai Y., Li Z., Zhang H., Zhang Q., Guo C., Zhang L., and Wang Q.. 2018. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 67: 235–247. [DOI] [PubMed] [Google Scholar]
- 71.Kang K., Reilly S. M., Karabacak V., Gangl M. R., Fitzgerald K., Hatano B., and Lee C. H.. 2008. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 7: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bolus W. R., Gutierrez D. A., Kennedy A. J., Anderson-Baucum E. K., and Hasty A. H.. 2015. CCR2 deficiency leads to increased eosinophils, alternative macrophage activation, and type 2 cytokine expression in adipose tissue. J. Leukoc. Biol. 98: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rao R. R., Long J. Z., White J. P., Svensson K. J., Lou J., Lokurkar I., Jedrychowski M. P., Ruas J. L., Wrann C. D., Lo J. C., et al. 2014. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 157: 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu Y., Nguyen K. D., Odegaard J. I., Cui X., Tian X., Locksley R. M., Palmiter R. D., and Chawla A.. 2014. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 157: 1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas D., and Apovian C.. 2017. Macrophage functions in lean and obese adipose tissue. Metabolism. 72: 120–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hasty A. H., and Yvan-Charvet L.. 2013. Liver X receptor alpha-dependent iron handling in M2 macrophages: The missing link between cholesterol and intraplaque hemorrhage? Circ. Res. 113: 1182–1185. [DOI] [PubMed] [Google Scholar]
- 77.Corna G., Campana L., Pignatti E., Castiglioni A., Tagliafico E., Bosurgi L., Campanella A., Brunelli S., Manfredi A. A., Apostoli P., et al. 2010. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 95: 1814–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boyle J. J., Harrington H. A., Piper E., Elderfield K., Stark J., Landis R. C., and Haskard D. O.. 2009. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am. J. Pathol. 174: 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boyle J. J., Johns M., Kampfer T., Nguyen A. T., Game L., Schaer D. J., Mason J. C., and Haskard D. O.. 2012. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ. Res. 110: 20–33. [DOI] [PubMed] [Google Scholar]
- 80.Finn A. V., Nakano M., Polavarapu R., Karmali V., Saeed O., Zhao X., Yazdani S., Otsuka F., Davis T., Habib A., et al. 2012. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J. Am. Coll. Cardiol. 59: 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Orr J. S., Kennedy A., Anderson-Baucum E. K., Webb C. D., Fordahl S. C., Erikson K. M., Zhang Y., Etzerodt A., Moestrup S. K., and Hasty A. H.. 2014. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes. 63: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hubler M. J., Erikson K. M., Kennedy A. J., and Hasty A. H.. MFehi adipose tissue macrophages compensate for tissue iron perturbations in mice. Am. J. Physiol. Cell Physiol. Epub ahead of print. May 16, 2018; doi:10.1152/ajpcell.00103.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gabrielsen J. S., Gao Y., Simcox J. A., Huang J., Thorup D., Jones D., Cooksey R. C., Gabrielsen D., Adams T. D., Hunt S. C., et al. 2012. Adipocyte iron regulates adiponectin and insulin sensitivity. J. Clin. Invest. 122: 3529–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu X., Grijalva A., Skowronski A., van Eijk M., Serlie M. J., and Ferrante A. W. Jr.. 2013. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 18: 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilson A. M., Shao Z., Grenier V., Mawambo G., Daudelin J. F., Dejda A., Pilon F., Popovic N., Boulet S., Parinot C., et al. 2018. Neuropilin-1 expression in adipose tissue macrophages protects against obesity and metabolic syndrome. Sci. Immunol. 3: eaan4626. [DOI] [PubMed] [Google Scholar]
- 86.Kranendonk M. E., Visseren F. L., van Balkom B. W., Nolte-’t Hoen E. N., van Herwaarden J. A., de Jager W., Schipper H. S., Brenkman A. B., Verhaar M. C., Wauben M. H., et al. 2014. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring). 22: 1296–1308. [DOI] [PubMed] [Google Scholar]
- 87.Kranendonk M. E., Visseren F. L., van Herwaarden J. A., Nolte-’t Hoen E. N., de Jager W., Wauben M. H., and Kalkhoven E.. 2014. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity (Silver Spring). 22: 2216–2223. [DOI] [PubMed] [Google Scholar]
- 88.Gao X., Salomon C., and Freeman D. J.. 2017. Extracellular vesicles from adipose tissue-a potential role in obesity and type 2 diabetes? Front. Endocrinol. (Lausanne). 8: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferrante S. C., Nadler E. P., Pillai D. K., Hubal M. J., Wang Z., Wang J. M., Gordish-Dressman H., Koeck E., Sevilla S., Wiles A. A., et al. 2015. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr. Res. 77: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jablonski K. A., Gaudet A. D., Amici S. A., Popovich P. G., and Guerau-de-Arellano M.. 2016. Control of the inflammatory macrophage transcriptional signature by miR-155. PLoS One. 11: e0159724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Winer S., and Winer D. A.. 2012. The adaptive immune system as a fundamental regulator of adipose tissue inflammation and insulin resistance. Immunol. Cell Biol. 90: 755–762. [DOI] [PubMed] [Google Scholar]
- 92.Rothenberg M. E., and Hogan S. P.. 2006. The eosinophil. Annu. Rev. Immunol. 24: 147–174. [DOI] [PubMed] [Google Scholar]
- 93.Lee J. J., Jacobsen E. A., McGarry M. P., Schleimer R. P., and Lee N. A.. Eosinophils in health and disease: the LIAR hypothesis. 2010. Clin. Exp. Allergy. 40: 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Molofsky A. B., Nussbaum J. C., Liang H. E., Van Dyken S. J., Cheng L. E., Mohapatra A., Chawla A., and Locksley R. M.. 2013. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 210: 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hams E., Locksley R. M., McKenzie A. N., and Fallon P. G.. 2013. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J. Immunol. 191: 5349–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nguyen K. D., Qiu Y., Cui X., Goh Y. P., Mwangi J., David T., Mukundan L., Brombacher F., Locksley R. M., and Chawla A.. 2011. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 480: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fischer K., Ruiz H. H., Jhun K., Finan B., Oberlin D. J., van der Heide V., Kalinovich A. V., Petrovic N., Wolf Y., Clemmensen C., et al. 2017. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 23: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Camell C. D., Sander J., Spadaro O., Lee A., Nguyen K. Y., Wing A., Goldberg E. L., Youm Y. H., Brown C. W., Elsworth J., et al. 2017. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature. 550: 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pirzgalska R. M., Seixas E., Seidman J. S., Link V. M., Sanchez N. M., Mahu I., Mendes R., Gres V., Kubasova N., Morris I., et al. 2017. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23: 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luo Y., Liu B., Yang X., Ma X., Zhang X., Bragin D. E., Yang X. O., Huang W., and Liu M.. 2017. Myeloid adrenergic signaling via CaMKII forms a feedforward loop of catecholamine biosynthesis. J. Mol. Cell Biol. 9: 422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spadaro O., Camell C. D., Bosurgi L., Nguyen K. Y., Youm Y. H., Rothlin C. V., and Dixit V. D.. 2017. IGF1 shapes macrophage activation in response to immunometabolic challenge. Cell Reports. 19: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orthgiess J., Gericke M., Immig K., Schulz A., Hirrlinger J., Bechmann I., and Eilers J.. 2016. Neurons exhibit Lyz2 promoter activity in vivo: Implications for using LysM-Cre mice in myeloid cell research. Eur. J. Immunol. 46: 1529–1532. [DOI] [PubMed] [Google Scholar]
- 103.Withers S. B., Forman R., Meza-Perez S., Sorobetea D., Sitnik K., Hopwood T., Lawrence C. B., Agace W. W., Else K. J., Heagerty A. M., et al. 2017. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Sci. Rep. 7: 44571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hussaarts L., Garcia-Tardon N., van Beek L., Heemskerk M. M., Haeberlein S., van der Zon G. C., Ozir-Fazalalikhan A., Berbee J. F., Willems van Dijk K., van Harmelen V., et al. 2015. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. 29: 3027–3039. [DOI] [PubMed] [Google Scholar]
- 105.Srivastava N., Iyer S., Sudan R., Youngs C., Engelman R. W., Howard K. T., Russo C. M., Chisholm J. D., and Kerr W. G.. 2016. A small-molecule inhibitor of SHIP1 reverses age- and diet-associated obesity and metabolic syndrome. JCI Insight. 1: e88544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duffen J., Zhang M., Masek-Hammerman K., Nunez A., Brennan A., Jones J. E. C., Morin J., Nocka K., and Kasaian M.. 2018. Modulation of the IL-33/IL-13 axis in obesity by IL-13Rα2. J. Immunol. 200: 1347–1359. [DOI] [PubMed] [Google Scholar]
- 107.Huang Z., Zhong L., Lee J. T. H., Zhang J., Wu D., Geng L., Wang Y., Wong C. M., and Xu A.. 2017. The FGF21-CCL11 axis mediates beiging of white adipose tissues by coupling sympathetic nervous system to type 2 immunity. Cell Metab. 26: 498–508.e4. [DOI] [PubMed] [Google Scholar]
- 108.Rozenberg P., Reichman H., Zab-Bar I., Itan M., Pasmanik-Chor M., Bouffi C., Qimron U., Bachelet I., Fulkerson P. C., Rothenberg M. E., et al. 2017. CD300f:IL-5 cross-talk inhibits adipose tissue eosinophil homing and subsequent IL-4 production. Sci. Rep. 7: 5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van den Berg S. M., van Dam A. D., Kusters P. J. H., Beckers L., den Toom M., van der Velden S., Van den Bossche J., van Die I., Boon M. R., Rensen P. C. N., et al. 2017. Helminth antigens counteract a rapid high-fat diet-induced decrease in adipose tissue eosinophils. J. Mol. Endocrinol. 59: 245–255. [DOI] [PubMed] [Google Scholar]
- 110.Bolus W. R., Peterson K. R., Hubler M. J., Kennedy A. J., Gruen M. L., and Hasty A. H.. 2018. Elevating adipose eosinophils in obese mice to physiologically normal levels does not rescue metabolic impairments. Mol. Metab. 8: 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berbudi A., Surendar J., Ajendra J., Gondorf F., Schmidt D., Neumann A. L., Wardani A. P., Layland L. E., Hoffmann L. S., Pfeifer A., et al. 2016. Filarial infection or antigen administration improves glucose tolerance in diet-induced obese mice. J. Innate Immun. 8: 601–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Molofsky A. B., Van Gool F., Liang H. E., Van Dyken S. J., Nussbaum J. C., Lee J., Bluestone J. A., and Locksley R. M.. 2015. Interleukin-33 and interferon-γ counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity. 43: 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chang S. K., Kohlgruber A. C., Mizoguchi F., Michelet X., Wolf B. J., Wei K., Lee P. Y., Lynch L., Duquette D., Ceperuelo-Mallafre V., et al. 2017. Stromal cell cadherin-11 regulates adipose tissue inflammation and diabetes. J. Clin. Invest. 127: 3300–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karsten C. M., Wiese A. V., Mey F., Figge J., Woodruff T. M., Reuter T., Scurtu O., Kordowski A., Almeida L. N., Briukhovetska D., et al. 2017. Monitoring C5aR2 expression using a floxed tdTomato-C5aR2 knock-in mouse. J. Immunol. 199: 3234–3248. [DOI] [PubMed] [Google Scholar]
- 115.Lee M. W., Odegaard J. I., Mukundan L., Qiu Y., Molofsky A. B., Nussbaum J. C., Yun K., Locksley R. M., and Chawla A.. 2015. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 160: 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suárez-Zamorano N., Fabbiano S., Chevalier C., Stojanovic O., Colin D. J., Stevanovic A., Veyrat-Durebex C., Tarallo V., Rigo D., Germain S., et al. 2015. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat. Med. 21: 1497–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brestoff J. R., Kim B. S., Saenz S. A., Stine R. R., Monticelli L. A., Sonnenberg G. F., Thome J. J., Farber D. L., Lutfy K., Seale P., et al. 2015. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 519: 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Amano S. U., Cohen J. L., Vangala P., Tencerova M., Nicoloro S. M., Yawe J. C., Shen Y., Czech M. P., and Aouadi M.. 2014. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 19: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nussbaum J. C., Van Dyken S. J., von Moltke J., Cheng L. E., Mohapatra A., Molofsky A. B., Thornton E. E., Krummel M. F., Chawla A., Liang H. E., et al. 2013. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 502: 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Satoh T., Kidoya H., Naito H., Yamamoto M., Takemura N., Nakagawa K., Yoshioka Y., Morii E., Takakura N., Takeuchi O., et al. 2013. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature. 495: 524–528. [DOI] [PubMed] [Google Scholar]
- 121.Moshkovits I., Shik D., Itan M., Karo-Atar D., Bernshtein B., Hershko A. Y., van Lookeren Campagne M., and Munitz A.. 2014. CMRF35-like molecule 1 (CLM-1) regulates eosinophil homeostasis by suppressing cellular chemotaxis. Mucosal Immunol. 7: 292–303. [DOI] [PubMed] [Google Scholar]
- 122.Kitamura H., Naoe Y., Kimura S., Miyamoto T., Okamoto S., Toda C., Shimamoto Y., Iwanaga T., and Miyoshi I.. 2013. Beneficial effects of Brazilian propolis on type 2 diabetes in ob/ob mice: Possible involvement of immune cells in mesenteric adipose tissue. Adipocyte. 2: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gutierrez D. A., Kennedy A., Orr J. S., Anderson E. K., Webb C. D., Gerrald W. K., and Hasty A. H.. 2011. Aberrant accumulation of undifferentiated myeloid cells in the adipose tissue of CCR2-deficient mice delays improvements in insulin sensitivity. Diabetes. 60: 2820–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Langouche L., Marques M. B., Ingels C., Gunst J., Derde S., Vander Perre S., D’Hoore A., and Van den Berghe G.. 2011. Critical illness induces alternative activation of M2 macrophages in adipose tissue. Crit. Care. 15: R245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Langouche L., Perre S. V., Thiessen S., Gunst J., Hermans G., D’Hoore A., Kola B., Korbonits M., and Van den Berghe G.. 2010. Alterations in adipose tissue during critical illness: an adaptive and protective response? Am. J. Respir. Crit. Care Med. 182: 507–516. [DOI] [PubMed] [Google Scholar]
- 126.Qin M., Wang L., Li F., Yang M., Song L., Tian F., Yukht A., Shah P. K., Rothenberg M. E., and Sharifi B. G.. 2017. Oxidized LDL activated eosinophil polarize macrophage phenotype from M2 to M1 through activation of CD36 scavenger receptor. Atherosclerosis. 263: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gouon-Evans V., Rothenberg M. E., and Pollard J. W.. 2000. Postnatal mammary gland development requires macrophages and eosinophils. Development. 127: 2269–2282. [DOI] [PubMed] [Google Scholar]
- 128.Heredia J. E., Mukundan L., Chen F. M., Mueller A. A., Deo R. C., Locksley R. M., Rando T. A., and Chawla A.. 2013. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 153: 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]