Abstract
Insulin resistance is a major risk factor for numerous diseases, including type 2 diabetes and cardiovascular disease. These disorders have dramatically increased in incidence with modern life, suggesting that excess nutrients and obesity are major causes of “common” insulin resistance. Despite considerable effort, the mechanisms that contribute to common insulin resistance are not resolved. There is universal agreement that extracellular perturbations, such as nutrient excess, hyperinsulinemia, glucocorticoids, or inflammation, trigger intracellular stress in key metabolic target tissues, such as muscle and adipose tissue, and this impairs the ability of insulin to initiate its normal metabolic actions in these cells. Here, we present evidence that the impairment in insulin action is independent of proximal elements of the insulin signaling pathway and is likely specific to the glucoregulatory branch of insulin signaling. We propose that many intracellular stress pathways act in concert to increase mitochondrial reactive oxygen species to trigger insulin resistance. We speculate that this may be a physiological pathway to conserve glucose during specific states, such as fasting, and that, in the presence of chronic nutrient excess, this pathway ultimately leads to disease. This review highlights key points in this pathway that require further research effort.
Keywords: insulin signaling, mitochondria, glucose transporter type 4, oxidants
Insulin resistance is a pathophysiological state where cells display reduced responsiveness to the glucose-lowering activity of insulin. While there are rare cases where mutations in genes associated with insulin signaling or lipodystrophy cause insulin resistance, for the most part, insulin resistance is associated with obesity and, thus, a state of positive energy balance. This form of insulin resistance is frequently associated with hyperinsulinemia, increased waist circumference or visceral adiposity, metabolic dyslipidemia with high triglycerides and low HDL, and hepatic steatosis, features collectively referred to as the metabolic syndrome. We refer to this as “common insulin resistance”. Here, both insulin-dependent glucose disposal and suppression of glucose output are impaired, albeit the relative degree of impairment in each process can vary between individuals (1–3).
In this review, we focus on the literature surrounding insulin resistance in muscle and adipose tissue, and specifically on insulin-stimulated glucose transport into myocytes and adipocytes within these tissues. Impaired insulin action in other tissues, most notably the liver (4, 5), brain (6, 7) and vasculature (8), also play a key role in whole-body insulin resistance, and we direct readers to reviews that explore insulin resistance at these sites in detail. We will examine the evidence that common insulin resistance, in the context of muscle and adipose tissue, results from a defect in “proximal” insulin signaling, which we define for the purposes of this review as the signaling intermediates that lead to the activation of Akt. We argue that common insulin resistance arises as a consequence of intracellular stress, specifically oxidative stress, which selectively targets the glucose transport arm of the insulin signaling network, and we discuss the role that impaired glucose transport into muscle and adipose tissue may play in the progression of whole-body insulin resistance. Finally, we explore the concept that insulin resistance, or impaired glucose disposal into muscle and adipose tissue, may be a normal physiological state that, under certain conditions, acts to prioritize glucose use to specific tissues, such as the brain. This pathway may be co-opted in obesity leading to a pathological state of chronic insulin resistance. We recommend several other reviews that have focused on other aspects of insulin resistance that will not be discussed in this review. Most notably, Czech (9) provided an elegant distillation of the complex relationship between hyperinsulinemia and insulin resistance, while others have presented evidence in support of a range of other factors as causes of insulin resistance, including diacylglycerols (DAGs) (10), ceramides (11), and inflammation (12).
ACUTE INSULIN ACTION IN MUSCLE AND ADIPOSE TISSUE
In considering mechanisms that contribute to insulin resistance, it is necessary to summarize the signaling events that are triggered upon engagement of the insulin receptor (IR), as well as the downstream metabolic consequences of this for muscle, adipose tissue, and the whole body.
Whole-body effects
Under fasting conditions, hepatic glucose output and release of fatty acids from triacylglycerol (TAG) stores in adipose tissue (lipolysis) provide substrates for oxidation and ATP production. In the fed state, increases in circulating amino acids, fatty acids, and glucose stimulate insulin secretion. Insulin suppresses hepatic glucose output and adipose tissue lipolysis, lowering blood glucose and fatty acid levels. It also increases hepatic lipid synthesis for subsequent storage in adipose tissue and stimulates glucose uptake into fat and muscle. The majority of glucose from a meal is deposited in muscle and liver with as little as 5% taken up by adipose tissue (13–15). However, as we describe in more detail below, while adipose tissue does not quantitatively account for much of the acute disposal of glucose at the whole-body level, glucose uptake into adipose tissue may indirectly influence both carbohydrate and lipid metabolism in other tissues, such as liver and muscle.
Proximal insulin signaling
Insulin elicits these metabolic changes by activating an intracellular signaling cascade largely comprising protein phosphorylation (16). This begins with activation of the IR, a tyrosine kinase that phosphorylates IR substrates (IRSs), such as IRS1/IRS2, on multiple tyrosine residues (Fig. 1A). These recruit proteins containing SH2 domains, including phosphoinositide 3-kinase (PI3K) and Grb2. This in turn activates the two major protein kinase signaling pathways found in most eukaryotic cells, those mediated by the Ser/Thr kinases, Akt and MAPK/ERK. In particular, Akt has been intensely studied in the context of metabolism, with its activation being both necessary and sufficient for insulin-stimulated glucose transport (17). Akt is recruited to the plasma membrane via binding of its PH domain to PI3K-produced phosphatidylinositol-3,4,5-phosphate (PIP3). It is activated by phosphorylation on Thr308 and Ser473 by PDK1 and mTORC2, respectively [reviewed in (18)]. Consequently, these sites are routinely used as markers of Akt activation and subsequently as a measure of the cell’s response to insulin. Beyond glucose transport, Akt has more than 100 substrates with a wide array of biological endpoints. These include regulating metabolism, protein synthesis (via mTORC1), transcription (e.g., via FOXO, SREBP1), and cellular proliferation. Here, we focus on the role of Akt in lipid and glucose metabolism.
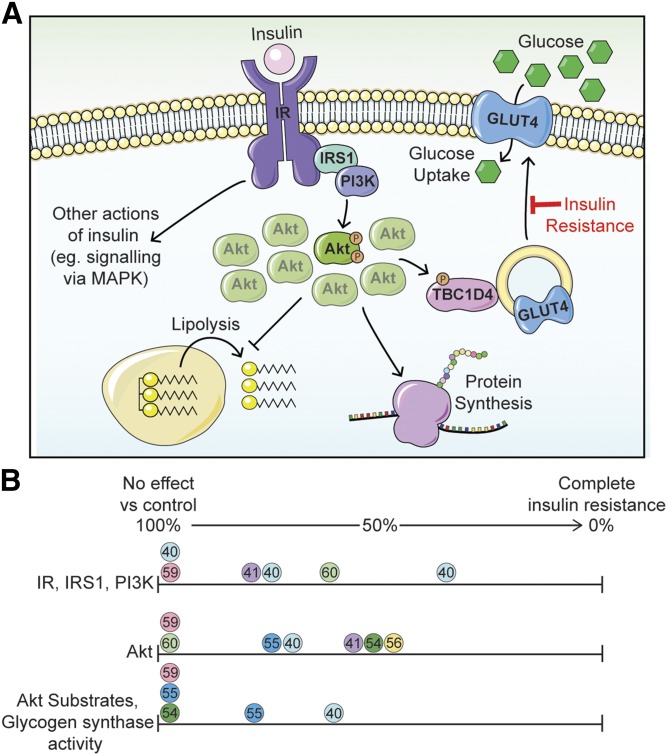
Fig. 1.
Insulin resistance is not due to a generalized impairment in insulin signaling. A: Insulin stimulates a signaling cascade via Akt kinase to regulate biological processes, such as lipolysis, protein synthesis, and glucose transport, the latter via GLUT4 translocation to the plasma membrane. See the main text for additional details. Only a small percentage of the total phosphorylatable pool of Akt is required to maximally regulate downstream biological processes. Insulin-regulated glucose transport is selectively impaired in common insulin resistance, while other insulin-regulated processes in the same cell are less affected. We refer to this as cis selective insulin resistance. B: Analysis of studies examining insulin signaling in the muscles of insulin-resistant subjects that underwent a hyperinsulinemic-euglycemic clamp. The phosphorylation or activity of signaling elements upstream of Akt (IR, IRS1, PI3K), at Akt (Akt), and downstream of Akt (Akt substrates, glycogen synthase) are expressed as a percentage of values from healthy control subjects. Circles of the same color represent data within a single study; reference numbers are included within the circles. References (54) and (60) (green circles) highlight that defects earlier in the pathway are often not translated downstream.
Glucose utilization in muscle and fat
Insulin rapidly increases glucose transport through the regulated trafficking of the glucose transporter, GLUT4, from intracellular stores to the cell surface in muscle and adipose cells (19, 20) (Fig. 1A). This is mediated by the phosphorylation of proteins that regulate GLUT4 trafficking, such as TBC1D4/AS160 (21). GLUT4 translocation is thought to be the rate-limiting step for insulin-dependent glucose utilization in these tissues (22). Once glucose enters muscle and adipose cells, it is rapidly phosphorylated, generating glucose 6-phosphate (G6P). The subsequent metabolism of G6P is coordinated by a series of allosteric and covalent regulatory steps. For example, activation of glycogen synthase (23) and ATP citrate lyase (24, 25) promotes glucose storage into glycogen and lipid, respectively. A recent analysis of the insulin-regulated phosphorylation network in adipocytes identified dozens of metabolic enzymes that undergo insulin-regulated phosphorylation (16, 26), and these likely play a key role in choreographing the ultimate metabolism of glucose in a manner that is more complex than originally anticipated. We recently presented evidence to show that the phosphorylation of these metabolic enzymes precedes the increased delivery of glucose into the cell, thus creating a demand-driven system that primes adipocytes to metabolize glucose in specific ways once glucose transport is fully activated (26).
Lipid metabolism in adipocytes
In addition to increasing glucose transport, insulin suppresses adipose tissue lipolysis (Fig. 1A). While this is one of the most important actions of insulin, our understanding of this process is relatively scant. The β-adrenergic receptor agonists activate lipolysis by increasing cAMP, leading to activation of protein kinase A (PKA) and phosphorylation of lipid droplet proteins to promote TAG hydrolysis (27). Insulin is thought to inhibit this via Akt-dependent phosphorylation and activation of the phosphodiesterase, PDE3B, lowering cAMP levels and inhibiting PKA (28, 29). However, recent studies have questioned the role of PDE3B activity and cAMP hydrolysis in this process (30). Insulin action in the brain is also reported to contribute to reduced adipose tissue lipolysis by dampening sympathetic innervation of adipose tissue (31). Insulin also stimulates adipocyte lipid storage through two concerted processes: 1) lipogenesis via activation of lipogenic enzymes, such as acetyl-CoA carboxylase (32) and ATP-citrate lyase (24, 25); and 2) formation of the TAG-glyceride backbone from glucose diverted from glycolysis (33). As described below, this latter step represents a key convergence point in metabolic regulation whereby fat cell glucose metabolism may have a profound influence on adipocyte lipid storage independently of de novo lipogenesis.
IS INSULIN RESISTANCE DUE TO A LESION IN PROXIMAL INSULIN SIGNALING?
One of the arguments in favor of the view that insulin resistance is due to a proximal signaling defect is that monogenic mutations in proximal components of the insulin signaling pathway in humans, including IR, IRS1, PI3K, and Akt2, are associated with profound insulin resistance (34). Moreover, insulin resistance and diabetes are major complications of cancer therapeutics targeting either PI3K or Akt (35). However, these monogenic lesions are extremely rare and the metabolic phenotype associated with these does not resemble that observed in common insulin resistance, most notably the absence of dyslipidemia and hepatic steatosis (34). Thus, major lesions of the insulin signaling pathway compromise insulin action, but are not synonymous with common insulin resistance.
Here, we argue that generalized impairments in insulin signaling as a result of a lesion in one of the proximal insulin signaling pathway components, for example, the IR, IRS1, or Akt, cannot explain common insulin resistance in muscle and adipose tissue. This is based on four arguments: 1) insulin resistance is observed in rodents and humans in the absence of decreased signal transduction; 2) most of the proximal insulin signaling components, like IR, IRS1, or Akt, operate at a threshold well below their maximum capacity such that modest changes in expression or impairments in function of these will have no significant impact on overall signaling (we define this concept as “spareness”); 3) circumventing the IR or IRS1 using alternate growth factors is not sufficient to block the defect observed in insulin resistance; and 4) insulin resistance in muscle and adipose tissue is quite selective for glucose transport.
Insulin resistance in the absence of decreased proximal signal transduction
Decreased IR expression, tyrosine phosphorylation, and kinase activity have been observed in a variety of tissues from insulin-resistant animals (36, 37) and humans (38–45). Similarly, alterations in IRS1 in insulin-resistant humans and animals have been reported, including altered phosphorylation (43, 46–50), expression (51), degradation (52), and protein-protein interactions (53). Studies in humans undergoing hyperinsulinemic-euglycemic clamps have also reported decreases in Akt phosphorylation by up to 50% in insulin-resistant subjects relative to controls (40, 41, 54–56). However, there are a number of cases in insulin-resistant humans and animals where no change in phosphorylation of the IR, IRS1, or Akt has been detected (48, 57–60) (Fig. 1B). Importantly, studies that have examined signaling downstream of Akt in insulin-resistant humans undergoing hyperinsulinemic-euglycemic clamps found no changes in TBC1D4/AS160 Thr642 phosphorylation (55), FoxO1 Ser256 phosphorylation (54), or glycogen synthase activity (59) (Fig. 1B). Therefore, although impaired signaling has been reported at proximal sites in insulin signaling in insulin resistance, there is ample evidence that insulin resistance occurs in the absence of such changes in signaling.
The spare insulin signaling hypothesis
Why is impaired signaling at proximal components of the insulin signaling pathway, like IR/IRS/Akt, not transmitted to distal elements, like FOXO or TBC1D4/AS160? To rationalize this, it is important to consider how the insulin dose response curve varies between these components. Decades ago, studies described a “Spare Receptor” hypothesis, where it was shown that maximal insulin responses require engagement of a relatively small subset of the available total IR pool at the cell surface (61, 62). More recent studies have shown that considerable spareness exists in many proximal components of the insulin signaling pathway, including IR, IRS, PI3K, and Akt. For example, in muscle cells and adipocytes, the IR and IRS1 are expressed at much higher levels than required for a maximal downstream response to insulin (63–65). This is exemplified by the fact that heterozygous deletion and resultant lower expression of each of these components has no effect on insulin-regulated metabolism and, in the case of PI3K, it was necessary to delete all major isoforms of PI3K simultaneously before reduced downstream signaling events or effects on metabolism were evident (66). Therefore, it is likely that only drastic changes in expression of proximal signaling elements could impair insulin signaling and drive insulin resistance.
In addition to spareness in protein expression, only a small proportion of Akt needs to be active in order for its substrates to be maximally phosphorylated. For instance, in L6 myotubes, insulin had a maximal effect on GLUT4 translocation at concentrations where only 5% of the total available Akt pool was phosphorylated (57) (Fig. 1A). In 3T3-L1 adipocytes, reducing insulin-simulated Akt phosphorylation by 85–90% using small molecule inhibitors only had modest effects on Akt substrate phosphorylation (67). Together, this implies a system whereby an excess of each signaling component enables a small change in the proximal signal to be amplified to the distal components, providing a rapid response to insulin and buffering minor decreases in activity in proximal components of the pathway. This has several implications for insulin resistance: 1) a severe defect in proximal signaling (e.g., a genetic mutation or saturating doses of signaling pharmaceuticals that are currently in use for cancer) may be required to translate to distal signaling components, where a more modest impairment, as observed in common insulin resistance, would not; 2) the inconsistent relationship between Akt and substrate phosphorylation in human studies (Fig. 1B) may be due to high doses of insulin obscuring subtle changes in substrate phosphorylation; and 3) the dose response characteristics of insulin action should be considered when interrogating insulin signaling in the context of insulin resistance.
Diminished signal transduction via IR/IRS is unlikely to drive common insulin resistance
One of the most prominent hypotheses for how insulin signaling may be impaired and so contribute to insulin resistance is via serine/threonine phosphorylation of IR or IRS1. Here, feedback signaling from distal kinases of the insulin signaling pathway (e.g., mTORC1/S6K) or lipid- or stress-activated kinases (e.g., PKC isoforms, JNK, p38 MAPK) leads to phosphorylation of the IR to reduce its kinase activity, or of IRS1 to elicit 14-3-3 protein binding and degradation. In support of this, ablation of a proposed PKCε-mediated feedback phosphorylation site on the IR protected mice from high-fat diet-induced insulin resistance in liver (68), implicating a role for feedback involving the IR in altering insulin responses in this tissue. However, recent studies have shown that liver-specific deletion of PKCε has no detectable effect on insulin resistance, questioning the relevance of this pathway (Schmitz-Peiffer, personal communication). The necessity for impaired signaling via IR/IRS1 in insulin resistance in adipose and muscle tissue was directly tested using mice ectopically expressing the PDGF receptor, which can facilitate GLUT4 translocation to the plasma membrane independently of the IR and IRS1. Although we cannot rule out the presence of feedback to ectopically expressed PDGF receptors in these experiments, a high-fat diet diminished the ability of both insulin and PDGF to activate glucose transport in skeletal muscle, suggesting that insulin resistance can occur independently of the IR or IRS1 (57). Similarly, disarming of the major negative feedback phosphorylation site, Ser307, in IRS1 by mutating it to Ala failed to protect mice against developing insulin resistance (69). As to whether other sites in IRS1 replace this negative feedback function remains to be investigated. Collectively, these data suggest that it is unlikely that common insulin resistance in different metabolic tissues is fully accounted for by feedback to IR or IRS1.
Selective insulin resistance
This term was originally used to describe the observation that under insulin-resistant conditions, MAPK signaling remains intact (70), implying that insulin resistance was not due to global inhibition of insulin-dependent signaling. The term has subsequently been repurposed to describe hepatic insulin resistance, where insulin-regulated suppression of hepatic glucose output, but not triglyceride synthesis, is impaired (71). In light of the emerging picture concerning non-cell autonomous effects of insulin in liver (72, 73), it is tempting to postulate that hepatic selective insulin resistance is due to selective impairments in the adipose tissue-lipolysis-hepatic glucose production axis, while cell-autonomous effects on hepatic lipogenesis remain intact (74).
Recently, an alternate form of selective insulin resistance has been reported. Although insulin/Akt signaling regulates several cellular processes (e.g., glucose transport, protein synthesis, antilipolysis), insulin resistance is characterized by selective downregulation of insulin/Akt-dependent processes within the same cell (Fig. 1A). We refer to this as “cis” selective insulin resistance. This contrasts with “trans” selective insulin resistance that may occur between cells or tissues, such as the adipose-lipolysis-liver-glucose output pathway described above. The cis selective insulin resistance challenges the notion that insulin resistance arises from a generalized impairment in insulin signaling, which would be expected to affect all insulin/Akt-regulated processes to a similar extent. Hence, we will summarize recent examples of cis selective resistance within adipose and muscle tissue.
Adipose tissue.
Impaired insulin-mediated inhibition of adipose tissue lipolysis, as measured by decreases in circulating nonesterified fatty acids during hyperinsulinemic-euglycemic clamps, is not always observed in insulin-resistant humans or rodents where insulin-stimulated glucose disposal is robustly downregulated by 30–50% (3, 75–78). Those studies that report decreased anti-lipolysis invariably find that the degree of impairment is much less than that observed in glucose disposal in the same subjects (3, 75, 79–81). Because insulin signaling in the brain can affect adipose tissue lipolysis in vivo (31), data from isolated cells or cell models provide a more direct assessment of adipocyte anti-lipolysis responses to insulin. Data from cell models and adipose tissue from mouse models show that insulin-mediated suppression of lipolysis is largely unaffected in insulin resistance despite impaired insulin-stimulated glucose transport, especially at higher doses of insulin (82). Indeed, the most striking change in lipolysis in some in vitro models of insulin resistance is in the basal rate of lipolysis, rather than in insulin responses (83–85).
Together, these data suggest that insulin-stimulated glucose transport is more severely blunted in insulin resistance than in anti-lipolysis responses. In addition, data from in vitro studies indicate that insulin-stimulated protein synthesis and nuclear exclusion of FOXO1, two other Akt-regulated processes, are unaffected in insulin-resistant adipocytes (82, 86).
Skeletal muscle.
There are reports of decreased insulin-regulated protein synthesis in skeletal muscle in insulin-resistant humans. However, again, the degree of impairment is markedly less than that observed in insulin-stimulated glucose disposal in the same subjects (75, 87), suggesting that these different insulin-regulated processes are differentially sensitive to insulin resistance. Similarly, insulin-dependent phosphorylation of FOXO in muscle was unaffected in common insulin resistance (54). Accordingly, FOXO target genes were suppressed in the muscle of these subjects, likely due to compensatory hyperinsulinemia.
At least in adipose and muscle tissue, the cell autonomous actions of insulin are differentially affected in insulin resistance (Fig. 1A). Therefore, a unifying model where common insulin resistance can be explained by attenuated insulin signaling to Akt seems unlikely. It has been suggested that Akt substrate specificity can be regulated by phosphorylation within the hydrophobic motif at Ser473 (88), as blockade of mTORC2, the major Akt Ser473 kinase, inhibited Akt-dependent phosphorylation of FOXO while phosphorylation of other substrates was unaffected (89). Furthermore, posttranslational modifications other than at Thr308 and Ser473 have been found to regulate Akt activity (90, 91). Thus, it is important to consider the possibility that insulin resistance may represent a subtler regulation of Akt that could somehow selectively influence the transmission of a signal to certain substrates, but not others.
HYPOTHESIS: IMPAIRED SIGNALING IN INSULIN RESISTANCE IS A CONSEQUENCE OF INSULIN RESISTANCE.
Although we argue here that reduced signaling via IR, IRS1, or Akt is unlikely to cause insulin resistance, impaired phosphorylation of these signaling intermediates is clearly observed, at least in certain models of insulin resistance, including obese insulin-resistant humans. One of the caveats of many such studies, particularly in humans, is that there is limited data on the timeline of insulin resistance. Dynamic studies in mice have shown that insulin resistance develops very rapidly in adipose tissue and liver (3–7 days), and more slowly in skeletal muscle (10–14 days) after exposure to high-fat diet (92, 93). Yet, there is no change in insulin-mediated Akt phosphorylation at these early times. Lower Akt phosphorylation was only observed after 42 days of exposure to the diet; but even here, this did not translate to lower TBC1D4 phosphorylation (57). It may be that insulin resistance drives systemic hyperinsulinemia, which in turn causes many of the changes in insulin signaling associated with insulin resistance, including downregulation of the IR, feedback inhibition of IRS1, reduced Akt activation, and loss of GLUT4 (described below). Alternatively, impaired glucose metabolism resulting from insulin resistance may impair insulin-stimulated Akt activation, as we have previously reported a link between glycolytic activity and Akt activation in a range of cell types (94). In support of this, adipose-specific GLUT4 knockout mice that are hyperinsulinemic have impaired insulin-responsive PI3K activation in liver and muscle (95), while overexpression of GLUT4 in muscle of db/db mice not only improved insulin action but also increased tyrosine phosphorylation of the IR and IRS1 (96). Together, these data raise the possibility that changes in insulin signaling in insulin resistance are a consequence rather than a cause of insulin resistance.
Accordingly, the molecular drivers of insulin resistance are likely either in a part of the signaling network not captured by current techniques (i.e., in phosphorylation-based signaling events that are not currently measurable by Western blotting techniques) or in the mechanics of the cellular processes that are regulated by insulin, but independent of insulin signaling (i.e., in GLUT4 trafficking). These ideas offer future directions for pursuing underlying causes of insulin resistance and suggest that current methods for monitoring the role of insulin signaling in insulin resistance may be, at best, insufficient, or at worst, misleading. For example, changes in Akt phosphorylation at Thr308 or Ser473, which are often used as markers for Akt activity, often do not correlate with Akt substrate phosphorylation (57, 67). Thus, in assessing Akt activity in cells or tissues, it is advised to examine the phosphorylation of a cadre of relevant Akt substrates. In addition, a temporal analysis can help to disentangle integrated systemic responses and suggest that more studies should include temporal data to shed further light on the association between changes in insulin action and insulin signaling.
GLUT4: A KEY NODE IN INSULIN RESISTANCE
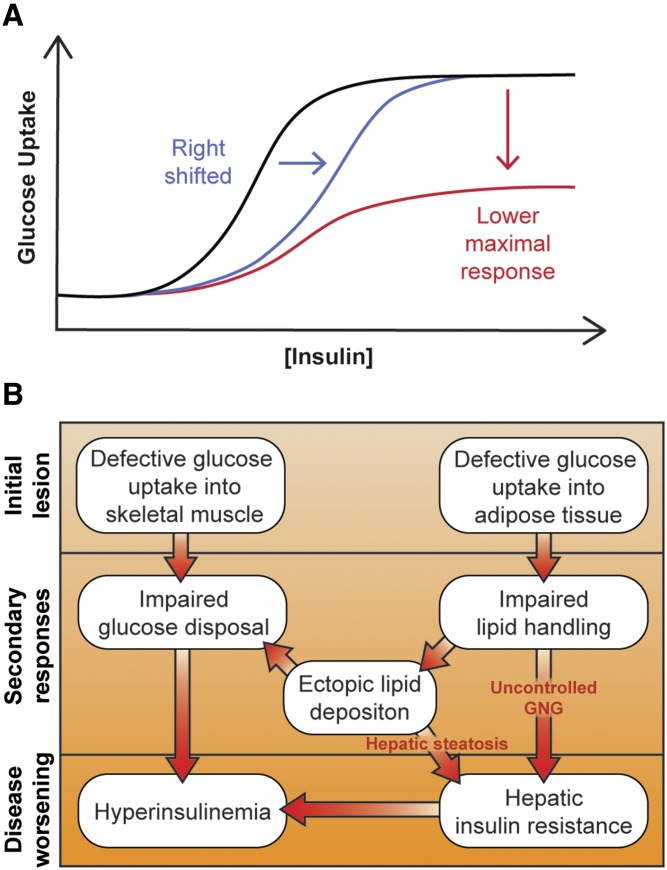
Up to this point, we have argued that insulin resistance is probably not due to inhibition of proximal components of the insulin signaling cascade. Major support for this comes from the observation that there is considerable spareness in these components, including IR, IRS, PI3K, and Akt. In obese insulin-resistant humans, the dose response of insulin-regulated whole-body glucose utilization is shifted both to the right (less sensitive) and down (lower maximal response) (44, 45, 97) (Fig. 2A). This is inconsistent with partial loss of function of a component with inbuilt spareness, as this would only likely affect the sensitivity of the response (Fig. 2A, blue line), not its maximum response (44, 45) (Fig. 2A, red line). Thus, the cause of the downward shift must be independent of proximal insulin signaling components where spareness has been demonstrated.
Fig. 2.
Impaired glucose transport at the center of common insulin resistance. A: Theoretical insulin dose response curves for glucose uptake in human subjects. A reduction in signal transmission via proximal signaling components (e.g., IR, IRS1, PI3K, Akt) would cause this curve to be right-shifted (blue line), requiring a higher insulin dose to achieve the same output. Alternatively, a “post-signaling” impairment would result in a lower maximal response (red line). Both are observed in common insulin resistance (45). B: Model for how initial reductions in glucose uptake into adipose and muscle tissue can precipitate hepatic and whole-body insulin resistance via impaired glucose disposal and lipid handling. Details are in the main text.
Reduced insulin-responsive glucose uptake more or less defines insulin resistance, at least in muscle and fat; whereas, other actions of insulin are either preserved or only modestly affected (see the section, Selective insulin resistance). Here, we consider the evidence that impaired insulin-regulated GLUT4 trafficking in these tissues could contribute to the panoply of metabolic changes attributed to the insulin-resistance syndrome. Either homozygous or heterozygous deletion of GLUT4 or specific ablation in adipose or muscle tissue or both, leads to whole-body insulin resistance and hyperinsulinemia (95, 98–102). Furthermore, a variant in TBC1D4, an Akt substrate that specifically controls GLUT4 translocation to the cell surface in muscle and fat cells, causes insulin resistance and predisposes humans to type 2 diabetes (103), and truncated TBC1D4 is associated with insulin resistance and postprandial hyperinsulinemia in humans (104). Conversely, a number of studies using GLUT4-overexpressing mice have reported improved insulin sensitivity, glycemia, and glucose tolerance, as well as insulin secretory function, in db/db mice (96, 105–110). It is of interest that studies comparing the effects of GLUT4 and GLUT1 overexpression, although limited by differences in the degree of GLUT overexpression, suggest that only GLUT4 confers metabolic benefits because GLUT1 overexpression had the opposite effect (105). Together, these data establish a causal relationship between GLUT4 responses and whole-body insulin sensitivity.
Considering adipose and muscle tissue individually, muscle is the major glucose consumer after a meal, supporting the argument that a block in muscle glucose uptake could lead to increased blood glucose and hyperinsulinemia (98). However, studies capturing the timeline of the progression of insulin resistance in mice fed a high-fat diet demonstrate that insulin resistance in adipose tissue precedes that in muscle (92, 93). Glucose uptake into adipocytes is essential for insulin-stimulated fatty acid synthesis and suppression of fatty acid oxidation [e.g., (111)], and evidence suggests that glucose metabolism suppresses NEFA-release from adipose lipolysis (112). Thus, although adipose tissue contributes relatively little to whole-body glucose disposal following a meal, reduced adipose glucose uptake could have a profound impact on lipid metabolism in the fat cell, leading to impaired suppression of adipose lipolysis by insulin, exacerbating release of NEFA into the circulation (Fig. 2B). This could prevent insulin-dependent suppression of hepatic glucose production (72, 73) and contribute to ectopic fat deposition in muscle and liver, thus contributing to latent insulin resistance in these tissues (Fig. 2B). This concept is supported by the observation that specific knockout of GLUT4 in adipose tissue leads to muscle and hepatic insulin resistance (95). Indeed, genetic lipodystrophies, characterized by prominent central adiposity, dyslipidemia, and hepatic steatosis, closely mimic common insulin resistance (34), while drugs, such as thiazolidinediones, that increase adipose tissue lipid storage capacity improve whole-body insulin sensitivity. This supports the notion, as suggested by others, that insulin resistance ensues from an inability to safely quarantine lipid in adipose tissue stores (113).
Thus, one can envisage a scenario whereby impaired GLUT4 function selectively in adipose tissue could disrupt the entire metabolic network (Fig. 2B). Intriguingly, although insulin resistance coincides with reduced adipose glucose uptake, this precedes a reduction in GLUT4 protein expression, suggesting that the cause of reduced glucose transport lies within the GLUT4 trafficking pathway and is not simply a consequence of less GLUT4. Therefore, we propose that lesions in the regulatory components of GLUT4 trafficking may play a role in the overall etiology of whole-body insulin resistance and associated metabolic derangements, yet how GLUT4 trafficking is perturbed in insulin resistance remains unknown.
WHAT CAUSES COMMON INSULIN RESISTANCE?
We have presented a case that a generalized impairment in insulin signaling is unlikely to explain common insulin resistance, and that reduced glucose transport alone may be sufficient to explain most of the metabolic disturbances associated with insulin resistance. But what is responsible for impaired glucose transport in common insulin resistance? Looking beyond insulin signaling, a number of intracellular perturbations have been reported to mediate insulin resistance: changes in the cellular lipid profile, including increased ceramides (114) or DAG (46); endoplasmic reticulum stress (115); altered mitochondrial function (116, 117); and increased reactive oxygen species (ROS) burden (76, 78, 83, 118–121). These intracellular stresses may be driven directly by diet, or indirectly via changes in the tissue microenvironment [e.g., hypoxia, see (122)] or inflammation [see (119, 123)]. There is evidence that interventions to alleviate any one of these intracellular stresses can alleviate obesity-linked insulin resistance, suggesting that insulin resistance may be a highly heterogeneous disorder, with many parallel pathways leading to the same outcome.
Mitochondrial ROS as a possible unifying factor
Alternatively, different stresses may conspire to disrupt insulin action by convergence upon a common stress or mechanism. Several laboratories, including our own, have reported an important role for increased production or lower scavenging of ROS in mitochondria in insulin resistance. This is based on three major lines of evidence. First, mitochondrial ROS are a characteristic feature of cells and tissues exposed to excess nutrients and a number of other models of insulin resistance (78, 83, 118–120), and oxidative stress is associated with the onset of insulin resistance in humans (76, 121). Second, interventions, either pharmacologic or genetic, that prevent increases in ROS specifically in mitochondria improve insulin sensitivity (78, 83, 118–120, 124). Third, acute induction of mitochondrial ROS causes insulin resistance in muscle and adipose tissue (118, 125). Further, the insulin-sensitizing drugs, thiazolidinediones, metformin, and berberine, may mediate part of their beneficial effects by modulating mitochondrial function (126, 127) and lowering ROS (119, 128, 129).
There is also evidence that many different intracellular stresses, including ceramides (130) and endoplasmic reticulum stress (131), converge upon mitochondria to cause increased ROS production. Hence, mitochondrial ROS production is intimately linked to many forms of cellular stress and thus poised to act as a sentinel of general stress. Based on the role of the mitochondrion in balancing cellular energy supply with demand, which is unbalanced in obesity where supply outweighs demand, and in mediating redox signaling, it represents an ideal candidate to coordinate insulin resistance in response to nutrient oversupply as occurs in diet/obesity-induced insulin resistance (132). Whether different stresses are linked linearly or cyclically is a point for debate and future research, but certainly if the latter, this could explain how disorders like insulin resistance could occur almost via an autocatalytic mechanism and why breaking the cycle at just one point (e.g., ROS, ceramides) may correct the entire cycle. There are currently no studies that have systematically assessed the temporal relationship between ROS, DAGs, ceramides, and ER stress, and such analyses have been invaluable in placing adipose tissue inflammation as a relatively late contributor to insulin resistance (93, 133). Understanding the interrelationship between stresses that cause insulin resistance is an important step in implementing rational therapies to overcome insulin resistance.
Why are mitochondrial ROS elevated in insulin resistance?
Increased availability of nutrients, such as fatty acids, which have a propensity to generate more ROS than other substrates, leads to increased mitochondrial ROS production (132, 134–136). Thus, at one level it seems logical that increased ROS will be a consequence of the increased fatty acid supply in obesity. However, mitochondria isolated from insulin-resistant adipose or muscle tissue generate higher rates of ROS production per unit of substrate (78), suggesting that there may be changes within mitochondria that promote ROS production or lower ROS scavenging. Defects in oxidative phosphorylation can increase ROS production, yet a number of studies have reported no change in or improved mitochondrial oxidative capacity in insulin resistance (78, 83, 140) [reviewed in (137–139)], suggesting that changes in the oxidative phosphorylation pathway are unlikely to be a primary driver of ROS under these conditions. We recently identified a deficiency in coenzyme Q, a cofactor that transfers electrons from complexes I and II to complex III of the electron transport chain, in insulin-resistant adipose and muscle tissue (83). This loss of coenzyme Q, specifically in mitochondria, drove increased mitochondrial ROS production and insulin resistance (83). The mechanism behind loss of coenzyme Q from mitochondria remains unclear, but these data highlight that there are chronic changes in mitochondria under insulin-resistant conditions, beyond substrate selection, that contribute to ROS production. Insulin resistance may also be accompanied by changes in substrate preference for oxidation, which remains a subject to future investigation.
In mitochondria, ROS, in the form of superoxide, are generated at several defined sites within the respiratory chain (141), and the overall ROS burden is dependent on the rates of production and scavenging. The proximal ROS from the electron transport chain is superoxide, but this is rapidly dismutated by SOD2 to hydrogen peroxide, and both superoxide and hydrogen peroxide are implicated in causing insulin resistance (132, 138). It is not clear whether a specific form of ROS or a specific site of ROS production in mitochondria is responsible for conferring insulin resistance. Because multiple interventions that scavenge either superoxide or hydrogen peroxide are insulin sensitizing and multiple sites of ROS production have been implicated in causing insulin resistance (78, 83, 125, 142), it may be that the overall redox state of mitochondria may be more important than the exact species of ROS or site of ROS production, as hypothesized by Fisher-Wellman and Neufer (132).
How do ROS cause insulin resistance?
In addressing this question, it is crucial to emphasize the importance of the site of ROS production, namely mitochondria, in the progression to insulin resistance. Indeed, cytosolic ROS production plays a central role in propagating critical signaling pathways like the insulin signaling pathway and, as such, these pathways positively influence insulin action [reviewed in (132)]. Moreover, the temporal nature of elevated ROS may also be a crucial determinant of the long-term outcome. It probably does not require long-term adaptive changes such as altered transcription (143) but rather either allosteric modifications possibly due to concomitant metabolic changes or posttranslational changes. In the case of the latter, ROS can react with a range of macromolecules, for instance, oxidizing exposed cysteine residues within proteins (132, 144). While the precise mechanism is unclear, ROS have been shown to activate a range of signaling molecules, particularly members of the MAPK family [reviewed in (145, 146)], such as JNK (147) and p38 MAPK (148). However, recent evidence suggests that increased JNK/p38 activity is associated with increased, rather than decreased, insulin sensitivity (149); so it seems unlikely that these molecules are major purveyors of insulin resistance. Similarly, ROS have been shown to activate other kinases, such as LYN and SYK, in mitochondria, but their role in insulin resistance has not been established (150). Hence, currently, the mechanism by which mitochondrial ROS trigger insulin resistance is not known.
In going forward, we propose that the following three questions might provide a useful framework to establish the link between mitochondrial ROS and insulin resistance:
Are mitochondrial ROS the most proximal driver of insulin resistance?
There are other aspects of mitochondrial biology that are linked to insulin resistance, such as mitochondrial quality control (151, 152), fission/fusion (153), and proteostasis (154) [reviewed in (155)], that are also regulated by ROS production [reviewed in (156)].
If ROS are the proximal driver, what is the form of the signal from mitochondria to cause insulin resistance?
The most direct way for increased oxidants to alter protein function is via cysteine oxidation. Although we have reported that global cytosolic ROS are unchanged in insulin resistance, perhaps localized release of ROS from mitochondria could oxidize and impair proteins that control GLUT4 trafficking responses. Alternatively, oxidants are well-known to impact signaling via initiation of kinase cascades or inactivation of phosphatases. Here, protein phosphorylation may convey the oxidant signal to perturb GLUT4 responses. Additionally, opening of the mitochondrial transition pore is required for ROS-induced insulin resistance in skeletal muscle (157), raising the possibility that mitochondrial metabolites (e.g., acetyl-coA) and/or ions (e.g., calcium) may act as intermediates between ROS and insulin resistance.
How does this “signal” affect how GLUT4 responds to insulin?
The most intuitive mechanism is that this signal intersects directly with regulators of GLUT4 or of insulin signaling that mediate GLUT4 responses; however, we cannot rule out an effect on Akt that somehow specifically impairs the GLUT4 arm of insulin signaling. For example, it has been reported that GLUT4 itself is carbonylated in response to short-term high calorie intake in humans (76) and, although the functional significance of these modifications was not investigated, it is tempting to speculate that such modifications on GLUT4 and other regulators of GLUT4 trafficking could initiate insulin resistance independently of insulin signaling. Given the strong link between mitochondrial ROS and insulin resistance, piecing together the pathway from mitochondria to insulin action is a key question for the field.
THE PHYSIOLOGICAL BENEFITS OF INSULIN RESISTANCE
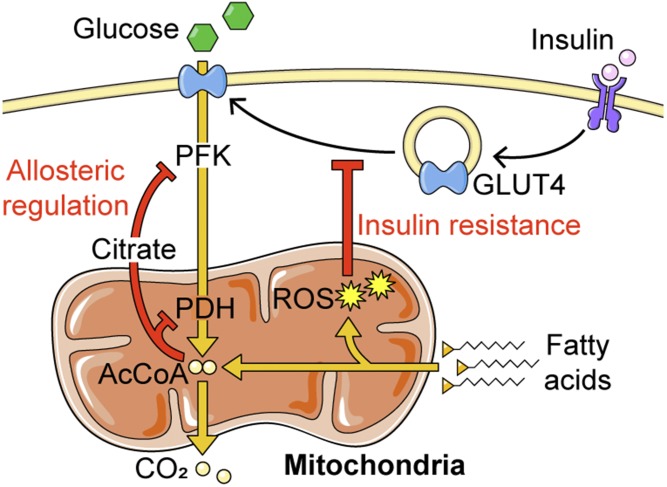
We now realize that insulin resistance is largely restricted to carbohydrate usage by adipose, muscle, and liver. Could this serve a normal physiological function? Insulin resistance is characteristic of starvation, pregnancy, and adolescent development, which may spare glucose for higher priority tissues, such as the brain during fasting or the fetus during pregnancy. The concept of fuel selection has been discussed since the early 1960s. Randle et al. (158) reported a mechanism whereby exposure of muscle to fatty acids rapidly shuts down carbohydrate metabolism. It was proposed that this was mediated by allosteric regulation of phosphofructokinase and pyruvate dehydrogenase by citrate and acetyl-CoA, respectively (158) (Fig. 3). However, fatty acids also inhibit insulin-stimulated GLUT4 translocation to the cell surface (118) and subsequent glucose transport (159), which cannot be explained by allosteric control. Fatty acids increase mitochondrial ROS via β-oxidation (118, 134–136), so ROS may provide an additional means by which fatty acids lower glucose metabolism by impairing GLUT4 translocation in fat and muscle tissue (160) (Fig. 3). Here, ROS can be seen as a form of “eustress,” facilitating metabolic switching. This complements the beneficial role of ROS in thermogenesis in brown adipose tissue through the activation of UCP1 via protein oxidation (161).
Fig. 3.
ROS mediate the interplay between fatty acid oxidation and GLUT4 translocation. ROS arising from fatty acid oxidation impair insulin-stimulated GLUT4 translocation to inhibit glucose uptake (insulin resistance) by an unknown mechanism. This complements the well-established mechanism by which fatty acid oxidation inhibits glucose metabolism, via targeting phosphofructokinase (PFK) and pyruvate dehydrogenase (PDH) by allosteric regulation.
In obesity there is a combination of increased fatty acid availability and diminished capacity for lipid storage leading to increased fat oxidation, as has been described in a model of lipodystrophy (162). This, in concert with structural changes in mitochondria to promote ROS production (e.g., loss of CoQ), may subvert this previously physiological ROS signal to continuously impair glucose transport, resulting in metabolic inflexibility. This is supported by data showing that acute inhibition of fatty acid oxidation enhanced whole-body glucose disposal during hyperinsulinemic-euglycemic clamps (77). Thus, the role of ROS shifts from eustress to stress.
GLUCOSE TRANSPORT AS A TARGET FOR INSULIN RESISTANCE?
We have argued that insulin resistance is not due to a widespread decrease in insulin signaling responses, but rather reflects a specific reduction in insulin-stimulated glucose uptake. In particular, GLUT4 translocation is impaired by ROS, a mechanism that appears to be a unifying driver of insulin resistance. Furthermore, genetic studies targeting GLUT4 have shown that disruption of glucose uptake in muscle or fat is sufficient to recapitulate the metabolic derangements observed in common insulin resistance, including unregulated hepatic glucose output.
Together, this places GLUT4 as a potential therapeutic target for common insulin resistance. Commonly used drugs like sulfonylureas work to restore glucose homeostasis by increasing insulin secretion, while metformin curbs hepatic gluconeogenesis. However, there are currently no treatments that directly increase glucose transport in muscle and adipose tissue, which might confer wider metabolic benefits beyond glucose metabolism. As proof of principle, GLUT4 overexpression in mice is insulin sensitizing (110, 163, 164), and even relatively limited GLUT4 overexpression appears to protect against diet-induced insulin resistance (163), although specific measures of insulin responses in muscle, adipose, and liver were not reported in this study. We envisage that targeting GLUT4 would impart several key benefits for overcoming insulin resistance, namely the restoration of: 1) metabolic flexibility between glucose and fat catabolism within adipose and muscle tissue, shifting the balance away from fatty acid oxidation and associated ROS production; 2) lipid handling in adipose tissue and subsequent cross-talk with hepatic glucose output; and 3) insulin sensitivity, minimizing hyperinsulinemia and its consequences (Fig. 2B). However, in order to target GLUT4 and find ways to restore glucose transport, we need to focus on understanding how the insulin-GLUT4 pathway is specifically impaired in insulin resistance. We present a case here that this does not involve a defect in proximal signaling; hopefully, this will provide an impetus to look beyond these components and to refocus efforts more specifically on the GLUT4 trafficking pathway, which remains mechanistically ill-defined.
Acknowledgments
The authors would like to thank the numerous researchers who have contributed to our understanding of insulin resistance over the decades and apologize to those whose work we could not discuss due to space limitations. The authors also thank Associate Professor Kyle Hoehn (University of New South Wales, Sydney, Australia), Professor Gregory Cooney (University of Sydney, Sydney, Australia), and Professor P. Darrell Neufer (East Carolina University, Greenville, NC) for critical reading of the manuscript and helpful comments
Footnotes
Abbreviations:
- DAG
- diacylglycerol
- IR
- insulin receptor
- IRS
- insulin receptor substrate
- PI3K
- phosphoinositide 3-kinase
- ROS
- reactive oxygen species
- TAG
- triacylglycerol
This work was supported by a Research Training Program Scholarship (A.L.K) and a National Health and Medical Research Council Senior Principal Research Fellowship (D.E.J.) (Grant number 1117078). J.R.K. is a National Health and Medical Research Council Early Career Fellow. The contents of the published material are solely the responsibility of the individual authors and do not reflect the view of the National Health and Medical Research Council.
REFERENCES
- 1.Abdul-Ghani M. A., Tripathy D., and DeFronzo R. A.. 2006. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 29: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 2.Meyer C., Pimenta W., Woerle H. J., Van Haeften T., Szoke E., Mitrakou A., and Gerich J.. 2006. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 29: 1909–1914. [DOI] [PubMed] [Google Scholar]
- 3.Chen D. L., Liess C., Poljak A., Xu A., Zhang J., Thoma C., Trenell M., Milner B., Jenkins A. B., Chisholm D. J., et al. 2015. Phenotypic characterization of insulin-resistant and insulin-sensitive obesity. J. Clin. Endocrinol. Metab. 100: 4082–4091. [DOI] [PubMed] [Google Scholar]
- 4.Samuel V. T., and Shulman G. I.. 2016. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J. Clin. Invest. 126: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen M. C., and Shulman G. I.. 2017. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol. Sci. 38: 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W., Balland E., and Cowley M. A.. 2017. Hypothalamic insulin resistance in obesity: effects on glucose homeostasis. Neuroendocrinology. 104: 364–381. [DOI] [PubMed] [Google Scholar]
- 7.Vogt M. C., and Bruning J. C.. 2013. CNS insulin signaling in the control of energy homeostasis and glucose metabolism - from embryo to old age. Trends Endocrinol. Metab. 24: 76–84. [DOI] [PubMed] [Google Scholar]
- 8.Wasserman D. H., Wang T. J., and Brown N. J.. 2018. The vasculature in prediabetes. Circ. Res. 122: 1135–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czech M. P. 2017. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 23: 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuel V. T., Petersen K. F., and Shulman G. I.. 2010. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 375: 2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavez J. A., and Summers S. A.. 2012. A ceramide-centric view of insulin resistance. Cell Metab. 15: 585–594. [DOI] [PubMed] [Google Scholar]
- 12.Saltiel A. R., and Olefsky J. M.. 2017. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 127: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng J. M., Azuma K., Kelley C., Pencek R., Radikova Z., Laymon C., Price J., Goodpaster B. H., and Kelley D. E.. 2012. PET imaging reveals distinctive roles for different regional adipose tissue depots in systemic glucose metabolism in nonobese humans. Am. J. Physiol. Endocrinol. Metab. 303: E1134–E1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFronzo R. A., and Tripathy D.. 2009. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 32 (Suppl. 2): S157–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraegen E. W., James D. E., Jenkins A. B., and Chisholm D. J.. 1985. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am. J. Physiol. 248: E353–E362. [DOI] [PubMed] [Google Scholar]
- 16.Humphrey S. J., Yang G., Yang P., Fazakerley D. J., Stockli J., Yang J. Y., and James D. E.. 2013. Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab. 17: 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng Y., Ramm G., Lopez J. A., and James D. E.. 2008. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3–L1 adipocytes. Cell Metab. 7: 348–356. [DOI] [PubMed] [Google Scholar]
- 18.Manning B. D., and Cantley L. C.. 2007. AKT/PKB signaling: navigating downstream. Cell. 129: 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James D. E., Brown R., Navarro J., and Pilch P. F.. 1988. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 333: 183–185. [DOI] [PubMed] [Google Scholar]
- 20.Stöckli J., Fazakerley D. J., and James D. E.. 2011. GLUT4 exocytosis. J. Cell Sci. 124: 4147–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sano H., Kane S., Sano E., Miinea C. P., Asara J. M., Lane W. S., Garner C. W., and Lienhard G. E.. 2003. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 278: 14599–14602. [DOI] [PubMed] [Google Scholar]
- 22.Bryant N. J., Govers R., and James D. E.. 2002. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 3: 267–277. [DOI] [PubMed] [Google Scholar]
- 23.Villar-Palasi C., and Larner J.. 1960. Insulin-mediated effect on the activity of UDPG-glycogen transglucosylase of muscle. Biochim. Biophys. Acta. 39: 171–173. [DOI] [PubMed] [Google Scholar]
- 24.Berwick D. C., Hers I., Heesom K. J., Moule S. K., and Tavare J. M.. 2002. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J. Biol. Chem. 277: 33895–33900. [DOI] [PubMed] [Google Scholar]
- 25.Pierce M. W., Palmer J. L., Keutmann H. T., Hall T. A., and Avruch J.. 1982. The insulin-directed phosphorylation site on ATP-citrate lyase is identical with the site phosphorylated by the cAMP-dependent protein kinase in vitro. J. Biol. Chem. 257: 10681–10686. [PubMed] [Google Scholar]
- 26.Krycer J. R., Yugi K., Hirayama A., Fazakerley D. J., Quek L. E., Scalzo R., Ohno S., Hodson M. P., Ikeda S., Shoji F., et al. 2017. Dynamic metabolomics reveals that insulin primes the adipocyte for glucose metabolism. Cell Reports. 21: 3536–3547. [DOI] [PubMed] [Google Scholar]
- 27.Duncan R. E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., and Sul H. S.. 2007. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 27: 79–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitamura T., Kitamura Y., Kuroda S., Hino Y., Ando M., Kotani K., Konishi H., Matsuzaki H., Kikkawa U., Ogawa W., et al. 1999. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol. Cell. Biol. 19: 6286–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi Y. H., Park S., Hockman S., Zmuda-Trzebiatowska E., Svennelid F., Haluzik M., Gavrilova O., Ahmad F., Pepin L., Napolitano M., et al. 2006. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J. Clin. Invest. 116: 3240–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiPilato L. M., Ahmad F., Harms M., Seale P., Manganiello V., and Birnbaum M. J.. 2015. The role of PDE3B phosphorylation in the inhibition of lipolysis by insulin. Mol. Cell. Biol. 35: 2752–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scherer T., O’Hare J., Diggs-Andrews K., Schweiger M., Cheng B., Lindtner C., Zielinski E., Vempati P., Su K., Dighe S., et al. 2011. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 13: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witters L. A., and Kemp B. E.. 1992. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J. Biol. Chem. 267: 2864–2867. [PubMed] [Google Scholar]
- 33.Beale E. G., Hammer R. E., Antoine B., and Forest C.. 2002. Glyceroneogenesis comes of age. FASEB J. 16: 1695–1696. [DOI] [PubMed] [Google Scholar]
- 34.Melvin A., O’Rahilly S., and Savage D. B.. 2018. Genetic syndromes of severe insulin resistance. Curr. Opin. Genet. Dev. 50: 60–67. [DOI] [PubMed] [Google Scholar]
- 35.Crouthamel M. C., Kahana J. A., Korenchuk S., Zhang S. Y., Sundaresan G., Eberwein D. J., Brown K. K., and Kumar R.. 2009. Mechanism and management of AKT inhibitor-induced hyperglycemia. Clin. Cancer Res. 15: 217–225. [DOI] [PubMed] [Google Scholar]
- 36.Kadowaki T., Kasuga M., Akanuma Y., Ezaki O., and Takaku F.. 1984. Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats. J. Biol. Chem. 259: 14208–14216. [PubMed] [Google Scholar]
- 37.Le Marchand-Brustel Y., Gremeaux T., Ballotti R., and Van Obberghen E.. 1985. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature. 315: 676–679. [DOI] [PubMed] [Google Scholar]
- 38.Maegawa H., Shigeta Y., Egawa K., and Kobayashi M.. 1991. Impaired autophosphorylation of insulin receptors from abdominal skeletal muscles in nonobese subjects with NIDDM. Diabetes. 40: 815–819. [DOI] [PubMed] [Google Scholar]
- 39.Nolan J. J., Freidenberg G., Henry R., Reichart D., and Olefsky J. M.. 1994. Role of human skeletal muscle insulin receptor kinase in the in vivo insulin resistance of noninsulin-dependent diabetes mellitus and obesity. J. Clin. Endocrinol. Metab. 78: 471–477. [DOI] [PubMed] [Google Scholar]
- 40.Pratipanawatr W., Pratipanawatr T., Cusi K., Berria R., Adams J. M., Jenkinson C. P., Maezono K., DeFronzo R. A., and Mandarino L. J.. 2001. Skeletal muscle insulin resistance in normoglycemic subjects with a strong family history of type 2 diabetes is associated with decreased insulin-stimulated insulin receptor substrate-1 tyrosine phosphorylation. Diabetes. 50: 2572–2578. [DOI] [PubMed] [Google Scholar]
- 41.Storgaard H., Song X. M., Jensen C. B., Madsbad S., Bjornholm M., Vaag A., and Zierath J. R.. 2001. Insulin signal transduction in skeletal muscle from glucose-intolerant relatives of type 2 diabetic patients [corrected]. Diabetes. 50: 2770–2778. [DOI] [PubMed] [Google Scholar]
- 42.Freidenberg G. R., Henry R. R., Klein H. H., Reichart D. R., and Olefsky J. M.. 1987. Decreased kinase activity of insulin receptors from adipocytes of non-insulin-dependent diabetic subjects. J. Clin. Invest. 79: 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cusi K., Maezono K., Osman A., Pendergrass M., Patti M. E., Pratipanawatr T., DeFronzo R. A., Kahn C. R., and Mandarino L. J.. 2000. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Invest. 105: 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., and Olefsky J. M.. 1981. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J. Clin. Invest. 68: 957–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolterman O. G., Insel J., Saekow M., and Olefsky J. M.. 1980. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J. Clin. Invest. 65: 1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu C., Chen Y., Cline G. W., Zhang D., Zong H., Wang Y., Bergeron R., Kim J. K., Cushman S. W., Cooney G. J., et al. 2002. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277: 50230–50236. [DOI] [PubMed] [Google Scholar]
- 47.Zick Y. 2005. Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci. STKE. 2005: pe4. [DOI] [PubMed] [Google Scholar]
- 48.Krook A., Bjornholm M., Galuska D., Jiang X. J., Fahlman R., Myers M. G. Jr., Wallberg-Henriksson H., and Zierath J. R.. 2000. Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes. 49: 284–292. [DOI] [PubMed] [Google Scholar]
- 49.Danielsson A., Ost A., Nystrom F. H., and Stralfors P.. 2005. Attenuation of insulin-stimulated insulin receptor substrate-1 serine 307 phosphorylation in insulin resistance of type 2 diabetes. J. Biol. Chem. 280: 34389–34392. [DOI] [PubMed] [Google Scholar]
- 50.Bouzakri K., Roques M., Gual P., Espinosa S., Guebre-Egziabher F., Riou J. P., Laville M., Le Marchand-Brustel Y., Tanti J. F., and Vidal H.. 2003. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 52: 1319–1325. [DOI] [PubMed] [Google Scholar]
- 51.Turnbow M. A., Keller S. R., Rice K. M., and Garner C. W.. 1994. Dexamethasone down-regulation of insulin receptor substrate-1 in 3T3-L1 adipocytes. J. Biol. Chem. 269: 2516–2520. [PubMed] [Google Scholar]
- 52.Wang Y., Nishina P. M., and Naggert J. K.. 2009. Degradation of IRS1 leads to impaired glucose uptake in adipose tissue of the type 2 diabetes mouse model TALLYHO/Jng. J. Endocrinol. 203: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caruso M., Ma D., Msallaty Z., Lewis M., Seyoum B., Al-janabi W., Diamond M., Abou-Samra A. B., Hojlund K., Tagett R., et al. 2014. Increased interaction with insulin receptor substrate 1, a novel abnormality in insulin resistance and type 2 diabetes. Diabetes. 63: 1933–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tonks K. T., Ng Y., Miller S., Coster A. C., Samocha-Bonet D., Iseli T. J., Xu A., Patrick E., Yang J. Y., Junutula J. R., et al. 2013. Impaired Akt phosphorylation in insulin-resistant human muscle is accompanied by selective and heterogeneous downstream defects. Diabetologia. 56: 875–885. [DOI] [PubMed] [Google Scholar]
- 55.Vind B. F., Pehmoller C., Treebak J. T., Birk J. B., Hey-Mogensen M., Beck-Nielsen H., Zierath J. R., Wojtaszewski J. F., and Hojlund K.. 2011. Impaired insulin-induced site-specific phosphorylation of TBC1 domain family, member 4 (TBC1D4) in skeletal muscle of type 2 diabetes patients is restored by endurance exercise-training. Diabetologia. 54: 157–167. [DOI] [PubMed] [Google Scholar]
- 56.Adams J. M. 2nd, Pratipanawatr T., Berria R., Wang E., DeFronzo R. A., Sullards M. C., and Mandarino L. J.. 2004. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 53: 25–31. [DOI] [PubMed] [Google Scholar]
- 57.Hoehn K. L., Hohnen-Behrens C., Cederberg A., Wu L. E., Turner N., Yuasa T., Ebina Y., and James D. E.. 2008. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 7: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoy A. J., Brandon A. E., Turner N., Watt M. J., Bruce C. R., Cooney G. J., and Kraegen E. W.. 2009. Lipid and insulin infusion-induced skeletal muscle insulin resistance is likely due to metabolic feedback and not changes in IRS-1, Akt, or AS160 phosphorylation. Am. J. Physiol. Endocrinol. Metab. 297: E67–E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyer M. M., Levin K., Grimmsmann T., Beck-Nielsen H., and Klein H. H.. 2002. Insulin signalling in skeletal muscle of subjects with or without Type II-diabetes and first degree relatives of patients with the disease. Diabetologia. 45: 813–822. [DOI] [PubMed] [Google Scholar]
- 60.Kim Y. B., Nikoulina S. E., Ciaraldi T. P., Henry R. R., and Kahn B. B.. 1999. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J. Clin. Invest. 104: 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kono T., and Barham F. W.. 1971. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J. Biol. Chem. 246: 6210–6216. [PubMed] [Google Scholar]
- 62.Olefsky J. M. 1976. The insulin receptor: its role in insulin resistance of obesity and diabetes. Diabetes. 25: 1154–1162. [DOI] [PubMed] [Google Scholar]
- 63.Cleasby M. E., Reinten T. A., Cooney G. J., James D. E., and Kraegen E. W.. 2007. Functional studies of Akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression. Mol. Endocrinol. 21: 215–228. [DOI] [PubMed] [Google Scholar]
- 64.Le Marchand-Brustel Y., Jeanrenaud B., and Freychet P.. 1978. Insulin binding and effects in isolated soleus muscle of lean and obese mice. Am. J. Physiol. 234: E348–E358. [DOI] [PubMed] [Google Scholar]
- 65.Rice K. M., Lienhard G. E., and Garner C. W.. 1992. Regulation of the expression of pp160, a putative insulin receptor signal protein, by insulin, dexamethasone, and 1-methyl-3-isobutylxanthine in 3T3–L1 adipocytes. J. Biol. Chem. 267: 10163–10167. [PubMed] [Google Scholar]
- 66.Taniguchi C. M., Kondo T., Sajan M., Luo J., Bronson R., Asano T., Farese R., Cantley L. C., and Kahn C. R.. 2006. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 3: 343–353. [DOI] [PubMed] [Google Scholar]
- 67.Tan S. X., Ng Y., Meoli C. C., Kumar A., Khoo P. S., Fazakerley D. J., Junutula J. R., Vali S., James D. E., and Stockli J.. 2012. Amplification and demultiplexing in insulin-regulated Akt protein kinase pathway in adipocytes. J. Biol. Chem. 287: 6128–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petersen M. C., Madiraju A. K., Gassaway B. M., Marcel M., Nasiri A. R., Butrico G., Marcucci M. J., Zhang D., Abulizi A., Zhang X. M., et al. 2016. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Invest. 126: 4361–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Copps K. D., Hancer N. J., Opare-Ado L., Qiu W., Walsh C., and White M. F.. 2010. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab. 11: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dib K., Whitehead J. P., Humphreys P. J., Soos M. A., Baynes K. C., Kumar S., Harvey T., and O’Rahilly S.. 1998. Impaired activation of phosphoinositide 3-kinase by insulin in fibroblasts from patients with severe insulin resistance and pseudoacromegaly. A disorder characterized by selective postreceptor insulin resistance. J. Clin. Invest. 101: 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown M. S., and Goldstein J. L.. 2008. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7: 95–96. [DOI] [PubMed] [Google Scholar]
- 72.Perry R. J., Camporez J. P., Kursawe R., Titchenell P. M., Zhang D., Perry C. J., Jurczak M. J., Abudukadier A., Han M. S., Zhang X. M., et al. 2015. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 160: 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Titchenell P. M., Quinn W. J., Lu M., Chu Q., Lu W., Li C., Chen H., Monks B. R., Chen J., Rabinowitz J. D., et al. 2016. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Cell Metab. 23: 1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferris H. A., and Kahn C. R.. 2016. Unraveling the paradox of selective insulin resistance in the liver: the brain-liver connection. Diabetes. 65: 1481–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burgos S. A., Chandurkar V., Tsoukas M. A., Chevalier S., Morais J. A., Lamarche M., and Marliss E. B.. 2016. Insulin resistance of protein anabolism accompanies that of glucose metabolism in lean, glucose-tolerant offspring of persons with type 2 diabetes. BMJ Open Diabetes Res. Care. 4: e000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boden G., Homko C., Barrero C. A., Stein T. P., Chen X., Cheung P., Fecchio C., Koller S., and Merali S.. 2015. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci. Transl. Med. 7: 304re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oakes N. D., Cooney G. J., Camilleri S., Chisholm D. J., and Kraegen E. W.. 1997. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes. 46: 1768–1774. [DOI] [PubMed] [Google Scholar]
- 78.Anderson E. J., Lustig M. E., Boyle K. E., Woodlief T. L., Kane D. A., Lin C. T., Price J. W. 3rd, Kang L., Rabinovitch P. S., Szeto H. H., et al. 2009. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 119: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kurioka S., Murakami Y., Nishiki M., Sohmiya M., Koshimura K., and Kato Y.. 2002. Relationship between visceral fat accumulation and anti-lipolytic action of insulin in patients with type 2 diabetes mellitus. Endocr. J. 49: 459–464. [DOI] [PubMed] [Google Scholar]
- 80.Korenblat K. M., Fabbrini E., Mohammed B. S., and Klein S.. 2008. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 134: 1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Howard B. V., Klimes I., Vasquez B., Brady D., Nagulesparan M., and Unger R. H.. 1984. The antilipolytic action of insulin in obese subjects with resistance to its glucoregulatory action. J. Clin. Endocrinol. Metab. 58: 544–548. [DOI] [PubMed] [Google Scholar]
- 82.Tan S. X., Fisher-Wellman K. H., Fazakerley D. J., Ng Y., Pant H., Li J., Meoli C. C., Coster A. C., Stockli J., and James D. E.. 2015. Selective insulin resistance in adipocytes. J. Biol. Chem. 290: 11337–11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fazakerley D. J., Chaudhuri R., Yang P., Maghzal G. J., Thomas K. C., Krycer J. R., Humphrey S. J., Parker B. L., Fisher-Wellman K. H., Meoli C. C., et al. 2018. Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance. eLife. •7: e32111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu C., He J., Jiang H., Zu L., Zhai W., Pu S., and Xu G.. 2009. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol. Endocrinol. 23: 1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Green A., Dobias S. B., Walters D. J., and Brasier A. R.. 1994. Tumor necrosis factor increases the rate of lipolysis in primary cultures of adipocytes without altering levels of hormone-sensitive lipase. Endocrinology. 134: 2581–2588. [DOI] [PubMed] [Google Scholar]
- 86.Gonzalez E., Flier E., Molle D., Accili D., and McGraw T. E.. 2011. Hyperinsulinemia leads to uncoupled insulin regulation of the GLUT4 glucose transporter and the FoxO1 transcription factor. Proc. Natl. Acad. Sci. USA. 108: 10162–10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chevalier S., Burgos S. A., Morais J. A., Gougeon R., Bassil M., Lamarche M., and Marliss E. B.. 2015. Protein and glucose metabolic responses to hyperinsulinemia, hyperglycemia, and hyperaminoacidemia in obese men. Obesity (Silver Spring). 23: 351–358. [DOI] [PubMed] [Google Scholar]
- 88.Beg M., Abdullah N., Thowfeik F. S., Altorki N. K., and McGraw T. E.. 2017. Distinct Akt phosphorylation states are required for insulin regulated Glut4 and Glut1-mediated glucose uptake. eLife. 6: e26896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S. Y., Huang Q., Qin J., and Su B.. 2006. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 127: 125–137. [DOI] [PubMed] [Google Scholar]
- 90.Liu P., Begley M., Michowski W., Inuzuka H., Ginzberg M., Gao D., Tsou P., Gan W., Papa A., Kim B. M., et al. 2014. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 508: 541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wani R., Qian J., Yin L., Bechtold E., King S. B., Poole L. B., Paek E., Tsang A. W., and Furdui C. M.. 2011. Isoform-specific regulation of Akt by PDGF-induced reactive oxygen species. Proc. Natl. Acad. Sci. USA. 108: 10550–10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burchfield J. G., Kebede M. A., Meoli C. C., Stockli J., Whitworth P. T., Wright A. L., Hoffman N. J., Minard A. Y., Ma X., Krycer J. R., et al. 2018. High dietary fat and sucrose results in an extensive and time-dependent deterioration in health of multiple physiological systems in mice. J. Biol. Chem. 293: 5731–5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turner N., Kowalski G. M., Leslie S. J., Risis S., Yang C., Lee-Young R. S., Babb J. R., Meikle P. J., Lancaster G. I., Henstridge D. C., et al. 2013. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia. 56: 1638–1648. [DOI] [PubMed] [Google Scholar]
- 94.Trefely S., Khoo P. S., Krycer J. R., Chaudhuri R., Fazakerley D. J., Parker B. L., Sultani G., Lee J., Stephan J. P., Torres E., et al. 2015. Kinome screen identifies PFKFB3 and glucose metabolism as important regulators of the insulin/insulin-like growth factor (IGF)-1 signaling pathway. J. Biol. Chem. 290: 25834–25846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abel E. D., Peroni O., Kim J. K., Kim Y. B., Boss O., Hadro E., Minnemann T., Shulman G. I., and Kahn B. B.. 2001. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 409: 729–733. [DOI] [PubMed] [Google Scholar]
- 96.Ishizuka T., Klepcyk P., Liu S., Panko L., Liu S., Gibbs E. M., and Friedman J. E.. 1999. Effects of overexpression of human GLUT4 gene on maternal diabetes and fetal growth in spontaneous gestational diabetic C57BLKS/J Lepr(db/+) mice. Diabetes. 48: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 97.Conte C., Fabbrini E., Kars M., Mittendorfer B., Patterson B. W., and Klein S.. 2012. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care. 35: 1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zisman A., Peroni O. D., Abel E. D., Michael M. D., Mauvais-Jarvis F., Lowell B. B., Wojtaszewski J. F., Hirshman M. F., Virkamaki A., Goodyear L. J., et al. 2000. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 6: 924–928. [DOI] [PubMed] [Google Scholar]
- 99.Kotani K., Peroni O. D., Minokoshi Y., Boss O., and Kahn B. B.. 2004. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J. Clin. Invest. 114: 1666–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stenbit A. E., Tsao T. S., Li J., Burcelin R., Geenen D. L., Factor S. M., Houseknecht K., Katz E. B., and Charron M. J.. 1997. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat. Med. 3: 1096–1101. [DOI] [PubMed] [Google Scholar]
- 101.Kim J. K., Zisman A., Fillmore J. J., Peroni O. D., Kotani K., Perret P., Zong H., Dong J., Kahn C. R., Kahn B. B., et al. 2001. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J. Clin. Invest. 108: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Katz E. B., Stenbit A. E., Hatton K., DePinho R., and Charron M. J.. 1995. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature. 377: 151–155. [DOI] [PubMed] [Google Scholar]
- 103.Moltke I., Grarup N., Jorgensen M. E., Bjerregaard P., Treebak J. T., Fumagalli M., Korneliussen T. S., Andersen M. A., Nielsen T. S., Krarup N. T., et al. 2014. A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. Nature. 512: 190–193. [DOI] [PubMed] [Google Scholar]
- 104.Dash S., Sano H., Rochford J. J., Semple R. K., Yeo G., Hyden C. S., Soos M. A., Clark J., Rodin A., Langenberg C., et al. 2009. A truncation mutation in TBC1D4 in a family with acanthosis nigricans and postprandial hyperinsulinemia. Proc. Natl. Acad. Sci. USA. 106: 9350–9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marshall B. A., and Mueckler M. M.. 1994. Differential effects of GLUT-1 or GLUT-4 overexpression on insulin responsiveness in transgenic mice. Am. J. Physiol. 267: E738–E744. [DOI] [PubMed] [Google Scholar]
- 106.Gnudi L., Shepherd P. R., and Kahn B. B.. 1996. Over-expression of GLUT4 selectively in adipose tissue in transgenic mice: implications for nutrient partitioning. Proc. Nutr. Soc. 55: 191–199. [DOI] [PubMed] [Google Scholar]
- 107.Shepherd P. R., Gnudi L., Tozzo E., Yang H., Leach F., and Kahn B. B.. 1993. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J. Biol. Chem. 268: 22243–22246. [PubMed] [Google Scholar]
- 108.Ren J. M., Marshall B. A., Mueckler M. M., McCaleb M., Amatruda J. M., and Shulman G. I.. 1995. Overexpression of Glut4 protein in muscle increases basal and insulin-stimulated whole body glucose disposal in conscious mice. J. Clin. Invest. 95: 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsao T. S., Katz E. B., Pommer D., and Charron M. J.. 2000. Amelioration of insulin resistance but not hyperinsulinemia in obese mice overexpressing GLUT4 selectively in skeletal muscle. Metabolism. 49: 340–346. [DOI] [PubMed] [Google Scholar]
- 110.Brozinick J. T. Jr., McCoid S. C., Reynolds T. H., Nardone N. A., Hargrove D. M., Stevenson R. W., Cushman S. W., and Gibbs E. M.. 2001. GLUT4 overexpression in db/db mice dose-dependently ameliorates diabetes but is not a lifelong cure. Diabetes. 50: 593–600. [DOI] [PubMed] [Google Scholar]
- 111.Bally P. R., Cahill G. F. Jr., Leboeuf B., and Renold A. E.. 1960. Studies on rat adipose tissue in vitro. V. Effects of glucose and insulin on the metabolism of palmitate-1-C14. J. Biol. Chem. 235: 333–336. [PubMed] [Google Scholar]
- 112.Chlouverakis C. 1967. The action of glucose on lipolysis. Metabolism. 16: 469–472. [DOI] [PubMed] [Google Scholar]
- 113.Lotta L. A., Gulati P., Day F. R., Payne F., Ongen H., van de Bunt M., Gaulton K. J., Eicher J. D., Sharp S. J., Luan J., et al. 2017. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 49: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Summers S. A., Garza L. A., Zhou H., and Birnbaum M. J.. 1998. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 18: 5457–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L. H., and Hotamisligil G. S.. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 306: 457–461. [DOI] [PubMed] [Google Scholar]
- 116.Kelley D. E., He J., Menshikova E. V., and Ritov V. B.. 2002. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 51: 2944–2950. [DOI] [PubMed] [Google Scholar]
- 117.Petersen K. F., Dufour S., Befroy D., Garcia R., and Shulman G. I.. 2004. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 350: 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hoehn K. L., Salmon A. B., Hohnen-Behrens C., Turner N., Hoy A. J., Maghzal G. J., Stocker R., Van Remmen H., Kraegen E. W., Cooney G. J., et al. 2009. Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl. Acad. Sci. USA. 106: 17787–17792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Houstis N., Rosen E. D., and Lander E. S.. 2006. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 440: 944–948. [DOI] [PubMed] [Google Scholar]
- 120.Paglialunga S., Ludzki A., Root-McCaig J., and Holloway G. P.. 2015. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia. 58: 1071–1080. [DOI] [PubMed] [Google Scholar]
- 121.Samocha-Bonet D., Campbell L. V., Mori T. A., Croft K. D., Greenfield J. R., Turner N., and Heilbronn L. K.. 2012. Overfeeding reduces insulin sensitivity and increases oxidative stress, without altering markers of mitochondrial content and function in humans. PLoS One. 7: e36320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Regazzetti C., Peraldi P., Gremeaux T., Najem-Lendom R., Ben-Sahra I., Cormont M., Bost F., Le Marchand-Brustel Y., Tanti J. F., and Giorgetti-Peraldi S.. 2009. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes. 58: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Holland W. L., Bikman B. T., Wang L. P., Yuguang G., Sargent K. M., Bulchand S., Knotts T. A., Shui G., Clegg D. J., Wenk M. R., et al. 2011. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 121: 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen L., Na R., Gu M., Salmon A. B., Liu Y., Liang H., Qi W., Van Remmen H., Richardson A., and Ran Q.. 2008. Reduction of mitochondrial H2O2 by overexpressing peroxiredoxin 3 improves glucose tolerance in mice. Aging Cell. 7: 866–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fazakerley D. J., Minard A. Y., Krycer J. R., Thomas K. C., Stockli J., Harney D. J., Burchfield J. G., Maghzal G. J., Caldwell S. T., Hartley R. C., et al. 2018. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J. Biol. Chem. 293: 7315–7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Turner N., Li J. Y., Gosby A., To S. W., Cheng Z., Miyoshi H., Taketo M. M., Cooney G. J., Kraegen E. W., James D. E., et al. 2008. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 57: 1414–1418. [DOI] [PubMed] [Google Scholar]
- 127.Zou M. H., Kirkpatrick S. S., Davis B. J., Nelson J. S., Wiles W. G. t., Schlattner U., Neumann D., Brownlee M., Freeman M. B., and Goldman M. H.. 2004. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J. Biol. Chem. 279: 43940–43951. [DOI] [PubMed] [Google Scholar]
- 128.Sun Y., Yuan X., Zhang F., Han Y., Chang X., Xu X., Li Y., and Gao X.. 2017. Berberine ameliorates fatty acid-induced oxidative stress in human hepatoma cells. Sci. Rep. 7: 11340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kelly B., Tannahill G. M., Murphy M. P., and O’Neill L. A.. 2015. Metformin inhibits the production of reactive oxygen species from NADH:ubiquinone oxidoreductase to limit induction of interleukin-1beta (IL-1beta) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J. Biol. Chem. 290: 20348–20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kogot-Levin A., and Saada A.. 2014. Ceramide and the mitochondrial respiratory chain. Biochimie. 100: 88–94. [DOI] [PubMed] [Google Scholar]
- 131.Malhotra J. D., and Kaufman R. J.. 2007. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 9: 2277–2293. [DOI] [PubMed] [Google Scholar]
- 132.Fisher-Wellman K. H., and Neufer P. D.. 2012. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol. Metab. 23: 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee Y. S., Li P., Huh J. Y., Hwang I. J., Lu M., Kim J. I., Ham M., Talukdar S., Chen A., Lu W. J., et al. 2011. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 60: 2474–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rindler P. M., Crewe C. L., Fernandes J., Kinter M., and Szweda L. I.. 2013. Redox regulation of insulin sensitivity due to enhanced fatty acid utilization in the mitochondria. Am. J. Physiol. Heart Circ. Physiol. 305: H634–H643. [DOI] [PubMed] [Google Scholar]
- 135.Anderson E. J., Yamazaki H., and Neufer P. D.. 2007. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J. Biol. Chem. 282: 31257–31266. [DOI] [PubMed] [Google Scholar]
- 136.Seifert E. L., Estey C., Xuan J. Y., and Harper M. E.. 2010. Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J. Biol. Chem. 285: 5748–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Montgomery M. K., and Turner N.. 2015. Mitochondrial dysfunction and insulin resistance: an update. Endocr. Connect. 4: R1–R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Di Meo S., Iossa S., and Venditti P.. 2017. Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. J. Endocrinol. 233: R15–R42. [DOI] [PubMed] [Google Scholar]
- 139.Muoio D. M., and Neufer P. D.. 2012. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab. 15: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fisher-Wellman K. H., Weber T. M., Cathey B. L., Brophy P. M., Gilliam L. A., Kane C. L., Maples J. M., Gavin T. P., Houmard J. A., and Neufer P. D.. 2014. Mitochondrial respiratory capacity and content are normal in young insulin-resistant obese humans. Diabetes. 63: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brand M. D. 2016. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 100: 14–31. [DOI] [PubMed] [Google Scholar]
- 142.Martin S. D., Morrison S., Konstantopoulos N., and McGee S. L.. 2014. Mitochondrial dysfunction has divergent, cell type-dependent effects on insulin action. Mol. Metab. 3: 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chaudhuri R., Krycer J. R., Fazakerley D. J., Fisher-Wellman K. H., Su Z., Hoehn K. L., Yang J. Y. H., Kuncic Z., Vafaee F., and James D. E.. 2018. The transcriptional response to oxidative stress is part of, but not sufficient for, insulin resistance in adipocytes. Sci. Rep. 8: 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chouchani E. T., James A. M., Fearnley I. M., Lilley K. S., and Murphy M. P.. 2011. Proteomic approaches to the characterization of protein thiol modification. Curr. Opin. Chem. Biol. 15: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Son Y., Kim S., Chung H. T., and Pae H. O.. 2013. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 528: 27–48. [DOI] [PubMed] [Google Scholar]
- 146.Torres M. 2003. Mitogen-activated protein kinase pathways in redox signaling. Front. Biosci. 8: d369–d391. [DOI] [PubMed] [Google Scholar]
- 147.Iordanov M. S., and Magun B. E.. 1999. Different mechanisms of c-Jun NH(2)-terminal kinase-1 (JNK1) activation by ultraviolet-B radiation and by oxidative stressors. J. Biol. Chem. 274: 25801–25806. [DOI] [PubMed] [Google Scholar]
- 148.Kulisz A., Chen N., Chandel N. S., Shao Z., and Schumacker P. T.. 2002. Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 282: L1324–L1329. [DOI] [PubMed] [Google Scholar]
- 149.Lawan A., Min K., Zhang L., Canfran-Duque A., Jurczak M. J., Camporez J. P. G., Nie Y., Gavin T. P., Shulman G. I., Fernandez-Hernando C., et al. 2018. Skeletal muscle-specific deletion of MKP-1 reveals a p38 MAPK/JNK/Akt signaling node that regulates obesity-induced insulin resistance. Diabetes. 67: 624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Patterson H. C., Gerbeth C., Thiru P., Vogtle N. F., Knoll M., Shahsafaei A., Samocha K. E., Huang C. X., Harden M. M., Song R., et al. 2015. A respiratory chain controlled signal transduction cascade in the mitochondrial intermembrane space mediates hydrogen peroxide signaling. Proc. Natl. Acad. Sci. USA. 112: E5679–E5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Henstridge D. C., Bruce C. R., Drew B. G., Tory K., Kolonics A., Estevez E., Chung J., Watson N., Gardner T., Lee-Young R. S., et al. 2014. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 63: 1881–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Drew B. G., Ribas V., Le J. A., Henstridge D. C., Phun J., Zhou Z., Soleymani T., Daraei P., Sitz D., Vergnes L., et al. 2014. HSP72 is a mitochondrial stress sensor critical for Parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes. 63: 1488–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jheng H. F., Tsai P. J., Guo S. M., Kuo L. H., Chang C. S., Su I. J., Chang C. R., and Tsai Y. S.. 2012. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell. Biol. 32: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kleinridders A., Lauritzen H. P., Ussar S., Christensen J. H., Mori M. A., Bross P., and Kahn C. R.. 2013. Leptin regulation of Hsp60 impacts hypothalamic insulin signaling. J. Clin. Invest. 123: 4667–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mottis A., Jovaisaite V., and Auwerx J.. 2014. The mitochondrial unfolded protein response in mammalian physiology. Mamm. Genome. 25: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Willems P. H., Rossignol R., Dieteren C. E., Murphy M. P., and Koopman W. J.. 2015. Redox homeostasis and mitochondrial dynamics. Cell Metab. 22: 207–218. [DOI] [PubMed] [Google Scholar]
- 157.Taddeo E. P., Laker R. C., Breen D. S., Akhtar Y. N., Kenwood B. M., Liao J. A., Zhang M., Fazakerley D. J., Tomsig J. L., Harris T. E., et al. 2013. Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Mol. Metab. 3: 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Randle P. J., Garland P. B., Hales C. N., and Newsholme E. A.. 1963. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1: 785–789. [DOI] [PubMed] [Google Scholar]
- 159.Dresner A., Laurent D., Marcucci M., Griffin M. E., Dufour S., Cline G. W., Slezak L. A., Andersen D. K., Hundal R. S., Rothman D. L., et al. 1999. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Invest. 103: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hue L., and Taegtmeyer H.. 2009. The Randle cycle revisited: a new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 297: E578–E591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chouchani E. T., Kazak L., Jedrychowski M. P., Lu G. Z., Erickson B. K., Szpyt J., Pierce K. A., Laznik-Bogoslavski D., Vetrivelan R., Clish C. B., et al. 2016. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature. 532: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Girousse A., Virtue S., Hart D., Vidal-Puig A., Murgatroyd P. R., Mouisel E., Sengenes C., and Savage D. B.. 2018. Surplus fat rapidly increases fat oxidation and insulin resistance in lipodystrophic mice. Mol. Metab. 13: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Atkinson B. J., Griesel B. A., King C. D., Josey M. A., and Olson A. L.. 2013. Moderate GLUT4 overexpression improves insulin sensitivity and fasting triglyceridemia in high-fat diet-fed transgenic mice. Diabetes. 62: 2249–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Carvalho E., Kotani K., Peroni O. D., and Kahn B. B.. 2005. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am. J. Physiol. Endocrinol. Metab. 289: E551–E561. [DOI] [PubMed] [Google Scholar]