Abstract
Context
Interest in identifying the effects of physical and mental activity on recovery after sport-related concussion is growing. Clinical studies of concussed athletes' activities require well-validated methods for tracking their intensity and timing.
Objective
To develop and validate a novel multimodal approach to monitoring activity postconcussion using mobile (mHealth) technologies.
Design
Cohort study.
Setting
Translational research unit.
Patients or Other Participants
A total of 40 high school and collegiate football players were evaluated at preseason and followed longitudinally after either concussion (n = 25; age = 17.88 ± 1.74 years, height = 182.07 ± 8.08 cm, mass = 98.36 ± 21.70 kg) or selection as a nonconcussed control (n = 15; age = 18.27 ± 1.83 years, height = 180.01 ± 7.19 cm, mass = 93.83 ± 24.56 kg).
Main Outcome Measure(s)
Participants wore a commercial actigraph and completed a daily mobile survey for 2 weeks. Analyses focused on comparisons between groups for actigraph-based physical activity and self-reported physical and mental activity during the follow-up period.
Results
For the first 2 days postinjury, objective measures showed fewer daily steps in concussed (6663 ± 2667 steps) than in control (11 148 ± 3381 steps) athletes (P < .001), and both objective and self-reported measures indicated less moderate to vigorous physical activity in concussed (27.6 ± 32.6 min/d and 25.0 ± 43.6 min/d, respectively) than in control (57.3 ± 38.6 min/d and 67.5 ± 40.1 min/d, respectively) athletes (both P values < .05). Correlations between objective and self-reported measures of moderate to vigorous physical activity were moderate across select 1-week and 2-week averages. We observed no group differences in self-reported mental activities.
Conclusions
Physical activity after sport-related concussion varied widely across athletes but on average was reduced during the acute and early subacute postinjury periods for both objective and self-reported measures. The lack of differences in mental activities between groups may reflect limited change in mental exertion postconcussion or difficulty accurately measuring mental activities. Assessing concussed athletes' activities using actigraphy and self-reported scales may help monitor their compliance with activity recommendations and be useful in studies aimed at better understanding the effects of physical activity on concussion recovery.
Keywords: traumatic brain injuries, fitness trackers, mobile applications
Key Points
On average, the concussed group demonstrated less physical activity than the control group on actigraphy and self-reported measures during the acute postinjury period.
Actigraphy and self-reported activity measures were moderately correlated for some metrics.
These approaches for quantifying activity in concussed athletes are feasible.
Acute reductions in moderate to vigorous physical activity postconcussion suggested that athletes broadly complied with current guidelines for acute rest postinjury, but the high variability in early activity levels implied variation in athletes' interpretations of rest.
Objective assessment may help confirm compliance with injury-management recommendations and enable researchers and clinicians to determine the most appropriate dose and timing of exercise for recovery.
Sport-related concussion (SRC) is estimated to affect as many as 1.6 to 3.8 million athletes each year.1 Current injury-management recommendations emphasize an individualized approach that includes a brief period of rest (24–48 hours) followed by a stepwise return to normal activities.2–4 Yet how rest should be defined and how active rehabilitation should be implemented are open to interpretation.5–7 Questions also remain about the optimal dose and timing of rest and activity for individual athletes and what the implications of different management strategies are on clinical and neurobiological recovery.8 Whereas preclinical studies supported a strong link between patient behaviors and neural recovery from mild head trauma, a lack of objective data on the postinjury activities of human patients limits advancement of evidence-based clinical management guidelines.6,7,9–12
Monitoring concussed athletes' activities more closely via subjective and objective measurement approaches may help us understand how SRCs are currently managed and develop informed hypotheses about the relationship between activity engagement and recovery postinjury. Validating activity-monitoring tools for clinical trials focused on exercise-based rehabilitation of concussed athletes would also be useful. To date, few investigators in prospective studies13 have collected detailed data on the activities of athletes immediately postconcussion. Whereas self-reported physical activity measures (eg, the International Physical Activity Questionnaire [IPAQ]) exist, they have not been specifically validated for this purpose.6,14–16 Self-reported measures have clear strengths (eg, cost efficiency), but more objective approaches to measuring activities (eg, commercially available actigraphs [CAs]) may circumvent concerns about athletes' truthfulness and insight when reporting their activities and provide other data not possible through self-report alone (eg, automatic capture of moment-to-moment changes in activity intensity, heart rate [HR], sleep). In addition to the adverse effects of concussion on academic performance, cognitive activity level may also be affected, resulting in a longer duration of postconcussion symptoms, and warrants further evaluation.17,18 Evaluating the degree to which athletes modify activity postinjury and how rapidly they return to normal levels using both subjective and objective measures during the acute and subacute windows is critical to understanding the effects of both physical and cognitive activity on recovery from SRC. Therefore, the purpose of our study was to develop and evaluate the feasibility and preliminary validity of a novel approach to monitoring athletes' activities after SRC. We outfitted acutely concussed and uninjured football players with CAs for 2 weeks. In addition, we developed a mobile application that collected daily ratings of athletes' mental and physical activities and recovery. We hypothesized that (1) both objective and subjective activity measures would suggest decreases in activity in the acute postinjury period with a gradual return to normal levels within the 2-week follow-up period and (2) we would find moderate concordance between objective and self-reported physical activity measures. Our second hypothesis was based on (1) the general psychometric principle that measures of a construct assessed via different measurement domains tend to correlate more modestly than measures from the same measurement domains19 and (2) the literature on physical activity measurement. In particular, the reported associations between objective and self-reported measures of physical activity have been variable and often modest (ie, −0.18 to 0.76).20 Consequently, researchers21 have suggested that correlations greater than 0.4 to 0.5 could be considered adequate to support the validity of self-reported physical activity measures.
METHODS
Participants
Forty males from southeastern Wisconsin who were actively participating in high school or National Collegiate Athletic Association Division III collegiate football were recruited from a larger ongoing study of SRC. Participants who were enrolled during fall 2016 in the parent study (Project Head to Head 2) were eligible for this activity-monitoring substudy. Concussed (n = 25) and uninjured teammate control (n = 15) athletes were enrolled, with control athletes selected to match the concussed group in school, sport team (and by extension sport and sex), estimated premorbid verbal intellectual ability (preseason Wechsler Test of Adult Reading performance), cumulative self-reported grade point average, and age (Table 1). All participants received compensation for preseason and follow-up assessments as a part of the parent study. In addition, participants enrolled in this substudy could keep the activity tracker given to them and were offered an additional $50 after study completion. Participants and their parents provided written informed assent or consent as appropriate, and the study was approved by the Medical College of Wisconsin Institutional Review Board.
Table 1.
Sample Characteristics
| Characteristic |
Groupa |
P Value |
t Value |
|
| Concussed (n = 25) |
Control (n = 15) |
|||
| Mean ± SD |
||||
| Age, y | 17.88 ± 1.74 | 18.27 ± 1.83 | .51 | −0.667 |
| Height, cm | 182.07 ± 8.08 | 180.01 ± 7.19 | .42 | 0.815 |
| Mass, kg | 98.36 ± 21.70 | 93.83 ± 24.56 | .55 | 0.608 |
| Body mass index, kg/m2 | 30.15 ± 5.30 | 29.57 ± 7.36 | .78 | 0.287 |
| Years of football participationb | 7.04 ± 2.90 | 8.33 ± 3.22 | .20 | −1.298 |
| Wechsler Test of Adult Reading, standard score | 94.72 ± 16.46 | 99.07 ± 13.00 | .39 | −0.871 |
| Valid mobile application daysc (N = 14) | 11.32 ± 1.95 | 12.20 ± 2.96 | .26 | −1.136 |
| Valid Fitbitd days (N = 14) | 10.68 ± 3.04 | 12.40 ± 3.27 | .10 | −1.685 |
| n (%) |
||||
| Race | .43 | 0.615 | ||
| White | 18 (72) | 9 (60) | ||
| Black or African American | 7 (28) | 6 (40) | ||
| Ethnicity | .60 | 1.893 | ||
| Not Hispanic or Latino | 22 (88) | 12 (80) | ||
| Hispanic or Latino | 1 (4) | 1 (7) | ||
| Not reported or unknown | 2 (8) | 2 (13) | ||
| Competition level | >.99 | 0.000 | ||
| College | 20 (80) | 12 (80) | ||
| High school | 5 (20) | 3 (20) | ||
| Previous history of concussion | 15 (60) | 3 (20) | .01 | 6.061 |
| Attention-deficit/hyperactivity disorder | 2 (8) | 1 (7) | .88 | 0.024 |
| Learning disability | 0 (0) | 0 (0) | >.99 | NA |
| n/N (%) |
χ2 Value |
|||
| Compliancee | ||||
| Mobile application | 283/327 (86.5) | 183/225 (81.3) | .63 | 0.234 |
| Fitbitd | 267/327 (81.7) | 186/225 (82.7) | .92 | 0.009 |
| Median (Interquartile Range) |
U Value |
|||
| Calculated Fitbitd wear time, h/df | 19.4 (15.5–20.2) | 19.6 (14.0–20.5) | .85 | 195.0 |
Abbreviation: NA, not applicable.
Group comparisons of continuous data were calculated using 2-sided t tests. Observed versus expected frequency comparisons were calculated using Pearson χ2 tests.
One concussed participant was missing data for years of participation.
Valid days is the number of days of valid data provided within the targeted 2-wk follow-up period.
Fitbit Charge HR version 122; Fitbit, San Francisco, CA.
Compliance is reported as the proportion of participant days with data out of the total number of participant days for which data could have been provided.
Evaluated using the Mann-Whitney test.
Study Design and Clinical Assessment Battery
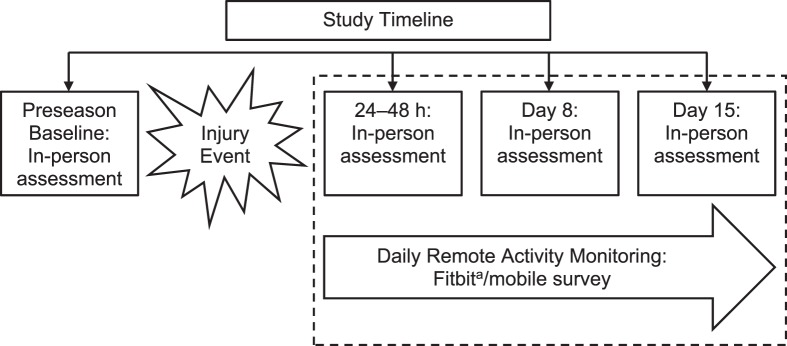
Athletes were enrolled in the parent study during the preseason (baseline). Concussed athletes were reassessed in person at <6 hours; 0 to 2 days (usually 24–48 hours); and days 8, 15, and 45 postinjury (Figure 1). The study protocol specified that participants were to enroll in the activity-monitoring substudy at the 0- to 2-day assessment and to provide daily activity metrics until their day-15 assessment. As soon as possible after identification, contact control athletes were enrolled in the same follow-up protocol while still in season and completed follow-up visits at the same intervals as concussed athletes. In-person assessments, except for the baseline and 6-hour visits, were conducted at the Medical College of Wisconsin Clinical and Translational Science Institute's Translational Research Unit. Baseline assessments involved demographics; medical history information; and a clinical assessment battery, including the following core measures to assess word reading (ie, estimated verbal intelligence), concussion symptoms, postural stability, oculomotor performance, and cognitive functioning: Wechsler Test of Adult Reading, Sport Concussion Assessment Tool 3 (SCAT3) symptom checklist,5 Standardized Assessment of Concussion (SAC),22 Balance Error Scoring System (BESS),23 King-Devick test, and Immediate Post-Concussion and Cognitive Testing (ImPACT) computerized neurocognitive test battery.24 Except for the Wechsler Test of Adult Reading, the same assessments were completed during follow-up visits; injury and recovery information were collected from the concussed athletes. Whereas not pertinent to our primary study aim of characterizing activity levels, a brief description of the clinical effects of concussion on the sample is provided in the Results section to illustrate to what degree and for how long the concussed sample was affected by injury, which might be expected to align with the course of changes in athletes' activity levels due to concussion.
Figure 1.
Study design timeline. The parent study included the assessment points depicted, as well as 6-hour and day-45 postinjury assessments. Concussed and nonconcussed control athletes enrolled in this substudy provided daily activity measures for 2 weeks from their 0- to 2-day assessment until their day-15 assessment. a Fitbit Charge HR version 122; Fitbit, San Francisco, CA.
Commercial Actigraphy
Participants were provided with a Fitbit Charge HR (firmware version 18.122; Fitbit, San Francisco, CA) and were instructed to wear this CA from their 0- to 2-day assessment through their day-15 in-person assessment. We selected a wrist-based device instead of one intended to be worn on the waist because we expected it to yield better compliance and because of our interest in continuous monitoring of HR. Research assistants entered the date of birth, height, weight, hand dominance, and device location (ie, dominant versus nondominant wrist) of each participant into the Fitbit mobile application during the initial setup. The dominant hand was defined as the hand used for fine motor skills, such as writing and eating. Athletes were instructed to wear the device as often as possible, including while sleeping and during athletic practices and games if allowed by their coaches or athletic trainers. Daily totals of physical activity, including steps, floors (ie, change in elevation of approximately 10 ft [3.1 m]), sleep, energy expenditure, and HR metrics, were collected for all participants. Step-based and HR-based physical activity were subdivided into activity-intensity zones based on standard thresholds as defined by the Fitbit software. Fitbit uses the common formula for maximum HR: 220 − age. The step-based activity-intensity zones were sedentary, lightly active, fairly active, and very active. The HR activity-intensity zones were categorized as peak (≥85% of maximum), cardio (70% to 84% of maximum), fat burn (50% to 69% of maximum), and out of zone (<50% of maximum). Whereas the CA allows for manual tracking of activities, only objectively recorded activities were collected.
Authors of previous studies25–28 addressing the validity of CAs have reported moderate to high correlations of both step count and HR with a reference standard (ie, ActiGraph or manually counted steps; electrocardiogram-recorded HR). Although more highly validated activity sensors, such as ActiGraph accelerometers (ActiGraph LLC, Pensacola, FL), are available, the CA was selected because it is a relatively affordable and popular activity tracker and, given that participants would receive the device as compensation, it was important to select an attractive consumer product.
The mHealth Survey
A mobile application (mHealth survey [MS]) was developed to survey participants daily about self-reported mental activity, physical activity (adapted from the IPAQ),29,30 stage of recovery, concussion symptoms, and sleep. The precise wording of the physical and mental activity questions is available in the Supplemental Figure (214.9KB, pdf) (available online at http://dx.doi.org/10.4085/1062-6050-93-18.S1). Mental activity was categorized as low (eg, watching television or movies, listening to music), moderate (eg, texting, online activities, video games), or high (eg, reading, doing homework, studying, test taking). Physical activity was categorized as time spent sitting (ie, sedentary), walking (for recreation, sport, or exercise), or doing moderate (eg, carrying light loads, biking at a regular pace) or vigorous (eg, heavy lifting, fast biking) activity. The MS was installed on each participant's smartphone by a research assistant during the 0- to 2-day visit or as soon as possible thereafter when hardware or software concerns arose at the time of enrollment. Participants were instructed to complete a survey each night and answer questions based on their activity throughout that day. Each day at 8:00 pm Central Time, the mobile application delivered a notification reminding participants to charge their activity trackers and complete the questionnaire. After the MS was completed, the data were automatically uploaded, and participants were unable to answer another survey until the next notification. Web-based surveys were used as an alternative data-collection method when technical difficulties arose.31
Data Management and Statistical Analysis
Data extraction of the CA and MS metrics were completed using a backend Web interface. Whereas the MS data were automatically uploaded from the application after completion, the CA data were pulled from the Fitbit Web server using the Fitbit developer application programming interface.
Actigraph Data Processing
Before analysis, the CA data were evaluated and screened for validity. Some data were suspected of being invalid (eg, extremely low daily step counts). Days suspected of providing invalid or incomplete data, which were determined a priori as <1000 steps or <1000 minutes of HR data for a 24-hour period, were dropped from the analyses; 6% of records were dropped due to these criteria. Next, although we obtained approval from most team athletic trainers to allow participants to wear the CA at practices, we were aware that some participants removed their CAs for practices or games. Most participants completed paper calendars at their days 8 and 15 visits for the parent study, reporting on which days they attended practices and games and whether they wore their CAs. This potentially confounding variable was expected to differentially affect concussed and control groups (control participants were likely to attend more practices and games than concussed participants). Therefore, the CA metrics were adjusted for days on which participants reported attending practices or games but not wearing the CA. In particular, we evaluated a subset of the sample (n = 14) who reported attending a practice or game while wearing a CA and compared them with a subset (n = 14) who also attended a practice or game but did not wear a CA. The difference between these means was used as an estimate of practice- and game-related activity and to prorate the activity metrics for participants on days when they reported attending a practice or game without wearing the CA. Participant records for missing data on practice and game attendance remained unadjusted. Unadjusted values for all CA metrics are included in Supplemental Table 1 (214.9KB, pdf) . Finally, given that the standard CA appeared to impute time not wearing the CA as sedentary activity, we estimated time in the sedentary activity zone by subtracting the duration of activity in the other activity zones from the total estimated daily wear time.
In addition to analyzing the 4 activity-intensity groups provided by the IPAQ (self-report) and Fitbit (CA), we computed a combined measure entitled moderate to vigorous physical activity (MVPA), which represented the sum of the highest 2 categories for each measure, to accommodate our interest in characterizing relatively intense activity and our assumption that the IPAQ and Fitbit activity categorizations may not be equivalent. Energy expenditure of physical activity has been commonly expressed in terms of metabolic equivalents (METs), which are roughly the energy cost of sitting at rest (consumption of 3.5 mL O2/kg/min).32 The IPAQ is structured so that walking is equivalent to 3.3 METs, moderate activity is equal to 4 METs, and vigorous activity is equal to 8 METs.33 In contrast, Fitbit uses a proprietary algorithm to categorize activity intensity. Therefore, we expected that the computation of MVPA may yield a more comparable metric of relatively intense activity.
Data Analyses
To inform our understanding of the feasibility of collecting these activity data, we reviewed the data for completeness and sources of missing values. Several metrics were derived to inform this goal. Valid days was defined as the number of days across the participants' targeted 2-week follow-up periods in which they provided valid MS or CA data (≥1000 steps or ≥1000 minutes of HR data for a 24-hour period). Reasons why participants did not provide valid data included procedural concerns (eg, enrollment in the MS/CA substudy after the 0- to 2-day assessment), technical difficulties, and participant noncompliance. Compliance was computed as the percentage of days for which participants provided MS or CA data compared with the days in which they were enrolled in the study (ie, same numerator as valid days, with the denominator adjusted for participants who were enrolled after the 0- to 2-day window). As described in the Results section, sometimes participants were not compliant due to technical difficulties. Finally, daily CA wear time was computed as the average number of hours per day that participants wore their CAs, which was estimated by summing the time recorded by the CA in the out-of-zone, fat-burn, cardio, and peak HR zones.
For comparisons of concussed and control groups with respect to continuous demographic, clinical assessment, and activity metrics, we used independent-samples t tests or comparable nonparametric tests when applicable. The magnitude of group differences (effect sizes) was quantified as Hedges g. The groups were compared on nominal variable frequencies using Pearson χ2 tests. Correlation analyses were performed using Pearson product moment correlations. The MS and CA data were grouped into multiday or weekly ranges to provide more stable activity estimates in the context of small sample sizes and some missing values. Frequency of and reasons for missing data are further described in the Results section. These ranges were from days 0 to 2, 3 to 6, 7 to 10, and 11 to 14 postinjury for concussed participants and from days 0 to 14 after enrollment (weeks 1 to 2) for controls. We aggregated the control sample's data across the 2-week monitoring period for several reasons. First, like concussed-group athletes, control-group athletes were enrolled at various times across the week, which meant that the labeling and interpretation of time (eg, day 0, day 8) for this group was arbitrary. Second, given the small sample of control participants, parsing their data into bins would have caused variability in the activity level, which can be greatly influenced by a few participants. Averaging these data over the 2-week window ensured the most stable estimate of a healthy football player's “typical” amount of in-season activity. The bins were not selected to be equivalent sizes to balance considerations, such as our a priori interest in evaluating activity in the first 48 hours postinjury (the recommended duration of rest for concussed athletes according to current consensus guidelines for managing SRC)4 and the desire to minimize the overall number of bins for analysis.
We used SPSS (version 22; IBM Corp, Armonk, NY) to conduct statistical analyses. The α level was set at .05.
RESULTS
Sample Characteristics and Group Matching
Sample characteristics are presented in Table 1. The concussed and control groups were closely matched for age, body mass index, years of participation in football, estimated premorbid verbal intellectual ability, race, ethnicity, and history of neurodevelopmental disorder. Concussed athletes more frequently reported a history of concussion at preseason baseline assessments (60% versus 20%; P = .02).
Data Compliance and Completion
Concussed participants conducted their 0- to 2-day assessment at an average of 35.2 ± 15.5 hours postinjury. Most concussed participants (n = 18, 72%) were enrolled at the target time of 0 to 2 days postinjury, whereas all but 1 of the remaining participants were enrolled within the following day. Five of 40 participants had technical problems at 1 or more times during the study that prevented them from being able to use the mobile application (eg, broken telephones, incompatible MS software and telephone operating system). Participants with known technical concerns were provided with a Web-based survey to minimize data loss.
We obtained valid MS and CA data for an average of about 11 of 13 to 15 targeted days per participant, assuming enrollment at 1 to 2 days postinjury and completion of the study at the day-15 postinjury visit and observed no difference between the concussed and control groups (Table 1). Participants provided 2 to 15 days of valid data (ie, met minimal activity thresholds for CA data) for both the CA and MS.
Daily compliance (ie, percentage of days enrolled in the study in which participants provided data) was 84.4 and 82.1% for the MS and CA, respectively, and was equivalent for the concussed and control groups (MS: P = .10; CA: P = .76). Of a total of 327 possible participant-days for concussed athletes, MS data were available for 283 (86.5%) days, and CA data were available for 267 (81.7%) days. A similar proportion of daily data were available for the control group, with MS data for 183 days (81.3%) and CA data for 186 days (82.7%) of 225 possible participant-days.
Across the entire sample, participants wore their CAs a median of 19.4 hours per day (interquartile range [IQR] = 15.46–20.36 hours per day), with equivalent wear times between groups (U = 195.0, P = .85). However, wear time for the concussed group (median = 11.83 hours, range = 2.1–22.6 hours) was lower in the 0- to 2-day postinjury window than the weeks 1 to 2 average for the control group (median = 19.6 hours, range = 5.4–21.9 hours). As we will describe, we secondarily analyzed findings that were different by comparing the average of days 0 to 2 for both groups, during which wear time between groups was not different, to understand the influence of this factor on the results.
Injury Characteristics, Clinical Assessments, and Return to Play
No concussed athletes reported a loss of consciousness or retrograde amnesia; 1 athlete reported posttraumatic amnesia. No athletes described other orthopaedic injuries at the time of study enrollment. The median duration of self-reported symptoms was approximately 1 week postinjury (median = 6 days, range = 1–45 days, IQR = 5.0–14.0 days). Of the 23 concussed participants, 21 reported enrollment in a graded exertion program, 1 quit the sport postinjury, and 1 was not symptom free until after the athletic season had ended. Participants began a graded exertion program at a median of 5 days postinjury (range = 0.5–11.0 days, IQR = 3.5–6.5 days). The median length of time lost from full sport participation was 11 days (range = 2–40 days, IQR = 9.0–15.0 days). Concussed athletes missed from 0 to 3 days (median = 0.5 day) of school postinjury and reported taking from 0 to 25 days (median = 1.0 day) to return to full academic performance. After their injuries, 8 (32%) athletes were provided academic modifications, including decreased homework (n = 1), excused absences from classes (n = 4), extended homework deadlines (n = 4), and extended examination deadlines or study time (n = 3).
The concussed and control groups were well matched at baseline on all symptom and performance metrics (SCAT3 symptom severity, SAC, BESS, King-Devick, ImPACT composite scores). At the 0- to 2-day postinjury time, the concussed group demonstrated more impairment than the control group on SCAT symptom severity (P = .001) and BESS (P = .02), King-Devick (P = .02), and ImPACT verbal memory (P = .048) scores. At day 8, the concussed group was not different from the control group on these clinical assessment measures (P > .09). The performance of the concussed and control groups on the clinical assessment metrics at each study time is provided in Supplemental Table 2 (214.9KB, pdf) .
Activity Measures by Injury Group
Objectively Recorded Physical Activity
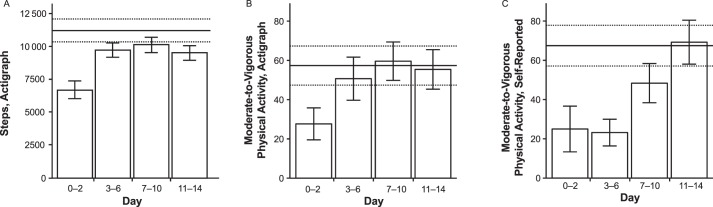
Changes in activity levels for the concussed group over time and between groups on various CA metrics are summarized in Table 2. The mean daily step count was lower during days 0 to 2 postinjury in the concussed group (6663 ± 2667 steps per day) than during weeks 1 to 2 in the control group (11 148 ± 3381 steps per day; P < .001; Figure 2A) postinjury. Variability across athletes was also striking: the mean daily steps for days 0 to 2 ranged from 2783 to 13 030 steps for the concussed group and 5438 to 19 602 steps for the control group. The concussed group also exhibited less time per day in the lightly active (P < .001) and fairly active (P < .001) activity zones from days 0 to 2. Whereas largely driven by differences in the fairly active zone, this effect was still significant (P = .03) when the top 2 activity zones, fairly active and very active (ie, MVPA) were combined at days 0 to 2 postinjury (Figure 2B).
Table 2.
Commercial Actigraph-Recorded Activity Metrics Adjusted for Game or Practice, Mean ± SD (Effect Size)a
| Activity Metric |
Group |
||||
| Concussed, d |
Control, wkb |
||||
| 0–2 (n = 16) |
3–6 (n = 23) |
7–10 (n = 23) |
11–14 (n = 21) |
1–2 (n = 15) |
|
| Step-based physical activity | |||||
| Min/d | |||||
| Sedentaryc | 485.8 ± 316.1g (−0.92) | 834.5 ± 230.3 (0.37) | 803.8 ± 249.6 (0.23) | 834.7 ± 237.0 (0.37) | 746.5 ± 245.8 |
| Lightly active | 148.6 ± 69.9h (−1.00) | 213.8 ± 72.4 (−0.10) | 210.5 ± 68.7 (−0.15) | 187.1 ± 70.6 (−0.47) | 221.3 ± 72.2 |
| Fairly active | 9.9 ± 10.3h (−1.00) | 23.0 ± 22.7 (−0.02) | 26.2 ± 21.0 (0.15) | 23.3 ± 18.9 (0.00) | 23.3 ± 15.4 |
| Very active | 17.7 ± 24.4 (−0.65) | 27.7 ± 31.9 (−0.21) | 33.4 ± 27.1 (−0.02) | 32.1 ± 29.7 (−0.07) | 34.0 ± 24.1 |
| Moderate to vigorousd | 27.6 ± 32.6g (−0.81) | 50.7 ± 52.7 (−0.14) | 59.6 ± 46.9 (0.05) | 55.4 ± 46.1 (−0.04) | 57.3 ± 38.6 |
| Count/d | |||||
| Step count | 6663 ± 2667h (−1.44) | 9669 ± 2720 (−0.48) | 10 074 ± 2805 (−0.35) | 9462 ± 2534 (−0.57) | 11 148 ± 3381 |
| Heart-rate–based physical activity | |||||
| Min/d | |||||
| Out of zonee | 606.5 ± 372.0h (−0.98) | 980.2 ± 334.2 (0.04) | 965.2 ± 310.5 (0.00) | 992.9 ± 249.0 (0.09) | 965.8 ± 342.3 |
| Fat burn | 47.2 ± 56.1 (−0.03) | 75.8 ± 80.6 (0.40) | 95.8 ± 119.2 (0.48) | 91.7 ± 95.4 (0.55) | 48.7 ± 32.0 |
| Cardio | 6.8 ± 23.4 (0.30) | 3.4 ± 9.0 (0.25) | 4.3 ± 15.3 (0.22) | 4.2 ± 8.6 (0.37) | 1.6 ± 2.2 |
| Peak | 0.0 ± 0.0 (−0.60) | 0.2 ± 0.4 (0.32) | 0.4 ± 1.4 (0.26) | 0.5 ± 1.4 (0.34) | 0.1 ± 0.2 |
| Moderate to vigorousf | 6.8 ± 23.4 (0.29) | 3.6 ± 9.3 (0.25) | 4.7 ± 16.6 (0.38) | 4.6 ± 9.7 (0.30) | 1.7 ± 2.2 |
| Beats/min | |||||
| Resting heart rate | 59.8 ± 9.3g (0.96) | 55.7 ± 7.3 (0.49) | 56.3 ± 6.6 (0.63) | 56.4 ± 7.2 (0.59) | 52.4 ± 5.4 |
Adjustments to physical activity were made by averaging the difference between recorded activity magnitudes on days when participants reported wearing the activity tracker during a practice or game and days when participants reported attending a practice or game but not wearing the tracker.
Control-group activity was averaged across the entire 2-week assessment period.
Duration was estimated by subtracting the time in the other 3 activity zones from the estimated wear time.
Combination of the highest 2 physical activity zones: fairly active and very active.
Time when heart rate was less than the fat-burn range.
Combination of the highest 2 physical activity zones: cardio and peak.
P < .05.
P < .01.
Figure 2.
Differences between concussed and nonconcussed control athletes in select physical activity metrics over time. Activity of the concussed group was stratified into 4 bins to illustrate changes in activity over time. Activity of the control group was averaged across the entire 2-week study period. A, Mean daily number of steps as recorded by the Fitbit (Fitbit Charge HR version 122; Fitbit, San Francisco, CA). B, Mean daily number of minutes spent in moderate to vigorous physical activity as recorded by the Fitbit. C, Mean daily number of minutes spent in moderate to vigorous physical activity as reported in the mobile survey. The error bars indicate the means and standard errors for the concussed-group activity. The solid horizontal lines indicate the means for control-group activity. The dotted horizontal lines indicate the standard errors of the means for control-group activity.
Acute variations in estimated wear time during days 0 to 2 postinjury for the concussed group (median = 11.8 hours) versus the weeks 1 to 2 average for the control group (median = 19.6 hours) may have resulted in overestimations in the effect of concussion on CA-recorded activity during this acute postinjury window. Given this imbalance, we compared a step count (10 253 ± 2897 steps per day) and CA MVPA (56.36 ± 38.03 min/d) in a subsample of control athletes with similar estimated wear time (control group wear time; median = 13.3 hours; U = 60.0, P = .33) across the first 2 days of enrollment against the concussed group during days 0 to 2 postinjury. Using these new estimates, a difference was sustained for both step count (P = .002, Hedges g = −1.29) and CA MVPA (P = .04, Hedges g = −0.80) at days 0 to 2.
For HR-based physical activity at days 0 to 2 postinjury, the concussed group demonstrated fewer out-of-zone minutes per day (P < .001). The concussed group demonstrated higher overall resting HR than the control group (P = .02).
Self-Reported Physical Activity
Changes in activity levels for the concussed group over time and compared with the control group on the MS are shown in Table 3. Self-reported vigorous-activity time was less in the concussed group at days 0 to 2 (6.4 ± 17.4 min/d; P < .001) and 3 to 6 (10.0 ± 25.6 min/d; P < .001) than the control group's weeks 1 to 2 average (51.2 ± 31.0 min/d). We observed similar reductions for self-reported MVPA in the concussed group during days 0 to 2 (25.0 ± 43.6 min/d; P = .01) and 3 to 6 (23.2 ± 34.0 min/d; P < .001) compared with the control group's weeks 1 to 2 average (67.5 ± 40.1 min/d; Figure 2C).
Table 3.
Self-Report Activity Metrics, Mean ± SD (Effect Size)
| Self-Report Metric, min/d |
Group |
||||
| Concussed, d |
Control, wka |
||||
| 0–2 (n = 14) |
3–6 (n = 25) |
7–10 (n = 25) |
11–14 (n = 24) |
1–2 (n = 15) |
|
| Physical activity | |||||
| Sitting | 272.4 ± 146.6 (−0.20) | 286.1 ± 120.0 (−0.13) | 305.9 ± 94.9 (0.02) | 296.3 ± 115.1 (−0.06) | 304.0 ± 157.0 |
| Walking | 38.6 ± 89.9 (0.26) | 32.6 ± 52.9 (0.25) | 30.1 ± 48.4 (0.22) | 24.1 ± 45.8 (0.09) | 20.5 ± 33.3 |
| Moderate | 18.6 ± 43.1 (0.07) | 13.2 ± 18.3 (−0.18) | 19.0 ± 29.7 (0.10) | 19.8 ± 30.1 (0.13) | 16.4 ± 16.6 |
| Vigorous | 6.4 ± 17.4c (−1.72) | 10.0 ± 25.6c (−1.46) | 29.4 ± 34.2 (−0.65) | 49.5 ± 56.7 (−0.03) | 51.2 ± 31.0 |
| Moderate to vigorousb | 25.0 ± 43.6d (−0.99) | 23.2 ± 34.0c (−1.19) | 48.4 ± 50.0 (−0.40) | 69.2 ± 54.8 (0.03) | 67.5 ± 40.1 |
| Mental activity | |||||
| Low | 115.5 ± 67.2 (−0.37) | 148.4 ± 85.8 (−0.02) | 150.8 ± 76.3 (0.00) | 160.0 ± 102.6 (0.09) | 150.8 ± 109.7 |
| Moderate | 154.6 ± 116.3 (−0.17) | 148.7 ± 63.5 (−0.27) | 158.0 ± 81.7 (−0.18) | 154.9 ± 98.5 (−0.19) | 179.4 ± 159.7 |
| High | 122.5 ± 138.7 (−0.04) | 130.8 ± 84.6 (0.04) | 179.0 ± 108.1 (0.50) | 159.2 ± 86.2 (0.36) | 127.5 ± 86.0 |
Control-group activity was averaged across the entire 1- to 2-week assessment period.
Combination of the highest 2 physical activity zones: moderate and vigorous.
P < .01.
P < .05.
Self-Reported Mental Activity
Self-reported engagement in mental activity was not different between groups at any time or for any mental-activity intensity level (Table 3).
Concordance Between Objective and Subjective Physical Activity Metrics
In addition to comparing patterns of change in different activity metrics over time, we directly evaluated ostensibly comparable CA and MS activity metrics using correlational analyses (Table 4). For concussed athletes, self-reported (MS) time spent participating in vigorous activity and the comparable CA measure of very active physical activity were moderately positively correlated across the weeks 1 to 2 average (n = 25; r = 0.45, P = .02). The week 2 (n = 22; r = 0.50, P = .02) and the weeks 1 to 2 (n = 25; r = 0.53, P = .006) averages for CA MVPA and MS MVPA were also moderately positively correlated. The control group displayed comparable correlations between MS vigorous and CA-recorded very active (n = 14; r = .62, P = .02) physical activity time at week 1, but these were not correlated at week 2. Similarly, the control group's self-reported and CA MVPA measures were moderately correlated (n = 14; r = .55, P = .044) only at week 1.
Table 4.
Correlations Between Commercial Actigraph and Self-Report Activity Metrics
| Actigraph Metric |
Self-Reported Metrica |
Group, wk |
|||||
| Concussed |
Control |
||||||
| 1 |
2 |
1–2 |
1 |
2 |
1–2 |
||
| Sedentary | Sitting | −0.04 | −0.12 | −0.06 | −0.40 | 0.07 | −0.15 |
| Lightly active | Walking | 0.27 | 0.14 | 0.17 | 0.08 | 0.19 | 0.18 |
| Fairly active | Moderate | 0.20 | 0.36 | 0.36 | 0.31 | −0.31 | 0.08 |
| Very active | Vigorous | 0.18 | 0.37 | 0.45b | 0.62b | −0.05 | 0.11 |
| Fairly active and very active | Moderate and vigorous | 0.30 | 0.50b | 0.53c | 0.55b | −0.26 | 0.13 |
Mobile survey data were matched to valid activity tracker days before averaging. Mobile survey days without a corresponding activity tracker data point were dropped.
P < .05.
P < .01.
Overall, concussed athletes slightly underestimated their step-based MVPA (86%), whereas control athletes slightly overestimated it (118%) in the weeks 1 to 2 average. Concussed athletes largely underestimated their step-based MVPA (56%) through week 1 postinjury but slightly overestimated it (112%) at week 2 postinjury.
DISCUSSION
Our study provided preliminary data for evaluating the feasibility of novel self-reported and objective methods of closely monitoring athletes' activities postconcussion. Consistent with our hypothesis, the concussed group demonstrated, on average, less physical activity than the control group, with a relatively rapid return to normal levels (by 3–6 days postinjury). We observed this for both the MS and CA measures of activity, which were moderately correlated for some metrics. Together, these findings support the feasibility of these approaches to quantifying activity in concussed athletes. The finding of acute reductions in MVPA postconcussion also suggested that athletes were broadly complying with current guidelines for acute rest postinjury, but the early high level of activity variability also implied variations in athletes' interpretations of rest. Objective activity measures may be clinically valuable due to individual variability in the accuracy of self-report measures. Assessing activity with standardized technologies also has important research implications, allowing for improved temporal resolution and validated measurements.
The sensitivity of different self-reported and objective indices of activity to concussion was not equivalent. For example, in the first week postinjury, the concussed group displayed more substantial and persistent reductions in MVPA than were detected using CA measures. This might indicate that recently concussed athletes tend to overestimate their amount of rest or the degree to which they have modified their behavior after injury. On the other hand, CAs may yield imprecise estimates of activity intensity or duration for several reasons that could affect their validity. For example, whereas we observed reduced estimates of MVPA in acutely concussed athletes via step-based activity estimates, we did not observe this hypothesized effect in the CA's HR-based activity measures. This could reflect imprecision in the HR-based intensity classification or simply inherent differences in the activity-related constructs assessed through these various measures (eg, self-reported exercise intensity, CA-recorded step-count intensity, and CA-recorded HR-based intensity). Step-based measures also inherently underestimate nongait-based activities, which may be better captured by self-reported and HR-based metrics.34 Overall, the best activity index likely depends on the research question or desired clinical application.
In a growing body of research,35–41 investigators have reported evidence of concussion-related impairments in cardiovascular and autonomic functioning as quantified by measures such as HR and HR variability. The higher resting HR observed in our sample acutely postconcussion is consistent with this literature, in which researchers noted cardiovascular autonomic dysfunction postconcussion as evidenced by increased resting HR and reduced HR variability with exercise.35–41 These results suggest that wearable actigraphs might provide useful information about the physiological effects of concussion in addition to allowing athletes' behaviors to be tracked. This effect of concussion on HR may affect the accuracy or interpretation of the CA's HR-based activity-intensity categorization. Specifically, a higher resting HR would be expected to result in categorizing recently concussed athletes as spending more time in higher activity-intensity zones than they would when not acutely concussed or than control athletes even when doing the same activities. Researchers need to decide if such categorization would be considered valid or invalid for their purposes and how they want to define activity intensity.
Differences in self-reported mental-activity duration between groups were not observed at any time, but the high variability in reported time spent in low, moderate, and high mental-activity zones suggested that interpretation of this question is highly subjective and influenced by individual interpretations. Whereas the MS attempted to qualify each category with specific examples, it was not exhaustive and may be too broad to detect these behavioral changes. More work is needed to determine how to best measure mental activities postconcussion.
LIMITATIONS
Our modestly sized sample comprised physically active male high school and collegiate football athletes. Such individuals may be highly motivated to return to sport participation quickly and do not represent the general population of people who experience concussions. In addition, the mild concussions in this sample and the rapid recovery course of the concussed group, which is typical of other youth SRC samples, does not represent the severity of mild traumatic brain injury in the general population. Furthermore, given that this substudy relied on the infrastructure of the parent study, enrollment of participants with SRC was typically completed from 24 to 48 hours postinjury, which limited the collection of MS and CA data before this timeframe. Baseline activity data were not available for the sample, so we relied on cross-sectional comparisons of concussed and uninjured athletes to estimate the effects of injury on activity. However, given that the concussed and control groups were well matched and demonstrated highly similar levels of activity after a brief postinjury period, we believe that the cross-sectional design was reasonable for addressing the research question. We also estimated CA wear time based on the total time spent in each HR zone, which may have underestimated actual wear time because a CA may not record HR when it is worn improperly (eg, too loosely or over a shirt sleeve). Also, intraday or single-day estimates of activity, which were not analyzed due to the small sample sizes in this study, may provide a clearer picture of actual daily wear time and enable a more detailed analysis of activity in the early recovery period after SRC. Whereas CA physical activity was adjusted for estimated practice and game effects for nearly three-quarters (n = 29) of all enrolled participants, the activity of athletes who did not provide practice- or game-attendance data and who attended these events but did not wear their CAs may be underestimated. As mentioned, step-based CA metrics may underestimate nongait-based activities (eg, cycling, weight training, rowing).34 The HR-based metrics may provide more valid estimates of these physical activities.
Several technical challenges may have adversely affected data collection. Three (7.5%) of the 40 participants experienced CA failures during the data-collection period (eg, broken band, stuck firmware update, blank or frozen CA, device caused skin irritation). These devices were replaced during the next scheduled in-person assessment but may have resulted in the loss of several subacute data points for these participants. Although the research assistants routinely reminded them, it also is likely that some athletes were not using their devices consistently (ie, forgetting to charge them or not “syncing” them correctly). The research assistants resolved these problems through text messages, telephone calls, or in person if necessary. Finally, while selected for convenience and compliance reasons, wrist-worn CAs may underperform waist-worn devices42 by misattributing upper extremity movements (eg, typing) as steps.
CONCLUSIONS
We assessed the feasibility of and correspondence between 2 novel approaches to recording athletes' activities after SRC: a mobile application that distributed daily surveys of physical and mental activities and an objective wearable physical activity monitor (CA). Objective assessment of athletes' physical activities after SRC may help confirm compliance with injury-management recommendations and inform research efforts to determine the most appropriate dose and timing of exercise for recovery. Evaluating differences between what athletes have been prescribed and their actual level of rest and activity may also improve our understanding of athletes' behavior postinjury. Future work to apply these activity-tracking techniques to larger samples and establish relationships between activities and outcomes may inform efforts to develop more evidence-based concussion-management recommendations.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by award W81XWH-14-1-0561 from the Defense Health Program under the Department of Defense Broad Agency Announcement for Extramural Medical Research. The REDCap database used in the study was supported by award UL1TR001436 from the National Center for Advancing Translational Sciences, National Institutes of Health. The content is solely the responsibility of the authors, and it does not necessarily represent the official views of the National Institutes of Health and is not necessarily endorsed by the Department of Defense.
We thank Dustin Hahn and the University of Wisconsin–Milwaukee's Mobile Innovation Lab (App Brewery) for their contributions to software development for this study.
REFERENCES
SUPPLEMENTAL MATERIAL
Supplemental Figure (214.9KB, pdf) . Mobile application activity questions.
Supplemental Tables 1 (214.9KB, pdf) and 2 (214.9KB, pdf) . Unadjusted commercial actigraph-recorded activity metrics, mean ± SD (effect size); Group comparisons of clinical assessments, mean ± SD.
Found at DOI: http://dx.doi.org/10.4085/1062-6050-93-18.S1
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Broglio SP, Cantu RC, Gioia GA, et al. National Athletic Trainers' Association position statement: management of sport concussion. J Athl Train. 2014;49(2):245–265. doi: 10.4085/1062-6050-49.1.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80(24):2250–2257. doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport: the 5th International Conference on Concussion in Sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–847. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 5.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250–258. doi: 10.1136/bjsports-2013-092313. [DOI] [PubMed] [Google Scholar]
- 6.Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135(2):213–223. doi: 10.1542/peds.2014-0966. [DOI] [PubMed] [Google Scholar]
- 7.Moser RS, Glatts C, Schatz P. Efficacy of immediate and delayed cognitive and physical rest for treatment of sports-related concussion. J Pediatr. 2012;161(5):922–926. doi: 10.1016/j.jpeds.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Schneider KJ, Leddy JJ, Guskiewicz KM, et al. Rest and treatment/rehabilitation following sport-related concussion: a systematic review. Br J Sports Med. 2017;51(12):930–934. doi: 10.1136/bjsports-2016-097475. [DOI] [PubMed] [Google Scholar]
- 9.Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil. 2016;31(4):233–241. doi: 10.1097/HTR.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taubman B, Rosen F, McHugh J, Grady MF, Elci OU. The timing of cognitive and physical rest and recovery in concussion. J Child Neurol. 2016;31(14):1555–1560. doi: 10.1177/0883073816664835. [DOI] [PubMed] [Google Scholar]
- 11.Carson JD, Lawrence DW, Kraft SA, et al. Premature return to play and return to learn after a sport-related concussion: physician's chart review. Can Fam Physician. 2014;60(6) :e310, e312–315. [PMC free article] [PubMed] [Google Scholar]
- 12.Elbin RJ, Sufrinko A, Schatz P, et al. Removal from play after concussion and recovery time. Pediatrics. 2016;138(3):e20160910. doi: 10.1542/peds.2016-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sufrinko AM, Howie EK, Elbin RJ, Collins MW, Kontos AP. A preliminary investigation of accelerometer-derived sleep and physical activity following sport-related concussion. J Head Trauma Rehabil. 2018;33(5):E64–E74. doi: 10.1097/HTR.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 14.Howell DR, Mannix RC, Quinn B, Taylor JA, Tan CO, Meehan WP., 3rd Physical activity level and symptom duration are not associated after concussion. Am J Sports Med. 2016;44(4):1040–1046. doi: 10.1177/0363546515625045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grool AM, Aglipay M, Momoli F, et al. Association between early participation in physical activity following acute concussion and persistent postconcussive symptoms in children and adolescents. JAMA. 2016;316(23):2504–2514. doi: 10.1001/jama.2016.17396. [DOI] [PubMed] [Google Scholar]
- 16.Majerske CW, Mihalik JP, Ren D, et al. Concussion in sports: postconcussive activity levels, symptoms, and neurocognitive performance. J Athl Train. 2008;43(3):265–274. doi: 10.4085/1062-6050-43.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ransom DM, Vaughan CG, Pratson L, Sady MD, McGill CA, Gioia GA. Academic effects of concussion in children and adolescents. Pediatrics. 2015;135(6):1043–1050. doi: 10.1542/peds.2014-3434. [DOI] [PubMed] [Google Scholar]
- 18.Brown NJ, Mannix RC, O'Brien MJ, Gostine D, Collins MW, Meehan WP., III Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics. 2014;133(2):e299–e304. doi: 10.1542/peds.2013-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol Bull. 1959;56(2):81–105. [PubMed] [Google Scholar]
- 20.Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terwee CB, Mokkink LB, van Poppel MN, Chinapaw MJ, van Mechelen W, de Vet HC. Qualitative attributes and measurement properties of physical activity questionnaires: a checklist. Sports Med. 2010;40(7):525–537. doi: 10.2165/11531370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.McCrea M, Kelly JP, Kluge J, Ackley B, Randolph C. Standardized assessment of concussion in football players. Neurology. 1997;48(3):586–588. doi: 10.1212/wnl.48.3.586. [DOI] [PubMed] [Google Scholar]
- 23.Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- 24.Iverson GL, Brooks BL, Collins MW, Lovell MR. Tracking neuropsychological recovery following concussion in sport. Brain Inj. 2006;20(3):245–252. doi: 10.1080/02699050500487910. [DOI] [PubMed] [Google Scholar]
- 25.Leth S, Hansen J, Nielsen OW, Dinesen B. Evaluation of commercial self-monitoring devices for clinical purposes: results from the Future Patient trial, phase I. Sensors (Basel) 2017;17(1):E211. doi: 10.3390/s17010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fokkema T, Kooiman TJ, Krijnen WP, Van Der Schans CP, De Groot M. Reliability and validity of ten consumer activity trackers depend on walking speed. Med Sci Sports Exerc. 2017;49(4):793–800. doi: 10.1249/MSS.0000000000001146. [DOI] [PubMed] [Google Scholar]
- 27.Voss C, Gardner RF, Dean PH, Harris KC. Validity of commercial activity trackers in children with congenital heart disease. Can J Cardiol. 2017;33(6):799–805. doi: 10.1016/j.cjca.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Wallen MP, Gomersall SR, Keating SE, Wisloff U, Coombes JS. Accuracy of heart rate watches: implications for weight management. PLoS One. 2016;11(5):e0154420. doi: 10.1371/journal.pone.0154420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(suppl 2):114–120. doi: 10.1080/02701367.2000.11082794. [DOI] [PubMed] [Google Scholar]
- 30.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jette M, Sidney K, Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13(8):555–565. doi: 10.1002/clc.4960130809. [DOI] [PubMed] [Google Scholar]
- 33.Sjostrom M, Ainsworth B, Bauman A, et al. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ): short and long forms. International Physical Activity Questionnaire Web site. 2018 http://www.ipaq.ki.se/scoring.pdf Accessed October 13.
- 34.Nelson MB, Kaminsky LA, Dickin DC, Montoye AH. Validity of consumer-based physical activity monitors for specific activity types. Med Sci Sports Exerc. 2016;48(8):1619–1628. doi: 10.1249/MSS.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 35.Gall B, Parkhouse W, Goodman D. Heart rate variability of recently concussed athletes at rest and exercise. Med Sci Sports Exerc. 2004;36(8):1269–1274. doi: 10.1249/01.mss.0000135787.73757.4d. [DOI] [PubMed] [Google Scholar]
- 36.Gall B, Parkhouse WS, Goodman D. Exercise following a sport induced concussion. Br J Sports Med. 2004;38(6):773–777. doi: 10.1136/bjsm.2003.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blake TA, McKay CD, Meeuwisse WH, Emery CA. The impact of concussion on cardiac autonomic function: a systematic review. Brain Inj. 2016;30(2):132–145. doi: 10.3109/02699052.2015.1093659. [DOI] [PubMed] [Google Scholar]
- 38.Hilz MJ, DeFina PA, Anders S, et al. Frequency analysis unveils cardiac autonomic dysfunction after mild traumatic brain injury. J Neurotrauma. 2011;28(9):1727–1738. doi: 10.1089/neu.2010.1497. [DOI] [PubMed] [Google Scholar]
- 39.Dobson JL, Yarbrough MB, Perez J, Evans K, Buckley T. Sport-related concussion induces transient cardiovascular autonomic dysfunction. Am J Physiol Regul Integr Comp Physiol. 2017;312(4):R575–R584. doi: 10.1152/ajpregu.00499.2016. [DOI] [PubMed] [Google Scholar]
- 40.Bishop S, Dech R, Baker T, Butz M, Aravinthan K, Neary JP. Parasympathetic baroreflexes and heart rate variability during acute stage of sport concussion recovery. Brain Inj. 2017;31(2):247–259. doi: 10.1080/02699052.2016.1226385. [DOI] [PubMed] [Google Scholar]
- 41.Senthinathan A, Mainwaring LM, Hutchison M. Heart rate variability of athletes across concussion recovery milestones: a preliminary study. Clin J Sport Med. 2017;27(3):288–295. doi: 10.1097/JSM.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 42.Tudor-Locke C, Barreira TV, Schuna JM., Jr Comparison of step outputs for waist and wrist accelerometer attachment sites. Med Sci Sports Exerc. 2015;47(4):839–842. doi: 10.1249/MSS.0000000000000476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.