Abstract
Background
Cellular immunity plays a crucial role in sepsis, and lymphocyte apoptosis is a key factor in immune homeostasis. Tumor necrosis factor-α (TNF-α)-induced protein 8-like 2 (TIPE2) is suggested to play a critical role in maintaining immune homeostasis. This study investigated the role of TIPE2 in CD4+ T lymphocyte apoptosis based on a mouse model of thermal injury.
Material/Methods
BALB/c male mice were randomized into 6 groups: sham, burn, burn with siTIPE2, burn with siTIPE2 control, burn with TIPE2, and burn with TIPE2 control groups. Splenic CD4+ T lymphocytes were collected by use of a magnetic cell sorting system.
Results
We found that TIPE2 downregulation reduced the CD4+ T lymphocytes apoptosis in the burn with siTIPE2 group, and the protein expression of P-smad2/P-Smad3 were remarkably downregulated. In the burn with siTIPE2 group, Bcl-2 expression was increased compared with that in the sham group (P<0.05), and Bim expression was reduced (P<0.05). In the burn with TIPE2 group, the mitochondrial membrane potential was markedly reduced (P<0.01), while cytochrome C expression was clearly higher than that in the other groups (P<0.01). Activities of caspase-3, -8, and -9 were notably higher in the burn with TIPE2 group relative to those for other groups (P<0.05).
Conclusions
Downregulation of TIPE2 in vivo can reduce the apoptosis of CD4+ T lymphocytes following thermal damage, and activate the TGFβ downstream signaling of Smad2/Smad3, upregulating Bim, and downregulating Bcl-2.
MeSH Keywords: Tumor necrosis factor-α-induced protein 8 like-2, Apoptosis, T lymphocytes, Thermal injury
Background
Severe burns, trauma, and surgical stress can induce sepsis and other infectious complications, which may finally result in septic shock or multiple organ dysfunction syndrome (MODS), and MODS is a major cause of death in the intensive care unit (ICU). Typically, the sepsis mortality rate remains high regardless of the remarkable progresses achieved in early fluid resuscitation, new antimicrobial drug therapy, nutrient metabolism, and organ support. Sepsis has greatly threatened patients and reduces the survival improvement in critically ill patients. Therefore, increasing importance has been attached theoretically and clinically to enhance the knowledge and treatment strategies for septic complications [1].
Notably, the extent of lymphocyte apoptosis is a key factor in the maintenance of immune homeostasis. Many lymphocytes are subject to apoptosis in both the central and peripheral lymphoid organs during severe trauma [2], and the increase in lymphocyte apoptosis is a major cause of immune suppression [3–5]. It has been found that the extent of apoptosis of circulating lymphocytes is positively correlated with sepsis severity [6], and preventing lymphocyte apoptosis can improve the host response against sepsis [7].
The Smad2/Smad3 proteins are transforming growth factor beta (TGF-β) ligands that can activate downstream receptor proteins. The activated TGF-β can then phosphorylate the Smad2/Smad3 proteins, which can subsequently regulate the pro-apoptotic and anti-apoptotic proteins of the Bcl-2 family. Subsequently, the endogenous mitochondrial apoptotic pathway is induced by releasing cytochrome C and activating caspase-9. Finally, the activated caspase-8 and caspase-9 allow for the catalytic maturation of caspase-3 and other caspases, which can eventually mediate the biochemical and morphological features of apoptosis. In resting cells, the pro-apoptotic proteins are endogenously neutralized by their anti-apoptotic counterparts. Specifically, the apoptin inhibitors in the Bcl-2 family, such as Bcl-xl and Bcl-2, play important roles in suppressing cell apoptosis, which can also maintain the mitochondrial integrity, thus hindering the release of mitochondrial cytochrome C. Notably, Bax, Bim, and other pro-apoptotic members can also promote the occurrence of this process.
TIPE2 is a member of the TIPE family and has been reported to have important roles in immunity, apoptosis, and tumorigenesis [8]. Overexpression of TIPE2 promotes lung cancer cell apoptosis through affecting the apoptosis-related molecules caspase-3, caspase-9, Bcl-2, and Bax by regulating P38 and Akt pathways [9]. TIPE2 can also inhibit the PI3K/Akt signaling pathway, which further suppresses proliferation, migration, and invasion in prostate cancer cells [10]. TIPE2 also regulates AKT and extracellular signal-regulated kinase 1/2 (ERK1/2) signaling. Adenovirus-directed expression of TIPE2 induces gastric cancer apoptosis by induction of apoptosis and inhibition of AKT and ERK1/2 signaling [11].
In recent years, accumulating evidence has shown that the tumor necrosis factor-α (TNF-α)-induced protein 8-like 2 (TIPE2) has a critical role in maintaining immune homeostasis. It has been found that the peripheral blood TIPE2 level within mononuclear cells of systemic lupus erythematosus (SLS) patients was decreased, and the pro-inflammatory cytokine levels, including IL-6, IL-12, and IFN-gamma, in serum were significantly increased [12]. Experimental evidence indicates that septic shock can be dramatically aggravated in TIPE2−/− animals relative to those in wild-type animals, which suggests the potential direct relationship of TIPE2 with suppression of septic shock [13]. TIPE2-deficient cells are hyper-responsive to activation of T cell receptor (TCR) and Toll-like receptor (TLR). Importantly, TIPE2 binds to caspase-8 and inhibits activation of protein-1 and nuclear factor-κB activation, while promoting Fas-induced apoptosis. Inhibiting caspase-8 significantly blocks the hyper-responsiveness of TIPE2-deficient cells [13].
TIPE2 is also reported to be predominantly expressed in immunocytes, including lymphoid and myeloid cells [14,15]. Furthermore, high TIPE2 protein expression is detected within the inflammatory nerve tissues but not in ordinary nerve tissues [16]. The present study assessed the underlying role of TIPE2 in CD4+ T lymphocyte apoptosis after severe burns, based on the critical role of apoptosis in the pathogenesis of severe burn-induced sepsis, which is of great importance to further understand the relationship of TIPE2 and lymphocytes apoptosis in the pathogenesis of severe burns. We also sought to provide new strategies to regulate inflammatory response and immune response for clinical transformation of sepsis treatment.
Material and Methods
Experimental animals and grouping
We obtained 90 male BALB/c mice (8 to 10 weeks old and weighing 20±2 g) from the Laboratory Animal Center of the Chinese Academy of Medical Sciences (Beijing, China). Small interfering RNA (siRNA) of TIPE2 was obtained from Genchem Co. (Shanghai, China). These 90 mice were randomly assigned to 6 groups: sham (n=15), burn (n=15), burn plus siTIPE2 (siTIPE2-burn, n=15), burn plus siTIPE2 control (negative-burn, n=15), burn plus TIPE2 overexpression (TIPE2-burn, n=15), and burn plus TIPE2 control (TIPE2-negative+burn, n=15).
Animal model
All mice were acclimatized to the new experimental environment for 1 week before the initiation of experiments, and were fasted overnight but with free access to water. Ether was used for anesthesia, and the dorsal and lateral skin of mice was prepared. The exposed mouse skin was scalded for 8 s with hot water (99°C) to establish the severe burn model involving the full-thickness skin of 15% of the total body surface area (TBSA). Mice in the sham group received the same anesthesia before surgery, followed by skin contact with water at room temperature. The number of mouse deaths in each group is shown in Table 1. Mice in the last 4 groups were given caudal venous injection of siTIPE2 overexpression and control mice were administered empty lentivirus vector, respectively. Two weeks later, the mice in each group were sacrificed 24 h after thermal injury.
Table 1.
The number of mice deaths in each group.
| Sham | Burn | siTIPE2-burn | Negative-burn | TIPE2-burn | TIPE2-negative+burn | |
|---|---|---|---|---|---|---|
| Total | 15 | 15 | 15 | 15 | 15 | 15 |
| Number of death | 0 | 3 | 2 | 3 | 4 | 3 |
Each experiment was carried out following the National Institute of Health Guide for the Care and Use of Laboratory Animals, which was approved by Scientific Investigation Board at the 960th Hospital of the PLA Joint Logistics Support Force (2016-47).
Isolation of CD4+ T lymphocytes from spleens
CD4+ T lymphocytes from spleens were prepared according to a previously described method [17,18]. Spleen samples were collected from the mice in each group, followed by gentle agitation with the RPMI 1640. Afterwards, gradient centrifugation was performed to separate the mononuclear cells at the Ficoll-Paque density, and CD4+ T lymphocytes were purified. Then, non-CD4+ T cells were stained with a biotin-antibody cocktail (107/10 ul total cells), followed by 10 min of incubation at 4°C. Cells were then subjected to magnetic labeling using the anti-biotin microbeads (107/20 ul total cells), followed by 15 min of incubation at 4°C. The cell suspension was passed using the MS/LD column of a magnetic-activated cell sorter (MACS) equipped within the MACS magnetic separator, following the manufacturer’s instructions, so as to separate the CD4+ T lymphocytes. Subsequently, the separated CD4+ T lymphocytes were subjected to FITC-labeled anti-CD4+ antibody incubation, and flow cytometry was performed to assess the CD4+ T cell purity after purification. Cells isolated from 1 animal were used for each experiment.
Flow cytometry
In brief, cells (5×105) were collected after centrifugation at 1200 rpm for 8 min and washed with the precooled PBS twice in accordance with the protocol of the PE annexin-V apoptosis kit. We used 100 μl diluents (1% binding buffer) to resuspend 200 μl cells, and 5 μl annexin-V and 7-AAD was then added to incubate cells for 20 min at 4°C in the dark, after which 300 μl binding buffer was added. Finally, cell apoptosis was determined by flow cytometry within 1 h.
Western blotting
The TIPE2, Smad2/Smad3, Bcl-2/Bim, and phosphorylation (P)-Smad2/P-Smad3 protein expression in CD4+ T lymphocytes was detected through Western blotting using the Bradford protein assay kit in accordance with the manufacturer’s protocol. Then, the protein supernatants were collected, mixed with SDS-loading buffer, heated to 96°C for 5 min, separated by 8% SDS-PAGE, and transferred to an Immobilon PVDF membrane. The PVDF membrane was then incubated with primary antibodies, including TIPE2 with specific polyclonal antibody (diluted at 1: 500), rabbit anti-mouse Smad2 monoclonal antibody (1: 2000), rabbit anti-mouse Smad3 monoclonal antibody (1: 2000), rabbit anti-mouse P-Smad2 polyclonal antibody (1: 500), rabbit anti-mouse Bcl-2 monoclonal antibody (1: 1000), rabbit anti-mouse P-Smad3 monoclonal antibody (1: 2000), and rabbit anti-mouse Bim polyclonal antibody (1: 500), and the expression of these proteins was detected through Western blotting. Then, horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody was added for incubation and the protein expression level was expressed as the expression ratio of protein loading/monoclonal anti-β-actin according to the Computer Image analysis. Each immunoblot was observed using the ECL Plus chemiluminescence kit and the protein levels were measured quantitatively by densitometry.
Measurement of mitochondrial membrane potential in CD4+ T lymphocytes by flow cytometry
The dyeing liquid was prepared following the instructions of the JC-1 reagent kit. Cells in each group were suspended in 0.5 ml cell culture fluid, and were reversed several times to mix with 0.5 ml JC-1 dyeing liquid. After 20 min of incubation at 37°C, the obtained homogenate was centrifuged for 4 min at 600 rpm, followed by cell precipitation. Finally, the membrane potential was measured by flow cytometry after washing twice with JCM staining buffer.
Cytochrome C expression detected by laser scanning confocal microscopy
CD4+ T lymphocytes (5×105) were harvested through centrifugation and washing 3 times for 10 min each time using PBS. Later, cells were subjected to 20 min of fixation and washing in 4% paraformaldehyde fixative solution supplemented with PBS, and the CD4+ T lymphocyte membrane after fixation was later subjected to 20 min of permeabilization using 0.2% Triton X-100 at room temperature. Later, 1% bovine serum albumin (BSA) contained within PBS-T was used for 30 min of section blocking, followed by staining using specific polyclonal anti-cytochrome C diluted at 1: 400 with PBS-T. Then, the sections were washed as mentioned earlier before the DyLight TM549-conjugated-labeled goat anti-rabbit IgG secondary antibody was added for 2 h of reaction at room temperature. The sections were then subjected 3 times to PBS-T washing, after which the nuclei were subjected to 5 min of 4,6-diamidino-2-phenylindole (DAPI) staining. Eventually, cytochrome C expression in CD4+ T lymphocytes was measured under a confocal laser scanning microscope.
Determination of caspase activity within CD4+ T lymphocytes through chemical chromatography
PBS was used to wash the collected CD4+ T lymphocytes (1×106) twice, after which the supernatant was discarded, 250 μl precooled lysis buffer was added, and the mixture was later placed in an ice bath for 10 min. The homogenate was centrifuged at 1000 rpm for 5 min, the supernatant was transferred to an EP tube, and the caspase activity was determined in the 96-well plate. The reaction system consisted of 100 μg protein, 50 μl reactive solution, and 5 μl caspase colorimetry for substrate. The homogenate was incubated for 2 h at 37°C in the dark, and the results were measured using a microplate spectrophotometer.
Statistical methods
Data are expressed as mean±standard deviation (SD). SPSS 16.0 software was used for all statistical analyses. Differences in the means between different groups were analyzed using one-way analysis of variance (ANOVA), and differences with a p-value of less than 0.05 were considered statistically significant.
Results
siTIPE2 reduced burn-induced CD4+ T cell apoptosis
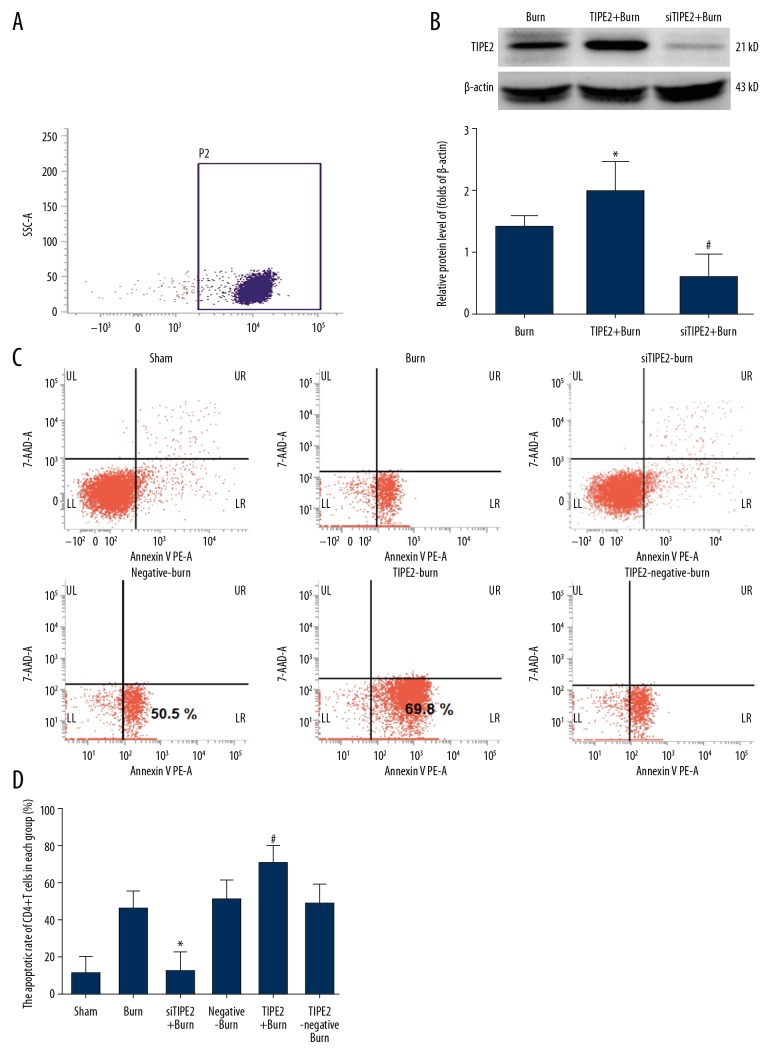
CD4+ T cell purity was first detected through flow cytometry to ensure the percentage of CD4+ T cells was more than 90% (Figure 1A). Two weeks after transfection, the interference or overexpression efficiency of lentivirus (LV)-TIPE2 within CD4+ T lymphocytes was measured through Western blotting (Figure 1B), which showed that TIPE2 expression in TIPE2 gene-silenced CD4+ T lymphocytes was obviously downregulated in mouse spleens. Moreover, high-efficiency transfection (above 70%) was obtained, and TIPE2 protein expression in the TIPE2-burn group was upregulated by over 50% in spleen CD4+ T lymphocytes in mice. Then, cell apoptosis was analyzed by Annexin-V-PE and 7-AAD staining. As shown in Figure 1C and 1D, the CD4+ T cell apoptosis rates of TIPE2-burn animals were higher than in the other groups but were lower in siTIPE2-burn animals relative to those of sham animals (P<0.01).
Figure 1.
siTIPE2 reduced burn-induced CD4+ T lymphocytes apoptosis. The purity of CD4+ T lymphocytes in mouse spleens was first assessed (A). The CD4+ T lymphocytes were transfected with the interference or overexpression lentivirus (LV)-TIPE2 and confirmed by Western blot (B). (C) Cells in each group were stained with Annexin-V-PE/7-AAD and the apoptotic rate was analyzed via flow cytometry. (D) Quantification of the percentage of apoptotic and living cells in each group. Error bars represent the mean±SD. * P<0.05 versus sham animals and # P<0.05 versus burn animals.
Bcl-2/Bim, P-Smad2/P-Smad3, and Smad2/Smad3 protein expression in CD4+ T cells
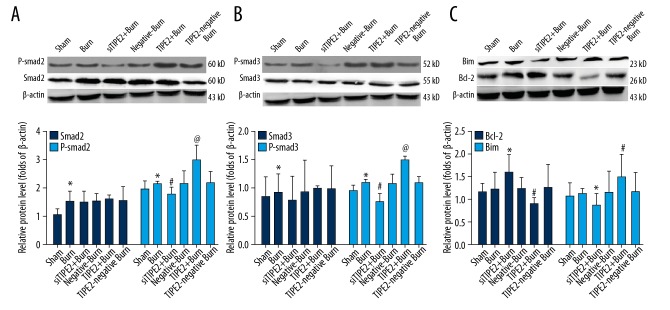
Figure 2 shows that Smad2/Smad3 and P-smad2/P-Smad3 protein levels of burn animals were higher than in sham and siTIPE2-burn groups. Levels of Smad2/Smad3 in the siTIPE2-burn group were not remarkably downregulated, while P-smad2/P-Smad3 protein levels were markedly decreased (P<0.01). Moreover, smad2/Smad3 protein expression levels in the TIPE2-burn group were not evidently higher than those in the other groups, but those of P-smad2/P-Smad3 were remarkably upregulated (P<0.01). The protein expression level of Bcl-2 in the siTIPE2-burn group was upregulated (P<0.05) while that of Bim was decreased (P<0.05), and the changes in Bcl-2/Bim expression levels were the opposite in the TIPE2-burn group.
Figure 2.
Bcl-2/Bim, Smad3/P-Smad3, and Smad2/P-Smad2 protein expression within CD4+ T lymphocytes. Cells had been transfected with siTIPE2 or TIPE2 overexpression lentivirus as well as the control vectors. The Smad2/P-Smad2 (A), Smad3/P-Smad3 (B), and Bcl-2/Bim (C) protein levels were detected by Western blot. Error bars represent mean±SD. * P<0.05 versus sham animals; @ P<0.05 versus burn animals; and # P<0.05 versus burn animals.
siTIPE2 maintained mitochondrial membrane potential (MMP) while reducing cytochrome C expression within CD4+ T lymphocytes
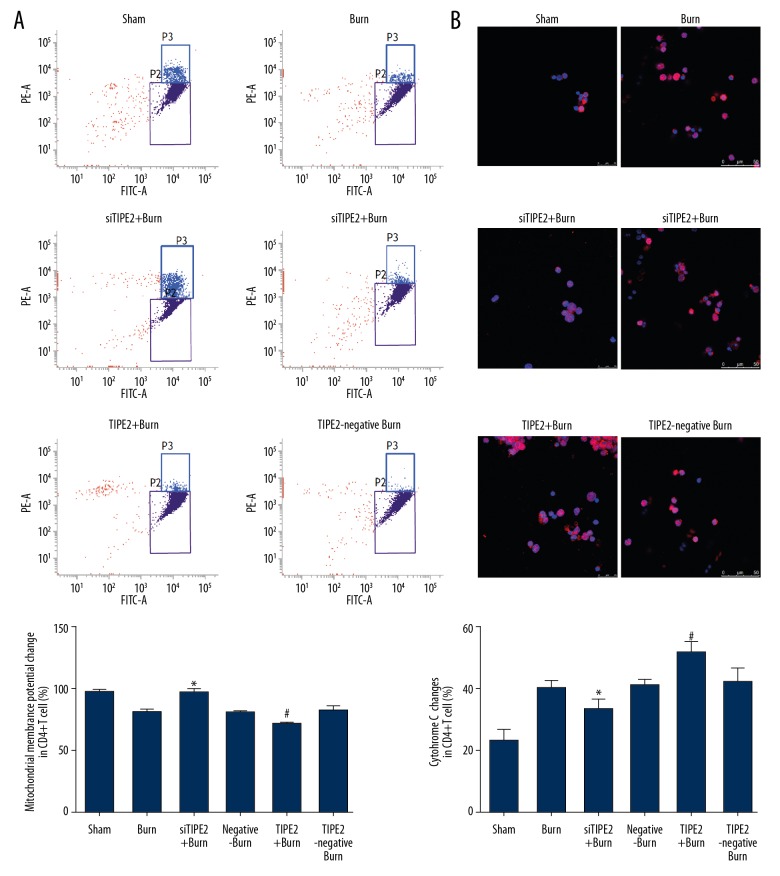
The mitochondrial membrane potential in each group was evaluated using the JC-1 probe. According to our results, the MMP of siTIPE2-burn animals was increased relative to that of the other 4 groups (P<0.05), whereas that in CD4+ T lymphocytes of the TIPE2-burn group was evidently decreased (Figure 3A, P<0.01). Additionally, we found that cytochrome C expression in the siTIPE2-burn group was markedly downregulated (P<0.05), while that in the TIPE2-burn group was obviously elevated (P<0.05, Figure 3B).
Figure 3.
siTIPE2 maintained MMP levels while reducing cytochrome C expression within CD4+ T lymphocytes. (A) The mitochondrial membrane potential in each group was then evaluated using the JC-1 probe and analyzed by flow cytometry. The percentage of mitochondrial membrane potential change was also calculated. (B) Cytochrome C expression in each group was detected by laser scanning confocal microscopy. Error bars represent the mean±SD. * P<0.05 versus sham animals and # P<0.05 versus burn animals.
siTIPE2 reduced the caspases activities of CD4+ T lymphocytes in various groups
As shown in Figure 4, the caspase-3 activity of CD4+ T lymphocytes in the siTIPE2-burn group was not evidently different relative to those in the sham, burn, burn with siTIPE2 control, and burn with TIPE2 control groups, but was decreased relative to that of TIPE2-burn animals (P<0.01). Besides, the activities of caspase-9 and caspase-8 in CD4+ T lymphocytes of siTIPE2-burn animals were obviously lower than those in the other groups (P<0.05). Compared with the other groups, the caspase-9, caspase-8, and caspase-3 activities of TIPE2-burn animals were dramatically higher (P<0.05).
Figure 4.
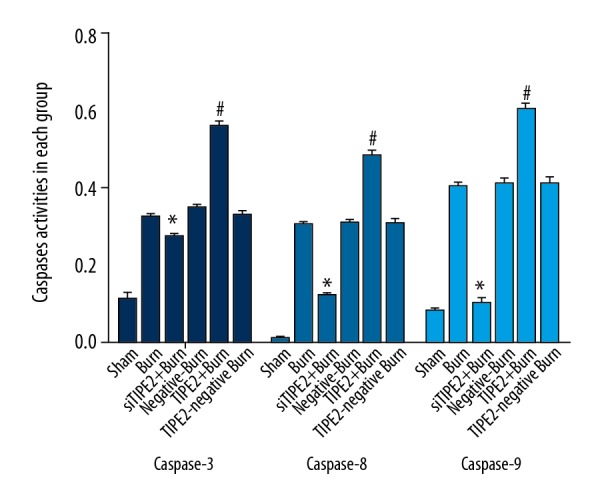
siTIPE2 reduced the caspase-3, -8, and -9 activities of CD4+ T lymphocytes in each group. Caspase-3, -8, and- 9 activities in CD4+ T lymphocytes in each group were detected by chemical chromatography assay. Error bars represent the mean±SD. * P<0.05 versus burn group; # P<0.05 versus siTIPE2+ burn group.
Discussion
Previous studies have mostly focused on inflammation regulation and anti-tumor effect of TIPE2 [17,19,20] and it is not clear whether TIPE2 participates in the pathophysiological process of cellular immune dysfunction after burn. Our preliminary work has shown the TIPE-2 functions in CD4+ lymphocytes apoptosis [21], but it was still unclear about the impact of TIPE-2 on the downstream factors related to cell apoptosis. Based on our previous study, our current work evaluated the role of TIPE2 in CD4+ T cell apoptosis in a mouse model of thermal injury. Our study demonstrated that siTIPE2 could markedly reduce the apoptosis of splenic T lymphocytes after severe burns, which was closely associated with the inhibition of Smad2/Smad3, the downregulation of Bim, and the upregulation of Bcl-2 in CD4+ T lymphocytes.
As shown by Sun et al. [13], TIPE2 plays an important role in adaptive and innate immune responses as a negative regulator by adjusting the signaling of Toll-like and T cell receptors. Interestingly, TIPE2 has high expression in inflamed tissues but has low or no expression in normal tissues [22,23]. Thus, TIPE2 is speculated to be an important regulator of immune system balance and prevents immune hyper-responsiveness. Additionally, it has been demonstrated that TIPE2 is greatly homologous to TNF-α induced protein 8 family members in terms of sequence, and it has been recognized to have a crucial role in regulating cell apoptosis. A previous study by our group showed TIPE2 protein expression within CD4+ T cytoplasm, and TIPE2 protein expression level in the spleen-derived CD4+ T lymphocytes was obviously enhanced from 24 h to 72 h at the termination of thermal injury exposure, which peaked at 24 h [24]. Therefore, 24 h after thermal injury was selected as the observation time point in the current study, with an aim to investigate the thermal injury-induced changes in TIPE2 expression and apoptosis of splenic CD4+ T lymphocytes. It has been found that after downregulation of the TIPE2 gene, the proliferation and apoptosis of splenic T lymphocytes in mice were significantly increased, suggesting that the expression of TIPE2 can promote apoptosis of T lymphocyte [25,26]. In addition, TIPE2 could be related to caspase-8, which also modulates the nuclear factor (NF)-κB pathway through apoptotic enzymes. In addition, it inactivates NF-κB and activator protein-1, but TIPE2-deleted cells have great reactivity to T cell receptor and Toll-like receptor signaling [13,27]. Our current study found that, consistent with previous studies regarding TIPE2 functions, overexpression of TIPE2 significantly increased CD4 + T cell apoptosis in the spleens of severely scalded mice, while CD4+ T cell apoptosis in siTIPE2-burn animals was lower than in the other groups, except for the sham group.
It was well established that regulatory T cells can enhance T cell apoptosis during sepsis, which is achieved through the membrane binding protein of TGFβ1, the Smads pathway, and the mitochondrial pathway [7,18,28]. However, the underlying mechanisms are complicated and involve activation of the downstream Smad2/Smad3 signaling pathway by TGFβ, which is then transferred to the nucleus to regulate the expression of the Bcl-2 family genes through upregulating the expression of pro-apoptotic protein Bim and reducing that of anti-apoptotic protein Bcl-2 [29]. Bax and Bak can combine with the mitochondrial outer membrane to enable apoptosis. Mitochondrial depolarization, permeabilization of the inner mitochondrial membrane, and loss of the mitochondrial potential were also observed, which resulted in release of cytochrome C into the cytoplasm to promote, activate, and cleave caspase-9, finally resulting in cell death [30]. Our results revealed that the phosphorylation of Smad2/Smad3 was remarkably enhanced in the burn group, while TIPE2 gene silencing inhibited the activation of Smad2/Smad3, suggesting that gene silencing of TIPE2 reduces the activation of TGFβ downstream signaling molecules Smad2/Smad3 in splenic T lymphocytes, which could thus markedly downregulate the expression of Bim while upregulating that of Bcl-2 in CD4+ T lymphocytes, thereby reducing the apoptosis of T lymphocytes in mice. In contrast, TIPE2 obviously inhibited the protein expression of Bcl-2 while enhancing that of Bim, and TIPE2 expression seems to promote the apoptosis of T lymphocytes. Notably, mitochondria play a key role in apoptosis by releasing apoptotic genes, and the Bcl-2 family can strictly regulate the permeability of the mitochondrial outer membrane [16]. Our flow cytometry observations revealed that the mitochondrial membrane potential in the siTIPE2-burn group was upregulated and cytochrome C expression was markedly decreased, while the opposite results were observed in the TIPE2-burn group. Meanwhile, the activities of caspase-3, caspase-8, and caspase-9 for TIPE2-burn animals were increased. Thus, in agreement with previous studies, our present study indicates that apoptosis can be initiated by TIPE2, which is transmitted to the mitochondria by the TGFβ pathway, eventually resulting in the release of cytochrome C, activation of caspases family, or release of apoptosis-inducing factors.
It has been demonstrated that the cellular immune state is closely related to the occurrence and development of sepsis, while immunocyte apoptosis is the main cause of immune suppression in the pathogenesis of septic complications [3,31,32]. Additionally, animal studies have confirmed that sepsis can result in extensive apoptosis of immunocytes, while T cells are the main constituents involved in cellular immunity, which plays a key role in the immune response to infection [33,34]. It was also reported that sepsis patients tend to have a sharp decrease in lymphocytes, and that the apoptosis degree of lymphocytes is positively correlated with sepsis severity [32]. As is well known, severe burns and trauma may lead to dysfunction of Th1 lymphocyte subtype, resulting in reduced proliferation of lymphocytes [24,32]. In addition, results of animal and human studies suggest that the apoptosis of peripheral and splenic lymphocytes accounts for the main mechanism of reduced lymphocyte count in sepsis. Therefore, preventing T lymphocyte apoptosis may contribute to enhancing host resistance against septic challenge. Typically, septic animal model studies have found that anti-apoptotic immune therapy can obviously improve the survival rate. Consequently, understanding the potential mechanism underlying T lymphocyte apoptosis and preventing apoptosis may provide a novel approach for research and treatment of severe sepsis [6,23,24,30]. Therefore, to reduce the mortality rate of sepsis, it is mandatory to investigate the precise mechanism of reducing the apoptosis of CD4+ T lymphocytes and to seek novel measures for the accelerated recovery of cell number and function while also blocking the immune suppression pathway [35, 36]. Our study shows the protective effect of siTIPE2 of splenic T lymphocytes after severe burns, suggesting the potential clinical use of TIPE2 interference drugs for sepsis induced by severe burns or trauma.
This study has some limitations. Although sepsis induced by severe burns can result in apoptosis of immunocytes, which play a key role in the immune response to infection and sepsis, and preventing T lymphocyte apoptosis could contribute to enhancing the ability of host resistance against septic challenge, our study was more focused on the effect of siTIPE2 on T lymphocytes apoptosis after burn injuries, and we do not provide direct evidence of the relationship between T lymphocyte apoptosis and sepsis, which is a limitation of our study. Our further research will focus more on the direct relationship between TIPE2 and sepsis after thermal injuries. In addition, our study suggests that siTIPE2 could reduce mortality after burn injuries (Table 1), but detailed mortality studies of the effect of TIPE2 on thermal injuries are still needed.
Conclusions
In the present research, we evaluated the role of TIPE2 in CD4+ T cell apoptosis in a mouse model of thermal injury. We found that TIPE2 is closely correlated with CD4+ T lymphocyte apoptosis during severe burn injuries, and siTIPE2 markedly reduces the apoptosis of splenic T lymphocytes after severe burns, which is closely associated with inhibition of Smad2/Smad3, downregulation of Bim, and upregulation of Bcl-2 in CD4+ T lymphocytes. Our findings suggest that TIPE2, an immunosuppressive protein, is involved in CD4+ T cell apoptosis and pathogenesis after thermal damage. As an important negative regulator, TIPE2 is highly correlated with abnormality in the immunity, and immunocyte apoptosis is the main cause of immune suppression in the pathogenesis of septic complications. Our study provides new insights into the relationship of TIPE2 with lymphocyte apoptosis in the pathogenesis of severe burns, and provides new strategies to regulate inflammatory response and immune response for clinical treatment of sepsis. Nonetheless, more studies are required to elucidate the precise mechanisms by which TIPE2 exerts its regulatory function, which may help to improve clinical ability to recognize and treat immune dysfunction in critically ill patient with postburn sepsis.
Acknowledgements
We thank Ning Dong and Xiaomei Zhu for expert technical assistance and Yingyi Luan for support and critical reading of the manuscript.
Footnotes
Source of support: Departmental sources
References
- 1.Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000–2007) Chest. 2011;140:1223–31. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 2.Zhang QH, Chen Q, Kang JR, et al. Treatment with gelsolin reduces brain inflammation and apoptotic signaling in mice following thermal injury. J Neuroinflammation. 2011;8:118. doi: 10.1186/1742-2094-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 4.Ayala A, Chaudry IH. Immune dysfunction in murine polymicrobial sepsis: Mediators, macrophages, lymphocytes and apoptosis. Shock (Augusta, Ga) 1996;6:S27–38. [PubMed] [Google Scholar]
- 5.Luan Y-Y, Dong N, Xie M, et al. The significance and regulatory mechanisms of innate immune cells in the development of sepsis. J Interferon Cytokine Res. 2014;34:2–15. doi: 10.1089/jir.2013.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang KC, Unsinger J, Davis CG, et al. Multiple triggers of cell death in sepsis: Death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708–19. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis – a potential treatment of sepsis? Clin Infect Dis. 2005;41:S465–S9. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- 8.Goldsmith JR, Chen YH. Regulation of inflammation and tumorigenesis by the TIPE family of phospholipid transfer proteins. Cell Mol Immunol. 2017;14:482–87. doi: 10.1038/cmi.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q-Q, Zhang F-F, Wang F, et al. TIPE2 inhibits lung cancer growth attributing to promotion of apoptosis by regulating some apoptotic molecules expression. PLoS One. 2015;10:e0126176. doi: 10.1371/journal.pone.0126176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Q, Liu Z, Li Z, et al. TIPE2 overexpression suppresses the proliferation, migration, and invasion in prostate cancer cells by inhibiting PI3K/Akt signaling pathway. Oncol Res. 2016;24:305–13. doi: 10.3727/096504016X14666990347437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Tao M, Wu J, et al. Adenovirus-directed expression of TIPE2 suppresses gastric cancer growth via induction of apoptosis and inhibition of AKT and ERK1/2 signaling. Cancer Gene Ther. 2016;23:98–106. doi: 10.1038/cgt.2016.6. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Song L, Fan Y, et al. Down-regulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Immunol. 2009;133:422–27. doi: 10.1016/j.clim.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Sun HH, Gong S, Carmody RJ, et al. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133:415–26. doi: 10.1016/j.cell.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Wang JW, Fan C, et al. Crystal structure of TIPE2 provides insights into immune homeostasis. Nat Struct Mol Biol. 2009;16:89–90. doi: 10.1038/nsmb.1522. [DOI] [PubMed] [Google Scholar]
- 15.Luan Y-y, Yao Y-m, Zhang L, et al. Expression of tumor necrosis factor-α induced protein 8 like-2 contributes to the immunosuppressive property of CD4+CD25+ regulatory T cells in mice. Mol Immunol. 2011;49:219–26. doi: 10.1016/j.molimm.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Gillies LA, Kuwana T. Apoptosis regulation at the mitochondrial outer membrane. J Cell Biochem. 2014;115:632–40. doi: 10.1002/jcb.24709. [DOI] [PubMed] [Google Scholar]
- 17.Jiang L, Yao Y, Zhu X, et al. The effect of tumor necrosis factor-α-induced protein 8 like-2 on immune response of CD4+ T lymphocytes in mice after thermal injury. Eur J Inflamm. 2013;11:87–102. [Google Scholar]
- 18.Luan Y-y, Yin C-f, Qin Q-h, et al. Effect of regulatory T cells on promoting apoptosis of T lymphocyte and its regulatory mechanism in sepsis. J Interferon Cytokine Res. 2015;35:969–80. doi: 10.1089/jir.2014.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gus-Brautbar Y, Johnson D, Zhang L, et al. The anti-inflammatory TIPE2 is an inhibitor of the oncogenic Ras. Mol Cell. 2012;45:610–18. doi: 10.1016/j.molcel.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Wei X, Liu L, et al. TIPE2, a novel regulator of immunity, protects against experimental stroke. J Biol Chem. 2012;287:32546–55. doi: 10.1074/jbc.M112.348755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H, Tian Z, Yao Y, Tanshi LI. [Tumor necrosis factor-α-induced protein 8 like-2 promotes apoptosis of CD4+T lymphocytes in mice with severe burn injury]. Nan Fang Yi Ke Da Xu Bao. 2016;36:1334–39. [in Chinese] [PubMed] [Google Scholar]
- 22.Zhang GZ, Hao CY, Lou YW, et al. Tissue-specific expression of TIPE2 provides insights into its function. Mol Immunol. 2010;47:2435–42. doi: 10.1016/j.molimm.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Zhang GZ, Zhao LY, Wang YY, et al. TIPE2 protein prevents injury-induced restenosis in mice. Bba-Mol Basis Dis. 2015;1852:1574–84. doi: 10.1016/j.bbadis.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Jiang LN, Yao YM, Zhu XM, et al. The effect of tumor necrosis factor-α-induced protein 8 like-2 on immune response of CD4+T lymphocytes in mice after thermal injury. Eur J Inflamm. 2013;11:87–102. [Google Scholar]
- 25.Ruan Q, Wang P, Wang T, et al. MicroRNA-21 regulates T-cell apoptosis by directly targeting the tumor suppressor gene Tipe2. Cell Death Dis. 2014;5:e1095. doi: 10.1038/cddis.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu M-W, Su M-X, Zhang W, et al. Rhodiola rosea suppresses thymus T-lymphocyte apoptosis by downregulating tumor necrosis factor-α-induced protein 8-like-2 in septic rats. Int J Mol Med. 2015;36:386–98. doi: 10.3892/ijmm.2015.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Shi Y, Wang Y, et al. The unique expression profile of human TIPE2 suggests new functions beyond its role in immune regulation. Mol Immunol. 2011;48:1209–15. doi: 10.1016/j.molimm.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, ten Dijke P. Immunoregulation by members of the TGFβ superfamily. Nat Rev Immunol. 2016;16:723–40. doi: 10.1038/nri.2016.112. [DOI] [PubMed] [Google Scholar]
- 29.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 30.Dijke PT, Hill CS. New insights into TGF-β-Smad signalling. Trends Biochem Sci. 2004;29:265–73. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–63. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 32.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotchkiss RS, Chang KC, Grayson MH, et al. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci. 2003;100:6724–29. doi: 10.1073/pnas.1031788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girardot T, Rimmelé T, Venet F, Monneret G. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis. 2017;22:295–305. doi: 10.1007/s10495-016-1325-3. [DOI] [PubMed] [Google Scholar]
- 35.Cabrera-Perez J, Condotta SA, Badovinac VP, Griffith TS. Impact of sepsis on CD4 T cell immunity. J Leukoc Biol. 2014;96:767–77. doi: 10.1189/jlb.5MR0114-067R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JS, Kim SJ, Lee SM. Genipin attenuates sepsis-induced immunosuppression through inhibition of T lymphocyte apoptosis. Int Immunopharmacol. 2015;27:15–23. doi: 10.1016/j.intimp.2015.04.034. [DOI] [PubMed] [Google Scholar]