Abstract
Background
Owing to the increased incidence of photodermatosis caused by ultraviolet light in recent years, it is necessary to clarify the mechanisms potential photodamage to the skin and reveal possible therapeutic targets. Heat shock protein 27 (Hsp27) is well known for suppressing apoptosis. The aim of present study was to elucidate possible photoprotective mechanism between Hsp27 and p21 on ultraviolet B (UVB)-induced photodamage.
Material/Methods
The Hsp27 gene was interfered to assess the expression of its downstream effectors, cell apoptosis, and cell proliferation ability. The cell apoptosis was tested using flow cytometry method. The cell proliferation ability was tested using Cell Counting Kit-8 (CCK-8) assay. The expression of protein was tested using western-blotting method. The expression of mRNA was detected using quantitative reverse transcription polymerase chain reaction (qRT-PCR). The subcellular localization was elucidated using immunofluorescence.
Results
Hsp27 knockdown decreased cell viability and increased the incidence of UVB-induced apoptosis. Compared with control group, activation of phosphorylated-Akt (p-Akt)-dependent pathway resulted in the nuclear accumulation of p21 and suppression of cell proliferation, while promoting apoptosis in Hsp27 knockdown group. In addition, Hsp27 knockdown increased p53 expression and the Bax: Bcl-2 ratio, which further accelerated the apoptotic process.
Conclusions
These findings complemented the mechanism of skin photodamage and demonstrated the photoprotective mechanisms of Hsp27 in HaCaT cells, which might implicate a potential therapeutic target of photodamage and photodermatosis.
MeSH Keywords: Apoptosis; Cyclin-Dependent Kinase Inhibitor p21; Dermatitis, Phototoxic; HSP27 Heat-Shock Proteins; Keratinocytes; Ultraviolet Rays
Background
The skin is the first defensive line against various environmental mutagens which is exposed to ultraviolet (UV) irradiation almost every day, thus the potential risk of UV-induced photodamage is easily neglected. UV irradiation not only causes acute and chronic photodermatosis, but also photoaging of the skin [1].
In our previous work, we found that p21 was closely related to apoptosis and G2 arrest of UVB-irradiated HaCaT cells [2]. The cyclin-dependent kinase (CDK) inhibitor p21 is a cell cycle inhibitor that binds to cyclin, CDK, or cyclin-CDK complexes to prevent progression of the cell cycle and provide extra time for the repair of cells with DNA damage. If damage cannot be restored, the cell will enter process of apoptosis or cell cycle arrest [3].
We also found that the protective function of Hsp27 controlling apoptosis from skin photoaging via the mitochondrial caspase-dependent apoptotic pathway [4,5]. Hsp27 is a small heat shock protein that responds to various stress signals. Hsp27 is well known for its function in inhibiting apoptosis.
Research indicates that HspB1-deficient fibroblasts exhibit increased expression of the CDK inhibitors p27kip1 and p21WAF1/CIP1 [6]. A report by Xu et al. suggested that Hsp27 inhibited p21 transcriptional activation and Hsp27 knockdown led to p21 accumulation in kidney 293A cells [7]. Moreover, some studies showed that Hsp27 regulated cell apoptosis or survival by influencing the subcellular localization of p21 [8,9]. Overall, according to these previous studies, there exists the possibility that Hsp27 and p21 are involved in protecting the skin from UV exposure through anti-apoptotic pathways.
The aims of this study were to elucidate the influence of Hsp27 on UVB-induced apoptosis and to investigate the possible photoprotective mechanisms between Hsp27 and p21 while there are only a few studies reveal it especially in the field of dermatology, which may provide a new therapeutic target for photodermatosis and photodamage.
Material and Methods
Cell culture
HaCaT cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin, and maintained at 37°C in an incubator under an atmosphere of 5% CO2/95% air. The cells were harvested upon reaching confluency of approximately 70%.
UVB irradiation
The UVB irradiation according to previously described methods [4]. HaCaT cells were washed twice after reaching 70% confluency. The UVB-irradiated HaCaT cells in a thin layer of phosphate-buffered saline (PBS) were exposed to UVB (dosage, 30 mJ/cm2; flux, 1.0 mW/cm2; distance, 10 cm) with the use of a UVB lamp (wavelength, 300–320 nm; Netherlands). The UVB dosage was measured using a UV-B 297 radiometer (Photoelectric Instrument Factory of Beijing Normal University, China). Under identical conditions, control HaCaT cells were sham irradiated without UVB irradiation.
Cell proliferation ability assay
Cell Counting Kit-8 (CCK-8) assay was implemented to test cell proliferation ability. Adherent cells (1000 cells/well) were exposed to UVB and cultured under standard conditions for various periods. Following CCK-8 reagent was added and cultured at 37°C for 2 hours. The absorbance at 450 nm was gauged.
Apoptosis analysis by flow cytometry
The cells were harvested after 24 hours (30 mJ/cm2) and diluted to 1×106 cells/mL. Annexin V-fluorescein isothiocyanate and propidium iodide were added (5 μL/well), and cultured for 15 minutes. Flow cytometry and CYTExpert software (Beckman Coulter, USA) were implemented to identify apoptotic cells and apoptotic rate.
Hsp27-lentiviral transfection
In our previous work, shHSP27-b was significantly reduction, so shHsp27-b was selected for lentiviral transfection. Briefly, 2×105 cells were seeded into the 6-well plates and cultured overnight. Polybrene at 6 μg/mL was applied to Hsp27-interference lentiviral vectors (Hanbio Biotechnology, China) at multiplicity of infection of 60. The medium inclusion 6 μg/mL of puromycin (NeoFROXX GmbH, Germany) were used for cultivating cells, and the stably transfected cell lines were achieved after 4 passages.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis
Derivatives of total mRNA using RNAiso Plus Total RNA extraction reagent (TaKaRa Biotechnology, China) and PrimeScriptTM RT reagent Kit (TaKaRa Biotechnology, China) was used for reverse-transcription. TB GreenTM Premix Ex Taq™ II polymerase (TaKaRa Biotechnology, China) was implemented to perform the qRT-PCR amplification. Total mRNA was estimated by qRT-PCR and relative mRNA expression was calculated by 2−ΔΔCt method. The sequences are listed: Akt1-forward/reverse (F/R), CCG AGG TGC TGG AGG ACA AT/TTG TAG AAG GGC AGG CGA CC; TP53-F/R, TGC GTG TTT GTG CCT GTC CT/AGT GCT CGC TTA GTG CTC CCT; p21-F/R, TGC CCA AGC TCT ACC TTC C/CAG GTC CAC ATG GTC TTC CT. The sequences of Hsp27, Bax, Bcl-2, and GAPDH were listed as previously described [4].
Western-blotting method
The cells were lysed on ice with radioimmunoprecipitation assay buffer (1% phenylmethylsulfonyl fluoride, 1% combined protease and phosphatase inhibitors; Boster Biological Technology, China). The BCA Protein Assay kit (Beyotime Institute of Biotechnology, China) was used for measuring the protein concentration. Approximately 30 mg of protein were separated on a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene difluoride (PVDF) membrane. The antibodies used in this study: Hsp27, cleaved caspase-3, Bax, Bcl-2 (Cell Signaling Technology, USA), Hsp27 (Ser82), AKT1/2/3, Akt1 (Ser473), p21 (Abcam, UK), p53, β-actin (Wanleibio, China).
Immunofluorescence staining
Cells were seeded on to coverslips, irradiated with UVB light, and harvested at various time points. Afterward, 4% formaldehyde solution (15 minutes, room temperature) was act on fixed cells, the cell membranes were permeated with 1% Triton X-100 for 10 minutes, blocked with 5% goat or donkey serum for 60 minutes at 37°C, and cultivated overnight at 4°C with primary antibodies. The next morning, the cells were reacted at 37°C for 1 hour with the fluorescent secondary antibodies after washing 3 times. Nuclei were counterstained with 4, 6-diamidino-2-phenylindole (DAPI; Beyotime Institute of Biotechnology, China) for 5 minutes in the dark. All cell samples were imaged using laser confocal microscope (LSM 800; Carl Zeiss AG, Germany). The antibodies in this study: Hsp27 (1: 200; Cell Signaling Technology, USA) and p21 (1: 200; Abcam, UK), Alexa Fluor 594-conjugated goat anti-mouse IgG (1: 200; catalog no. SA00006-3; Proteintech, China), Dylight 488 goat anti-rabbit IgG (1: 200; catalog no. A23230-1; Abbkine Scientific, China), and Dylight 549 goat anti-rabbit IgG (1: 200; catalog no. A23320-1; Abbkine Scientific, China.).
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, USA) and IBM SPSS Statistics 20.0 (IBM Corporation, USA) were used for data analysis. The Student’s t-test was used for testing the differences between 2 groups. One-way analysis of variance was used for analyzing the differences among groups. P<0.05 indicated that the difference was statistically significant.
Results
UVB irradiation inhibited viability and increased apoptosis of HaCaT cells
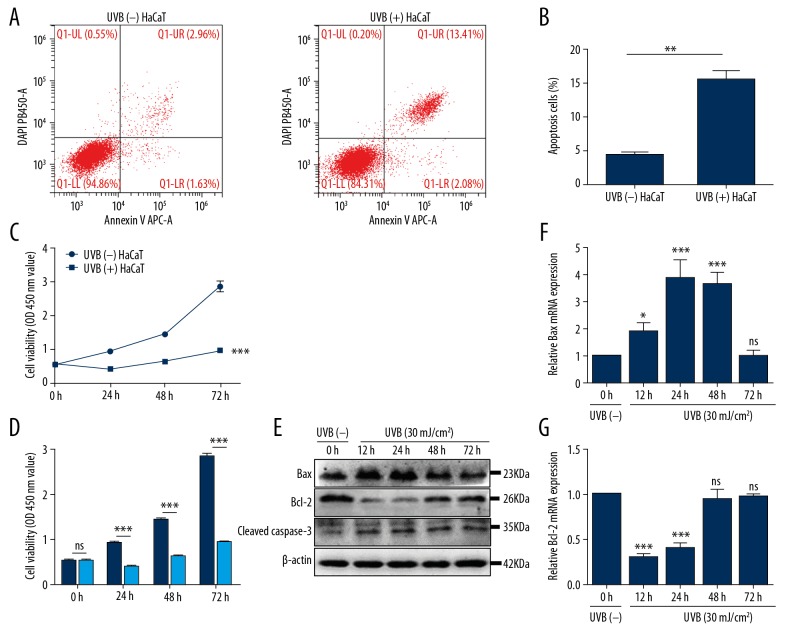
Flow cytometry was performed to identify the influence of UVB irradiation on apoptosis of HaCaT cell. The data suggested that the degree of apoptosis after UVB exposure was significantly greater than sham irradiated HaCaT cells (P=0.0044) (Figure 1A, 1B). Next, CCK-8 assay was implemented to test cell proliferation ability. Our data revealed that the cell proliferation ability after UVB exposure was significantly decreased as compared with sham irradiated HaCaT cells (P<0.0001) (Figure 1C, 1D).
Figure 1.
UVB irradiation inhibited cell proliferation ability and increased apoptosis. (A, B) The proportion of UVB-induced apoptosis was measured by flow cytometry. Bars stand for mean ±SD. ** P<0.01 versus sham-irradiated HaCaT cells [UVB (−) HaCaT], n=3/group. (C, D) At 6, 12, 24, 48, and 72 hours post-UVB (30 mJ/cm2), the cell proliferation ability of UVB-irradiated HaCaT cells was tested using CCK-8 assay. Bars stand for the mean ±SD. *** P<0.001, ns P>0.05 versus UVB (−) HaCaT cells, n=3/group. (E) HaCaT cells were harvested at different time point after UVB irradiation. The protein expression of Bax, Bcl-2, and cleaved caspase-3 were tested using western blotting method; data versus UVB (−) HaCaT cells, n=3/group. (F, G) The relative mRNA expression levels of Bax and Bcl-2 to GAPDH were tested by qRT-PCR at 6, 12, 24, 48, and 72 hours after UVB irradiation. Bars stand for the mean ±SD. *** P<0.001, * P<0.05, ns P>0.05 versus UVB (−) HaCaT cells, n=3/group. SD – standard deviation; UVB – ultraviolet B; CCK-8 – Cell Counting Kit-8; qRT-PCR – quantitative reverse transcription polymerase chain reaction.
To explore the possible apoptotic pathway of UVB-induced apoptosis, the expression of apoptosis-associated protein and gene were detected. Western-blotting revealed that expression of Bax peaked at 12–24 hours, while Bcl-2 was obviously decreased at 12–24 hours and that of cleaved caspase-3 continued to increase at 72 hours (Figure 1E). The results of qRT-PCR analysis of Bax and Bcl-2 were basically the same as those revealed by western-blotting (Figure 1F, 1G). These findings clarified that UVB irradiation induced apoptosis of HaCaT cells through activating the Bax/Bcl-2-dependent mitochondrial apoptotic pathway.
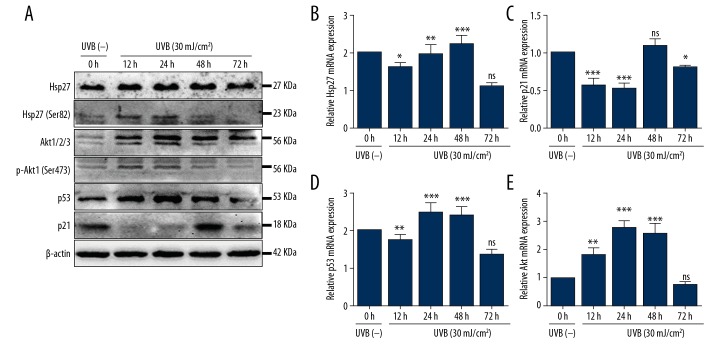
UVB irradiation increased the expression of Hsp27 and decreased that of p21 in HaCaT cells
Western-blotting and qRT-PCR analyses were implemented to test the expression of Hsp27 and p21. Western-blotting showed that Hsp27 expression had gradually increased after UVB exposure, the p-Hsp27 (Ser82) increased after UVB irradiation and maximal expression was detected at 12–24 hours. The p21 was significantly decreased at 12, 24, and 72 hours after UVB irradiation (Figure 2A). Results of qRT-PCR analysis revealed that mRNA of Hsp27 was increased after UVB irradiation (P<0.01 and 0.001 respectively; Figure 2B), while that of p21 decreased at 12, 24, and 72 hours (P<0.0001, 0.0001, and 0.05, respectively; Figure 2C), which was approximately consistent with the expression patterns of the proteins (Figure 2A).
Figure 2.
UVB irradiation induced changes in protein and mRNA expression. (A) Protein expression was measured by western blotting. UVB irradiation (30 mJ/cm2) induced changes in proteins expression profiles versus UVB (−) HaCaT cells, n=3/group. (B–E) The relative mRNA expression levels of Hsp27, Akt, p53, and p21 to GAPDH were detected by qRT-PCR at 6, 12, 24, 48, and 72 hours after UVB exposure. Bars stand for the mean ±SD. *** P<0.001, ** P<0.01, * P<0.05, ns P>0.05 versus UVB (−) HaCaT cells, n=3/group. UVB – ultraviolet B; qRT-PCR – quantitative reverse transcription polymerase chain reaction; SD – standard deviation.
p21 is the specific downstream gene of p53. The classical p53/p21 dependent pathway can result in cell cycle arrest or lead to cell senescence or apoptosis. It has been reported that Hsp27 may contribute to regulation of cellular senescence or apoptosis by modulating p53/p21 pathway [10,11]. So, in this study, we detected the protein and mRNA expression of p53, respectively. The western blotting revealed that p53 was increased after UVB irradiation (Figure 2A). The qRT-PCR results showed that mRNA of p53 were in accordance with that of the protein level (Figure 2D). The data proved that p53 did not increase the expression of p21 after UVB irradiation. As we know, HaCaT cells are immortalized human keratinocytes that harbor 2 point mutations of p53 [12], our results demonstrating that mutated p53 failed to transcriptionally activate the p21 promotor, in contrast to that observed with wild type p53 in HaCaT cells [13].
To further explore the mechanism of Hsp27 in the regulation of apoptosis of HaCaT cells caused by photodamage, Akt expression was tested by western-blotting and qRT-PCR analyses. The results demonstrated that protein and mRNA expression of Akt had significantly increased. Protein expression of p-Akt (Ser473) was obviously increased, the maximal expression was tested at 12–24 hours after UVB irradiation, which was consistent with the expression of p-Hsp27 (Ser82).
Subcellular localization of Hsp27 and p21 in UVB-irradiated HaCaT cells
To determine the connection between Hsp27 and p21, co-localization of Hsp27 and p21 at 24 hours after UVB radiation was elucidated by immunofluorescence. The results showed that the Hsp27 and p21 were observed in both the nuclei and cytoplasm of sham irradiated HaCaT cells, and p21 was detectable in the nuclei for 24 hours after UVB irradiation while Hsp27 was mainly localized in the cytoplasm (Figure 3A). These results indicate that Hsp27 and p21 did not co-localize, and the function of p21 was closely related with the subcellular localization of photodamage caused by UVB irradiation in HaCaT cells.
Figure 3.
Co-localization of Hsp27 and p21, and knockdown of Hsp27 affected cell proliferation ability and apoptosis of UVB-irradiated HaCaT cells. (A) Hsp27 and p21 did not colocalized in UVB-irradiated HaCaT cells. Hsp27 (1: 200) is shown in red, p21 (1: 200) in green, and DAPI nuclear counterstaining in blue. Scale bars=5 μm, n=3/group. (B, C) Protein and mRNA expression of Hsp27 relative to GAPDH were detected by western blotting and qRT-PCR analyses, respectively. Bars stand for the mean ±SD. ** P<0.01, n=3/group. (D, E) Apoptosis of UVB-irradiated shHsp27 cells [UVB (+) shHsp27] was increased as detected by flow cytometry. Bars stand for the mean ±SD. * P<0.05 versus UVB-irradiated shNC cells [UVB (+) shNC], n=3/group. (F) Cell proliferate viability of UVB (+) shHsp27 was decreased as detected with the CCK-8 assay. Bars stand for the mean ±SD. ** P<0.01, *** P<0.001 versus UVB (+) shNC, n=3/group. UVB – ultraviolet B; qRT-PCR – quantitative reverse transcription polymerase chain reaction; DAPI – 4, 6-diamidino-2-phenylindole; SD – standard deviation; CCK-8 – Cell Counting Kit-8.
Knockdown of Hsp27 inhibited cell viability and enhanced apoptosis
Hsp27 gene was interfered by lentivirus-delivered shRNA (Figure 3B, 3C) to demonstrate the photoprotective activities of Hsp27 by suppressing UVB-induced photodamage. Cell proliferation ability was evaluated by the CCK-8 assay. The data indicated that cell proliferation ability was significantly inhibited in shHsp27 cells, as compared with shNC cells (P<0.0001) (Figure 3F). Flow cytometry was performed to determine whether Hsp27 contributes to the UVB-induced apoptosis. Results suggested that proportion of apoptotic shHsp27 cells was greater than that of shNC cells (Figure 3D, 3E) (P=0.0409). Hsp27 inhibition promoted growth inhibition and apoptosis, indicating that Hsp27 plays a photoprotective role in UVB-irradiated HaCaT cells through an anti-apoptotic mechanism.
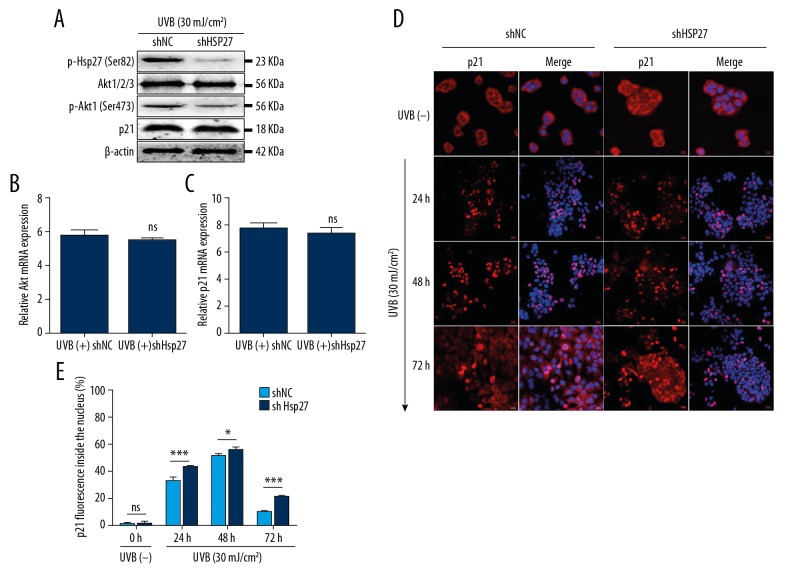
Knockdown of Hsp27 contributed to apoptosis through the regulation of the subcellular localization of p21 by inhibiting the activation of Akt
It has been reported that Hsp27 might induce activation of p-Akt to promote cytoplasmic localization of p21 [9,14]. To determine whether this phenomenon exists in UVB-irradiated HaCaT cells, protein and mRNA expression were tested by western-blotting and qRT-PCR. The present study revealed that protein and mRNA expression of Akt and p21 were unaffected by Hsp27 inhibition after UVB irradiation (Figures 4A–4C), but p-Akt1 (Ser473) was closely related to p-Hsp27 (Ser82) in HaCaT cells (Figures 2A, 4A). After exposure to UVB (30 mJ/cm2), the expression of p-Akt (Ser473) was significantly decreased in shHsp27 cells, as compared to shNC cells.
Figure 4.
Knockdown of Hsp27 induced nuclear accumulation of p21 through the Akt/p21-dependent pathways. (A) The expression of p-Hsp27 (Ser82), Akt, p-Akt (Ser473), and p21 in UVB (+) shHsp27, as compared to UVB (+) shNC, were measured by western-blotting method, n=3/group. (B, C) The relative mRNA expression levels of Akt and p21 to GAPDH were detected by qRT-PCR. Bars stand for the mean ±SD. ns P>0.05 versus UVB (+) shNC. (D) p21 (1: 200) is shown in red and DAPI nuclear counterstaining in blue. Scale bars=20 μm, n=3/group. (E) The statistic of p21 fluorescence inside the nucleus (%). Bars stand for the mean ±SD. *** P<0.001, * P<0.05, ns P>0.05 versus shNC cells, n=3/group. UVB – ultraviolet B; qRT-PCR – quantitative reverse transcription polymerase chain reaction; SD – standard deviation.
Then, the localization of p21 at different times after UVB irradiation was elucidated by immunofluorescence. Our results showed that p21 was observed in the nuclei of shNC cells after UVB irradiation, but disappeared at 72 hours. In UVB-irradiated shHsp27 cells, p21 continued to be present in the nuclei even at 72 hours after UVB irradiation (Figure 4D, 4E). These results indicate that Hsp27 inhibition induced nuclear accumulation of p21 by inhibiting the activation of Akt, which resulted in the failure of cells to enter the proliferation process as well as increased apoptosis.
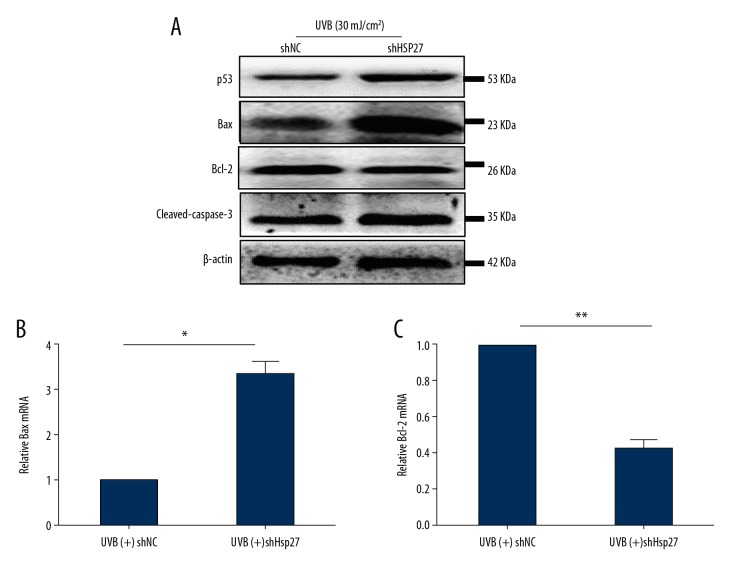
Hsp27 inhibition promoted apoptosis of HaCaT cells by regulation of the p53/Bax/Bcl-2-dependent pathway
Studies have shown that the mutated p53 gene still contribute to apoptosis and differentiation [15]. Bcl-2 and Bax are downstream genes of p53[16]. To determine whether the anti-apoptosis effect of Hsp27 on UVB-induced photodamage is regulated by p53/Bax/Bcl-2-dependent pathways, lentivirus-delivered shRNA was used for interfering with the expression of Hsp27. The results revealed that protein expression of p53, Bax, and cleaved caspase-3 were increased, while that of Bcl-2 was decreased, as compared to UVB-irradiated shNC cells (Figure 5A). The data of qRT-PCR revealed that mRNA expression of Bax and Bcl-2 relative to GAPDH in UVB-irradiated shHsp27 cells were consistent with the trends of protein expression (Figure 5B, 5C). These results demonstrated that knockdown of Hsp27 increased apoptosis of photodamaged HaCaT cells, which might be related to the increases in p53 expression, the Bax: Bcl-2 ratio, and cleaved caspase-3.
Figure 5.
Knockdown of Hsp27 increased apoptosis via activation of the p53/Bax/Bcl-2-dependent apoptotic pathway in HaCaT cells. (A) The protein expression of p53, Bax, Bcl-2, and cleaved-caspase-3 in UVB (+) shHsp27, as compared to UVB (+) shNC, were calculated using western-blotting method, n=3/group. (B, C) The mRNA expression levels of Bax and Bcl-2 mRNA relative to GAPDH in UVB (+) shHsp27 were detected by qRT-PCR. Bars stand for the mean ±SD. * P<0.05, ** P<0.01 versus UVB (+) shNC, n=3/group. UVB – ultraviolet B; qRT-PCR – quantitative reverse transcription polymerase chain reaction; SD – standard deviation.
Discussion
UV irradiation can cause acute or chronic photodermatosis and even skin tumors. Although these diseases vary with the dosage and duration of UV exposure, the physiopathology of them is characterized by UV-induced apoptosis [17,18].
Apoptosis, or programmed cell death, is a key factor of cell growth and homeostasis that maintains normal cellular proliferation [18]. The mechanisms of apoptosis are highly sophisticated and involve 2 main pathways: the death receptor pathway and the mitochondrial pathway. Our study indicated that UVB-induced apoptosis of HaCaT cells was caused by activating the Bax/Bcl-2-dependent mitochondrial apoptotic pathway.
There are 2 forms of Hsp27 in cells: constitutively expressed Hsp27, which is mainly induced under physiological conditions and associated with cell differentiation and development, and stress-inducible expression of Hsp27, which is mainly responds to different stress signals that regulate the death receptor pathway and the mitochondrial pathway of apoptosis, thereby inhibiting the occurrence of apoptosis and playing an important role in cell protection [19,20]. Our study suggested that Hsp27 play a protective role against UVB-induced photodamage via regulation of the anti-apoptotic pathway which have been confirmed in rat skin and HaCaT cells in our previously published article [4,21].
The CDK inhibitor p21 is a cell cycle inhibitor which was the marker of wild type p53 activity [3]. Several recent studies have reported that p21-mediated apoptosis was associated with the subcellular localization of p21, localization in cytoplasmic signify survival while in nuclear always indicate the inhibition of growth [22,23]. Our results demonstrated that p21 was obviously transported to the nuclear region of the UVB-irradiated cells (Figure 3A). Therefore, we concluded that nuclear localization of p21 also contributes to UVB-induced apoptosis in HaCaT cells.
Furthermore, our data indicated that UVB irradiation induced increase in Hsp27 expression, while the p21 was decreased at both the protein and mRNA levels (Figures 2A–2E), in contrast to findings of a previous study [13]. Lei et al. ascribed the reduced expression of the p21 protein to its degradation both in the nuclei and cytoplasm, which promoted UVB-induced apoptosis [24]. However, in opposition to our findings, an obvious increase in the translocation of p21 to the nuclear region was observed (Figure 3A), which indicated that the function of p21 in UVB-induced apoptosis was closely related to its nuclear localization. Prolonged UVB exposure (12, 24, 48, and 72 hours) was employed in the present study to confirm the findings of Xia et al., while shorter durations (0.5, 1.5, 3, 6, and 9 hours) were used for promoting UVB-induced apoptosis. Furthermore, mRNA expression of p21 was measured by qRT-PCR analysis, unlike the study by Xia et al. The present study demonstrated that UVB irradiation not only decreased the expression of p21 at the protein level, but also decreased the expression of p21 at the mRNA level (Figure 2A, 2C), in accordance with a report by Datto et al. [25] which pointed out the possibly reason of reduced p21 expression was associated with the loss of p53 function.
Although it remains uncertain whether Hsp27 exerts anti-apoptosis effects through the p21-dependent pathway in response to UVB-induced photodamage, but some research indicated that Hsp27 regulated cell apoptosis or survival by influencing the subcellular localization or transcriptional activation of p21. In the present study, immunofluorescence staining showed that there was no co-localization of Hsp27 and p21 after UVB irradiation, suggesting that there might be no direct interaction between these proteins (Figure 3A).
It has been reported that Hsp27 might induced activation of p-Akt to promote cytoplasmic localization of p21 [9,14]. The serine/threonine kinase protein kinase B (Akt) is a crucial regulator of cell growth and proliferation. Lu et al. suggested that the activation of Akt increased cytoplasmic localization of p21 to promote cell survival [8]. Meanwhile, Wu et al. demonstrated that silencing of Hsp27 inhibited Akt phosphorylation and activation, and induced apoptosis of HK-11 cells [26]. These studies suggest that the anti-apoptotic or pro-survival effect of Hsp27 might be related to the activation of p-Akt, which further affects the subcellular localization of p21 after UVB irradiation. Data of present study revealed that protein and mRNA expression of Akt and p21 were unaffected by Hsp27 inhibition after UVB irradiation (Figures 4A–4C), but p-Akt1 (Ser473) was closely related to p-Hsp27 (Ser82) in HaCaT cells (Figure 2A, 4A). Then subcellular localization of p21 at different time points was detected by immunofluorescence. Knockdown of Hsp27 induced p21 protein accumulation in the nucleus, which forced the cells to undergo apoptosis. Meanwhile, the nuclear localization of p21 was significantly reduced at 72 hours after UVB irradiation in the shNC group. These results confirmed our hypothesis that the inhibition of Hsp27 did not affect the expression of Akt and p21, but decreased the activation of p-Akt and induced the nuclear accumulation of p21, which suppressed cell proliferation and increased apoptosis. Ultimately, these results suggested that Hsp27 might regulate survival of UVB-irradiated HaCaT cells through Akt/p21-dependent pathways.
UV irradiation activates apoptotic pathways through eliciting DNA damage with the activation of the tumor suppressor gene p53 [27–29]. p21 is the specific downstream gene of p53. It has been reported that Hsp27 contribute to regulation of cellular senescence or apoptosis by modulating the p53/p21 pathway [10,11]. However, this phenomenon was not observed in photodamaged HaCaT cells caused by UVB irradiation. As we know, HaCaT cells are immortalized human keratinocytes that harbor 2 point mutations of p53, which are typical of UVB-induced DNA damage [12]. Hence, we speculated that activation of the mutated p53 gene failed to increase the transcriptional activity of the p21 gene and the translocation of the p21 protein after UVB irradiation in HaCaT cells, in conformity with a report by Datto et al. [25]. Therefore, we considered that Hsp27 knockdown-induced nuclear localization of p21 might not occur through the classical p53/p21-dependent pathway after UVB irradiation in HaCaT cells.
Furthermore, although the mutated p53 gene was not able to regulate apoptosis of HaCaT cells via the p53/p21-dependent pathway, other studies have demonstrated that mutated p53 still contribute to apoptosis and differentiation [15]. According to our results, knockdown of Hsp27 increased the expression of p53 in UVB-irradiated HaCaT cells. O’Callaghan-Sunol et al. indicated that Hsp27 promoted MDM-2 activity and accelerated the degradation of p53 in drug-treated cancer cells, which is a possible reason for the increase in p53 expression in present study [11]. Subsequently, the expression profiles of Bax and Bcl-2 were modified following the upregulation of p53. Overall, the data revealed that apoptosis was induced in Hsp27-deficient HaCaT cells by activating p53/Bax/Bcl-2-dependent mitochondrial apoptotic pathway after UVB irradiation.
Conclusions
This study selected 2 possible pathways for verification, and found that the classical p53/p21-dependent pathway did not work in photodamaged HaCaT cells. The study intuitively showed the translocation of p21 which accurate explained the regulation mechanism between Hsp27 and p21. Hsp27 exerted photoprotection by modulation of the subcellular localization of p21 through activating the phosphorylated-Akt-dependent pathway and inhibition of the p53/Bax/Bcl-2-dependent mitochondrial apoptotic pathway in HaCaT cells. These findings complemented the mechanism of skin photodamage and demonstrated the photoprotective mechanisms of Hsp27 in HaCaT cells, which might implicate a potential therapeutic target of photodermatosis.
Acknowledgements
We thank the Laboratory Research Center of the First Affiliated Hospital of Chongqing Medical University for Technical Support.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (No. 81573027 and No. 81874238) and Natural Science Foundation Project of CQ (No. cstc2016jcyjA0430)
Conflict of interests
None.
References
- 1.Gilchrest BA. Photoaging. J Invest Dermatol. 2013;133:E2. doi: 10.1038/skinbio.2013.176. [DOI] [PubMed] [Google Scholar]
- 2.Chen A, Huang X, Xue Z, et al. The Role of p21 in apoptosis, proliferation, cell cycle arrest, and antioxidant activity in UVB-irradiated human HaCaT keratinocytes. Med Sci Monit Basic Res. 2015;21:86–95. doi: 10.12659/MSMBR.893608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellegata NS, Antoniono RJ, Redpath JL, Stanbridge EJ. DNA damage and p53-mediated cell cycle arrest: Areevaluation. Proc Natl Acad Sci USA. 1996;93:15210–14. doi: 10.1073/pnas.93.26.15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Huang X, Pan Y, et al. Resveratrol protects HaCaT cells from ultraviolet B-induced photoaging via upregulation of HSP27 and modulation of mitochondrial caspase-dependent apoptotic pathway. Biochem Biophys Res Commun. 2018;499:662–68. doi: 10.1016/j.bbrc.2018.03.207. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Huang X, Wang P, et al. The effects of HSP27 against UVB-induced photoaging in rat skin. Biochem Biophys Res Commun. 2019;512:435–40. doi: 10.1016/j.bbrc.2019.03.076. [DOI] [PubMed] [Google Scholar]
- 6.Crowe J, Aubareda A, Mcnamee K, et al. Heat shock protein B1-deficient mice display impaired wound healing. PLoS One. 2013;8:e77383. doi: 10.1371/journal.pone.0077383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Diao Y, Qi S, et al. Phosphorylated Hsp27 activates ATM-dependent p53 signaling and mediates the resistance of MCF-7 cells to doxorubicin-induced apoptosis. Cell Signal. 2013;25:1176–85. doi: 10.1016/j.cellsig.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Lu H, Sun C, Zhou T, et al. HSP27 Knockdown Increases cytoplasmic p21 and cisplatin sensitivity in ovarian carcinoma cells. Oncol Res. 2016;23:119. doi: 10.3727/096504015X14496932933656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanagasabai R, Karthikeyan KK, Qien W, et al. Hsp27 protects adenocarcinoma cells from UV-induced apoptosis by Akt and p21-dependent pathways of survival. Mol Cancer Res. 2010;8:1399–412. doi: 10.1158/1541-7786.MCR-10-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatakrishnan CD, Dunsmore K, Wong H, et al. HSP27 regulates p53 transcriptional activity in doxorubicin-treated fibroblasts and cardiac H9c2 cells: p21 upregulation and G2/M phase cell cycle arrest. Am J Physiol Heart Circ Physiol. 2008;294:H1736–44. doi: 10.1152/ajpheart.91507.2007. [DOI] [PubMed] [Google Scholar]
- 11.O’Callaghan-Sunol C, Gabai VL, Sherman MY. Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res. 2007;67:11779. doi: 10.1158/0008-5472.CAN-07-2441. [DOI] [PubMed] [Google Scholar]
- 12.Lehman TA, Modali R, Boukamp P, et al. p53 mutations in human immortalized epithelial cell lines. Carcinogenesis. 1993;14:833. doi: 10.1093/carcin/14.5.833. [DOI] [PubMed] [Google Scholar]
- 13.Wagner AJ, Kokontis JM, Hay N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 1994;8:2817–30. doi: 10.1101/gad.8.23.2817. [DOI] [PubMed] [Google Scholar]
- 14.Rui W, Hina K, Paul J, et al. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem. 2007;282:21598–608. doi: 10.1074/jbc.M611316200. [DOI] [PubMed] [Google Scholar]
- 15.Paramio JM, Segrelles C, Laín S, et al. p53 is phosphorylated at the carboxyl terminus and promotes the differentiation of human HaCaT keratinocytes. Mol Carcinog. 2000;29:251–62. doi: 10.1002/1098-2744(200012)29:4<251::aid-mc1007>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Strasser A, Harris AW, Jacks T, et al. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994;79:329–39. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 17.Pustisek N, Situm M. UV-radiation, apoptosis and skin. Coll Antropol. 2011;35(Suppl 2):339–41. [PubMed] [Google Scholar]
- 18.Raj D, Brash DE, Grossman D. Keratinocyte apoptosis in epidermal development and disease. J Invest Dermatol. 2006;126:243–57. doi: 10.1038/sj.jid.5700008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8:61–70. doi: 10.1023/a:1021601103096. [DOI] [PubMed] [Google Scholar]
- 20.Huot J, Lambert H, Lavoie JN, et al. Characterization of 45-kDa/54-kDa HSP27 kinase, a stress-sensitive kinase which may activate the phosphorylation-dependent protective function of mammalian 27-kDa heat-shock protein HSP27. FEBS J. 2010;227:416–27. doi: 10.1111/j.1432-1033.1995.tb20404.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Huang X, Wang P, et al. The effects of HSP27 against UVB-induced photoaging in rat skin. Biochem Biophys Res Commun. 2019;512(3):435–40. doi: 10.1016/j.bbrc.2019.03.076. [DOI] [PubMed] [Google Scholar]
- 22.Asada M, Yamada T, Ichijo H, et al. Apoptosis inhibitory activity of cytoplasmic p21Cip1/WAF1 in monocytic differentiation. EMBO J. 2014;18:1223–34. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schepers H, Geugien M, Eggen BJL, Vellenga E. Constitutive cytoplasmic localization of p21Waf1|[sol]|Cip1 affects the apoptotic process in monocytic leukaemia. Leukemia. 2003;17:2113–21. doi: 10.1038/sj.leu.2403106. [DOI] [PubMed] [Google Scholar]
- 24.Lei X, Liu B, Han W, et al. UVB-Induced p21 degradation promotes apoptosis of human keratinocytes. Photochem Photobiol Sci. 2010;9:1640–48. doi: 10.1039/c0pp00244e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Datto MB, Li Y, Panus JF, et al. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA. 1995;92:5545–49. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu R, Kausar H, Johnson P, et al. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem. 2007;282:21598. doi: 10.1074/jbc.M611316200. [DOI] [PubMed] [Google Scholar]
- 27.Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis – the p53 network. J Cell Sci. 2003;116:4077–85. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- 28.Roos WP, Bernd K. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–50. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Kulms D, Schwarz T. Molecular mechanisms of UV-induced apoptosis. Photodermatol Photoimmunol Photomed. 2010;16:195–201. doi: 10.1034/j.1600-0781.2000.160501.x. [DOI] [PubMed] [Google Scholar]