Abstract
TCF3, also known as E2A, is a well-studied transcription factor that plays an important role in stem cell maintenance and hematopoietic development. The TCF3 gene encodes two related proteins, E12 and E47, which arise from mutually exclusive alternative splicing (MEAS). Since these two proteins have different DNA binding and dimerization domains, this AS event must be strictly regulated to ensure proper isoform ratios. Previously, we found that heterogeneous nuclear ribonucleoprotein (hnRNP) H1/F regulates TCF3 AS by binding to exonic splicing silencers (ESSs) in exon 18b. Here, we identify conserved intronic splicing silencers (ISSs) located between, and far from, the two mutually exclusive exons, and show that they are essential for MEAS. Further, we demonstrate that the hnRNP PTBP1 binds the ISS and is a regulator of TCF3 AS. We also demonstrate that hnRNP H1 and PTBP1 regulate TCF3 AS reciprocally, and that position-dependent interactions between these factors are essential for proper TCF3 MEAS. Our study provides a new model in which MEAS is regulated by cooperative actions of distinct hnRNPs bound to ISSs and ESSs.
Keywords: TCF3, alternative splicing, hnRNPH, PTBP1, intronic splicing silencer
INTRODUCTION
Alternative splicing (AS) is an important mechanism that contributes to the regulation of gene expression and generation of proteomic diversity (Wang et al. 2008; Baralle and Giudice 2017). Whether an alternative exon is included or excluded during splicing is often determined by RNA elements that are recognized by regulatory RNA binding proteins (RBPs) (Chen and Manley 2009). The two groups of classical AS regulators are the hnRNP proteins, which often act as splicing repressors, and serine/arginine-rich (SR) proteins, which usually function as splicing activators. hnRNP proteins bind to exonic splicing silencers (ESSs) or intronic splicing silencers (ISSs) to inhibit splicing, whereas SR proteins function by recognizing exonic splicing enhancers (ESEs). Orchestrating these and/or other splicing regulators/elements results in the major distinct subtypes of AS: exon skipping, intron retention, alternative 3′/5′ splice site usage, and the relatively rare mutually exclusive AS (MEAS).
TCF3, also known as E2A, is a member of the E box protein (class I) family of HLH transcription factors (Murre et al. 1989). Many studies have revealed that TCF3 plays important roles in a variety of developmental processes (Bohrer et al. 2015; Cunningham et al. 2017; Miyazaki et al. 2017). The TCF3 gene encodes two major isoforms that arise from MEAS. These isoforms, E12 and E47, are related transcription factors that differ only in their bHLH DNA binding regions and have different dimerization preferences and hence different DNA binding properties (Sun and Baltimore 1991). This can lead to different functional consequences. For example, one study using knockout mice deficient for E12 or E47 revealed that E47 is essential for developmental progression at the prepro-B cell stage, whereas E12 is dispensable for early B cell development, commitment and maintenance (Beck et al. 2009). In cortical neurogenesis, E47 is required for proper neuronal differentiation and layer-specific localization, whereas E12 is dispensable for early corticogenesis (Pfurr et al. 2017). In addition, we recently reported that E12 and E47 differentially regulate expression of E cadherin during human embryonic stem cell (hESC) differentiation (Yamazaki et al. 2018). These reports together suggest that TCF3 MEAS plays an important role in a variety of developmental processes.
How TCF3 MEAS is regulated is not fully understood. We recently showed that the highly related and developmentally regulated hnRNP H1 and F proteins contribute to TCF3 MEAS regulation during hESC differentiation by binding to ESSs in exon 18b of TCF3 (Yamazaki et al. 2018). However, how hnRNP H1/F function and what other RBPs might be involved are unknown. Indeed, as multiple proteins bind the ESSs identified in our previous study (Yamazaki et al. 2018), it is likely that TCF3 MEAS is regulated by additional RBPs. This possibility is also supported by previous studies by our laboratory, which demonstrated that the hnRNPs PTBP1 and hnRNP A1/A2 function together to regulate MEAS of pyruvate kinase M (PKM) pre-mRNAs (David et al. 2010; Chen et al. 2012).
In this study, we provide additional insights into how TCF3 MEAS is regulated. We identify evolutionarily conserved distal ISSs located between the two mutually exclusive exons and show that they are involved in TCF3 MEAS. RNA affinity assays show that PTBP1 and DDX21, a DEAD-box RNA helicase, bind to these conserved ISSs. We also demonstrate that cooperative actions of PTBP1 bound to these newly identified ISSs and hnRNP H1 bound to ESSs in exon 18b control TCF3 MEAS. Our study thus reveals the details of TCF3 MEAS and suggests a new mechanism of MEAS regulation.
RESULTS
A distal intronic conserved region is involved in TCF3 MEAS
As described above, our previous study identified ESSs in exon 18b that are essential for TCF3 MEAS. However, given the complexity of MEAS, we suspected that additional sequence elements and regulatory RBPs are involved in control of TCF3 AS. To gain additional insight into the mechanism of TCF3 MEAS, we first set out to identify additional cis-acting sequence elements in the TCF3 pre-mRNA. As an initial approach, we searched for evolutionary conservation throughout the genomic region spanning exon 17 to exon 19. Intriguingly, a distal intronic conserved region (ICR) was found between exons 18a and 18b [>500 nucleotides (nt) from either exon and ∼400 nt in length; Fig. 1A]. We hypothesized that this ICR functions as an intronic splicing regulatory element. To test this idea, we used a previously developed TCF3 minigene system that recapitulates TCF3 MEAS changes (Yamazaki et al. 2018). We initially created two mutated TCF3 minigene constructs, ICRdel, in which only the ICR has been deleted, and Intdel, in which the entire intronic region other than the ICR was deleted (Fig. 1B). These constructs and the unmodified parental vector were transfected into HeLa cells and the resultant transcripts were analyzed by semi-quantitative 32P RT-PCR. As shown in Figure 1C, deletion of the ICR essentially eliminated exon 18b inclusion (or increased exon 18a inclusion) and also gave rise to low levels of a double inclusion (DI) product that included both exons. Interestingly, deletion of other parts of the intron did not significantly affect TCF3 splicing (Fig. 1C). These data suggest that the sequences within the ICR are essential for proper TCF3 MEAS.
FIGURE 1.
The conserved distal intronic region of TCF3 functions as a cis-acting element. (A) Conservation analysis of sequences surrounding TCF3 human exons 18a and 18b across 46 vertebrate species. The TCF3 gene contains a distal ICR between exons 18a and 18b, indicated by the red dashed box. Conservation levels are shown by Phylop and PhastCons. Conserved sequences are shown by Multiz at the bottom. All conservation plots were generated from the UCSC browser using the hg19 genome assembly. (B) Schematic presentations of wild type (WT) and mutated TCF3 minigenes. (C) Splicing of the transfected minigenes (depicted in panel B) in HeLa cells by 32P RT-PCR, using T7 primer and Ex19R. PCR products were digested with PstI and resolved on 5% PAGE gel. Three independent experiments were performed and the representative result was shown.
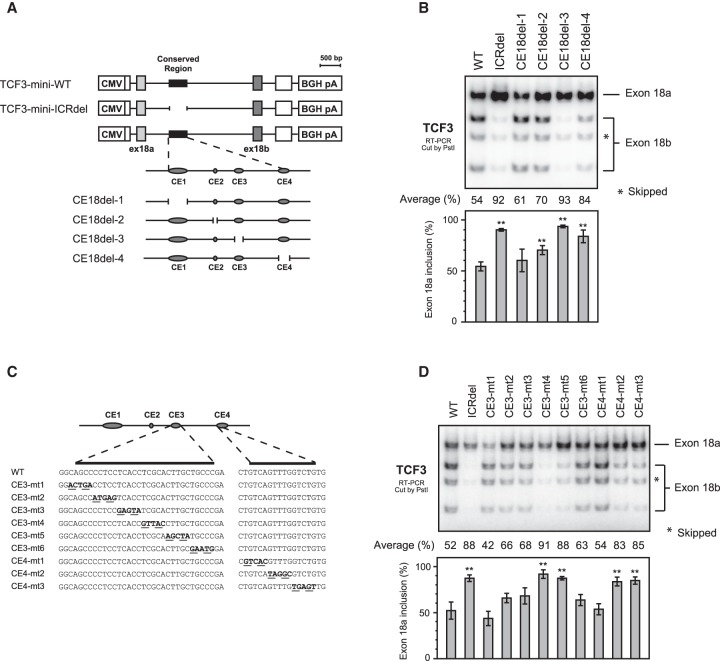
The conservation analysis described above revealed that this intronic region contains four highly conserved distinct motifs, which we named CE1, CE2, CE3, and CE4 (Figs. 1A, 2A). To determine which motif(s) contain(s) the splicing regulatory element(s), we generated four mutated TCF3 minigenes, each devoid of one of the four motifs (CE18del -1, -2, -3 and -4) (Fig. 2A) and used them in transfection assays as above. We quantified percent exon 18a inclusion by dividing the exon 18a signal by the sum of exons 18a and 18b. (Note that the double-skipped isoform was not included, since for unknown reasons the levels of this species displayed relatively high variation from experiment to experiment, whereas the E12/E47 ratios were much more consistent.) Deletion of CE3 or CE4 sharply reduced exon 18b inclusion (percent exon 18a was increased from 54% to 93% or 84%, respectively). Although its effect was relatively mild, CE2 deletion also reduced exon 18b inclusion (from 54% to 70%). On the other hand, deletion of CE1 did not affect the splicing pattern (Fig. 2B). This result indicates that CE3 and CE4 contain sequences that are critical for proper TCF3 MEAS.
FIGURE 2.
Identification of cis-acting elements that mediate TCF3 alternative splicing. (A) Schematic presentations of TCF3 exon-intron structure around the two exon 18s and the minigenes with mutated conserved element (CE). The four distinctive CEs are indicated by gray ovals. (B) Splicing of the transfected TCF3 minigenes (depicted in panel A) in HeLa cells by 32P RT-PCR. PCR products were digested by PstI and resolved on 5% PAGE gel. (C) Schematic presentations of CE3 and CE4 mutated minigenes. (D) Splicing of the transfected TCF3 minigenes (depicted in panel C) in HeLa cells by 32P RT-PCR. (B,D) The exon 18a inclusion percentages were calculated by dividing exon 18a signal by the sum of exons 18a and 18b signals. The results are indicated in bar graph with means ± SD from three independent experiments. Averages of 18a inclusion ratio are indicated on the top. (**) P < 0.01.
Since deletion of CE3 and CE4 had strong effects on TCF3 MEAS, we next set out to characterize the CE3 and CE4 elements further. Specifically, we constructed additional minigenes by mutating CE3 or CE4 in 5 nt blocks (Fig. 2C) and analyzed them as above. In this series of mutations, mutants 4 and 5 in CE3 increased exon 18a inclusion, from 52% to 91% and 88%, respectively. Increases of exon 18a inclusion (from 52% to 83% and 85%) were also observed in mutants 2 and 3 in CE4 (Fig. 2D). These experiments indicate that these two 10 nt motifs, CE3 (TCGCACTTGC) and CE4 (GTTTGGTCTG), constitute intronic regulatory elements that are essential for exon18b inclusion.
DDX21 and PTBP1 bind to the conserved intronic splicing regulatory elements
Having identified intronic RNA elements that are necessary for exon 18b inclusion, we next wished to identify RBPs that bind these sequences. To this end, we performed RNA affinity assays using nuclear extracts (NEs) prepared from HeLa cells and four biotin-labeled 18-nt RNA oligonucleotides containing either the WT CE3 and CE4 regulatory motifs identified above or mutated derivatives (Fig. 3A, left; Fig. 3B, top). Precipitated proteins were resolved by SDS-PAGE and were visualized by silver-staining. This experiment revealed two proteins of molecular weights ∼87 and 55 kDa that bound specifically to WT CE3 and CE4 sequences, respectively, but not to the mutated sequences (Fig. 3A, left; Fig. 3B, left). Mass spectrometry analysis identified the 87 kDa protein as the DEAD-box protein DDX21 and the 55 kDa protein as the RBP PTBP1. Western blots confirmed the identities of the two proteins and their loss of binding to the mutated sequences (Fig. 3A, right; Fig. 3B, right). These data suggest that DDX21 and PTBP1 function in TCF3 MEAS.
FIGURE 3.
PTBP1 and hnRNPH1 reciprocally regulate TCF3 alternative splicing. (A,B) RNA oligonucleotide sequences of WT and mutated (mt) regulatory motifs from CE3 and CE4 (top). Isolated proteins were resolved on 10% SDS-PAGE and were stained by silver staining for visualization (bottom left). The indicated bands were analyzed by mass spectrometry. Protein levels were analyzed by western blot using the indicated antibodies (bottom right). (C) Splicing of the endogenous TCF3 in HeLa cells by 32P RT-PCR after the indicated siRNA treatments. PCR products were digested by PstI and resolved by 5% PAGE. (D) Splicing of the transfected WT TCF3 minigene in HeLa cells by 32P RT-PCR using T7 primer and Ex19R after overexpression of the indicated exogenous genes. DDX21v1 and DDX21v2 are isoforms encoded by the DDX21 gene. (C,D) The exon 18a inclusion percentages are calculated by dividing exon 18a signal by the sum of exons 18a and 18b signals. The results are indicated in bar graph with means ± SD from at least three independent experiments. Averages of 18a inclusion ratio are indicated on the top. (*) P < 0.05. (**) P < 0.01.
PTBP1 and hnRNPH1 reciprocally regulate TCF3 AS
Since the above results suggest that DDX21 and PTBP1 are involved in TCF3 MEAS regulation, we next tested whether depletion or overexpression of either protein affects the splicing pattern of TCF3. We first transfected HeLa cells with siRNAs targeting DDX21 or PTBP1 (see Fig. 3C, bottom). siRNA targeting hnRNP H1, which we used in our previous study (Yamazaki et al. 2018), was used as positive control. Consistent with what we reported previously, hnRNP H1 knockdown (KD) increased exon 18b inclusion (exon 18a in total was decreased from 45% to 28%), likely due to loss of repression that resulted from binding the ESSs in exon 18b (Fig. 3C). In contrast to hnRNP H1 KD, PTBP1 KD increased exon 18a inclusion, from 45% to 53% (Fig. 3C). Next, we cotransfected HeLa cells with DDX21, PTBP1, or hnRNP H1 expression vectors together with the WT TCF3 minigene. As we showed in our previous study (Yamazaki et al. 2018), overexpression of hnRNP H1 suppressed exon 18b inclusion (exon 18a inclusion levels increased from 46% to 65%) (Fig. 3D). The resulting overexpression of PTBP1 suppressed exon 18a inclusion, from 46% to 27% (Fig. 3D). These results indicate that PTBP1 binding to the ICR we identified represses exon 18a inclusion. Unexpectedly, both depletion and overexpression of DDX21 slightly increased exon 18b inclusion (Fig. 3C,D). While our results together suggest a role for DDX21 in TCF3 MEAS, these and other results suggest that it might be complex. We therefore have not pursued DDX21 function further, but discuss its possible role in TCF3 MEAS below.
The ICR represses either exons 18a or 18b
Our results indicate that deletion of the ICR we identified resulted in decreased 18b inclusion and/or increased exon 18a inclusion. To determine whether the ICR functions by repressing exon 18a or by facilitating exon 18b usage, we constructed additional mutated TCF3 minigenes by precisely deleting either exon 18a or 18b, with or without ICR deletion (Fig. 4A), and then analyzed splicing as above. Interestingly, simultaneous deletion of exon 18a and the ICR resulted in more exon 18b inclusion compared to deletion of exon 18a alone, from 34% to 77% (Fig. 4B). However, this phenomenon was also observed following exon 18b deletion: Simultaneous deletion of the ICR enhanced exon 18a inclusion, from 41% to 95% (Fig. 4B). Thus, rather than targeting a specific exon, the ICR appears to repress inclusion of either exon 18a or exon 18b. This raises the possibility that interplay between the ICR and the ESSs we identified previously (Yamazaki et al. 2018) may function to control TCF3 MEAS.
FIGURE 4.
The conserved intronic elements act as splicing silencers against both exons 18a and 18b. (A) Schematic presentations of mutated TCF3 minigenes with one of the two exon 18s deleted, with or without ICR deletion. (B) Splicing of the transfected TCF3 minigenes (depicted in panel A) in HeLa by 32P RT-PCR, using T7 primer and Ex19R. PCR products were digested with PstI. The percentages of all spliced are indicated in bar graph with means ± SD from three independent experiments. (**) P < 0.01. Averages are indicated at the bottom of the RT-PCR image.
Positional relationship of the ESSs and the ICR are essential for TCF3 MEAS
We next asked how the PTBP1-dependent repressive function of the ICR might cooperate with the hnRNP H1/F-dependent ESSs in exon 18b to coordinate TCF3 MEAS. Previously, we observed that swapping the positions of the two ME exons in a TCF3 minigene resulted in the exclusive inclusion of exon 18a, whereas the inclusion ratio of exon 18a to 18b was approximately 1:1 in the WT construct (Yamazaki et al. 2018).
In light of our current results, we considered the possibility that there might be a position-dependent interaction between the ICR and the ESSs. To test this idea, we generated additional mutated TCF3 minigenes with duplicated exon 18a or duplicated exon 18b, by replacing exon 18b with exon 18a, or vice versa (Fig. 5A). To differentiate the two repeated exons, a PstI site was either introduced into the second exon or removed from the first exon by a single nt substitution (CTCCAC to CTGCAG to introduce, or vice versa, to remove). These minigenes thus contain or lack the hnRNP H1/F ESSs in each duplicated exon 18. Because the two exons are highly similar (74% identical) and the two ESSs in exon 18b are the only functional differences between the two, these constructs allow us to determine the effects of the ESSs in a position-dependent manner. The mutated minigenes were transfected into HeLa cells and splicing patterns were determined by RT-PCR. The results showed that the second (distal) exon 18a, located in the original exon 18b position, was exclusively included with the duplicated exon 18a minigene (Fig. 5B, lanes 1 in both panels). This finding is consistent with our previous results that mutation or deletion of the ESSs in exon 18b strongly increased exon 18b inclusion (Yamazaki et al. 2018). On the other hand, in addition to exclusive inclusion of the second exon 18b, we observed a substantial amount of the double-skipped form with the duplicated exon 18b minigene (Fig. 5B, lanes 3 in both panels).
FIGURE 5.
The conserved intronic elements and the exonic silencers prevent DI of the two exon 18s. (A) Schematic presentations of mutated TCF3 minigenes containing double exon 18a or double exon 18b, with or without ICR deletion. (B) Splicing of the transfected TCF3 minigenes (depicted in panel A) in HeLa by RT-PCR, using T7 primer and Ex19R. PCR products were resolved by 5% PAGE before digestion (left) and after digestion with PstI (right).
We next wished to examine the effect of the ICR in the context of the duplicated exons containing or lacking the ESSs. To this end, we deleted the ICR from the double exon 18a/b minigenes and analyzed splicing as above (Fig. 5A). Strikingly, deletion of the ICR in the duplicated exon 18a minigene gave rise to a substantial amount of the DI form, in contrast to the inclusion of only the distal exon 18a observed in the presence of the ICR (Fig. 5B, lanes 2 in both panels). This result supports the idea that the ICR functions to prevent exon 18a inclusion, although the ICR's repressive function is not limited to this exon (see Fig. 4), and is also consistent with our finding that reduced levels of PTBP1, which binds to the ICR, increased exon 18a inclusion (Fig. 3). Furthermore, deletion of the ICR in the duplicated exon 18a minigene prevented single inclusion of the first (natural) exon 18a (Fig. 5B). This result indicates that the ESSs in exon 18b are essential for the sole inclusion of exon 18a. In addition, the finding that deleting the ICR created the DI form, not only in the duplicated exon 18a construct but also in the WT minigene, indicates that the repressive function of the ICR is important to prevent DI even when the ESSs are present in exon 18b. In contrast to the duplicated exon 18a minigenes, removing the ICR from the double exon 18b minigene resulted in the same splicing pattern as that of the same minigene with the ICR (Fig. 5, lanes 3 and 4 in both panels). Since these two duplicated exon 18b constructs both have exon 18b in the original exon 18a position, the presence of the additional ESSs likely completely repressed inclusion of the first exon 18b. Taken together, our data indicate that position-dependent cooperative actions of the ICR and the ESSs are essential for TCF3 MEAS. We discuss below more details of the underlying mechanism.
DISCUSSION
TCF3 MEAS was first described 30 yr ago (Murre et al. 1989). Since then, accumulating evidence has suggested that this process has a great impact on multiple biological processes. However, how TCF3 MEAS is controlled has not been fully elucidated. Recently, we found that hnRNP H1/F regulates TCF3 isoform expression by binding to ESSs in exon 18b in hESCs (Yamazaki et al. 2018). In this study, we provide additional insights into how this process is regulated. We showed that evolutionarily conserved ISSs (which we refer to as the ICR) located between the two mutually exclusive exons are essential for proper TCF3 MEAS. RNA affinity purification showed that PTBP1 and DDX21 bind the ICR, and functional assays showed that PTBP1 and hnRNP H1 determine which exon is included in a reciprocal manner. That is, when PTBP1 levels are high exon 18b is preferentially included, whereas when hnRNP H1 levels are high exon 18a is included. In addition, our data indicate that position-dependent cooperative interactions between PTBP1/ICR and hnRNP H1/ESSs are essential for proper MEAS regulation. Below we discuss these new insights into the molecular mechanism of TCF3 MEAS in more detail, as well as the implications of our results with respect to alternative splicing control more generally.
The main finding of our work is that bindings of the ESSs by hnRNP H1 and the ICR by PTBP1 cooperate in TCF3 MEAS, in a manner regulated by the intracellular levels of the two proteins. Based on our data, we propose the following two-step model for control of TCF3 MEAS (Fig. 6). The first step is binding of PTBP1 to the ICR (Fig. 6A). Even though the ICR's repressive function need not be specific to exon 18a, our observations that removing it leads to single inclusion of exon 18a or to the production of the DI form suggests that the natural target of the ICR is exon 18a. This may be simply because exon 18a is transcribed first and exon or intron definition would be prevented by ICR-bound PTBP1 until exon 18b is transcribed. Supporting this view, multiple studies have shown that PTBP1 indeed induces skipping of exons by inhibiting exon and intron definition (Wagner and Garcia-Blanco 2001; Izquierdo et al. 2005; Sharma et al. 2005, 2008, 2011; Wongpalee et al. 2016). The second step is selection of exon 18a or b, which depends on hnRNP H1 availability. When hnRNP H1 levels are low, the inclusion of exon 18b is favored because exon 18a is repressed by PTBP1 bound to the ICR (Fig. 6B). Conversely, when highly expressed, hnRNP H1 binds to the ESSs in exon 18b and represses 18b inclusion. We suspect that hnRNP H1 might “transfer” the repressive effect of the ICR from exon 18a to exon 18b by physically interacting with PTBP1 under the above conditions (Fig. 6C). This idea arose from our data that ICR deletion increases not only single inclusion of exon 18a but also DI, suggesting that TCF3 AS would not be mutually exclusive if ICR function is solely suppression of exon 18a. The fact that the repressive function of the ICR does not target specific exon 18a or b sequences also supports this model. And importantly, multiple studies have pointed to the existence of physical, cooperative interactions between hnRNP H1 and PTBP1 (Min et al. 1995; Chou et al. 1999; Markovtsov et al. 2000; Rooke et al. 2003). Together, this mode of action successfully explains the mutually exclusive nature of TCF3 AS.
FIGURE 6.
A model for TCF3 mutually exclusive alternative splicing. (A) Exon 18a is usually repressed by PTBP1 bound to the ICR. (B) Exon 18b is included under low hnRNP H1 level, because exon 18a is excluded by PTBP1. (C) Exon 18a is included under high hnRNP H1 level, because exon 18b is repressed by hnRNP H1 and the repressive function of the ICR is transferred to exon 18b (blue arrow) by physical interaction between PTBP1 and hnRNP H1 (red double headed arrow).
Distinct mechanisms of MEAS regulation have been documented in previous studies. Early work with α-tropomyosin pre-mRNA revealed that an unusual far upstream branch site utilized by the downstream ME exon (exon 3) is incompatible with splicing of the upstream exon (Smith and Nadal-Ginard 1989), leading to selective use of exon 3. Exon 2 usage is induced by repression of exon 3, which interestingly is mediated by PTB binding to repressive elements around that exon (Gooding et al. 1998). Our laboratory previously showed that PKM MEAS is controlled by PTBP1 and hnRNP A1/A2 in a concentration-dependent manner (David et al. 2010; Chen et al. 2012). However, the TCF3 MEAS mechanism revealed by the current study is different from the PKM MEAS mechanism, even though PTBP1 functions in both. For example, inclusion or exclusion of one of the two ME exons in PKM AS is independent of exon position and is dominantly determined by ESSs in exon 9 (the upstream exon), whereas TCF3 MEAS is dependent on the position of the two exons for proper cooperative actions between the ESSs and ICR. Additionally, PKM MEAS requires many cis-elements in the ME exons and flanking introns, while TCF3 MEAS appears to be mostly regulated by the ESSs in exon18b and the ICR. These differences in regulatory mechanisms between different MEAS events provide important insights into how interactions between multiple splicing factors/elements create diverse modes of AS regulation. Interestingly, PTBP1, hnRNP H1, and hnRNP A have been implicated in the regulation of multiple MEAS events (Llorian et al. 2010; Huelga et al. 2012). It will be interesting to learn how widely each of the above mechanisms, or related ones, is/are used for MEAS regulation.
An additional question is how DDX21 is involved in TCF3 MEAS. DDX21 has been implicated in transcription and ribosomal RNA processing (Calo et al. 2015), but its functions in splicing regulation have not been reported. As with other DDX proteins that are known to function in pre-mRNA splicing (e.g., DDX5, DDX17, DDX23, DDX39; Mathew et al. 2008; Dardenne et al. 2014; Nakata et al. 2017; Lee et al. 2018), DDX21 also has RNA helicases activity (Gustafson and Wessel 2010). In addition, DDX21 indeed copurified with hnRNP H1 and PTBP1 in messenger ribonucleoprotein (mRNP) complexes isolated using an hnRNP A antibody (Close et al. 2012), and was isolated with RNA oligonucleotides corresponding to a characterized exonic splicing regulatory sequence (Hall et al. 2013). These findings support the view that DDX21 functions in some way in splicing regulation. However, our gain and loss of functions experiments did not clarify its function. Either RNAi-mediated depletion or overexpression of DDX21 resulted in a very modest increase in exon 18b inclusion. Interestingly, and consistent with our findings, data from the ENCODE project (https://www.encodeproject.org) showed that DDX21 KD did not have strong effects on the TCF3 MEAS, in HepG2 and K562 cells, although DDX21 binding was detected on the ICR (precisely on CE3) by eCLIP in K562 cells. One possible explanation for these results is that when DDX21 is depleted with RNAi, another DDX family member may function redundantly, and this may in fact mimic the DDX21 overexpression effect we observed.
Another interesting question is how might DDX21 function in TCF3 MEAS. Interestingly, a recent study revealed that DDX21 binds and unwinds RNA G-quadruplex structures (G-Qs) (McRae et al. 2017). G-Qs are known to be an important secondary structure formed from some G-rich sequences and are involved in multiple gene expression steps including alternative splicing (Verma and Das 2018; Weldon et al. 2018). Furthermore, hnRNP H1 is known to bind RNAs that form G-Qs although it displays somewhat higher affinity when the ability of the RNA to form G-Qs is reduced (Conlon et al. 2016). Notably, both ESSs in exon18b, which are recognized by hnRNP H1, are predicted to form G-Qs, as simulated by QGRS mapper (Kikin et al. 2006). Thus, an attractive idea is that DDX21 is involved in TCF3 MEAS by modulating RNA structure to facilitate hnRNP H1 binding.
In addition to the above possibility, the RNA helicase activity of DDX21 is interesting with respect to possible regulatory mechanisms used by distal intronic cis-elements. Although previous studies have described examples of AS regulation with distal intronic regulatory sequences, the mechanisms by which a splicing factor might act on a distant exon have been largely obscure (Guo and Kawamoto 2000; Coté et al. 2001; Baraniak et al. 2003; Dirksen et al. 2003; Lenasi et al. 2006). Interestingly, however, a relatively recent genome-wide study on Rbfox has shown that more than half of Rbfox binding sites that are conserved through evolution in mammalian brain are located distally (>500 nt) from exons, and these intronic elements affect distal exon inclusion by modulating the distance between these elements, affecting exons via long-range RNA–RNA secondary bridge structures (Lovci et al. 2013). The ICR we identified in TCF3 AS has similar characteristics with such distal cis-elements, that is, it is located >500 nt from either exon and evolutionally conserved, at least in mammals. Interestingly, our results showed that deletion of all intronic sequences between exons 18a and b except for the ICR slightly increased production of the DI isoform, consistent with a possible role for long-range interactions in ensuring accurate exon inclusion. While our data has shown that the primary regulatory mechanism for TCF3 MEAS involves interactions between the ESSs/hnRNP H1 and ISSs/PTBP1, it may also be that RNA–RNA secondary bridge structures function in TCF3 MEAS. This suggests another possible function for DDX21 in modulating RNA–RNA secondary bridge structures. In any case, it will be interesting to determine the exact function of DDX21 in future studies.
As indicated above, we found that the ratio of PTBP1 and hnRNP H1 expression levels is important for control of TCF3 MEAS. An intriguing question then is how do levels of these RBPs change during TCF3 AS-related developmental processes. We previously observed the switching of TCF3 MEAS from exon 18a to 18b during hESC differentiation reflected a corresponding decrease in hnRNP H1/F levels (Yamazaki et al. 2018). We speculated that the ratio of PTBP1/hnRNP H/F would increase during hESC differentiation, in keeping with this switch in splicing. However, our data (not shown) suggests a small decrease in PTBP1 and only very modest changes in this ratio, likely insufficient by itself to drive the TCF3 MEAS switch. One possible explanation for this is that tissue-specific paralogs of PTBP1 (such as nPTB and ROD1), which have some redundant functions with PTBP1 (Spellman et al. 2007), increase during differentiation. Indeed, studies have shown that nPTB levels do increase when PTBP1 levels decrease during neural differentiation (Boutz et al. 2007; Linares et al. 2015). This might serve to increase the PTB/hnRNP H/F ratio, and thereby drive the exon 18a to 18b switch. Further investigation will be required to understand how PTBP1 and hnRNP H1/F, perhaps with the help of paralogs such as nPTB and hnRNP H2, function together to control TCF3 MEAS.
In conclusion, we have provided detailed insights into the mechanism of TCF3 MEAS. We found that this process is regulated by interactions between PTBP1 bound to an ICR containing ISS elements and hnRNP H1 bound to ESSs in one of the two ME exons, and that a DEAD-box helicase that binds specifically to an ISS in the same ICR may facilitate MEAS by an unknown mechanism.
MATERIALS AND METHODS
Plasmid constructs
hnRNP H1 and hnRNP F cDNAs were amplified from HeLa cDNA and cloned into pcDNA3 (Invitrogen) with 3×Flag-tag using either BamHI and XbaI or BamH1 and XhoI for mammalian expression. Construction of TCF3 minigene reporters is described in Yamazaki et al. (2018). All mutations of TCF3 minigene were introduced by PCR-based site-directed mutagenesis as previously described (Yamazaki et al. 2018). Primer sequences are available upon requests.
RT-PCR and 32P RT-PCR
Total RNA was extracted from tissue culture samples using the TRIzol (Invitrogen) according to the manufacturer's instructions. Total RNA (1 µg) was reverse-transcribed using Maxima RT (Thermo Scientific) primed with random hexamers (Invitrogen). RT-PCR was performed using Taq DNA polymerase (Invitrogen). For 32P RT-PCR, 3 µCi [32P] dCTP was added per 50 µL PCR reaction. Primers used in the PCR reactions were described in Yamazaki et al. (2018).
Minigene splicing assays
One hundred nanograms of WT or mutated minigene plasmids were transfected into HeLa cells. To examine the effects of exogenous expression of PTBP1, hnRNP H1, 500 ng of empty vector and/or expression vectors were transfected with 50 ng of TCF3 minigene. Forty-eight hours after transfection, cells were collected and the E12/E47 ratio was analyzed using 32P RT-PCR followed by PstI digestion. Digested PCR products were separated on a 5% PAGE gel. ImageQuant TL (GE healthcare) was used for quantification.
Western blotting
Cells were lysed with SDS sample buffer (50 mM Tris pH 7.4, 100 mM NaCl, 1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate, and 1× proteinase inhibitor cocktail [Biotools]) and resolved by SDS-PAGE. Proteins were then transferred to nitrocellulose membranes and incubated with primary antibodies detecting PTBP1 (BB7) (Sigma, 1:8000), DDX21 (Bethyl Laboratories, 1:1000), hnRNPH1 (Bethyl Laboratories, 1:1500), or ACTB (Sigma, 1:30,000) overnight at 4°C. Secondary HRP-conjugated anti-mouse and anti-rabbit IgGs (Sigma) were used at 1:10,000 for 2 h at room temperature. Following washes with PBST (1× PBS, 0.1% Tween-20), ECL-Prime (GE Healthcare) was used to visualize the chemiluminescence signal, and Image J software was used for quantification.
RNA affinity purification
The 5′ biotinylated 18 RNA oligonucleotides were purchased from IDT. NEs from HeLa cells were prepared as previously described (Kleiman and Manley 2001). Three nanomoles biotinylated RNA were incubated with 100 µL of streptavidin-agarose beads (Sigma) overnight at 4°C with rotation. Incubated beads were then washed with binding buffer (10 mM Tris pH7.4, 0.5 mM EDTA, 500 mM NaCl) twice and buffer D (20 mM Hepes pH7.9, 20% glycerol, 300 mM KCl, 0.2 mM EDTA, 0.5 mM DTT) twice. RNA coated beads were then mixed and rotated with NEs from HeLa cells for 4 h at 4°C. After washing beads with buffer D twice and with buffer D without glycerol twice, precipitated proteins were eluted by SDS sample buffer and analyzed by WB.
Silver staining
Isolated proteins were resolved with SDS-PAGE. Gels were fixed by soaking with fixation buffer (40% ethanol, 10% acetic acid) for 30 min. Fixed gels were soaked with sensitizing buffer (0.02% sodium thiosulfate, 0.8 M sodium acetate, 0.1% glutaraldehyde, 30% ethanol) for 30 min. After washing with water for 5 min three times, gels were soaked with reaction buffer (0.2% silver nitrate, 0.04% formaldehyde) for 30 min. Gels were next quickly rinsed with water, then soaked with developing buffer (2% sodium carbonate, 0.04% formaldehyde) for 10 min. The developing step was stopped by soaking with 0.05 M EDTA solution (pH 8.0).
ACKNOWLEDGMENTS
We thank Amr Al-Zain for help with RT-PCR and members of the Manley laboratory for discussions. We also thank Lewis Brown for help with mass spectrometry. This work was supported by US National Institutes of Health (NIH) R35 GM118136 (J.L.M.). L.L. is supported by the National Science Foundation Graduate Research Fellowship Program, grant no. DGE - 1644869.
Footnotes
Article is online at http://www.rnajournal.org/cgi/doi/10.1261/rna.072298.119.
REFERENCES
- Baralle FE, Giudice J. 2017. Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol 18: 437–451. 10.1038/nrm.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraniak AP, Lasda EL, Wagner EJ, Garcia-Blanco MA. 2003. A stem structure in fibroblast growth factor receptor 2 transcripts mediates cell-type-specific splicing by approximating intronic control elements. Mol Cell Biol 23: 9327–9337. 10.1128/MCB.23.24.9327-9337.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K, Peak MM, Ota T, Nemazee D, Murre C. 2009. Distinct roles for E12 and E47 in B cell specification and the sequential rearrangement of immunoglobulin light chain loci. J Exp Med 206: 2271–2284. 10.1084/jem.20090756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer C, Pfurr S, Mammadzada K, Schildge S, Plappert L, Hils M, Pous L, Rauch KS, Dumit VI, Pfeifer D, et al. 2015. The balance of Id3 and E47 determines neural stem/precursor cell differentiation into astrocytes. EMBO J 34: 2804–2819. 10.15252/embj.201591118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutz PL, Stoilov P, Li Q, Lin C-H, Chawla G, Ostrow K, Shiue L, Ares M, Black DL. 2007. A post-transcriptional regulatory switch in polypyrimidine tract-binding proteins reprograms alternative splicing in developing neurons. Genes Dev 21: 1636–1652. 10.1101/gad.1558107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo E, Flynn RA, Martin L, Spitale RC, Chang HY, Wysocka J. 2015. RNA helicase DDX21 coordinates transcription and ribosomal RNA processing. Nature 518: 249–253. 10.1038/nature13923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Manley JL. 2009. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol 10: 741–754. 10.1038/nrm2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, David CJ, Manley JL. 2012. Concentration-dependent control of pyruvate kinase M mutually exclusive splicing by hnRNP proteins. Nat Struct Mol Biol 19: 346–354. 10.1038/nsmb.2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MY, Rooke N, Turck CW, Black DL. 1999. hnRNP H is a component of a splicing enhancer complex that activates a c-src alternative exon in neuronal cells. Mol Cell Biol 19: 69–77. 10.1128/MCB.19.1.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close P, East P, Dirac-Svejstrup AB, Hartmann H, Heron M, Maslen S, Chariot A, Söding J, Skehel M, Svejstrup JQ. 2012. DBIRD complex integrates alternative mRNA splicing with RNA polymerase II transcript elongation. Nature 484: 386–389. 10.1038/nature10925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon EG, Lu L, Sharma A, Yamazaki T, Tang T, Shneider NA, Manley JL. 2016. The C9ORF72 GGGGCC expansion forms RNA G-quadruplex inclusions and sequesters hnRNP H to disrupt splicing in ALS brains. Elife 5: e17820 10.7554/eLife.17820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coté J, Dupuis S, Jiang Z, Wu JY. 2001. Caspase-2 pre-mRNA alternative splicing: identification of an intronic element containing a decoy 3′ acceptor site. Proc Natl Acad Sci 98: 938–943. 10.1073/pnas.98.3.938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Yu MS, McKeithan WL, Spiering S, Carrette F, Huang C-T, Bushway PJ, Tierney M, Albini S, Giacca M, et al. 2017. Id genes are essential for early heart formation. Genes Dev 31: 1325–1338. 10.1101/gad.300400.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne E, Polay Espinoza M, Fattet L, Germann S, Lambert M-P, Neil H, Zonta E, Mortada H, Gratadou L, Deygas M, et al. 2014. RNA helicases DDX5 and DDX17 dynamically orchestrate transcription, miRNA, and splicing programs in cell differentiation. Cell Rep 7: 1900–1913. 10.1016/j.celrep.2014.05.010 [DOI] [PubMed] [Google Scholar]
- David CJ, Chen M, Assanah M, Canoll P, Manley JL. 2010. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 463: 364–368. 10.1038/nature08697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen WP, Mohamed SA, Fisher SA. 2003. Splicing of a myosin phosphatase targeting subunit 1 alternative exon is regulated by intronic cis-elements and a novel bipartite exonic enhancer/silencer element. J Biol Chem 278: 9722–9732. 10.1074/jbc.M207969200 [DOI] [PubMed] [Google Scholar]
- Gooding C, Roberts GC, Smith CW. 1998. Role of an inhibitory pyrimidine element and polypyrimidine tract binding protein in repression of a regulated α-tropomyosin exon. RNA 4: 85–100. [PMC free article] [PubMed] [Google Scholar]
- Guo N, Kawamoto S. 2000. An intronic downstream enhancer promotes 3′ splice site usage of a neural cell-specific exon. J Biol Chem 275: 33641–33649. 10.1074/jbc.M005597200 [DOI] [PubMed] [Google Scholar]
- Gustafson EA, Wessel GM. 2010. DEAD-box helicases: posttranslational regulation and function. Biochem Biophys Res Commun 395: 1–6. 10.1016/j.bbrc.2010.02.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MP, Nagel RJ, Fagg WS, Shiue L, Cline MS, Perriman RJ, Donohue JP, Ares M. 2013. Quaking and PTB control overlapping splicing regulatory networks during muscle cell differentiation. RNA 19: 627–638. 10.1261/rna.038422.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelga SC, Vu AQ, Arnold JD, Liang TY, Liu PP, Yan BY, Donohue JP, Shiue L, Hoon S, Brenner S, et al. 2012. Integrative genome-wide analysis reveals cooperative regulation of alternative splicing by hnRNP proteins. Cell Rep 1: 167–178. 10.1016/j.celrep.2012.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo JM, Majós N, Bonnal S, Martínez C, Castelo R, Guigó R, Bilbao D, Valcárcel J. 2005. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol Cell 19: 475–484. 10.1016/j.molcel.2005.06.015 [DOI] [PubMed] [Google Scholar]
- Kikin O, D'Antonio L, Bagga PS. 2006. QGRS Mapper: a web-based server for predicting G-quadruplexes in nucleotide sequences. Nucleic Acids Res 34: W676–W682. 10.1093/nar/gkl253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman FE, Manley JL. 2001. The BARD1-CstF-50 interaction links mRNA 3′ end formation to DNA damage and tumor suppression. Cell 104: 743–753. 10.1016/S0092-8674(01)00270-7 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Wang Q, Rio DC. 2018. Coordinate regulation of alternative pre-mRNA splicing events by the human RNA chaperone proteins hnRNPA1 and DDX5. Genes Dev 32: 1060–1074. 10.1101/gad.316034.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenasi T, Peterlin BM, Dovc P. 2006. Distal regulation of alternative splicing by splicing enhancer in equine β-casein intron 1. RNA 12: 498–507. 10.1261/rna.7261206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares AJ, Lin C-H, Damianov A, Adams KL, Novitch BG, Black DL. 2015. The splicing regulator PTBP1 controls the activity of the transcription factor Pbx1 during neuronal differentiation. Elife 4: e09268 10.7554/eLife.09268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorian M, Schwartz S, Clark TA, Hollander D, Tan L-Y, Spellman R, Gordon A, Schweitzer AC, de la Grange P, Ast G, et al. 2010. Position-dependent alternative splicing activity revealed by global profiling of alternative splicing events regulated by PTB. Nat Struct Mol Biol 17: 1114–1123. 10.1038/nsmb.1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovci MT, Ghanem D, Marr H, Arnold J, Gee S, Parra M, Liang TY, Stark TJ, Gehman LT, Hoon S, et al. 2013. Rbfox proteins regulate alternative mRNA splicing through evolutionarily conserved RNA bridges. Nat Struct Mol Biol 20: 1434–1442. 10.1038/nsmb.2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovtsov V, Nikolic JM, Goldman JA, Turck CW, Chou MY, Black DL. 2000. Cooperative assembly of an hnRNP complex induced by a tissue-specific homolog of polypyrimidine tract binding protein. Mol Cell Biol 20: 7463–7479. 10.1128/MCB.20.20.7463-7479.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Hartmuth K, Möhlmann S, Urlaub H, Ficner R, Lührmann R. 2008. Phosphorylation of human PRP28 by SRPK2 is required for integration of the U4/U6-U5 tri-snRNP into the spliceosome. Nat Struct Mol Biol 15: 435–443. 10.1038/nsmb.1415 [DOI] [PubMed] [Google Scholar]
- McRae EKS, Booy EP, Moya-Torres A, Ezzati P, Stetefeld J, McKenna SA. 2017. Human DDX21 binds and unwinds RNA guanine quadruplexes. Nucleic Acids Res 45: 6656–6668. 10.1093/nar/gkx380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H, Chan RC, Black DL. 1995. The generally expressed hnRNP F is involved in a neural-specific pre-mRNA splicing event. Genes Dev 9: 2659–2671. 10.1101/gad.9.21.2659 [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Miyazaki K, Chen K, Jin Y, Turner J, Moore AJ, Saito R, Yoshida K, Ogawa S, Rodewald H-R, et al. 2017. The E-Id protein axis specifies adaptive lymphoid cell identity and suppresses thymic innate lymphoid cell development. Immunity 46: 818–834.e4. 10.1016/j.immuni.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C, McCaw PS, Baltimore D. 1989. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell 56: 777–783. 10.1016/0092-8674(89)90682-X [DOI] [PubMed] [Google Scholar]
- Nakata D, Nakao S, Nakayama K, Araki S, Nakayama Y, Aparicio S, Hara T, Nakanishi A. 2017. The RNA helicase DDX39B and its paralog DDX39A regulate androgen receptor splice variant AR-V7 generation. Biochem Biophys Res Commun 483: 271–276. 10.1016/j.bbrc.2016.12.153 [DOI] [PubMed] [Google Scholar]
- Pfurr S, Chu Y-H, Bohrer C, Greulich F, Beattie R, Mammadzada K, Hils M, Arnold SJ, Taylor V, Schachtrup K, et al. 2017. The E2A splice variant E47 regulates the differentiation of projection neurons via p57(KIP2) during cortical development. Development 144: 3917–3931. 10.1242/dev.145698 [DOI] [PubMed] [Google Scholar]
- Rooke N, Markovtsov V, Cagavi E, Black DL. 2003. Roles for SR proteins and hnRNP A1 in the regulation of c-src exon N1. Mol Cell Biol 23: 1874–1884. 10.1128/MCB.23.6.1874-1884.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Falick AM, Black DL. 2005. Polypyrimidine tract binding protein blocks the 5′ splice site-dependent assembly of U2AF and the prespliceosomal E complex. Mol Cell 19: 485–496. 10.1016/j.molcel.2005.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Kohlstaedt LA, Damianov A, Rio DC, Black DL. 2008. Polypyrimidine tract binding protein controls the transition from exon definition to an intron defined spliceosome. Nat Struct Mol Biol 15: 183–191. 10.1038/nsmb.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Maris C, Allain FH-T, Black DL. 2011. U1 snRNA directly interacts with polypyrimidine tract-binding protein during splicing repression. Mol Cell 41: 579–588. 10.1016/j.molcel.2011.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CW, Nadal-Ginard B. 1989. Mutually exclusive splicing of α-tropomyosin exons enforced by an unusual lariat branch point location: implications for constitutive splicing. Cell 56: 749–758. 10.1016/0092-8674(89)90678-8 [DOI] [PubMed] [Google Scholar]
- Spellman R, Llorian M, Smith CWJ. 2007. Crossregulation and functional redundancy between the splicing regulator PTB and its paralogs nPTB and ROD1. Mol Cell 27: 420–434. 10.1016/j.molcel.2007.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XH, Baltimore D. 1991. An inhibitory domain of E12 transcription factor prevents DNA binding in E12 homodimers but not in E12 heterodimers. Cell 64: 459–470. 10.1016/0092-8674(91)90653-G [DOI] [PubMed] [Google Scholar]
- Verma SP, Das P. 2018. G-quadruplex structure at intron 2 of TFE3 and its role in Xp11.2 translocation and splicing. Biochim Biophys Acta 1862: 630–636. 10.1016/j.bbagen.2017.11.011 [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Garcia-Blanco MA. 2001. Polypyrimidine tract binding protein antagonizes exon definition. Mol Cell Biol 21: 3281–3288. 10.1128/MCB.21.10.3281-3288.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. 2008. Alternative isoform regulation in human tissue transcriptomes. Nature 456: 470–476. 10.1038/nature07509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon C, Dacanay JG, Gokhale V, Boddupally PVL, Behm-Ansmant I, Burley GA, Branlant C, Hurley LH, Dominguez C, Eperon IC. 2018. Specific G-quadruplex ligands modulate the alternative splicing of Bcl-X. Nucleic Acids Res 46: 886–896. 10.1093/nar/gkx1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongpalee SP, Vashisht A, Sharma S, Chui D, Wohlschlegel JA, Black DL. 2016. Large-scale remodeling of a repressed exon ribonucleoprotein to an exon definition complex active for splicing. Elife 5: e19743 10.7554/eLife.19743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Liu L, Lazarev D, Al-Zain A, Fomin V, Yeung PL, Chambers SM, Lu C-W, Studer L, Manley JL. 2018. TCF3 alternative splicing controlled by hnRNP H/F regulates E-cadherin expression and hESC pluripotency. Genes Dev 32: 1161–1174. 10.1101/gad.316984.118 [DOI] [PMC free article] [PubMed] [Google Scholar]