Alterations in the DNA damage response (DDR) pathway contribute to chemotherapy and radiotherapy resistance. The relevance of DDR defects in gastrointestinal cancers (GI) is understudied. This article characterizes DDR‐defective GI malignancies, exploring genomic context and tumor mutational burden to provide a foundation for future studies.
Keywords: DNA damage response, Tumor mutational burden, Poly (ADP‐ribose) polymerase, Immunotherapy, Gastrointestinal cancers
Abstract
Background.
Alterations in the DNA damage response (DDR) pathway confer sensitivity to certain chemotherapies, radiation, and other DNA damage repair targeted therapies. BRCA1/2 are the most well‐studied DDR genes, but recurrent alterations are described in other DDR pathway members across cancers. Deleterious DDR alterations may sensitize tumor cells to poly (ADP‐ribose) polymerase inhibition, but there are also increasing data suggesting that there may also be synergy with immune checkpoint inhibitors. The relevance of DDR defects in gastrointestinal (GI) cancers is understudied. We sought to characterize DDR‐defective GI malignancies and to explore genomic context and tumor mutational burden (TMB) to provide a platform for future rational investigations.
Materials and Methods.
Tumor samples from 17,486 unique patients with advanced colorectal, gastroesophageal, or small bowel carcinomas were assayed using hybrid‐capture‐based comprehensive genomic profiling including sequencing of 10 predefined DDR genes: ARID1A, ATM, ATR, BRCA1, BRCA2, CDK12, CHEK1, CHEK2, PALB2, and RAD51. TMB (mutations per megabase [mut/Mb]) was calculated from up to 1.14 Mb of sequenced DNA. Clinicopathologic features were extracted and descriptive statistics were used to explore genomic relationships among identified subgroups.
Results.
DDR alterations were found in 17% of cases: gastric adenocarcinoma 475/1,750 (27%), small bowel adenocarcinoma 148/666 (22%), esophageal adenocarcinoma 467/2,501 (19%), and colorectal cancer 1,824/12,569 (15%). ARID1A (9.2%) and ATM (4.7%) were the most commonly altered DDR genes in this series, followed by BRCA2 (2.3%), BRCA1 (1.1%), CHEK2 (1.0%), ATR (0.8%), CDK12 (0.7%), PALB2 (0.6%), CHEK1 (0.1%) and RAD51 (0.1%). More than one DDR gene alteration was found in 24% of cases. High microsatellite instability (MSI‐H) and high TMB (TMB‐H, ≥20 mut/Mb) were found in 19% and 21% of DDR‐altered cases, respectively. Of DDR‐altered/TMB‐H cases, 87% were also MSI‐H. However, even in the microsatellite stable (MSS)/DDR‐wild‐type (WT) versus MSS/DDR‐altered, TMB‐high was seen more frequently (0.4% vs. 3.3%, P < .00001.) Median TMB was 5.4 mut/Mb in the MSS/DDR‐altered subset versus 3.8 mut/Mb in the MSS/DDR‐WT subset (P ≤ .00001), and ATR alterations were enriched in the MSS/TMB‐high cases.
Conclusion.
This is the largest study to examine selected DDR defects in tubular GI cancers and confirms that DDR defects are relatively common and that there is an association between the selected DDR defects and a high TMB in more than 20% of cases. Microsatellite stable DDR‐defective tumors with elevated TMB warrant further exploration.
Implications for Practice.
Deleterious DNA damage response (DDR) alterations may sensitize tumor cells to poly (ADP‐ribose) polymerase inhibition, but also potentially to immune checkpoint inhibitors, owing to accumulation of mutations in DDR‐defective tumors. The relevance of DDR defects in gastrointestinal (GI) cancers is understudied. This article characterizes DDR‐defective GI malignancies and explores genomic context and tumor mutational burden to provide a platform for future rational investigations.
摘要
背景 DNA 损伤修复 (DDR) 通路的改变与某些化疗、放疗和其他 DNA 损伤修复靶向治疗的敏感性密切相关。BRCA1/2 是研究最深入的 DDR 基因,但也有研究描述癌症的其他 DDR 通路成员反复出现突变。DDR 基因的毒化改变可能使肿瘤细胞对多聚 ADP 核糖聚合酶抑制敏感,但也有越来越多的数据表明,也可能与免疫检查点抑制剂具有协同作用。DDR 缺陷在胃肠道 (GI) 肿瘤中的相关性研究尚不充分。本文旨在描述 DDR 缺陷的 GI 恶性肿瘤,探讨其基因组结构和肿瘤突变负荷 (TMB),为未来的合理研究提供基础。
材料和方法 采用基于杂交捕获综合基因组分析 17 486 例晚期结直肠癌、胃食管癌或小肠癌患者的肿瘤样本,包括 10 个预先确定的 DDR 基因序列:ARID1A、ATM、ATR、BRCA1、BRCA2、CDK12、CHEK1、CHEK2、PALB2 以及 RAD51。通过1.14 Mb 的测序 DNA计算出TMB[每百万碱基的突变数(mut/Mb)]。提取临床病理特征,采用描述性统计方法探讨筛选出的亚群之间的基因组关系。
结果 17% 的患者发现 DDR 改变:胃腺癌 475/1 750 (27%)、小肠腺癌 148/666 (22%)、食管腺癌 467/2 501 (19%)、结直肠癌 1 824/12 569 (15%)。ARID1A (9.2%) 与 ATM (4.7%) 是本系列中最常见的 DDR 基因改变,其次为 BRCA2 (2.3%)、BRCA1 (1.1%)、CHEK2 (1.0%)、ATR (0.8%)、CDK12 (0.7%)、PALB2 (0.6%)、CHEK1 (0.1%) 和 RAD51 (0.1%)。24% 的患者出现了一种以上的 DDR 基因改变。DDR 改变的患者中,高微卫星不稳定性 (MSI‐H) 和高 TMB (TMB‐H, ≥20 mut/Mb) 分别占 19% 和 21%。DDR 改变/TMB‐H 患者 87% 为 MSI‐H。在微卫星稳定 (MSS)/DDR‐野生型 (WT) 与 MSS/DDR‐改变的患者中,TMB‐H 同样更为常见(0.4% vs. 3.3%,P < 0.000 01.)。MSS/DDR‐改变组的 TMB 平均值为 5.4 mut/Mb,MSS/DDR‐WT 组 TMB 平均值为 3.8 mut/Mb (P ≤ 0.000 01),而 MSS/TMB‐H 患者的 ATR 改变相对富集。
结论 本文是对 GI 管状腺癌经选 DDR 缺陷规模最大的研究,结果证实 DDR 缺陷相对比较常见,并且超过 20% 的患者经选 DDR 缺陷与高 TMB 相关。微卫星稳定的 DDR 缺陷肿瘤 TMB 升高,值得进一步研究。
实践意义:DDR 基因的毒化改变可能使肿瘤细胞对多聚 ADP 核糖聚合酶抑制敏感,但也可能由于 DDR 缺陷肿瘤中突变的积累,而对免疫检查点抑制剂敏感。DDR 缺陷在胃肠道 (GI) 肿瘤中的相关性研究尚不充分。本文描述了 DDR 缺陷的 GI 恶性肿瘤,探讨了其基因组结构和肿瘤突变负荷,为未来的合理研究提供了基础。
Introduction
The fundamental ability to accurately copy DNA, sense and correct replication errors, and repair potentially damaging defects is central to normal cellular and organismal function. Deleterious alterations in genes important to the DNA damage response (DDR) impact genomic integrity and increase the rates of cancer risk. Both germline and somatic loss of function genomic alterations (GAs) in several DNA damage genes can lead to the inability of cells to repair single‐stranded or double‐stranded DNA breaks, causing cell death [1], [2]. There are nearly 200 genes directly involved in the repair of DNA damage as well as several caretaker genes that may help with DNA damage repair [3]. The most well‐studied examples include BRCA1 and BRCA2, which are required for the repair of double‐stranded DNA breaks through the homologous recombination repair pathway [4]. Although the BRCA genes may be the most well‐described genes involved with homologous repair, there are several other genes and their associated proteins such as ATM, ATR, CHK2, PALB2, and other Fanconi genes that are critically involved with the DDR and found in gastrointestinal (GI) cancers such as pancreatic cancers [5]. Tumor‐specific studies in pancreatic cancer identified germline BRCA mutations and other DDR defects in up to 10% of patients with pancreatic ductal adenocarcinoma (PDAC) and microsatellite instability (MSI) in about 1% [6], [7], [8], [9], [10]. Within pancreatic adenocarcinomas, DDR defects (dDDR) are associated with patterns of genomic structural variation [9].
The therapeutic implications of dDDR and genomic instability are highlighted by the success and U.S. Food and Drug Administration approval of poly (ADP‐ribose) polymerase (PARP) inhibitors in breast and ovarian cancers and most recently in patients with germline BRCA mutations in metastatic PDAC [11], [12], [13], [14], [15], [16]. There are also data supporting sensitivity to certain DNA‐damaging agents such as platinum‐based chemotherapy for some inherited pancreatic cancers, which may be suggestive of defects in DNA repair [17]. Similarly, the extreme instability observed in mismatch repair deficiency is associated with a high tumor mutational burden (TMB), a known positive predictive biomarker for immune checkpoint inhibitors [18], [19], [20]. Supporting the possible expansion of immune‐responsive patients is the observation that dDDR has been shown to be associated with a higher TMB [21], [22], [23]. The scientific rationale between PARP inhibitors and immunotherapy is related to immune activation such that error‐prone repair may cause more point mutations leading to neoantigen formation but also that innate cytosolic DNA can lead to a type I immune‐activating response via the STING path. Furthermore, certain checkpoints such as ATM, ATR, and CHK1 can lead to upregulation of programmed death‐ligand 1 (PD‐L1) [24], [25], [26], [27], [28]. Another example is that of ARID1 deficiency and synergy with immunotherapy [29]. The synergy between immunotherapy and PARP inhibitors has now shown early activity independent of BRCA status in both ovarian and breast cancer [30], [31], [32]. Given this association between DNA damage, genomic instability, and TMB, we sought to determine the prevalence of select DDR defects in tubular GI cancers including esophageal, gastric, small bowel, and colorectal cancer (CRC) and to evaluate the correlation between DDR, TMB, and MSI.
Materials and Methods
Hybrid‐capture‐based comprehensive genomic profiling was performed prospectively on submitted tumor tissue in a Clinical Laboratory Improvement Amendments‐certified, College of American Pathologists‐accredited, New York State‐regulated reference laboratory (Foundation Medicine Inc., Cambridge, MA) to identify genomic alterations, including base substitutions, insertions/deletions, copy number alterations, and rearrangements [33]. At least 50 ng of DNA per specimen was extracted from clinical formalin‐fixed, paraffin‐embedded and consecutively submitted specimens. Next‐generation sequencing (NGS) was performed on hybridization‐captured, adaptor ligation‐based libraries to high, uniform coverage (>500×) for all coding exons of 236 (version 1) or 315 (version 2) cancer‐related genes plus 19 (version 1) or 28 (version 2) genes frequently rearranged in cancer [33]. Further analysis was performed on cases with GAs in 10 genes selected based on an adequate representation of the DDR pathways and coverage by the Foundation Medicine assay. Deleterious alterations in DDR genes included any protein truncating mutations (nonsense and/or frameshift indels), any mutations in the consensus splice donor or acceptor sequence that disrupts the consensus, including insertions and deletions, and any missense or nonframeshift mutations that had been confirmed somatic as described in the COSMIC database. TMB was calculated as the total number of relevant mutations divided by the coding region target territory of the test (0.83 Mb for version 1 and 1.14 Mb for version 2) and is characterized as the number of somatic base substitution or indel alterations per megabase (Mb) after filtering to remove known somatic and deleterious mutations [34]. High tumor mutational burden was defined as greater than 20 mutations per DNA megabase (mut/Mb). MSI was measured by evaluating the changes to 114 loci selected from a total set of 1,897 that have adequate coverage on both the version 1 and version 2 bait sets [35]. Each chosen locus was intronic and had hg19 reference repeat length of 10–20 bp. This range of repeat lengths was chosen so that the microsatellites are long enough to produce a high rate of DNA polymerase slippage but short enough that they are well within the 49 bp read length of NGS to facilitate alignment to the human reference genome. Using the 114 loci, for each sample, we calculated the repeat length in each read that spans the locus. We recorded the means and variances of repeat lengths across the reads, forming 228 data points per sample. In a large training set of data from clinical specimens, we then used principal components analysis to project the 228‐dimension data onto a single dimension (the first principal component) that maximizes the data separation, producing an NGS‐based “MSI score.” Ranges of the MSI score were assigned MSI‐High (MSI‐H) or microsatellite stable (MSS). For the purposes of this study, the MSS designation includes a small number of cases with an ambiguous MSI score. Approval for this study, including a waiver of informed consent and a Health Insurance Portability and Accountability Act waiver of authorization, was obtained from the Western Institutional Review Board (protocol 20152817). Differences between the demographic characteristics of different cohorts of patients were examined using t tests or Fisher's exact test, with two‐sided p value <.05 considered statistically significant. Differences between TMB scores were examined using Mann‐Whitney U test, with one‐sided p value <.05 considered statistically significant. Differences between the prevalence of MSI‐H in different patient cohorts were examined using Fisher's exact test, with one‐sided p value <.05 considered statistically significant.
Results
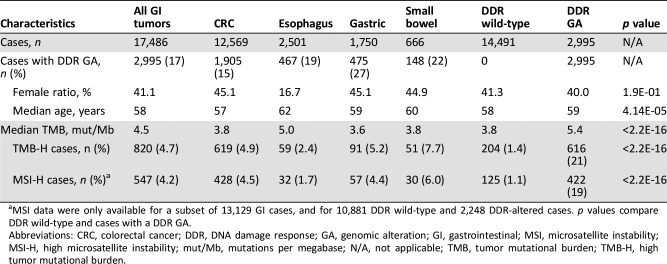
Deleterious DDR GAs in ARID1A, ATM, ATR, BRCA1, BRCA2, CDK12, CHEK1, CHEK2, PALB2, or RAD51 were found in 17.1% (2,995/17,486) of all cases. DDR GAs were most frequent in gastric adenocarcinoma (GC; 475/1,750, 27.1%), followed by small bowel adenocarcinoma (SBA; 148/666, 22.2%), esophageal adenocarcinoma (EA; 467/2,501, 18.7%), and colorectal cancer (CRC; 1,905/12,569, 15.2%; Table 1). The gender distribution for patients with GI cancers harboring DDR GA was 60% male and 40% female, with a median age of 59 years (Table 1). High TMB (TMB‐H; ≥20 mut/Mb) was observed in 20.6% (616/2,995) of DDR‐altered GI cases versus 1.4% (204/10,881) of DDR‐wild‐type (WT) cases. MSI status was available for 2,248/2,995 (75%) of DDR‐altered GI cases, and of those, MSI‐H was observed in 18.8% (422/2,248) versus 1.1% (125/10,881) of DDR‐WT GI cases (Table 1). Of DDR‐altered cases with available MSI data, 78.6% (1,766/2,248) were both MSS and TMB‐Low (TMB <20 mut/MB). Of the DDR‐altered/TMB‐H cases, 87.0% (403/463) were MSI‐H (Fig. 1A, 1B). Of DDR‐altered cases, 13.3% (298/2,248) also had one or more GAs in a mismatch repair (MMR) gene (MLHI, MSH2, MSH6, or PMS2). MMR GAs were present in 58.3% (270/463) of DDR‐altered/TMB‐H cases (Fig. 1B). Among MSI‐H GI cases, the median TMB was significantly higher in cases with a DDR alteration than in DDR‐WT cases (50.5 vs. 35.1 mutations/Mb, p < .0001).
Table 1. Overview of clinical characteristics and genomic features of patients with GI tumors by tumor type and presence of DDR alteration.
MSI data were only available for a subset of 13,129 GI cases, and for 10,881 DDR wild‐type and 2,248 DDR‐altered cases. p values compare DDR wild‐type and cases with a DDR GA.
Abbreviations: CRC, colorectal cancer; DDR, DNA damage response; GA, genomic alteration; GI, gastrointestinal; MSI, microsatellite instability; MSI‐H, high microsatellite instability; mut/Mb, mutations per megabase; N/A, not applicable; TMB, tumor mutational burden; TMB‐H, high tumor mutational burden.
Figure 1.
Venn diagram showing overlap of gastrointestinal (GI) cases defined by the indicated molecular features. (A): MSI‐H cases, TMB‐H cases, and DDR‐altered cases within the GI cohort (n = 13,129 with available microsatellite instability [MSI] data). (B): MSI‐H cases, TMB‐H cases, and cases with GA in MMR genes (MLH1, MSH2, MSH6, PMS2) within the DDR‐altered GI cohort (n = 2,248 with available MSI data).
Abbreviations: DDR, DNA damage response; GA, genomic alteration; MMR, mismatch repair; MSI‐H, high microsatellite instability; mut, mutations; pts, patients; TMB‐H, high tumor mutational burden.
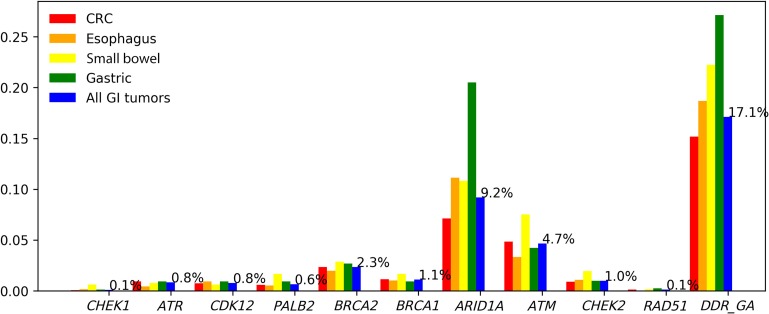
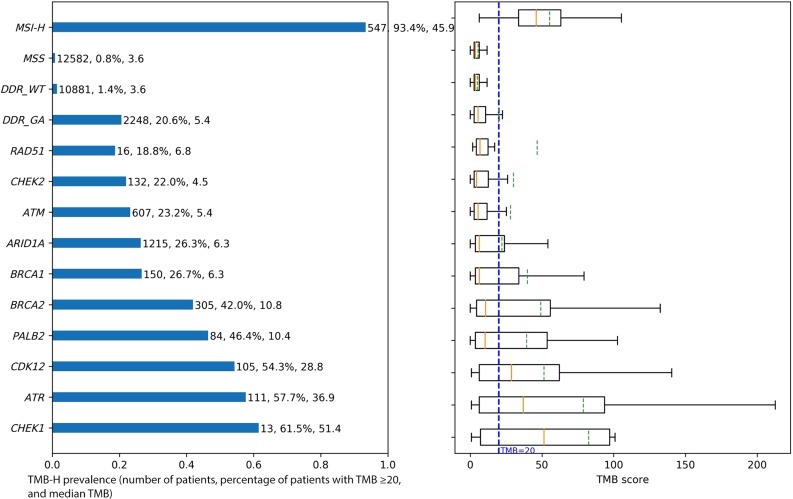
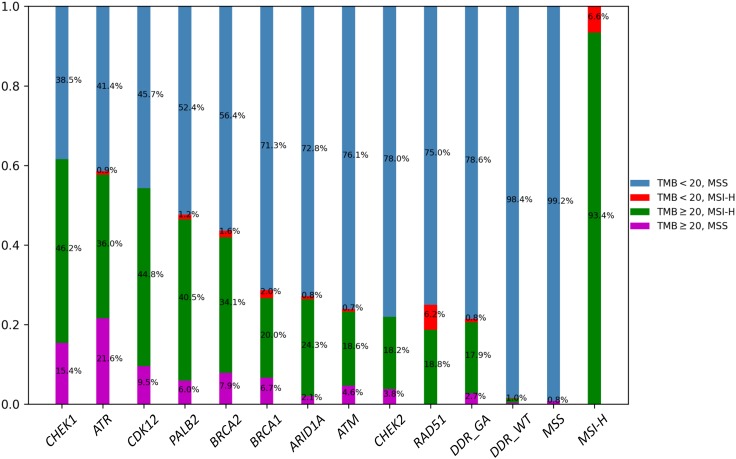
In terms of specific DDR genes, ARID1A (9.2%) and ATM (4.7%) were the most commonly altered DDR genes, followed by BRCA2 (2.3%), BRCA1 (1.1%), CHEK2 (1.0%), ATR (0.8%), CDK12 (0.7%), PALB2 (0.6%), CHEK1 (0.1%), and RAD51 (0.1%; Fig. 2). More than 50% of cases with CHEK1, ATR, or CDK12 GA were TMB‐H, with median TMB of 51.4, 36.9, and 28.8 mutations/Mb, respectively (Fig. 3). We also looked at the overlap of TMB‐H and MSI‐H status within cases with alterations in each of the DDR genes. For MSI‐H cases, the TMB was high (≥20 mutations/Mb for 93.4% of cases and ≥10 mutations/Mb for 100% of cases; Fig. 4).
Figure 2.
Percentage of patients with tumors harboring at least one DDR GA.
Abbreviations: DDR, DNA damage response; CRC, colorectal cancer; GA, genomic alteration; GI, gastrointestinal.
Figure 3.
TMB‐H prevalence and TMB score box plot for subgroup with GA in each gene or feature.
Abbreviations: DDR, DNA damage response; GA, genomic alteration; MSI‐H, high microsatellite instability; MSS, microsatellite stable; TMB, tumor mutational burden; TMB‐H, high tumor mutational burden; WT, wild‐type.
Figure 4.
TMB and MSI distribution in cases with GA in each indicated gene or genomic feature.
Abbreviations: DDR, DNA damage response; GA, genomic alteration; MSI‐H, high microsatellite instability; MSS, microsatellite stable; WT, wild‐type.
Given that the majority of DDR alterations were found in an MSI‐H setting, we also performed a focused analysis specifically on the MSS/DDR‐altered subset. In 12,582 MSS GI cases, DDR alterations were present in 14.5% (n = 1,826) of cases and included GA in ARID1A (7.2%), ATM (3.9%), BRCA2 (1.6%), BRCA1 (0.9%), CHEK2 (0.9%), ATR (0.6%), CDK12 (0.5%), PALB2 (0.4%), RAD51 (0.1%), and CHEK1 (0.1%). In MSS GI cases, TMB‐H was seen more frequently in the DDR‐altered versus DDR‐WT subset (3.3% vs. 0.4%, p < .00001; Fig. 5). DDR‐altered/TMB‐high/MSS cases were found across disease histologies including SBA (1.0%), CRC (0.5%), EA (0.3%), and GC (0.1%). The majority (87.0%) of DDR‐altered TMB‐H cases were also MSI‐H, but for each DDR gene, there was a subset of cases that were MSS but with high TMB, with the notable exception of RAD51. Cases with CHEK1 (15.4%) or ATR (21.6%) GA had the largest enrichment of TMB‐H/MSS cases (Fig. 4). Given the enrichment of high‐TMB/MSS in ATR‐altered cases, we looked at coalterations in other known genomic drivers; however, there were no significant differences in alteration frequencies of EGFR, ERBB2, BRAF, or KRAS in ATR‐altered/MSS cases versus ATR‐WT/MSS cases.
Figure 5.
MSS samples only: TMB‐H prevalence and TMB score box plot for subgroup with GA in each gene or feature.
Abbreviations: DDR, DNA damage response; GA, genomic alteration; MSS, microsatellite stable; TMB, tumor mutational burden; TMB‐H, high tumor mutational burden; WT, wild‐type.
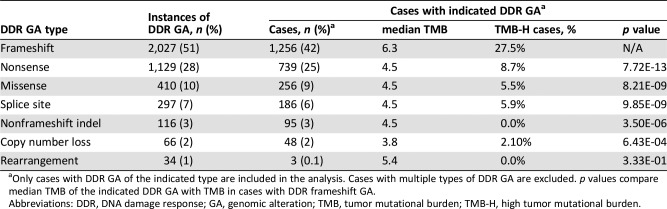
Most DDR GAs in this series were frameshift mutations (50.5%), followed by nonsense mutations (28.1%) and missense mutations (10.2%). In cases with only DDR frameshift mutations, the median TMB was 6.3 mutations/Mb, and 27.5% of cases were TMB‐H. In cases with only one type of DDR GA, the TMB in cases with frameshift mutation was significantly higher than in cases with any other type of DDR GA, with the exception of cases with only DDR rearrangements, likely due to the low occurrence of this class of cases (n = 3; Table 2). Of DDR‐altered cases, 24% (704/2,995) had more than one DDR GA.
Table 2. TMB in cohorts with different types of DDR GA.
Only cases with DDR GA of the indicated type are included in the analysis. Cases with multiple types of DDR GA are excluded. p values compare median TMB of the indicated DDR GA with TMB in cases with DDR frameshift GA.
Abbreviations: DDR, DNA damage response; GA, genomic alteration; TMB, tumor mutational burden; TMB‐H, high tumor mutational burden.
Discussion
Herein we report the largest analysis of DDR pathway genes in tubular GI malignancies to our knowledge and identify recurrent deleterious alterations in 17% of cases as well as a notable relationship with TMB. Dysfunctional DDR machinery identified here has both prognostic and therapeutic implications. We focused on 10 prespecified DDR genes for which therapeutic targeting is ongoing to maximize clinical relevance of our findings. It is well described that BRCA1/2 deficiency in pancreatic cancer portends a better prognosis; these patients show sensitivity to platinum analogues and PARP inhibitors, and there may be other non‐BRCA gene alterations that confer a similar phenotype [36]. Early reporting has noted overall response rates of roughly 20% with single‐agent PARP inhibitors in BRCA1/2‐mutated pancreatic cancer, highlighting the actionability of dDDR [37]. Our findings that 21% of dDDR cases exhibited high mutational burden is consistent with the role of DDR in maintaining genomic stability and is in stark contrast to the 1.4% TMB‐H rate in DDR‐WT cases. TMB‐H DDR altered appears to be largely driven by microsatellite instability, although there was a small, but clinically relevant, subset of DDR‐altered/TMB‐H cases that are MSS. The high mutational burden induced by MMR protein deficiency (dMMR) enriches the tumor with other random mutations, including those in the DDR pathway, hence the strong correlation with MSI‐H suggesting the driver is likely dMMR rather than DDR. However, the notable presence of a subgroup of cases that are TMB‐H but MSS suggested that DDR without dMMR can indeed lead to a hypermutated status, and this subgroup could have important clinical implications. The DDR‐altered/MSS/TMB‐H subset was most commonly seen in cases with GA in CHEK1 or ATR. This is likely a function of ATR’s role in protecting the integrity of the genome; with impaired ATR, cells are unable to cope with genomic breaks or mutations, and normal ATR stress response is to phosphorylate transducers such as CHK1 [38]. The optimal genomic context for targeting ATR remains unknown, and we may postulate that ATR‐mutant/TP53 mutant with elevated TMB may be optimal candidates for ATR inhibitors in combination with immunotherapies [39].
As expected, the majority of DDR alterations were frameshift mutations, as frameshifts are more likely to cause functional deficit as opposed to single‐nucleotide variants, and frameshifts alterations are a characteristic of dMMR. Cases with frameshift DDR alterations also had significantly higher TMB than those with other types of nonframeshift DDR alterations [40]. Notably, in MSI‐H cases, the presence of a DDR alteration correlated with a significantly higher TMB as compared with DDR‐WT MSI‐H cases. This observation may be particularly relevant in light of emerging data suggesting that TMB is predictive as a continuous variable of response to immunotherapies within the MSI‐H CRC population [41]. However, it is unclear whether the DDR alteration is causative of the higher TMB or a result of the high TMB phenotype.
Conclusion
Although these are important preliminary findings contributing to the literature on DNA damage repair in GI cancers, this study was limited in that only cases with a GA in 1 of 10 DDR genes were analyzed, and the patient pool may expand should the gene list be expanded. Given the association between DNA damage GA and higher TMB in tubular GI cancers, these results may have implications for clinical trials with DNA repair inhibitors and checkpoint inhibitors, as there is a cohort of patients in whom both DNA damage repair agents and immunotherapy approaches may be indicated perhaps as monotherapy but also in combination [42]. Independent of a hypermutated phenotype associated with alterations in genes such as POLE or MSH2, defects in this pathway may have therapeutic implications, as emerging data suggest that certain DNA damage events and/or an impaired ability to repair DNA portends susceptibility to immune checkpoint blockade. For example, ovarian cancers with BRCA1 or BRCA2 alterations had a higher neoantigen load than HR‐proficient tumors [23]. Moreover, there are data showing that combining checkpoint blockade with DNA repair targets has rationale, as PARP inhibitors have been shown to increase CD8 T cells and illicit an increased interferon gamma and tumor necrosis factor alpha response in BRCA1‐deficient ovarian tumors [22], [27]. Furthermore, in combination with cytotoxic T‐lymphocyte‐associated protein 4 blockade, this further enhanced the interferon response and activated T cells more than just PARP inhibition alone, and PARP inhibition, independent of an existing DDR defect, seems to upregulate checkpoints such as PD‐L1 [25], [27], [43]. Trials are underway in gynecologic, breast, and genitourinary cancers with signals of activity [31], [44], [45]. The importance of DNA damage and the DNA damage repair process and the effects on the immune response are complex but are increasingly being recognized as effects that have therapeutic implications. This first came to light with defects in MMR repair proteins and response to checkpoint blockade. However, there are many other defective DNA repair processes that may similarly impact increasing mutational burden and neoantigen load; however, there seems to be more complex immunology at play beyond just a response to increased mutational burden and neoantigen load, as shown by the synergistic effects of drugs such as PARP inhibitors and checkpoint inhibitors. Further work should explore whether there is a specific subset of DDR alterations that are predictive biomarkers for benefit from DNA damage agents, immune checkpoint inhibitors, or their combination.
Contributed equally.
Author Contributions
Conception/design: Aparna R. Parikh, Yuting He, Ted S. Hong, Ryan B. Corcoran, Siraj M. Ali, Alexa B. Schrock
Provision of study material or patients: Aparna R. Parikh, Yuting He, Ted S. Hong, Ryan B. Corcoran, Jeff W. Clark, David P. Ryan, Lee Zou, David T. Ting, Daniel V. Catenacci, Joseph Chao, Marwan Fakih, Samuel J. Klempner, Jeffrey S. Ross, Garrett M. Frampton, Vincent A. Miller, Siraj M. Ali, Alexa B. Schrock
Collection and/or assembly of data: Aparna R. Parikh, Yuting He, Ted S. Hong, Ryan B. Corcoran, Jeff W. Clark, David P. Ryan, Lee Zou, David T. Ting, Daniel V. Catenacci, Joseph Chao, Marwan Fakih, Samuel J. Klempner, Jeffrey S. Ross, Garrett M. Frampton, Vincent A. Miller, Siraj M. Ali, Alexa B. Schrock
Data analysis and interpretation: Aparna R. Parikh, Yuting He, Ted S. Hong, Ryan B. Corcoran, Jeff W. Clark, David P. Ryan, Lee Zou, David T. Ting, Daniel V. Catenacci, Joseph Chao, Marwan Fakih, Samuel J. Klempner, Jeffrey S. Ross, Garrett M. Frampton, Vincent A. Miller, Siraj M. Ali, Alexa B. Schrock
Manuscript writing: Aparna R. Parikh, Yuting He, Ted S. Hong, Ryan B. Corcoran, Jeff W. Clark, David P. Ryan, Lee Zou, David T. Ting, Daniel V. Catenacci, Joseph Chao, Marwan Fakih, Samuel J. Klempner, Jeffrey S. Ross, Garrett M. Frampton, Vincent A. Miller, Siraj M. Ali, Alexa B. Schrock
Final approval of manuscript: Aparna R. Parikh, Yuting He, Ted S. Hong, Ryan B. Corcoran, Jeff W. Clark, David P. Ryan, Lee Zou, David T. Ting, Daniel V. Catenacci, Joseph Chao, Marwan Fakih, Samuel J. Klempner, Jeffrey S. Ross, Garrett M. Frampton, Vincent A. Miller, Siraj M. Ali, Alexa B. Schrock
Disclosures
Aparna R. Parikh: Eisai (C/A), Foundation Medicine, Inc., Puretech (SAB); Ryan B. Corcoran: Amgen, Array Biopharma, Astex Pharmaceuticals, Avidity Biosciences, Bristol‐Myers Squibb, Chugai, Fog Pharma, Genentech, LOXO, Merrimack, N‐of‐one, Novartis, nRichDx, Roche, Roivant, Shire, Spectrum Pharmaceuticals, Symphogen, Taiho, Warp Drive Bio (C/A), Avidity Biosciences, nRichDx (OI), Asana, AstraZeneca, Sanofi (RF); David P. Ryan: MPM Capita, Acworth Pharmaceuticals, Oncorus, Gritstone Oncology, Maverick Therapeutics, Inc (C/A), MPM Capital, Acworth Pharmaceuticals (OI), MPM Capita, Acworth Pharmaceuticals, Oncorus, Gritstone Oncology, Maverick Therapeutics, Inc (C/A); David T. Ting: EMD Millipore Sigma, Ventana Roche, Merrimack Pharmaceuticals (C/A), ACD Bio‐Techne (RF), PanTher Therapeutics (OI); Daniel V. Catenacci: Genentech/Roche, Amgen (C/A), Genentech/Roche, Amgen, Eli Lilly and Company, Five Prime, Merck, Bristol‐Myers Squibb, Taiho, Astellas, Gritstone Oncology, Guardant Health, Foundation Medicine, Inc. (H); Joseph Chao: Eli Lilly and Company, Merck, Foundation Medicine, Inc., AstraZeneca, Boston Biomedical, Daiichi‐Sankyo, Taiho (C/A), Merck (RF); Samuel J. Klempner: Foundation Medicine, Inc., Eli Lilly and Company, Astellas (C/A), Leap Therapeutics, Astellas (RF), Foundation Medicine, Inc., Merck (H), TP Therapeutics (OI); Jeffrey S. Ross: Foundation Medicine, Inc. (E, OI); Garrett M. Frampton: Foundation Medicine, Inc. (E); Vincent A. Miller: Foundation Medicine, Inc. (E, OI), Revolution Medicines (SAB), EGFR T790M testing assigned to Sloan Kettering Institute for Cancer Research (IP); Siraj M. Ali: Foundation Medicine, Inc. (E, OI, IP), Incysus Therapeutics, Inc. (SAB), Revolution Medicines (C/A); Alexa B. Schrock: Foundation Medicine, Inc. (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Hoppe MM, Sundar R, Tan DSP et al. Biomarkers for homologous recombination deficiency in cancer. J Natl Cancer Inst 2018;110:704–713. [DOI] [PubMed] [Google Scholar]
- 2.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature 2001;411:366–374. [DOI] [PubMed] [Google Scholar]
- 3.Knijnenburg TA, Wang L, Zimmermann MT et al. Genomic and molecular landscape of DNA damage repair deficiency across The Cancer Genome Atlas. Cell Rep 2018;23:239–254.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer 2012;12:801–817. [DOI] [PubMed] [Google Scholar]
- 5.Heeke AL, Pishvaian MJ, Lynce F et al. Prevalence of homologous recombination‐related gene mutations across multiple cancer types. JCO Precis Oncol 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shindo K, Yu J, Suenaga M et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol 2017;35:3382–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts NJ, Norris AL, Petersen GM et al. Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov 2016;6:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts NJ, Jiao Y, Yu J et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov 2012;2:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waddell N, Pajic M, Patch AM et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu ZI, Shia J, Stadler ZK et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: Challenges and recommendations. Clin Cancer Res 2018;24:1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inglis SC, Clark RA, Dierckx R et al. Structured telephone support or non‐invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev 2015:CD007228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margolis KL, Asche SE, Bergdall AR et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: A cluster randomized clinical trial. JAMA 2013;310:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirza MR, Monk BJ, Herrstedt J et al. Niraparib maintenance therapy in platinum‐sensitive, recurrent ovarian cancer. N Engl J Med 2016;375:2154–2164. [DOI] [PubMed] [Google Scholar]
- 14.Coleman RL, Oza AM, Lorusso D et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2017;390:1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AstraZeneca . Lynparza significantly delayed disease progression as 1st‐line maintenance treatment in germline BRCA‐mutated metastatic pancreatic cancer. Available at https://www.astrazeneca.com/media‐centre/press‐releases/2019/lynparza‐significantly‐delayed‐disease‐progression‐as‐1st‐line‐maintenance‐treatment‐in‐germline‐brca‐mutated‐metastatic‐pancreatic‐cancer‐26022019.html. Accessed March 1, 2019.
- 16.Guo XX, Wu HL, Shi HY et al. The efficacy and safety of olaparib in the treatment of cancers: A meta‐analysis of randomized controlled trials. Cancer Manag Res 2018;10:2553–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fogelman D, Sugar EA, Oliver G et al. Family history as a marker of platinum sensitivity in pancreatic adenocarcinoma. Cancer Chemother Pharmacol 2015;76:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le DT, Durham JN, Smith KN et al. Mismatch‐repair deficiency predicts response of solid tumors to PD‐1 blockade. Science 2017;357:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson DB, Frampton GM, Rioth MJ et al. Targeted next generation sequencing identifies markers of response to PD‐1 blockade. Cancer Immunol Res 2016;4:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg JE, Hoffman‐Censits J, Powles T et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: A single arm, multicentre, phase 2 trial. Lancet 2016;387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouw KW, Goldberg MS, Konstantinopoulos PA et al. DNA damage and repair biomarkers of immunotherapy response. Cancer Discov 2017;7:675–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strickland KC, Howitt BE, Shukla SA et al. Association and prognostic significance of BRCA1/2‐mutation status with neoantigen load, number of tumor‐infiltrating lymphocytes and expression of PD‐1/PD‐L1 in high grade serous ovarian cancer. Oncotarget 2016;7:13587–13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hugo W, Zaretsky JM, Sun L et al. Genomic and transcriptomic features of response to anti‐PD‐1 therapy in metastatic melanoma. Cell 2016;165:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Wang L, Cong Z et al. The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1(‐/‐) murine model of ovarian cancer. Biochem Biophys Res Commun 2015;463:551–556. [DOI] [PubMed] [Google Scholar]
- 25.Brown JS, Sundar R, Lopez J. Combining DNA damaging therapeutics with immunotherapy: More haste, less speed. Br J Cancer 2018;118:312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mouw KW, Konstantinopoulos PA. From checkpoint to checkpoint: DNA damage ATR/Chk1 checkpoint signalling elicits PD‐L1 immune checkpoint activation. Br J Cancer 2018;118:933–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao S, Xia W, Yamaguchi H et al. PARP inhibitor upregulates PD‐L1 expression and enhances cancer‐associated immunosuppression. Clin Cancer Res 2017;23:3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS‐STING pathway of cytosolic DNA sensing. Nat Immunol 2016;17:1142–1149. [DOI] [PubMed] [Google Scholar]
- 29.Shen J, Ju Z, Zhao W et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med 2018;24:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konstantinopoulos PA, Waggoner SE, Vidal GE et al. TOPACIO/Keynote‐162 (NCT02657889): A phase 1/2 study of niraparib + pembrolizumab in patients (pts) with advanced triple‐negative breast cancer or recurrent ovarian cancer (ROC)—Results from ROC cohort. J Clin Oncol 2018;36(suppl 15):106a. [Google Scholar]
- 31.Vinayak S, Tolaney SM, Schwartzberg LS et al. TOPACIO/Keynote‐162: Niraparib + pembrolizumab in patients (pts) with metastatic triple‐negative breast cancer (TNBC), a phase 2 trial. J Clin Oncol 2018;36(suppl 15):1011a. [Google Scholar]
- 32.Lee JM, Cimino‐Mathews A, Peer CJ et al. Safety and clinical activity of the programmed death‐ligand 1 inhibitor durvalumab in combination with poly (ADP‐ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1‐3 inhibitor cediranib in women's cancers: A dose‐escalation, phase I study. J Clin Oncol 2017;35:2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frampton GM, Fichtenholtz A, Otto GA et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013;31:1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalmers ZR, Connelly CF, Fabrizio D et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall MJ, Gowen K, Sanford EM et al., Evaluation of microsatellite instability (MSI) status in 11,573 diverse solid tumors using comprehensive genomic profiling (CGP). J Clin Oncol 2016;34(suppl 15):1523a. [Google Scholar]
- 36.Golan T, Kanji ZS, Epelbaum R et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 2014;111:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golan T, Varadhachary GR, Sela T et al. Phase II study of olaparib for BRCAness phenotype in pancreatic cancer. J Clin Oncol 2018;36(suppl 4):297a. [Google Scholar]
- 38.Yazinski SA, Zou L. Functions, regulation, and therapeutic implications of the ATR checkpoint pathway. Annu Rev Genet 2016;50:155–173. [DOI] [PubMed] [Google Scholar]
- 39.Minchom A, Aversa C, Lopez J. Dancing with the DNA damage response: Next‐generation anti‐cancer therapeutic strategies. Ther Adv Med Oncol 2018;10:1758835918786658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turajlic S, Litchfield K, Xu H et al. Insertion‐and‐deletion‐derived tumour‐specific neoantigens and the immunogenic phenotype: A pan‐cancer analysis. Lancet Oncol 2017;18:1009–1021. [DOI] [PubMed] [Google Scholar]
- 41.Oncology Pro . Lower tumor mutational burden (TMB) and hepatic metastases may predict for lack of response to PD‐1 blockade in MSI‐H metastatic colorectal cancer. Available at https://oncologypro.esmo.org/Meeting‐Resources/ESMO‐2018‐Congress/Lower‐Tumor‐Mutational‐Burden‐TMB‐and‐Hepatic‐Metastases‐May‐Predict‐for‐Lack‐of‐Response‐to‐PD‐1‐Blockade‐in‐MSI‐H‐Metastatic‐Colorectal‐Cancer‐MCRC. Accessed December 30, 2018.
- 42.Chae YK, Anker JF, Carneiro BA et al. Genomic landscape of DNA repair genes in cancer. Oncotarget 2016;7:23312–23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higuchi T, Flies DB, Marjon NA et al. CTLA‐4 blockade synergizes therapeutically with PARP inhibition in BRCA1‐deficient ovarian cancer. Cancer Immunol Res 2015;3:1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karzai F, Madan RA, Owens H et al. A phase II study of the anti‐programmed death ligand‐1 antibody durvalumab (D; MEDI4736) in combination with PARP inhibitor, olaparib (O), in metastatic castration‐resistant prostate cancer (mCRPC). J Clin Oncol 2017;35(suppl 6):162a. [Google Scholar]
- 45.Rauh‐Hain JA, Brewster WR, Behbakht K. Society of Gynecologic Oncology 2018 Annual Meeting on Women's Cancer: Meeting report. Gynecol Oncol 2018;151:e7–e9. [DOI] [PubMed] [Google Scholar]