Abstract
Lessons Learned.
The NEO‐CLASSIC study provided valuable insight for the clinical efficacy and tolerability profiles of perioperative chemotherapy with oxaliplatin and capecitabine, plus gastrectomy, in patients with localized resectable gastric cancer.
The study was designed to explore the potential survival benefits of an eight‐cycle, perioperative oxaliplatin and capecitabine (XELOX) schedule in the above‐mentioned setting and to explore the feasibility of prolonging the cycles of preoperative chemotherapy. The projected endpoint was not met.
Background.
This multicenter, open‐label study (NEO‐CLASSIC) evaluated the efficacy and safety of oxaliplatin and capecitabine (XELOX), plus D2 gastrectomy, in localized resectable gastric cancer.
Methods.
Patients aged 18–75 years with histologically‐confirmed gastric adenocarcinoma (stage T2–4a/N+M0) were given eight cycles of XELOX (four preoperatively, four postoperatively). Each 3‐week cycle comprised capecitabine 1,000 mg/m2 twice daily on days 1–14 and oxaliplatin 130 mg/m2 on day 1. Curative D2 gastrectomy was scheduled 2–4 weeks after the last preoperative cycle. The primary objective of the study was to determine the objective response rate (ORR) of XELOX in the preoperative setting. Sample size was calculated by assuming that a minimum of 47 cases would be required to increase the ORR by 15% (from 40% to 55%). With an estimated 10% dropout rate, 55 patients would have to be recruited.
Results.
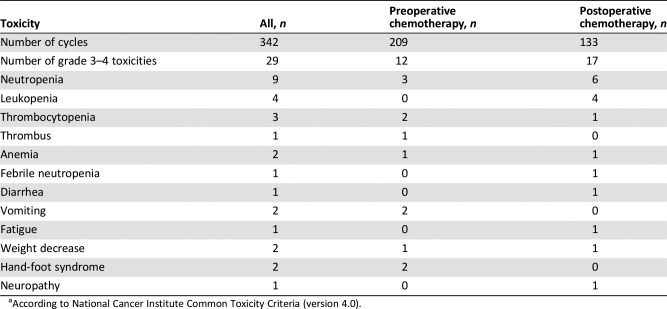
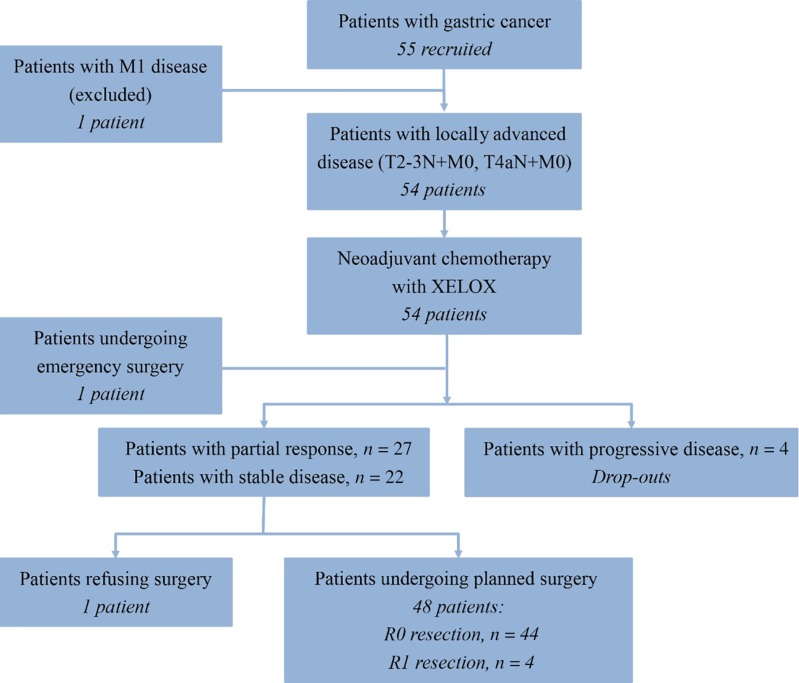
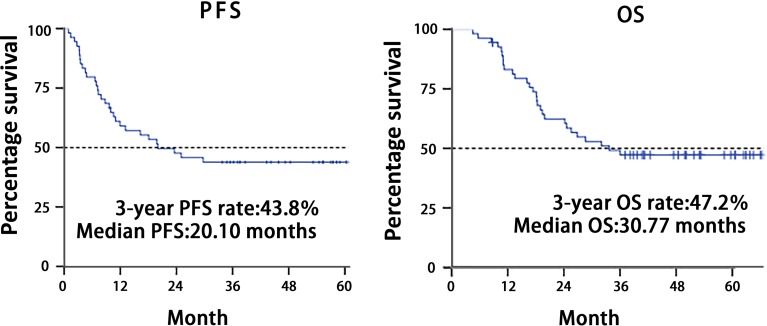
Fifty‐five patients were enrolled, and one was excluded because of screening failure. R0 resections were achieved in 45 of 54 intent‐to‐treat patients (83.3%), and four patients received R1 resections (Fig. 1). There were no complete responses, 27 (50.0%) partial responses, 22 cases (40.7%) of stable disease, and 4 (7.4%) of progressive disease. The objective response rate was 50.0%. Median follow‐up was 52.97 months; 30 patients (55.6%) had disease progression (Table 1), and median progression‐free survival was 20.10 (95% confidence interval: 4.31—35.89) months; median overall survival was 30.77 months (95% confidence interval was not yet available) (Fig. 2). Fifty‐four patients completed 209 cycles of preoperative chemotherapy; 42 patients received 133 cycles of postoperative chemotherapy (Table 3). The rate of grade 3–4 adverse events was 8.5% (29/342 cycles); the most frequent events were neutropenia (9/342 cycles) and leukopenia (4/342 cycles).
Conclusion.
These findings suggest that combination therapy with capecitabine and oxaliplatin as perioperative chemotherapy, followed by D2 gastrectomy, is effective and safe in late‐stage, locally advanced gastric cancer. Although enrollment exceeded the 47 patients required to identify an increase in the ORR by 15% (from 40% to 55%), results did not meet the primary endpoint.
Abstract
经验总结
• NEO‐CLASSIC 研究针对局部可切除性胃癌患者采用围手术期化疗(奥沙利铂联合卡培他滨)并结合胃切除术的临床疗效和耐受性特性提供了宝贵见解
• 本研究旨在研究八个周期的围手术期奥沙利铂联合卡培他滨 (XELOX) 方案在上述情况下的潜在生存优势,并研究延长围手术期化疗周期的可行性。预计的终点并未实现。
摘要
背景。此项多中心、开放标签研究 (NEO‐CLASSIC) 对奥沙利铂联合卡培他滨 (XELOX) 并结合 D2 胃切除术治疗局部可切除性胃癌的疗效和安全性进行了评估。
方法。针对经组织学检查证实患有胃腺癌的 18–75 岁的患者(T2–4a/N+M0 期)实施了八个周期的 XELOX(术前四个周期,术后四个周期)治疗。在每个为期 3 周的治疗周期内,具体用药包括卡培他滨(第 1–14 天,剂量为 1 000 mg/m2,每日两次)和奥沙利铂(第 1 天,剂量为 130 mg/m2)。在术前最后一个治疗周期结束后 2–4 周内实施治疗性 D2 胃切除术。本研究的主要目的是确定 XELOX 方案在术前治疗期间的客观缓解率 (ORR)。通过假设至少 47 例需将ORR提升 15%(从 40% 提升至 55%)的方式,计算样本量。据估计,退出率为 10%,因此需招募 55 名患者。
结果。共招募了 55 名患者,其中一名因筛查失败而被排除。在 54 例意向性治疗患者中,有 45 例 (83.3%) 接受了 R0 切除,4 例患者接受了 R1 切除(图 1)。完全缓解病例为零,27 例 (50.0%) 部分缓解,22 例 (40.7%) 疾病稳定,4 例 (7.4%) 疾病进展。客观缓解率为 50.0%。中位随访52.97 个月;30 名患者 (55.6%) 出现了疾病进展(表 1),中位无进展生存期为 20.10 个月(95% 置信区间:4.31—35.89);中位总生存期为 30.77 个月(95% 置信区间尚不适用)(图 2)。54 名患者完成了 209 个术前化疗周期;42 名患者接受了 133 个术后化疗周期(表 3)。3‐4 级不良事件发生率为 8.5%(29/342 个周期);最常见的事件是中性粒细胞减少症(9/342 个周期)和白细胞减少症(4/342 个周期)。
结论。这些结果表明,在治疗晚期局部晚期胃癌时,卡培他滨联合奥沙利铂作为围手术期化疗并辅以 D2 胃切除术的方法安全有效。尽管入组患者超过 47 名,需确认ORR是否提升 15%(从 40% 提升至 55%),但结果并未实现主要终点。
Discussion
The NEO‐CLASSIC study was designed to investigate the efficacy and safety of perioperative chemotherapy. Thus, with laparoscopic staging, we enrolled patients with T2–4/N+M0 disease. Among the 54 intent‐to‐treat patients, 14.8% of patients were stage II, and the other 85.2% of patients were stage III.
Table 1. Clinical response in the intent‐to‐treat population (n = 54).
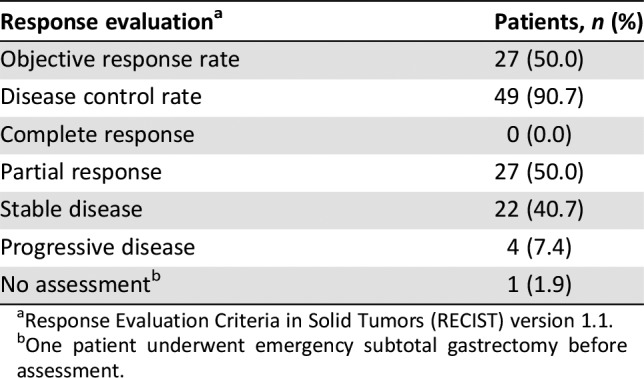
Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
One patient underwent emergency subtotal gastrectomy before assessment.
The ORR was 50%. Although the ORR did not achieve the primary endpoint, the disease control rate was 90.7% (49/54 patients), which created possibilities for radical surgery. The R0 resection rate was one of the secondary study objectives, with 49 patients proceeding to any surgical resection. R0 resection of the primary tumor was achieved in 83.3% of patients (45/54), which was similar to that in previous studies. The survival analysis revealed a 3‐year progression‐free survival (PFS) rate of 43.8%, and a 3‐year overall survival (OS) rate of 47.2%. The median PFS was 20.1 months, with an event rate of 55.6%. However, median OS was 30.77 months. The survival disparity between the CLASSIC study (adjuvant capecitabine and oxaliplatin, with its 78% 5‐year overall survival) and the FLOT4 trial (fluorouracil plus leucovorin, oxaliplatin, and docetaxel) trial and our study (50 months vs. 30.77 months overall survival, respectively) may have resulted from population differences. However, patients in our study had much later disease (T2–4/N+M0) and were typically in stage III.
The proportion of grade 3–4 adverse events (AEs) was 8.5% during perioperative chemotherapy. The most frequent grade 3–4 AEs were neutropenia and leukopenia, and no treatment‐related deaths occurred. All these rates were significantly lower than the FLOT4 trial. Our study also found that XELOX can be delivered with higher dose intensity and better feasibility in the preoperative than in the postoperative setting. No deaths occurred during hospitalization or 30 days after surgery. The median hospitalization time was 9.6 days. Thus, because of its good tolerability profile, XELOX might also be a promising regimen for adjuvant chemotherapy.
Trial Information
- Disease
Gastric cancer
- Stage of Disease/Treatment
Neoadjuvant
- Prior Therapy
None
- Type of Study – 1
Phase II
- Type of Study – 2
Single arm
- Primary Endpoint
Overall response rate
- Secondary Endpoints
-
Progression‐free survival
Overall survival
Pathologic response
Safety
Toxicity
- Additional Details of Endpoints or Study Design
- The primary objective of the study was to determine the ORR of XELOX in the preoperative setting. Sample size was calculated by assuming that a minimum of 47 cases would be required to increase the ORR by 15% (from 40% to 55%). With an estimated 10% dropout rate, 55 patients would have to be recruited.
- Investigator's Analysis
Inactive because results did not meet primary endpoint
Drug Information
- Drug 1
- Generic/Working Name
XELOX
- Schedule of Administration
- The treatment plan was for all patients to receive eight cycles of perioperative XELOX chemotherapy (four cycles preoperatively, four postoperatively). Each 3‐week cycle comprised capecitabine 1,000 mg/m2 twice daily on days 1–14, and oxaliplatin 130 mg/m2 administered on day 1. Dose reductions or interruptions were allowed to manage potentially serious or life‐threatening AEs. Curative D2 gastrectomy was scheduled within 2–4 weeks after completion of the last cycle of preoperative chemotherapy in patients without progressive disease. Postoperative chemotherapy was started within 8 weeks of surgery.
- Drug 2
- Generic/Working Name
Oxaliplatin
- Drug Type
Small molecule
- Drug Class
Platinum compound
- Dose
130 milligrams (mg) per squared meter (m2)
- Route
IV
- Schedule of Administration
On day 1
Patient Characteristics
- Number of Patients, Male
38
- Number of Patients, Female
16
- Stage
- Clinically diagnosed stage T2–4a/N+M0 disease, according to computed tomography and magnetic resonance imaging, and resectable disease according to laparoscopic exploration. Details of patient characteristics are shown in Table 2.
- Age
Median (range): 65 (39∼71)
- Number of Prior Systemic Therapies
Median: 0
- Performance Status: ECOG
-
0 — 5
1 — 49
2 — 0
3 — 0
Unknown —
- Cancer Types or Histologic Subtypes
-
Intestinal, 26
Diffuse, 14
Mixed, 10
Unknown, 4
Table 2. Baseline patient characteristics (n = 54).
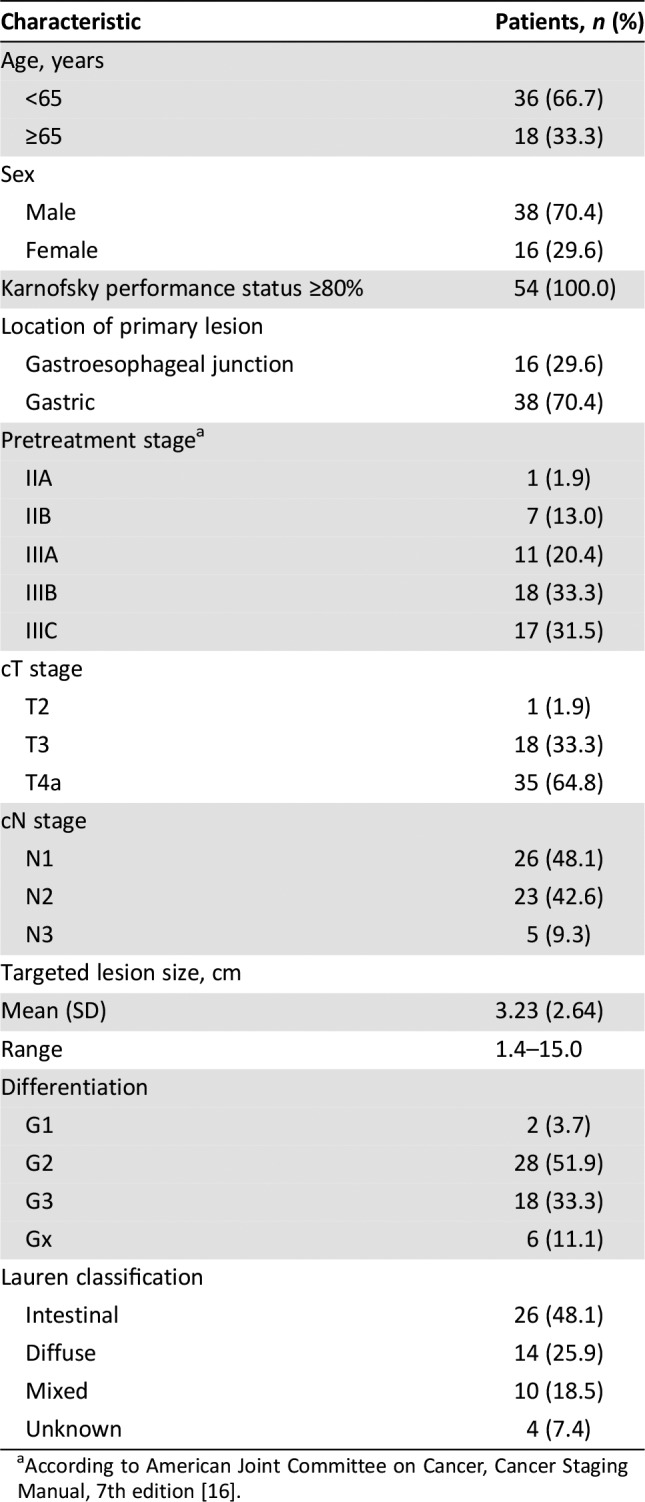
According to American Joint Committee on Cancer, Cancer Staging Manual, 7th edition [16].
Primary Assessment Method
- Number of Patients Screened
55
- Number of Patients Enrolled
54
- Number of Patients Evaluable for Toxicity
54
- Number of Patients Evaluated for Efficacy
54
- Evaluation Method
RECIST 1.1
- Response Assessment CR
n = 0 (0%)
- Response Assessment PR
n = 27 (50%)
- Response Assessment SD
n = 22 (40.7%)
- Response Assessment PD
n = 4 (7.4%)
- (Median) Duration Assessments PFS
20.1 months
- (Median) Duration Assessments OS
30.77 months
- Outcome Notes
No deaths occurred during hospitalization or 30 days after surgery. Median hospitalization time was 9.6 days.
Adverse Events
- Grade 3–4 toxicities of chemotherapy are shown in Table 5. Details related to complications and safety of surgery are shown in Table 6.
Table 5. Grade 3–4 toxicities of chemotherapya.
According to National Cancer Institute Common Toxicity Criteria (version 4.0).
Table 6. Complications and safety of surgery (n = 49a).
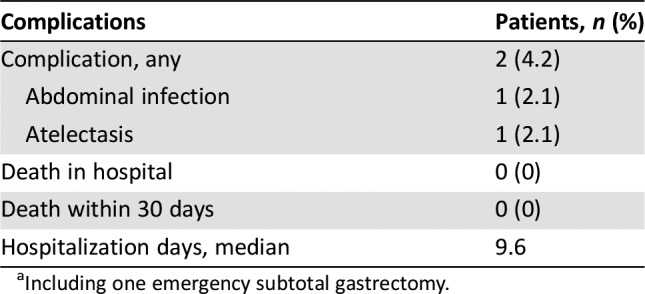
Including one emergency subtotal gastrectomy.
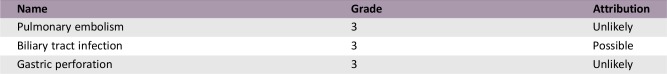
Serious Adverse Events
Assessment, Analysis, and Discussion
- Completion
Study completed
- Investigator's Assessment
Inactive because results did not meet primary endpoint
The NEO‐CLASSIC study was designed to investigate the efficacy and safety of perioperative chemotherapy in patients with gastric cancer. Thus, with laparoscopic staging, we enrolled patients with T2–4/N+M0 disease. Among the 54 intent‐to‐treat patients, 14.8% of patients were stage II, and the other 85.2% of patients were stage III. Although the use of diagnostic laparoscopy for staging patients with gastric cancer is not adopted universally, it has substantial value because of its high accuracy and effectiveness, avoidance of unnecessary laparotomy, and its reduced recovery time. Therefore, laparoscopy can improve treatment decision‐making in advanced gastric cancer, and is recommended for patients with resectable gastric cancer [1].
The objective response rate (ORR) of oxaliplatin and capecitabine (XELOX) as perioperative chemotherapy was evaluated as the primary endpoint of NEO‐CLASSIC, with clinical response evaluated using RECIST (version 1.1). The ORR was 50.0%. The R0 resection rate was one of the secondary study objectives, with 49 patients proceeding to any surgical resection. R0 resection of the primary tumor was achieved in 83.3% of patients (45/54), which was similar to that in previous studies. The R0 resection rate was 84% in the NeoFLOT study [2], 79% in the MAGIC trial [3], 84% in the ACCORD trial [4], and with epirubicin, cisplatin, 5‐fluorouracil or epirubicin, cisplatin, and capecitabine (ECF/ECX) and 57% with fluorouracil plus leucovorin, oxaliplatin, and docetaxel (FLOT) in the FLOT4 trial [5]. Pathologic response was another important objective of the current trial, and a pathologic complete response (pCR) was reported in 3 of 48 patients (6.3%) from the per‐protocol population who underwent surgery; this was consistent with the 0%–16% rate reported by previous prospective studies [6], [7], [8]. The rate of pathologic response, defined as a complete response or <10% of the residual cancer remaining, was 20.8% (10/48 patients). According to Japanese Classification of Gastric Cancer criteria [9], 31.2% of patients (15/45) achieved histologic grade 2–3, which means less than 33.3% of viable tumor cells observed.
We believed that the reasons for the failure to achieve the primary endpoint may be attributed to the following reasons. First, in locally advanced gastric cancer, perigastric lymph nodes were the only target lesions according to RECIST 1.1 criteria, and spiral computed tomography had some limitations in evaluating the primary lesions and lymph nodes. Second, ORR was a widely used preoperative evaluation criterion currently, but it was not an ideal therapeutic indicator of neoadjuvant therapy in gastric cancer.
Although ORR did not achieve the primary endpoint, the disease control rate was 90.7% (49/54 patients), which created possibilities for radical surgery.
An R0 resection rate of 84.3% (59/70 patients) was achieved in the European Organisation for Research and Treatment of Cancer 40954 trial, which used a prolonged (two 48‐day cycles) neoadjuvant cisplatin/5‐fluorouracil‐based regimen [10]. Several recent studies reported an improved complete resection rate with neoadjuvant chemotherapy, and R0 resection rate was associated with long‐term survival after neoadjuvant therapy in gastric cancer [11]. The present study showed a satisfactory R0 resection rate, but pCR (ypT0N0M0) was reported in 3 of 48 per‐protocol patients (6.3%) who underwent surgery. Comparison with initial clinical and pathologic stages revealed downstaging of T stage in 66.7% of patients (32/48) and downstaging of N stage in 45.8% of patients (22/48) (Table 4). Although there is still no universally accepted grading system for pathologic regression, numerous studies have shown that the degree of pathologic response is a vital prognostic marker of local recurrence, distant metastases, and long‐term outcomes in locally advanced gastric cancer after neoadjuvant therapy [12]. More importantly, pathologic response can guide clinical decisions about surgical strategies, adjuvant therapy, and long‐term surveillance [13], [14].
Table 4. Pathologic response in patients who underwent planned surgery (n = 48).
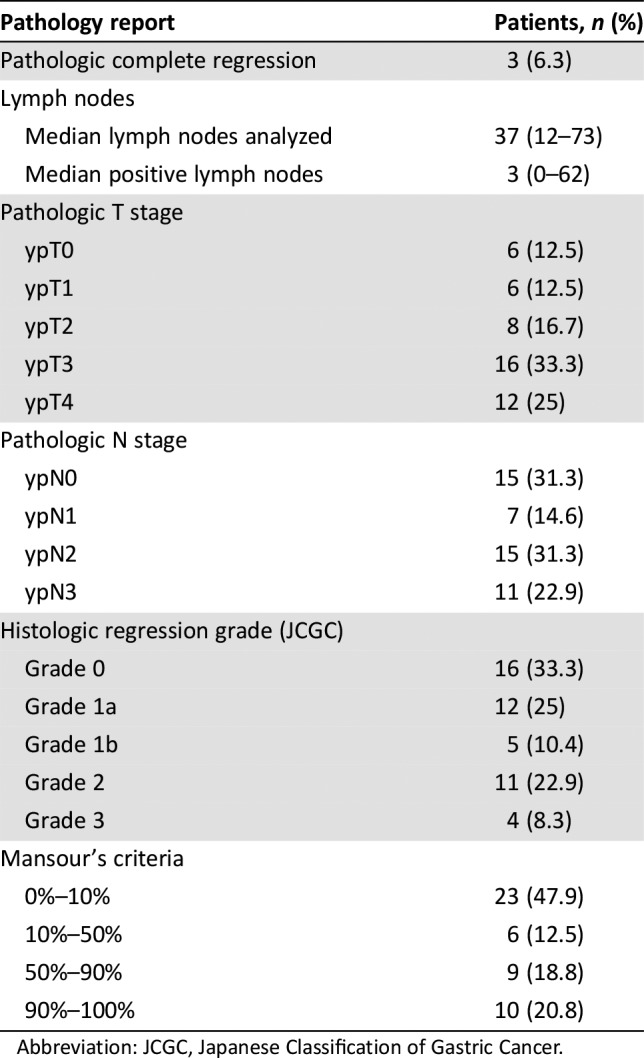
Abbreviation: JCGC, Japanese Classification of Gastric Cancer.
The survival analysis revealed a 3‐year progression‐free survival (PFS) rate of 43.8%, and a 3‐year overall survival (OS) rate of 47.2%. The median PFS was 20.1 months, with an event rate of 55.6%. However, median OS was 30.77 months. In the CLASSIC trial, 3‐year disease‐free survival was 74% in the chemotherapy group, and 59% in the surgery‐only group; corresponding values for 3‐year OS were 83% and 78% [15]. In FLOT4 trial, the median OS was 35 months with ECX/ECF and 50 months with FLOT. The PFS was 18 months with ECX/ECF and 30 months with FLOT. 3‐year OS rate was 48% with ECF/ECX and 57% with FLOT. The survival disparity between the CLASSIC, the FLOT4 trial, and our study may have resulted from population differences. The CLASSIC trial enrolled patients with stage II (T2N1, T1N2, T3N0), IIIA (T3N1, T2N2, T4N0), or IIIB (T3N2) disease according to American Joint Committee on Cancer criteria (6th edition). However, patients in our study had much later disease (T2–4/N+M0) and were typically in stage III [16]: 20.4% of patients were stage IIIA, 33.3% were stage IIIB, and 31.5% were stage IIIC. In the FLOT4 trial, the rate of node‐positive patients was 79.4%. However, the rate in our study was 100%, even though the 3‐year OS rate in our study was comparable to the rate of ECF/ECX group in the FLOT4 study.
Two‐drug and three‐drug regimens are both recommended for patients with ≥ stage IB resectable gastric cancer, and there is no current evidence showing a significant difference in efficacy between these regimens. According to National Comprehensive Cancer Network guidelines [17], treatment regimens should be chosen after considering performance status, medical comorbidities, and toxicity profiles. Therefore, a balance between efficacy and tolerability is important when selecting treatment. The recommended treatment duration is usually 2–4 cycles, but this could be modified depending on the circumstances [16], [17]. Our study indicated that eight cycles of XELOX (i.e., four cycles preoperatively, and four postoperatively) was effective as a perioperative chemotherapy schedule.
Usually, two‐drug chemotherapy regimens are preferred for patients with unresectable, locally advanced, recurrent or metastatic disease because of lower toxicity, whereas three‐drug regimens are reserved for medically fit patients with good performance status and access to frequent toxicity evaluations [17]. The current study confirmed the good tolerability of XELOX as a perioperative chemotherapy regimen. The frequency, severity, and type of adverse events (AEs) documented with XELOX were consistent with the safety profile reported in the CLASSIC study [15]. The proportion of grade 3–4 AEs was 8.5% during perioperative chemotherapy. The most frequent grade 3–4 AEs were neutropenia and leukopenia, and no treatment‐related deaths occurred. All these rates were significantly lower than the FLOT4 trial [5]. Our study also found that XELOX can be delivered with higher dose intensity and better feasibility in the preoperative than the postoperative setting: 49 of 54 patients (90.7%) completed the four planned preoperative cycles, but only 27 of 42 patients (62.3%) completed the four planned postoperative cycles. The mean dose intensity of oxaliplatin was 840 mg preoperatively and 577 mg postoperatively. Moreover, only 2 of 49 patients (4.2%) experienced mild operative complications. No deaths occurred during hospitalization or 30 days after surgery. The median hospitalization time was 9.6 days. Thus, because of its good tolerability profile, XELOX might also be a promising regimen for adjuvant chemotherapy.
Figures and Tables
Figure 1.
Study flowchart.
Abbreviation: XELOX, oxaliplatin and capecitabine.
Figure 2.
Kaplan‐Meier plots of progression‐free survival and overall survival.
Abbreviations: OS, overall survival; PFS, progression‐free survival.
Table 3. Chemotherapy administration.
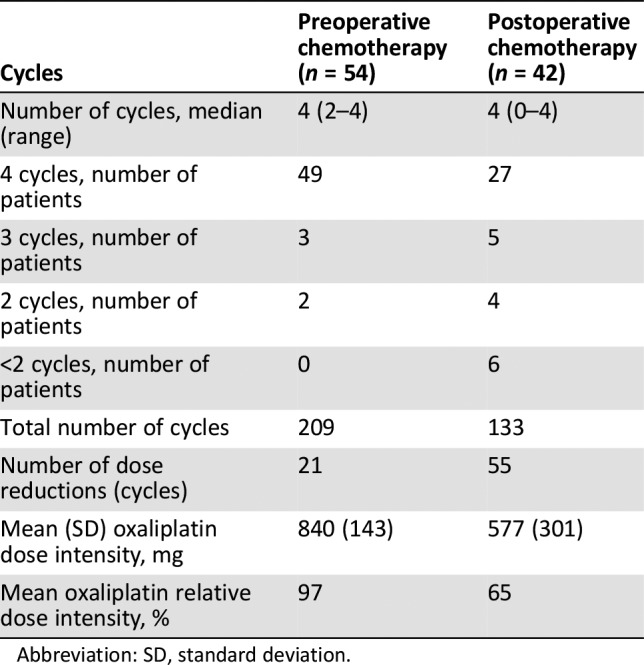
Abbreviation: SD, standard deviation.
Acknowledgments
This investigator‐initiated study was supported by Shanghai Roche Pharmaceuticals Ltd., which provided capecitabine and partial funding. Medical writing and editorial support was provided by Content Ed Net, with funding from Shanghai Roche Pharmaceuticals Ltd. This research is supported by the Shanghai Science and Technology Commission (15411961900).
Contributed equally.
Footnotes
ClinicalTrials.gov Identifier: NCT01880632
Sponsor(s): Zhongshan Hospital, Fudan University
Principal Investigators: Tianshu Liu, Yihong Sun
IRB Approved: Yes
Disclosures
Tianshu Liu: Novartis, Merck KGaA, Merck Sharp & Dohme, Roche, Bayer, Hengrui (C/A, H), Merck KGaA, Merck Sharp & Dohme, Roche, Bayer, Hengrui (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Smyth EC, Verheij M, Allum W et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016;27:v38–v49. [DOI] [PubMed] [Google Scholar]
- 2.Schulz C, Kullmann F, Kunzmann V et al. NeoFLOT: Multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinoma ‐ Very good response predominantly in patients with intestinal type tumors. Int J Cancer 2015;137:678–685. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- 4.Ychou M, Boige V, Pignon JP et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715–1721. [DOI] [PubMed] [Google Scholar]
- 5.Al‐Batran SE, Pauligk C, Homann N et al. LBA‐008Docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) as perioperative treatment of resectable gastric or gastro‐esophageal junction adenocarcinoma: The multicenter, randomized phase 3 FLOT4 trial (German Gastric Group at AIO). Ann Oncol 2017;28(suppl 3):152–153. [Google Scholar]
- 6.Al‐Batran SE, Hofheinz RD, Pauligk C et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro‐oesophageal junction adenocarcinoma (FLOT4‐AIO): Results from the phase 2 part of a multicentre, open‐label, randomised phase 2/3 trial. Lancet Oncol 2016;17:1697–1708. [DOI] [PubMed] [Google Scholar]
- 7.Ostwal V, Sahu A, Ramaswamy A et al. Perioperative epirubicin, oxaliplatin, and capecitabine chemotherapy in locally advanced gastric cancer: Safety and feasibility in an interim survival analysis. J Gastric Cancer 2017;17:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshikawa T, Sasako M, Yamamoto S et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg 2009;96:1015–1022. [DOI] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101–112. [DOI] [PubMed] [Google Scholar]
- 10.Schuhmacher C, Gretschel S, Lordick F et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong BH, Cheng Y, Ma L et al. An updated meta‐analysis of randomized controlled trial assessing the effect of neoadjuvant chemotherapy in advanced gastric cancer. Cancer Invest 2014;32:272–284. [DOI] [PubMed] [Google Scholar]
- 12.Blackham AU, Greenleaf E, Yamamoto M et al. Tumor regression grade in gastric cancer: Predictors and impact on outcome. J Surg Oncol 2016;114:434–439. [DOI] [PubMed] [Google Scholar]
- 13.Mansour JC, Tang L, Shah M et al. Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann Surg Oncol 2007;14:3412–3418. [DOI] [PubMed] [Google Scholar]
- 14.MacGregor TP, Maughan TS, Sharma RA. Pathological grading of regression following neoadjuvant chemoradiation therapy: The clinical need is now. J Clin Pathol 2012;65:867–871. [DOI] [PubMed] [Google Scholar]
- 15.Bang YJ, Kim YW, Yang HK et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open‐label, randomised controlled trial. Lancet 2012;379:315–321. [DOI] [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC et al. American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edition New York, NY: Springer, 2010. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer. 2017, Version 5. October 13, 2017. Available from https://www.nccn.org/professionals/physician_gls/pdf/gastric_blocks.pdf. Accessed March 21, 2018.