Abstract
There are no officially approved therapies for metastatic pheochromocytomas apart from ultratrace 131I-metaiodbenzylguanidine therapy, which is approved only in the United States. We have, therefore, investigated the antitumor potential of molecular-targeted approaches in murine pheochromocytoma cell lines [monocyte chemoattractant protein (MPC)/monocyte chemoattractant protein/3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)], immortalized mouse chromaffin Sdhb−/− cells, three-dimensional pheochromocytoma tumor models (MPC/MTT spheroids), and human pheochromocytoma primary cultures. We identified the specific phosphatidylinositol-3-kinase α inhibitor BYL719 and the mammalian target of rapamycin inhibitor everolimus as the most effective combination in all models. Single treatment with clinically relevant doses of BYL719 and everolimus significantly decreased MPC/MTT and Sdhb−/− cell viability. A targeted combination of both inhibitors synergistically reduced MPC and Sdhb−/− cell viability and showed an additive effect on MTT cells. In MPC/MTT spheroids, treatment with clinically relevant doses of BYL719 alone or in combination with everolimus was highly effective, leading to a significant shrinkage or even a complete collapse of the spheroids. We confirmed the synergism of clinically relevant doses of BYL719 plus everolimus in human pheochromocytoma primary cultures of individual patient tumors with BYL719 attenuating everolimus-induced AKT activation. We have thus established a method to assess molecular-targeted therapies in human pheochromocytoma cultures and identified a highly effective combination therapy. Our data pave the way to customized combination therapy to target individual patient tumors.
Pheochromocytomas (PCCs) and paragangliomas (PGLs) are rare neuroendocrine, catecholamine-producing tumors that originate from the adrenal medulla or extra-adrenal paraganglia. About 10% of PCCs and 35% to 40% of PGLs are metastatic (1–9). The prognosis of patients with metastatic PCC/PGL is poor, with a 5-year survival rate of 63% (10). Current therapeutic approaches for metastatic PCC/PGL, such as radionuclide therapy with 131I-metaiodbenzylguanidine, peptide receptor radionuclide therapy (11), or chemotherapy, are (apart from ultratrace 131I-metaiodbenzylguanidine, which has been approved only in the United States) not officially approved and are limited in their effectiveness, as recently reviewed (12). Therefore, novel therapeutic strategies are urgently needed.
The standard chemotherapy regimen for PCC/PGLs is CVD (cyclophosphamide, vincristine, dacarbazine). Inhibitors of the DNA repair enzyme poly(ADP-ribose) polymerase (PARP) have been demonstrated to potentiate DNA damaging effects of alkylating chemotherapeutic agents, such as dacarbazine or temozolomide (13–15), both being metabolized to the same active metabolite (16). Therefore, combining standard chemotherapy with a PARP inhibitor might be a promising novel therapeutic approach.
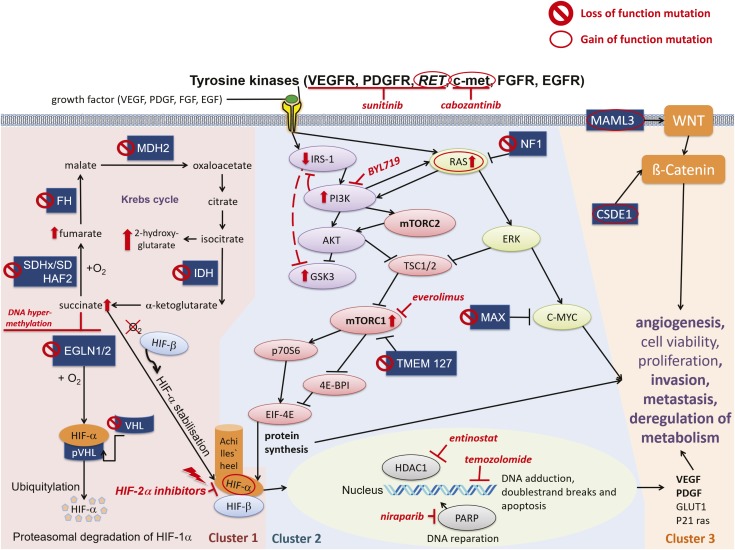
Another therapeutic approach is to define the aberrant molecular pathways in such tumors, which can then be targeted by specific therapy. Germline mutations can be identified in ∼30% to 40% of patients with PCC/PGL, with an equal number showing somatic mutations in more than 20 well-characterized susceptibility genes (17, 18). These different mutations can be separated into three main molecular clusters [summarized in Fig. 1 and recently reviewed (19)]:
The pseudohypoxic signaling cluster (cluster-1) is related to mutations of genes encoding for proteins that are associated with significant regulation of the hypoxia signaling pathway; these include mutations in genes encoding for hypoxia-inducible factor-2α (HIF2A), Krebs cycle enzymes such as succinate dehydrogenase subunits [SDHx (SDHA, SDHB, SDHC, SDHD)], succinate dehydrogenase complex assembly factor-2 (SDHAF2), fumarate hydratase (FH), malate dehydrogenase 2 (MDH2), and isocitrate dehydrogenase 1 (IDH1). This pathway includes mutations in von Hippel–Lindau tumor suppressor (VHL) and egl-9 prolyl hydroxylase-1 and -2 (EGLN1/2) genes. Such mutations promote HIF-α stabilization and accumulation resulting in, among other effects, increased angiogenesis via changes in vascular endothelial growth factor-1 and -2 receptors (VEGFR1/2) and platelet-derived growth factor-β receptor transcription.
The kinase signaling cluster (cluster-2) is related to mutations of genes encoding for proteins that belong to the phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTORC1) pathway/receptor kinase signaling and comprises mutations in the rearranged-during-transfection (RET) proto-oncogene, neurofibromin 1 (NF1) tumor suppressor, H-RAS and K-RAS proto-oncogenes, transmembrane protein 127 (TMEM127), and Myc-associated factor X (MAX).
Most recently, the Wnt signaling cluster (cluster-3) has been described as being of pathological significance.
Figure 1.
Cluster-1, cluster-2, and cluster-3, with molecular-targeted therapeutic options. Cluster-1: The pseudo-hypoxic signaling cluster includes mutations in genes encoding for hypoxia-inducible factor 2-α (HIF2A), Krebs-cycle enzymes such as succinate dehydrogenase subunits [SDHx (SDHA, SDHB, SDHC, SDHD)], succinate dehydrogenase complex assembly factor-2 (SDHAF2), fumarate hydratase (FH), malate dehydrogenase 2 (MDH2), and isocitrate dehydrogenase (IDH), and including von Hippel–Lindau tumor suppressor (VHL) and egl-9 prolyl hydroxylase 1 and 2 (EGLN1/2). Cluster-2: The kinase signaling cluster comprises mutations in the rearranged-during-transfection (RET) proto-oncogene, neurofibromin 1 (NF1) tumor suppressor, H-RAS and K-RAS proto-oncogenes, transmembrane protein 127 (TMEM127), and Myc-associated factor X (MAX). Receptor tyrosine kinases (among others, RET, VEGFR, c-met) activate insulin receptor substrate 1 (IRS-1), which recruits the phosphatidylinositol-3-kinase (PI3K). PI3K activates AKT, which inhibits tuberous sclerosis proteins 1/2 (TSC1/2), leading to disinhibition/activation of the mammalian target of rapamycin (mTORC1); mTORC1 phosphorylates and activates various proteins, including p70S6 kinase (p70S6K), by which p70S6 is phosphorylated. Activated p70S6 promotes cell growth, proliferation, cell survival, and leads, among other effects, to the protein synthesis of HIF-1α, which favors angiogenesis [VEGF/platelet-derived growth factor (PDGF)] transcription, among others], invasion, and metastasis under hypoxic or pseudo-hypoxic conditions. AKT also inhibits glycogen synthase kinase 3 (GSK3). Persistent activation of PI3K leads to feedback downregulation of IRS-1, which may lead to GSK3 disinhibition/activation. The RAS/RAF/ERK pathway is also activated by tyrosine kinases (RET, among others) and activates mTORC1. NF1 mutations lead to disinhibition/activation of RAS. TMEM127 mutations lead to disinhibition/activation of mTORC1. The tumor suppressor MAX antagonizes Myc-dependent cell survival, proliferation, and angiogenesis: mutations lead to increased cell proliferation and angiogenesis. Cluster-3: The Wnt signaling cluster comprises somatic mutations in cold shock domain containing E1 (CSDE1) and the mastermind-like transcriptional coactivator 3 (MAML3) fusion genes. 🛇, pheochromocytoma-promoting loss-of-function mutation of a tumor suppressor gene; ○, pheochromocytoma-promoting gain-of-function mutation of a proto-oncogene. ↑, increase/upregulation; ⊥, inhibition; ↓, activation.
The multitargeted receptor tyrosine kinase inhibitor (TKI) sunitinib has antiangiogenic and antiproliferative potential due to inhibition of VEGFR1/2, platelet-derived growth factor-β receptor, and RET and has been approved for the treatment of renal cell carcinomas, pancreatic neuroendocrine tumors (NETs), and gastrointestinal stromal tumors. Sunitinib is currently being investigated in a randomized placebo-controlled phase II clinical trial in patients with PCC/PGL, for which recruitment has been completed (FIRST-MAPPP, clinical trial no. NCT01371201).
The mTORC1 inhibitor everolimus, currently used in the treatment of breast cancer, renal cell carcinoma, and NETs, showed poor efficacy when used alone in PCC/PGLs (20). However, one patient with an SDHB mutation who was treated with the combination of low doses of the mTORC1 inhibitor rapamycin and the TKI sunitinib showed long-term disease control (21).
In an in vivo breast cancer mouse model, the mTORC1 inhibitor everolimus has shown synergistic effects together with the PI3Kα inhibitor BYL719 (alpelisib) and overcame BYL719 resistance (22). The clinical phase III approval study SOLAR-1 investigating BYL719 in patients with breast cancer has reached its final endpoints (23). Moreover, BYL719 overcame everolimus resistance in a stably everolimus-resistant pancreatic NET cell line model (24). Therefore, combining different targeted drugs may strongly enhance their therapeutic potential and prevent the development of resistance.
Based on these data, we have investigated the antitumor potential of a variety of both targeted and chemotherapeutic agents and their combinations and their impact on cellular signaling pathways in well-validated murine PCC cell line models and a murine Sdhb knock-out (Sdhb−/−) cell line (the best available cell line models for highly aggressive SDHB-mutated paragangliomas), three-dimensional murine PCC spheroids, and human PCC primary cultures to identify novel therapeutic options.
Materials and Methods
Cell lines
Murine monocyte chemoattractant protein (MPC) (MPC 4/30/PRR) (25) and 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (26) cell lines were cultured as described previously (27) in DMEM/F12 (1:1) (Life Technologies, Karlsruhe, Germany) supplemented with 10% fetal bovine serum (Biochrom, Berlin, Germany), 1% penicillin/streptomycin (Life Technologies), and 0.4% amphotericin B (Biochrom) at 5% CO2 and 37°C. Cells were counted by an automated cell counter (Countess; Invitrogen, Darmstadt, Germany). MPC/MTT cells were confirmed for species identity by cytochrome C oxidase I DNA barcoding and for strain identity by comparing short tandem repeats from these cell lines with short tandem repeats of identity-confirmed counterparts as described previously (28). These analyses were performed by the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany).
Immortalized mouse chromaffin cells both wild-type (WT) and Sdhb−/− (29) were cultured in DMEM Glutamax (Gibco) with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco) at 37°C in 5% CO2.
Inhibitors
Everolimus, BYL719, sunitinib, temozolomide, niraparib (PARP inhibitor), cabozantinib (a TKI inhibiting c-met/VEGFR2/c-KIT/FLT3/RET/TIE2), and entinostat (a histone deacetylase 1/3 inhibitor) were purchased from Selleckchem (Munich, Germany). All drugs were dissolved in dimethyl sulfoxide (DMSO) (D8418; Sigma) and diluted in 0.2% BSA/PBS. DMSO was used as the vehicle (DMSO control) equal to the highest dose of each single experiment and had no effect on cell viability up to concentrations of 0.2% (1:500) DMSO.
Dose finding: Clinically relevant doses
Treatment doses of 10 to 25 nM everolimus were chosen because the plasma levels in patients on daily therapy with 10 mg everolimus range between ∼8 nM (cmin after 2 weeks of daily exposure to 10 mg everolimus) and 59 nM (c2h after 12 weeks of daily exposure to 10 mg everolimus) (30).
Concentrations of 2.5 to 10 µM BYL719 used in our experiments are also clinically relevant: In patients with advanced solid malignancies, median BYL719 plasma concentrations between 2 and 11 µM were found 2 to 8 hours post dose with 1-month daily oral standard therapy with 300 to 450 mg BYL719 (31).
We used 0.5 to 2 µM sunitinb in MPC/MTT cells and in human PCC cultures. Administration of 50 mg sunitinib per day leads to plasma concentrations of 0.1 to 0.2 μM (32). Thus, we only used a slightly higher dose as our starting concentration.
We used 0.5 to 2 µM cabozantinib in MPC/MTT cells and 1 to 10 µM cabozantinib in human PCC cultures. Administration of 20 to 60 mg of cabozantinib per day leads to plasma concentrations of 0.7 to 2.2 µM (33).
Human PCC primary cultures
This study was approved by the European Network for the Study of Adrenal Tumors, and informed consent was obtained from each patient. Primary tumor tissues were isolated from adrenal glands of six patients with PCC, and lymph node/uterus metastases were isolated from one patient with metastatic PGL (n = 7). Specimens were taken immediately after surgery and placed into Petri dishes filled with PBS. Tissue was separated into 0.5-mm pieces. Diced tissue was resuspended in 10 mL collagenase (Biochrom) and incubated at 37°C for 60 minutes. Subsequently, 2 mL pure fetal calf serum were added to inactivate the collagenase, and tumor tissue was centrifuged at 1100 rpm for 5 minutes. The supernatant was removed, and the pellet was gently resuspended in erythrocyte lysis buffer. After incubation for 7 minutes, centrifugation was repeated, the supernatant was removed, and the pellet was resuspended in DMEM/F12 supplemented with 10% fetal calf serum, 1% penicillin/streptomycin, and 0.4% amphotericin B. The suspension was filtered through a 70-µm sieve, and cells were counted and seeded into 96- or 6-well plates. After 3 to 5 days of incubation, cells were treated with everolimus, BYL719, sunitinib, temozolomide, niraparib, cabozantinib, or entinostat. After 48 or 72 hours, cell viability was analyzed. To confirm chromaffin cell origin, we used Western blot analysis of synaptophysin after 24 hours, as described below.
Cell viability
MPC/MTT cells were seeded into 96-well plates at densities of 2000 cells per well, grown for 24 hours, and treated with different concentrations of sunitinib, BYL719, and cabozantinib, either alone or in combination with everolimus. Furthermore, cells were treated with niraparib alone or in combination with temozolomide or with entinostat alone. Metabolic activity was assessed by the Cell Titer Blue® cell viability assay (no. G8081; Promega, Madison, WI) after 48, 72, and 120 hours of drug incubation. Cells were incubated for 4 hours with Cell Titer Blue® solution; fluorescence was measured at 560/590 nm using a GLOMAX plate reader (Promega).
Cell cycle analysis
The cell cycle was analyzed (Accuri C6 Analysis; BD Biosciences, San Jose, CA) as previously described (24). For flow cytometric analysis, cells were incubated for 24 hours with BYL719, everolimus, and sunitinib alone or in combination.
Apoptosis
Apoptosis was measured after 24 hours of treatment with BYL719, everolimus, and sunitinib, alone or in combination, using the Apo-One homogeneous caspase3/7-Assay kit (no. G7790; Promega) as previously described (24).
Cytotoxicity by crystal violet assay
A total of 2.5 × 103 cells per well were seeded into 48-well plates and treated for 72 hours (everolimus, BYL719, combination or vehicle). Crystal violet assay was then performed as previously described (34). Values were standardized to the vehicle absorbance at 24 hours and expressed as cell viability. Cell viability curves were established. Experiments were performed three times in duplicate.
Spheroid cultivation and treatment
Generation and cultivation of MPC/MTT cell spheroids was conducted as described previously (35, 36). Four days after spheroid generation, spheroids were treated with sunitinib or BYL719 alone or in combination with everolimus. Afterward, spheroid growth was monitored for 14 days (single treatment). During cultivation, medium was replaced every 3 to 4 days. A fractionated treatment regimen was used by additional treatment of the spheroids on days 7, 11, and 14.
Protein extraction and Western blot analysis
Cells were seeded into 10-cm plates and grown for 24 hours in complete medium before incubation with sunitinib and BYL719, either alone or in combination with everolimus, for 24 or 48 hours. Duplicate wells were used for each concentration of drug. Western blotting was conducted as described previously (37) using the following primary and secondary antibodies and their dilutions: pAktT (Ser473) (dilution: 1:20,000) (RRID: AB_2315049) (38), AKT (dilution: 1:5000) (RRID: AB_329827) (39), p4EBP1 (Ser65) (dilution: 1:2000) (RRID: AB_330947) (40), 4EBP1 (dilution: 1:1000) (RRID: AB_2097841) (41), p53 (dilution: 1:1000) (RRID: AB_10695803) (42), cyclin D1 (dilution: 1:1000) (RRID: AB_2259616) (43), cyclin D3 (dilution: 1:2000) (RRID: AB_2070801) (44), pp70S6K (Thr389) (dilution: 1:1000) (RRID: AB_330944) (45), p70S6K (dilution: 1:5000) (RRID: AB_390722) (46), pS6 (Ser240/244) (dilution: 1:200,000) (RRID: AB_10694233) (47), S6 (dilution: 1:20,000) (RRID: AB_331355) (48), pGSK3α/β (Ser21/9) (dilution: 1:5000) (RRID: AB_329830) (49), GSK3α/β (dilution: 1:1000) (RRID: AB_10547140) (50), CDK1/cdc2 (POH1) (dilution: 1:1000) (RRID: AB_2074795) (51), Chk1 (2G1D5) (dilution: 1:1000) (RRID: AB_2080320) (52) (Cell Signaling, Danvers, MA), IRS-1 (dilution: 1:5000) (RRID: AB_2536328) (53), pIRS-1 (Ser312) (dilution: 1:5000) (RRID: AB_2533767) (54) (Invitrogen, Carlsbad, CA), actin (dilution: 1:10,000) (RRID: AB_476744) (55) (Sigma-Aldrich, St. Louis, MO), and synaptophysin clone SY38 (dilution: 1:1000) (RRID: AB_95187) (56) (Merck-Millipore, Darmstadt, Germany). Western blot quantification was performed, and a representative blot of three independent experiments is shown.
Multigene panel next-generation sequencing
Testing for disease-causing variants in PCC/PGL-associated genes was performed by targeted next-generation sequencing (NGS), as described previously (57), using a custom-designed multigene panel based on the TruSeq® Nano DNA Library Prep Kit (Illumina, San Diego, CA).
The panel comprises 84 genes, including more than 20 genes associated with PCC/PGL (58), and was shown to be suitable for analysis of blood and formalin-fixed paraffin-embedded or fresh frozen tumor tissue. DNA was isolated from formalin-fixed paraffin-embedded tumor tissue samples. Library preparation was done according to the manufacturer’s instructions, and 150 nt paired-end sequencing was carried out with a minimum median coverage of 1000-fold on a NextSeq500 sequencer (Illumina). Data analysis was performed using the Biomedical Genomics Workbench 5.0 (Qiagen) as described previously (59). Settings for variant calling in tumor tissue were adapted using a low-frequency variant detection algorithm with a required minimum frequency of 5%, three reads supporting the variant, and a required minimum coverage of 10 reads.
Statistical analysis
Results are displayed as mean ± SD of at least three independent experiments. Each cell viability experiment consisted of at least six samples per concentration and incubation time. Cell viability in the monocyte chemoattractant protein/3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide experiments was modeled via a linear mixed model with random effects for trial and fixed effects for substances and their interactions. For the human primary cultures, we used a linear mixed model for the natural logarithm of the viability. Synergism was assessed as described previously (60) and was confirmed when both single effects were significant, the interaction effect was significant, and all regression coefficients were negative. When both single effects were significant and the interaction effect was not significant, it was defined as an “additive” effect.
For spheroid data, the diameter of the spheroids was modeled via a quasi-Poisson mixed model with random effects for trial and spheroid. Temporal correlation was modeled via a continuous AR(1) correlation structure. Besides the main effects, the model included a quadratic term for the day since starting and interactions between time and substances. All significance tests were performed within the respective model.
Calculations were performed using R 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Significance was assessed at P < 0.05. Due to the exploratory character of this work, we did not adjust any P values for multiple testing.
Results
MPC/MTT cells
Cell viability after different drug treatments
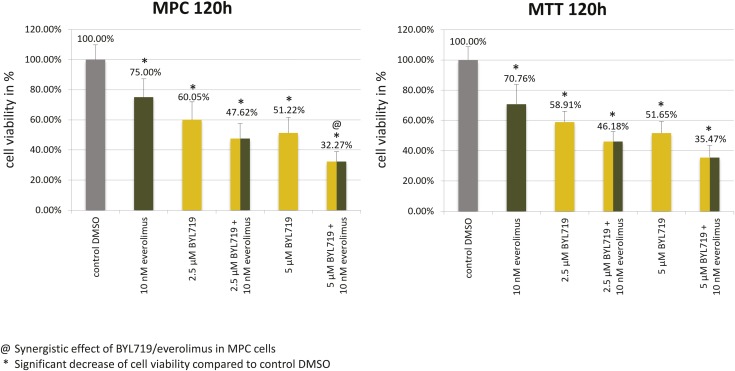
Treatment (120 hours) with clinically relevant doses of BYL719 (2.5 µM/5 µM) and everolimus (10 nM) significantly diminished MPC/MTT cell viability compared with the control (Fig. 2); combination treatment with 5 μM BYL719 and 10 nM everolimus synergistically reduced MPC and additively MTT reduced cell viability (Fig. 2).
Figure 2.
Significant MPC/MTT cell viability reduction by clinically relevant doses of BYL719, everolimus, and by the combination of both after 120 h. The arithmetic means and standard deviation of at least three independent experiments are shown. @The BYL719/everolimus combination treatment was statistically synergistic in MPC cells and additive in MTT cells. *Statistically significant differences in comparison with the DMSO control (P < 0.05).
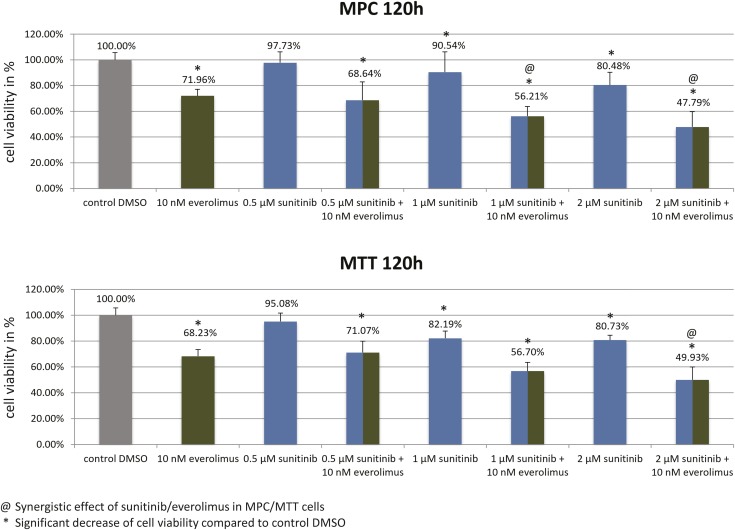
Treatment with sunitinib (1 µM/2 µM) and everolimus (10 nM) significantly reduced MPC/MTT cell viability compared with the control (Fig. 3). Combination treatment synergistically reduced MPC (1 µM/2 µM sunitinib plus 10 nM everolimus) and MTT (2 µM sunitinib plus 10 nM everolimus) cell viability (Fig. 3).
Figure 3.
Significant MPC/MTT cell viability reduction by sunitinib, everolimus, and by the combination of both after 120 h. The arithmetic means and SD of at least three independent experiments are shown. @Sunitinib/everolimus combination treatment was statistically synergistic in both cell lines. *Statistically significant differences in comparison with the DMSO control (P < 0.05).
Cabozantinib (1 μM/2 µM) significantly reduced MPC/MTT cell viability (58) but was not synergistic with everolimus (data not shown).
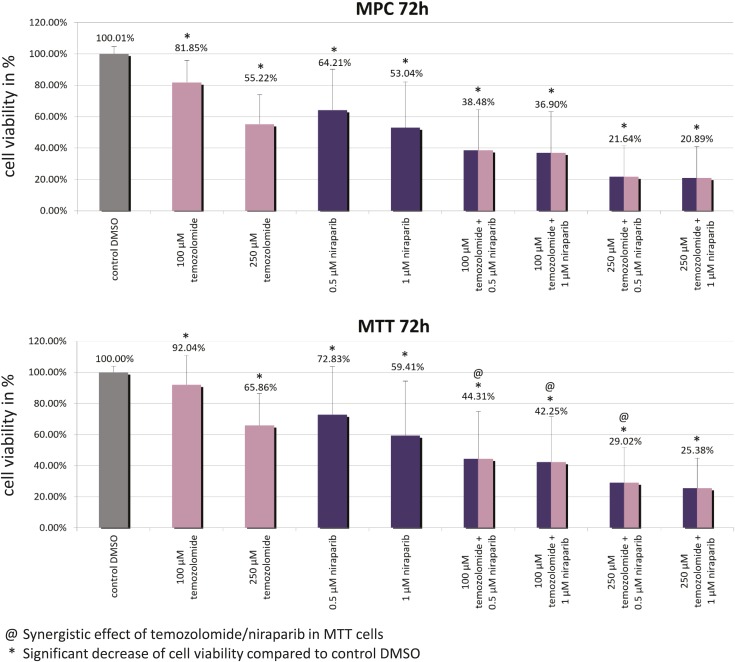
Niraparib (0.5 μM/1 μM) and temozolomide (100 μM/250 μM) singly significantly reduced MPC/MTT cell viability compared with the control (Fig. 4). Combination of 0.5 to 1.0 μM niraparib plus 100 µM temozolomide, or 0.5 µM niraparib plus 250 µM temozolomide, synergistically diminished MTT, but not MPC, cell viability (Fig. 4).
Figure 4.
Significant MPC/MTT cell viability reduction by niraparib, by temozolomide, and by the combination of both after 72 h. @The niraparib/temozolomide combination was statistically synergistic in MTT cells but not in MPC cells. *Statistically significant differences in comparison with the DMSO control (P < 0.05).
For all the following experiments in MPC/MTT cells and spheroids, we investigated only the most promising combinations (BYL719/everolimus and sunitinib/everolimus).
Cell cycle and apoptosis
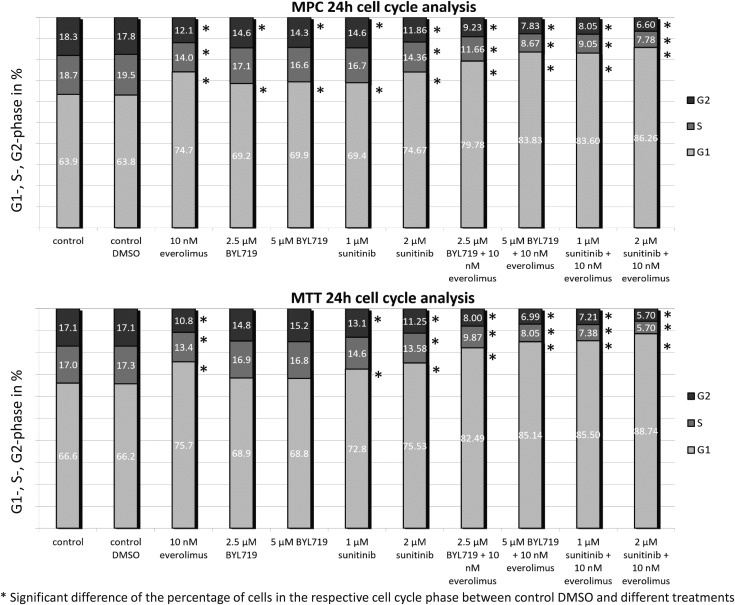
BYL719 (5 µM), everolimus (10 nM), and sunitinib (1 µM/2 µM) significantly increased the G1 cell cycle phase in MPC cells compared with the control (Fig. 5). In MTT cells, everolimus (10 nM) and sunitinib (1 µM/2 µM) significantly increased the G1 cell cycle phase. In both cell lines, BYL719/everolimus or sunitinib/everolimus combination treatments further enhanced G1-phase cell cycle arrest compared with single treatments (Fig. 5). The sub-G1 phase and Western blot quantification can be viewed in materials saved to an online repository (58).
Figure 5.
Cell cycle analysis via flow cytometric analysis. Significant induction of a G1-phase cell cycle arrest in MPC cells by BYL719, everolimus, or sunitinib alone and (even stronger) by the BYL719/everolimus and sunitinib/everolimus combination treatments at 24 h of treatment. Significant induction of a G1-phase cell cycle arrest in MTT cells by everolimus (10 nM) and sunitinib (1 µM/2 µM) and (even stronger) by the BYL719/everolimus and sunitinib/everolimus combination treatment at 24 h of treatment. The arithmetic means and SD of at least three independent experiments are shown. *Statistically significant differences in comparison with the DMSO control (P < 0.05).
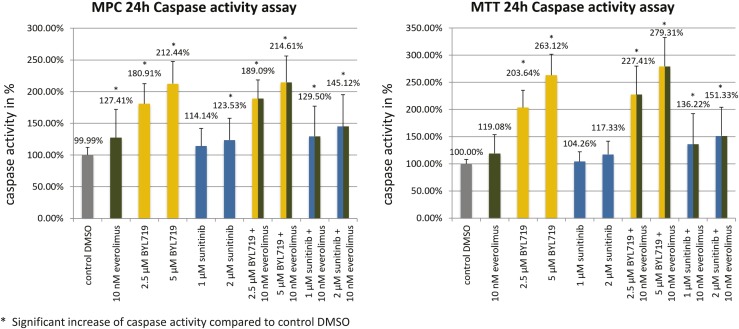
Apoptosis was investigated by caspase assay. In both cell lines, significant apoptosis induction was observed after BYL719 treatment alone (2.5 µM/5 µM), after BYL719/everolimus combination treatment, and (to a lesser extent) after the sunitinib/everolimus combination treatment (Fig. 6).
Figure 6.
Mean caspase-3/-7 activity in percent ± SD in MPC/MTT cell lines after 24 h of incubation with different drugs. In both cell lines, significant apoptosis induction was observed after BYL719 treatment alone, after BYL719/everolimus combination treatment, and (to a lesser extent) after sunitinib/everolimus combination treatment. The arithmetic means and SD of at least three independent experiments are shown. *Statistically significant differences in comparison with the DMSO control (P < 0.05).
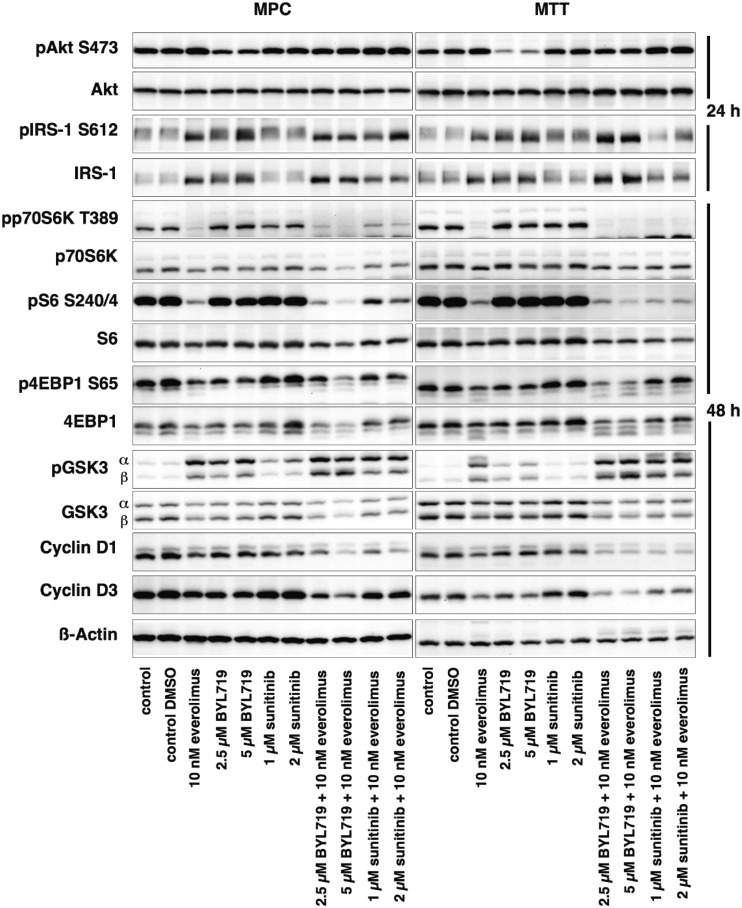
Western blot analysis
Western blot results of MPC/MTT cells are shown in Fig. 7. Western blot quantification can be viewed in materials saved to an online repository (58). Everolimus is known to inhibit mTORC1/p70S6K and thereby activate AKT. Indeed, everolimus and each combination treatment inhibited p70S6K and S6 in MPC/MTT cells; 4EBP1, another mTORC1 effector, was inhibited by everolimus, BYL719, and each combination treatment. PI3K/AKTS473 was activated in response to everolimus, inhibited by BYL719, and unaffected by sunitinib. The BYL719/everolimus combination attenuated everolimus-induced PI3K/AKTS473 activation to the level of the untreated control. The sunitinib/everolimus combination did not effectively attenuate everolimus-induced PI3K/AKTS473 activation. Although most of the effects were seen at the highest doses tested, these are still low clinically relevant doses (clinically relevant doses of BYL719 are 2 to 11 µM and of everolimus are 8 to 59 nM).
Figure 7.
Western blot analysis of MPC/MTT cell lines after 24 or 48 h of incubation with different drugs. Inhibition of p70S6K, S6, 4EBP1, and GSK3 signaling, attenuation of everolimus-induced AKT activation, IRS-1 upregulation, and cyclin D1/D3 downregulation after BYL719/everolimus combination treatment. A representative blot of three independently performed experiments is shown.
PI3K/AKT phosphorylates and thereby inhibits GSK3 (61). Persistent activation of PI3K leads to feedback downregulation of IRS-1, which may lead to GSK3 disinhibition/activation (62). GSK3α/β phosphorylation and thereby inhibition led to a significant decrease in NET cell viability (63). In this study BYL719 and everolimus alone led to GSK3 inhibition in MPC/MTT cells, and combination treatment even more strongly inhibited GSK3. The sunitinib/everolimus combination also led to GSK3 inhibition, compared with the control and single treatment in MTT cells, but inhibited GSK3β to a lesser extent than the BYL719/everolimus combination. BYL719 alone, everolimus alone, and the BYL719/everolimus combination treatment promoted IRS-1 upregulation in MPC/MTT cells as a potential mechanism of GSK3 inhibition.
Consistent with induction of G1 cell cycle arrest, Cyclin D1 and D3 decreased after BYL719/everolimus and sunitinib/everolimus combination treatments in MPC/MTT cells.
Sdhb −/− immortalized mouse chromaffin cells
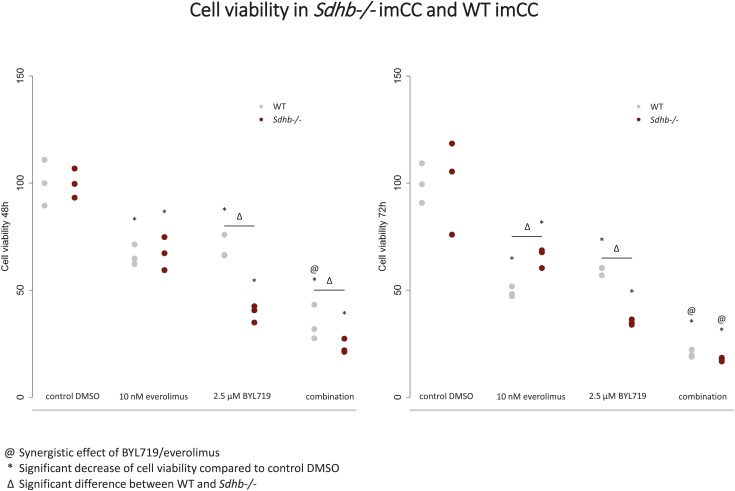
Patients with PCC/PGL carrying mutations in SDHB often show increased incidence of an early-onset, increased metastatic risk and thus a poor prognosis (64). Effective treatment strategies for these patients are missing. Therefore, we have investigated the impact of BYL719 (2.5 µM) and everolimus (10 nM) alone or in combination on immortalized mouse chromaffin cells (imCCs) with and without expression of Sdhb. Sdhb−/− imCCs carry a true knockout of the Sdhb gene, leading to the absence of SDHB protein and complete inhibition of SDH activity (29). Both everolimus and BYL719 alone significantly reduced Sdhb−/− cell viability compared with the vehicle, with a synergistic effect of the combination at 72 hours (Fig. 8). Moreover, BYL719 treatment alone had a significantly stronger inhibitory effect on Sdhb−/− imCC viability compared with WT imCC viability after 48 and 72 hours of treatment, but the combination treatment was no more effective in Sdhb−/− than in the WT cells after 72 hours of treatment (Fig. 8).
Figure 8.
Significant reduction of cell viability after BYL719 and everolimus treatment in immortalized mouse chromaffin WT and Sdhb−/− cells at 48 and 72 h, with a synergistic effect (@) of the combination in both cell lines at 72 h and a substantially stronger effect of BYL719 alone in the Sdhb−/− compared with the WT cells. ∆Significant difference between WT and Sdhb−/−. *Statistically significant differences in comparison with the DMSO control (P < 0.05).
MPC/MTT cell spheroids
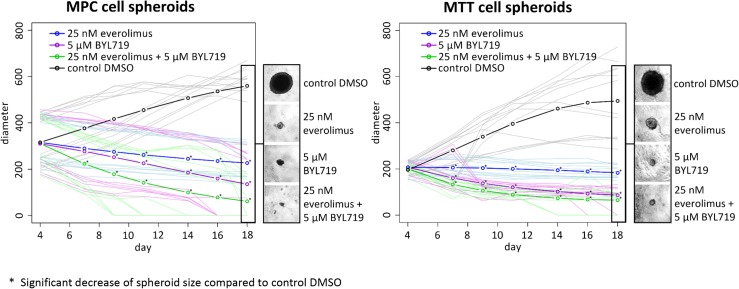
Tumor cell spheroids are characterized by an oxygen and nutrient gradient and provide an excellent in vitro model for drug screening regarding their antitumor activity. Fractionated treatment with clinically relevant doses of BYL719 (5 µM) led to significant shrinkage of MPC/MTT spheroids, compared with the spheroid sizes at the beginning (day 4), and in several cases led to complete spheroid collapse (day 16) (Fig. 9) (58). Fractionated treatment with clinically relevant doses of everolimus (25 nM) significantly inhibited MPC/MTT spheroid growth (Fig. 9) (58). These effects were enhanced by the BYL719/everolimus combination, leading to a significant shrinkage or an early complete collapse of MPC/MTT spheroids in several experiments (days 9 and 14, respectively) (Fig. 9). Although the DMSO control had an average diameter of 585 ± 50.2 µm (MPC) and 500 ± 166.3 µm (MTT) after 18 days, fractionated treatment with 25 nM everolimus plus 5 µM BYL719 led to a significantly smaller average spheroid diameters of 59.5 ± 68.8 µm (MPC) and 62.7 ± 42.3 µm (MTT), respectively. Fractionated treatment with sunitinib only slowed spheroid growth down (58). In MPC spheroids, sunitinib enhanced the efficacy of everolimus (58), but this effect was not seen in MTT spheroids after fractionated treatment (58). Fractionated treatment was more effective than single treatment. No spheroid regrowth was seen after BYL719 single treatment.
Figure 9.
Significant MPC/MTT spheroid shrinkage or complete spheroid collapse after fractionated treatment with 5 µM BYL719, even stronger shrinkage, as compared with BYL719 alone, or an early complete collapse of the spheroids in several experiments after fractionated treatment with the BYL719/everolimus combination and spheroid growth inhibition after fractionated treatment with 25 nM everolimus. Spheroids were treated with different concentrations of BYL719 and everolimus after completed spheroid formation (day 4) and on days 7, 11, and 14 (fractionated treatment). Growth was monitored over 18 d. The arithmetic means and all single values of each experiment of at least three independent experiments are shown. *Statistically significant different results in comparison with the DMSO control (P < 0.05).
Human primary cultures
Clinical, histological, and genetic characteristics
We generated primary cultures from seven patients with PCC/PGL and confirmed tumor identity by detecting synaptophysin as a well-established PCC/PGL marker (30).
Tumors from patients 1, 2, 4, and 6 showed criteria associated with an adverse prognosis, including capsule invasion, vascular invasion, a size of >5 cm, or a Ki-67 of 5% (Table 1), although they were not metastatic. Patient 7 had a metastatic PGL.
Table 1.
Clinical and Histological Characteristics of the Human PCC/PGL Primary Cultures From Seven Different Patients
| Patient | Age | Sex | Tumor | Metastatic | Histology and Criteria of Malignancy | Ki-67 (%) | NGS of the Tumors |
|---|---|---|---|---|---|---|---|
| 1 | 82 | M | Adrenal pheochromocytoma | No | Tumor size: 1.5 cm; capsule invasion | 1–2 | No known susceptibility mutation |
| 2 | 50 | M | Adrenal pheochromocytoma | No | Tumor size: 12.9 cm; nuclear pleomorphism | 1–2 | NF1 mutation |
| 3 | 73 | F | Adrenal pheochromocytoma | No | Tumor size: 3.9 cm; nuclear and cell pleomorphism | 2 | NF1 mutation |
| 4 | 26 | M | Adrenal pheochromocytoma | No | Tumor size: 3.2 cm; vascular invasion, anisonucleosis, cell inclusions | 5 | No known susceptibility mutation |
| 5 | 65 | M | Adrenal pheochromocytoma | No | Tumor size: 5 cm | <1 | Not assessed |
| 6 | 60 | M | Adrenal pheochromocytoma | No | Tumor size: 5.5 cm; capsule invasion, vascular invasion | <1 | Not assessed |
| 7 | 30 | F | Uterus metastasis | Yes | Tumor size: 5.3 cm | 20 | Variant of unknown significance in TET1 |
| Lymph node metastasis | Yes | Tumor size: 2.5 cm | 20 |
Abbreviations: F, female; M, male.
Targeted NGS (Table 1) showed disease-causing NF1 mutations in tumors of patients 2 and 3, in line with cluster-2 tumors. These were likely somatic mutations; in neither patient was germline testing available, but neither patient showed clinical stigmata of neurofibromatosis. However, to exclude the possibility of a hereditary PPGL syndrome, pathogenic variants identified in tumor tissue would need to be analyzed in corresponding blood samples as well.
In tumor tissue from patient 7, we identified a missense variant of unknown significance in the tet methylcytosine dioxygenase (TET1) gene, a demethylase involved in epigenetic regulation and gene activation. In the tumor tissues from patients 1 and 4, no known disease-causing susceptibility mutation was identified by NGS. NGS was not performed on tumor tissues from patients 5 and 6.
Primary cell culture viability after different drug treatments
We have statistically combined all human tumor cultures in which combination treatments were tested (tumor cultures from six patients, n = 6).
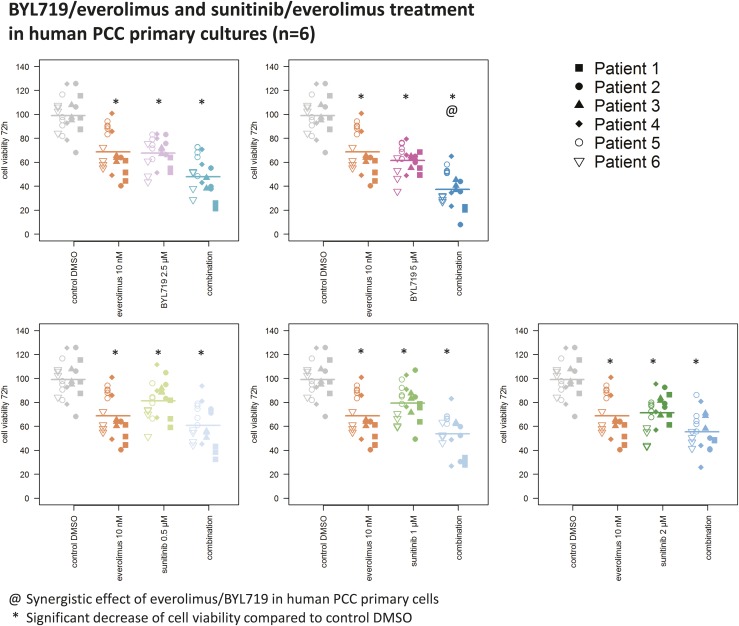
In all combination-treated human primary cultures (n = 6), clinically relevant doses of BYL719 (2.5 µM/5 µM) and everolimus (10 nM) significantly reduced viability, and the combination of 5µM BYL719 and 10 nM everolimus was highly effective and synergistic (P < 0.05) (Fig. 10).
Figure 10.
Significant reduction of human PCC primary culture viability (n = 6) by clinically relevant doses of BYL719, everolimus, and the combination of BYL719 plus everolimus at 72 h of treatment, showing a synergistic effect (@) of 5 µM BYL719 plus 10 nM everolimus. A significant reduction of human PCC primary culture viability (n = 6) by sunitinib and the combination of sunitinib plus everolimus is seen at 72 h. Sunitinib and everolimus did not act synergistically. The arithmetic means and all single values of six human PCC cultures from six different patients are shown. *Statistically significant different results in comparison with the DMSO control (P < 0.05).
Sunitinib (2 µM) significantly decreased primary culture viability but was not synergistic with everolimus (Fig. 10).
We could not test combination treatments in the primary cultures from patient 7 because there was insufficient tissue material. Sunitinib (1 to 8 µM) and clinically relevant doses of BYL719 (2.5 to 10 µM) separately decreased viability of the primary cultures from patient 7 (58).
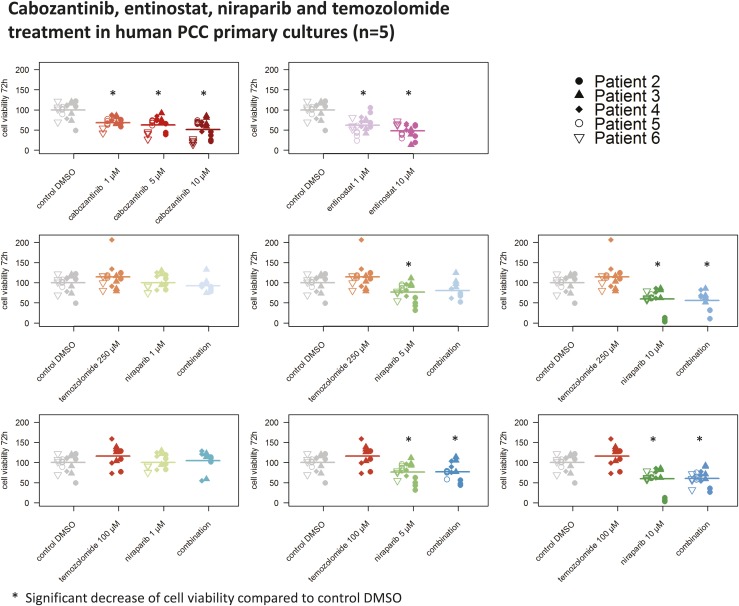
Furthermore, we tested entinostat (1 to 10 µM), cabozantinib (1 to 10 µM), niraparib (5 to 10 µM), and temozolomide (100 to 250 µM) in primary cultures from five patients with PCC. Entinostat (1 to 10 µM), cabozantinib (1 to 10 µM), and niraparib (5 to 10 µM) significantly decreased primary culture viability, but temozolomide had no effect (Fig. 11).
Figure 11.
Substantial reduction of human PCC primary culture viability (n = 5) by cabozantinib, entinostat, and niraparib, but not temozolomide, at 72 h of treatment. The arithmetic means and all single values of five human PCC primary cultures from five patients are shown. *Statistically significant different results in comparison with the DMSO control (P < 0.05).
Western blot analysis
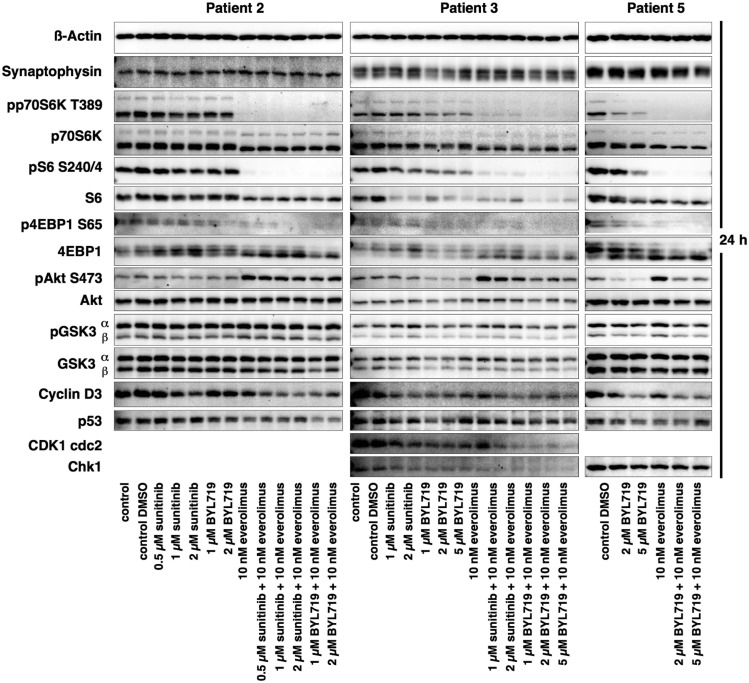
We had sufficient human primary culture material from patients 2, 3, and 5 for performing detailed Western blot analysis of these primary cultures; the results are shown in Fig. 12. Western blot quantification of the treatments performed identically in all three primary cultures (control DMSO, 10 nM everolimus, 2 µM BYL719, BYL719/everolimus combination) can be viewed in materials saved to an online repository (58). Supplemental Table 2 (58) shows all the values of the Western blot quantification of all primary culture Western blots. The mTORC1 effector proteins p70S6K and S6 were strongly inhibited by everolimus and by each combination treatment at 24 hours; 4EBP1, another mTORC1 effector protein, was inhibited by everolimus and the BYL719/everolimus combination treatment. AKTS473 was activated in response to everolimus, and this activation was attenuated by the addition of BYL719 in the primary cultures from patients 3 and 5. In contrast to the mouse cell lines, inhibition of GSK3 by BYL719 or by combination treatments could not be shown, possibly due to a shorter treatment time of 24 hours. However, after a longer incubation time of 48 hours, the protein concentration of the primary cultures was too low for Western blot detection. P53 was moderately downregulated by the everolimus/BYL719 combination. Consistent with a G1-phase cell cycle arrest, combination treatments were associated with downregulation of the cell cycle promoting markers cyclin D3, CDK1 cdc2 (patient 3), and chk1 (patients 3 and 5).
Figure 12.
Western blot analysis of the human primary cultures from patients 2, 3, and 5 after 24 h of incubation with different drugs. There was strong synaptophysin expression in all primary cultures. There was strong inhibition of mTORC1/p70S6K and 4EBP1 signaling by everolimus and all combination treatments, attenuation of everolimus-induced AKT activation by the combination treatment in the primary cultures from patients 3 and 5 and downregulation of cyclin D3, CDK1 cdc2 (patient 3), chk1 (patients 3 and 5), and p53 by the combination treatment. GSK3 showed no clear differences, most likely due to suboptimal incubation times.
Discussion
We have shown that targeted combination treatment with clinically relevant doses of the PI3Kα inhibitor BYL719 and the mTORC1 inhibitor everolimus exhibited strong synergistic antitumor potential in PCC cell lines and especially Sdhb−/− cells and in human PCC primary cultures. In MPC/MTT three-dimensional spheroids, this combination treatment led to dramatic spheroid shrinkage or to a complete spheroid collapse in several experiments. The BYL719/everolimus combination was the most effective treatment we have tested in PCC spheroids.
Everolimus has been approved for the treatment of NETs in many countries (65–67). Similarly to PCC/PGL with increased PI3K/AKT/mTORC1 activation (68), gastroenteropancreatic NETs show frequent PI3K/AKT/mTORC1 overactivation (69, 70). However, everolimus alone showed disappointing results in a small study of patients with PCC/PGL (20). Compensatory IRS-1/PI3K/AKT disinhibition/activation in response to mTORC1/p70S6K inhibition is the known short-term mechanism of everolimus resistance (71–75). Indeed, we found strong everolimus-induced PI3K/AKT activation in PCC cell lines and human primary cultures. In different NET cell lines, the PI3Kα inhibitor BYL719 showed synergism with everolimus at clinically relevant doses (24, 76) and overcame long-term everolimus resistance in human pancreatic NET cells (24). In a breast cancer model, everolimus also acted synergistically with BYL719 and delayed the development of resistance to BYL719 in vitro and in vivo (22). Consistently, we showed in our murine PCC cell lines and in two different human PCC cultures that addition of BYL719 attenuated everolimus-induced AKT activation, which may contribute to the synergism of both drugs. As also observed in human NET cells (24, 76), BYL719/everolimus combination treatment led to synergistic inhibition of GSK3 in PCC cell lines, possibly as a consequence of IRS-1 upregulation. IRS-1 has previously been shown to inhibit GSK3 via different signaling pathways (61, 77, 78), and GSK3 inhibition has shown antitumor potential in human NET cell lines (63).
In our murine cell lines, the BYL719/everolimus combination induced a substantial G1-phase cell cycle arrest that was more prominent than in each single-drug treatment. Consistently, the BYL719/everolimus combination led to cyclin D1/D3 downregulation in PCC cell lines and human primary cultures.
In contrast to other PI3K inhibitors, such as NVP-BEZ235 or BKM120, BYL719 is most likely to become clinically available. The phase III approval study SOLAR-1 evaluating BYL719 (alpelisib) in metastatic breast cancer has reached its final endpoints (23). Another phase IB trial is evaluating the safety of BYL719 (alpelisib) plus everolimus in advanced breast, renal cell, and pancreatic cancer, including pancreatic NETs (clinical trial no. NCT02077933).
Sunitinib is being evaluated in a randomized, prospective, placebo-controlled clinical phase II trial in PCC/PGL (FIRST-MAPPP, NCT01371202), which has completed recruitment. A synergistic effect of sunitinib and everolimus was shown in MPC/MTT cell lines at doses exceeding clinical relevance but not in human primary cultures. This may be in part due to additional antiangiogenic activity of sunitinib in vivo, which cannot be mimicked by cell culture. However, BYL719/everolimus combination treatment seems to be more promising, as compared with sunitinib/everolimus in vitro. Nevertheless, one case in the literature notes a patient with the SDHB mutation who received the mTORC1 inhibitor rapamycin in combination with sunitinib and experienced long-term disease control (21).
The combination of niraparib with temozolomide was synergistic in the MTT cells but not in human primary cultures. PARP inhibition by niraparib suppresses DNA repair and sensitizes tumors to alkylating DNA-damaging agents (14, 79, 80), thus synergizing with temozolomide in the murine PCC cell lines. Consistent with this, in an allograft PCC mouse model, a PARP inhibitor sensitized PCCs to temozolomide (15). However, temozolomide showed an absence of efficacy in human primary cultures, whereas niraparib and entinostat were effective in primary cultures. This implicates fundamental differences of drug effects between murine cell lines and human primary cultures, rendering additional confirmatory drug testing in human primary cultures even more important.
Cabozantinib showed significant efficacy in PCC cell lines and human primary cultures at clinically relevant doses but no synergism with everolimus. The PCC cell lines and human primary cultures displayed a weaker response to cabozantinib alone, as compared with everolimus/BYL719 combination treatment. Our data suggest that dual PI3K/mTORC1 inhibition might be more effective at lower doses with less toxicity. As a next step, we will investigate the most promising combination of everolimus/BYL719 in vivo in a suitable PCC allograft model.
The success of the different molecular-targeted therapies alone and in combination depends on the individual molecular aberrations of each tumor, as shown in other tumor types (81). For example, the tumors from patients 2 and 3 are associated with NF1 mutations leading to MEK/RAS/ERK and mTORC1 overactivation (82–84). Accordingly, both tumor cultures responded well to mTORC1 and PI3Kα inhibition by everolimus and BYL719, respectively, and especially to the combination, with attenuation of everolimus-induced AKT activation by BYL719. This suggests that, among others, NF1-mutated tumors, which are frequently present among sporadic PCCs (85), should respond to these targeted drugs. In the PGL of patient 7, who had an unusual metastatic phenotype, no disease-causing variants were identified in any of the known PCC/PGL susceptibility genes; however, a variant of unknown significance in TET1, a DNA demethylase, was found, which presents a potential candidate gene that needs further investigation in the future.
In summary, we have established a method to investigate molecular-targeted agents in human PCC primary cultures of individual patient tumors and have correlated drug efficacy with signaling pathway alterations. The established protocol for ex vivo treatment of human PCC/PGL primary cultures with different molecular-targeted drugs allows screening for appropriate treatment approaches for each patient. In addition, we identified dual mTORC1/PI3Kα inhibition by the everolimus/BYL719 combination treatment as clinically relevant, highly effective synergistic targeted therapy in all the PCC models investigated, including human tumor cultures.
Acknowledgments
The authors thank Susanne Schmid (study nurse) for her excellent work and Isabel Poser and Daniela Stanke for excellent technical assistance.
Financial Support: This work was funded by Deutsche Forschungsgemeinschaft within the CRC/Transregio 205/1 “The Adrenal: Central Relay in Health and Disease” (to N.B., B.K., C.G.Z., M.F., F.B., S.R.B., G.E., and M.R.) and by the Intramural Research Program of the National Institutes of Health, National Institute of Child Health and Human Development.
Clinical Trial Information: ClinicalTrials.gov nos. NCT01371201 (registered 10 June 2011) and NCT02077933 (registered 14 March 2014).
Additional Information
Disclosure Summary: A.B.G. has received research funding from AAA and lecture fees from Ipsen, Roche, and HRA Pharma. C.J.A. has received research contracts from Ipsen, Novartis, and ITM Solucin; lecture honorarium from Ipsen, Novartis, and Falk Foundation; and advisory board honorarium from Novartis. S.N. has received research contracts from Novartis and lecture fees from Ipsen. The remaining authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- CVD
cyclophosphamide, vincristine, dacarbazine
- DMSO
dimethyl sulfoxide
- imCC
immortalized mouse chromaffin cells
- MPC
monocyte chemoattractant protein
- mTORC1
mammalian target of rapamycin
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NET
neuroendocrine tumor
- NGS
next-generation sequencing
- PARP
poly(ADP-ribose) polymerase
- PCC
pheochromocytoma
- PGL
paraganglioma
- PI3K
phosphatidylinositol-3-kinase
- TET1
tet methylcytosine dioxygenase
- TKI
tyrosine kinase inhibitor
- VEGFR
vascular endothelial growth factor receptor
- WT
wild-type
References and Notes
- 1. Remine WH, Chong GC, Van Heerden JA, Sheps SG, Harrison EG Jr. Current management of pheochromocytoma. Ann Surg. 1974;179(5):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Proye CA, Vix M, Jansson S, Tisell LE, Dralle H, Hiller W. “The” pheochromocytoma: a benign, intra-adrenal, hypertensive, sporadic unilateral tumor. Does it exist? World J Surg. 1994;18(4):467–472. [DOI] [PubMed] [Google Scholar]
- 3. Goldstein RE, O’Neill JA Jr, Holcomb GW III, Morgan WM III, Neblett WW III, Oates JA, Brown N, Nadeau J, Smith B, Page DL, Abumrad NN, Scott HW Jr. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755–764, discussion 764–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mannelli M, Ianni L, Cilotti A, Conti A. Pheochromocytoma in Italy: a multicentric retrospective study. Eur J Endocrinol. 1999;141(6):619–624. [DOI] [PubMed] [Google Scholar]
- 5. John H, Ziegler WH, Hauri D, Jaeger P. Pheochromocytomas: can malignant potential be predicted? Urology. 1999;53(4):679–683. [DOI] [PubMed] [Google Scholar]
- 6. Edström Elder E, Hjelm Skog AL, Höög A, Hamberger B. The management of benign and malignant pheochromocytoma and abdominal paraganglioma. Eur J Surg Oncol. 2003;29(3):278–283. [DOI] [PubMed] [Google Scholar]
- 7. Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005;90(4):2110–2116. [DOI] [PubMed] [Google Scholar]
- 8. Feng F, Zhu Y, Wang X, Wu Y, Zhou W, Jin X, Zhang R, Sun F, Kasoma Z, Shen Z. Predictive factors for malignant pheochromocytoma: analysis of 136 patients. J Urol. 2011;185(5):1583–1590. [DOI] [PubMed] [Google Scholar]
- 9. Park J, Song C, Park M, Yoo S, Park SJ, Hong S, Hong B, Kim CS, Ahn H. Predictive characteristics of malignant pheochromocytoma. Korean J Urol. 2011;52(4):241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamidi O, Young WF Jr, Gruber L, Smestad J, Yan Q, Ponce OJ, Prokop L, Murad MH, Bancos I. Outcomes of patients with metastatic phaeochromocytoma and paraganglioma: A systematic review and meta-analysis. Clin Endocrinol (Oxf). 2017;87(5):440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mak IYF, Hayes AR, Khoo B, Grossman A. Peptide receptor radionuclide therapy as a novel treatment for metastatic and invasive phaeochromocytoma and paraganglioma. Neuroendocrinology. (in press). [DOI] [PubMed] [Google Scholar]
- 12. Nölting S, Grossman A, Pacak K. Metastatic phaeochromocytoma: spinning towards more promising treatment options. Exp Clin Endocrinol Diabetes. 2019;127(2-3):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18(10):610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Murcia JM, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, Oliver FJ, Masson M, Dierich A, LeMeur M, Walztinger C, Chambon P, de Murcia G. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci USA. 1997;94(14):7303–7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pang Y, Lu Y, Caisova V, Liu Y, Bullova P, Huynh TT, Zhou Y, Yu D, Frysak Z, Hartmann I, Taïeb D, Pacak K, Yang C, Yang C. Targeting NAD+/PARP DNA repair pathway as a novel therapeutic approach to SDHB-mutated cluster I pheochromocytoma and paraganglioma. Clin Cancer Res. 2018;24(14):3423–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koumarianou A, Kaltsas G, Kulke MH, Oberg K, Strosberg JR, Spada F, Galdy S, Barberis M, Fumagalli C, Berruti A, Fazio N. Temozolomide in advanced neuroendocrine neoplasms: pharmacological and clinical aspects. Neuroendocrinology. 2015;101(4):274–288. [DOI] [PubMed] [Google Scholar]
- 17. Luchetti A, Walsh D, Rodger F, Clark G, Martin T, Irving R, Sanna M, Yao M, Robledo M, Neumann HP, Woodward ER, Latif F, Abbs S, Martin H, Maher ER. Profiling of somatic mutations in phaeochromocytoma and paraganglioma by targeted next generation sequencing analysis. Int J Endocrinol. 2015;2015:138573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burnichon N, Vescovo L, Amar L, Libé R, de Reynies A, Venisse A, Jouanno E, Laurendeau I, Parfait B, Bertherat J, Plouin PF, Jeunemaitre X, Favier J, Gimenez-Roqueplo AP. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet. 2011;20(20):3974–3985. [DOI] [PubMed] [Google Scholar]
- 19. Jochmanova I, Pacak K. Genomic landscape of pheochromocytoma and paraganglioma. Trends Cancer. 2018;4(1):6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Druce MR, Kaltsas GA, Fraenkel M, Gross DJ, Grossman AB. Novel and evolving therapies in the treatment of malignant phaeochromocytoma: experience with the mTOR inhibitor everolimus (RAD001). Horm Metab Res. 2009;41:697–702. [DOI] [PubMed] [Google Scholar]
- 21. Ayala-Ramirez M, Chougnet CN, Habra MA, Palmer JL, Leboulleux S, Cabanillas ME, Caramella C, Anderson P, Al Ghuzlan A, Waguespack SG, Deandreis D, Baudin E, Jimenez C. Treatment with sunitinib for patients with progressive metastatic pheochromocytomas and sympathetic paragangliomas. J Clin Endocrinol Metab. 2012;97(11):4040–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, Tao J, Campos AB, Rodon J, Ibrahim YH, Serra V, Rodrik-Outmezguine V, Hazra S, Singh S, Kim P, Quadt C, Liu M, Huang A, Rosen N, Engelman JA, Scaltriti M, Baselga J. mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med. 2013;5(196):196ra99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, Yamashita T, Lu YS, Inoue K, Takahashi M, Pápai Z, Longin AS, Mills D, Wilke C, Hirawat S, Juric D; SOLAR-1 Study Group. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–1940. [DOI] [PubMed] [Google Scholar]
- 24. Aristizabal Prada ET, Spöttl G, Maurer J, Lauseker M, Koziolek EJ, Schrader J, Grossman A, Pacak K, Beuschlein F, Auernhammer CJ, Nölting S. The role of GSK3 and its reversal with GSK3 antagonism in everolimus resistance. Endocr Relat Cancer. 2018;25(10):893–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powers JF, Evinger MJ, Tsokas P, Bedri S, Alroy J, Shahsavari M, Tischler AS. Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell Tissue Res. 2000;302(3):309–320. [DOI] [PubMed] [Google Scholar]
- 26. Martiniova L, Lai EW, Elkahloun AG, Abu-Asab M, Wickremasinghe A, Solis DC, Perera SM, Huynh T-T, Lubensky IA, Tischler AS, Kvetnansky R, Alesci S, Morris JC, Pacak K. Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clin Exp Metastasis. 2009;26(3):239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nölting S, Garcia E, Alusi G, Giubellino A, Pacak K, Korbonits M, Grossman AB. Combined blockade of signalling pathways shows marked anti-tumour potential in phaeochromocytoma cell lines. J Mol Endocrinol. 2012;49(2):79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Almeida JL, Hill CR, Cole KD. Mouse cell line authentication. Cytotechnology. 2014;66(1):133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Letouzé E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, Janin M, Menara M, Nguyen AT, Benit P, Buffet A, Marcaillou C, Bertherat J, Amar L, Rustin P, De Reyniès A, Gimenez-Roqueplo AP, Favier J. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23(6):739–752. [DOI] [PubMed] [Google Scholar]
- 30. Budde K, Zonnenberg BA, Frost M, Cheung W, Urva S, Brechenmacher T, Stein K, Chen D, Kingswood JC, Bissler JJ. Pharmacokinetics and pharmacodynamics of everolimus in patients with renal angiomyolipoma and tuberous sclerosis complex or lymphangioleiomyomatosis. Br J Clin Pharmacol. 2016;81(5):958–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Buck SS, Jakab A, Boehm M, Bootle D, Juric D, Quadt C, Goggin TK; Buck SSd. Population pharmacokinetics and pharmacodynamics of BYL719, a phosphoinositide 3-kinase antagonist, in adult patients with advanced solid malignancies. Br J Clin Pharmacol. 2014;78(3):543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand J-P, Scigalla P, Raymond E. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24(1):25–35. [DOI] [PubMed] [Google Scholar]
- 33. Miles D, Jumbe NL, Lacy S, Nguyen L. Population pharmacokinetic model of cabozantinib in patients with medullary thyroid carcinoma and its application to an exposure-response analysis. Clin Pharmacokinet. 2016;55(1):93–105. [DOI] [PubMed] [Google Scholar]
- 34. Feoktistova M, Geserick P, Leverkus M.. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc. 2016;2016(4):pdb.prot087379. [DOI] [PubMed] [Google Scholar]
- 35. Bechmann N, Ehrlich H, Eisenhofer G, Ehrlich A, Meschke S, Ziegler CG, Bornstein SR. Anti-tumorigenic and anti-metastatic activity of the sponge-derived marine drugs aeroplysinin-1 and isofistularin-3 against pheochromocytoma in vitro. Mar Drugs. 2018;16(5):E172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bechmann N, Poser I, Seifert V, Greunke C, Ullrich M, Qin N, Walch A, Peitzsch M, Robledo M, Pacak K, Pietzsch J, Richter S, Eisenhofer G. Impact of extrinsic and intrinsic hypoxia on catecholamine biosynthesis in absence or presence of Hif2α in pheochromocytoma cells. Cancers (Basel). 2019;11(5):594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reuther C, Heinzle V, Spampatti M, Vlotides G, de Toni E, Spöttl G, Maurer J, Nölting S, Göke B, Auernhammer CJ. Cabozantinib and tivantinib, but not INC280, induce antiproliferative and antimigratory effects in human neuroendocrine tumor cells in vitro: evidence for ‘off-target’ effects not mediated by c-Met inhibition. Neuroendocrinology. 2016;103(3-4):383–401. [DOI] [PubMed] [Google Scholar]
- 38. RRID:AB_2315049, https://scicrunch.org/resolver/RRID:AB_2315049.
- 39. RRID:AB_329827, https://scicrunch.org/resolver/RRID:AB_329827.
- 40. RRID:AB_330947, https://scicrunch.org/resolver/RRID:AB_330947.
- 41. RRID:AB_2097841, https://scicrunch.org/resolver/RRID:AB_2097841.
- 42. RRID:AB_10695803, https://scicrunch.org/resolver/RRID:AB_10695803.
- 43. RRID:AB_2259616, https://scicrunch.org/resolver/RRID:AB_2259616.
- 44. RRID:AB_2070801, https://scicrunch.org/resolver/RRID:AB_2070801.
- 45. RRID:AB_330944, https://scicrunch.org/resolver/RRID:AB_330944.
- 46. RRID:AB_390722, https://scicrunch.org/resolver/RRID:AB_390722.
- 47. RRID:AB_10694233, https://scicrunch.org/resolver/RRID:AB_10694233.
- 48. RRID:AB_331355, https://scicrunch.org/resolver/RRID:AB_331355.
- 49. RRID:AB_329830, https://scicrunch.org/resolver/RRID:AB_329830.
- 50. RRID:AB_10547140, https://scicrunch.org/resolver/RRID:AB_10547140.
- 51. RRID:AB_2074795, https://scicrunch.org/resolver/RRID:AB_2074795.
- 52. RRID:AB_2080320, https://scicrunch.org/resolver/RRID:AB_2080320.
- 53. RRID:AB_2536328, https://scicrunch.org/resolver/RRID:AB_2536328.
- 54. RRID:AB_2533767, https://scicrunch.org/resolver/RRID:AB_2533767.
- 55. RRID:AB_476744, https://scicrunch.org/resolver/RRID:AB_476744.
- 56. RRID:AB_95187, https://scicrunch.org/resolver/RRID:AB_95187.
- 57. Gieldon L, William D, Hackmann K, Jahn W, Jahn A, Wagner J, Rump A, Bechmann N, Nölting S, Knösel T, Gudziol V, Constantinescu G, Masjkur J, Beuschlein F, Timmers HJ, Canu L, Pacak K, Robledo M, Aust D, Schröck E, Eisenhofer G, Richter S, Klink B. Optimizing genetic workup in pheochromocytoma and paraganglioma by integrating diagnostic and research approaches. Cancers (Basel). 2019;11(6):E809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fankhauser M, Bechmann N, Lauseker M, Goncalves J, Favier J, Klink B, William D, Gieldon L, Maurer J, Spöttl G, Rank P, Knösel T, Orth M, Ziegler CG, Aristizabal Prada ET, Rubinstein G, Fassnacht M, Spitzweg C, Grossman AB, Pacak K, Beuschlein F, Bornstein SR, Eisenhofer G, Auernhammer CJ, Reincke M, Nölting S Data from: Synergistic highly potent targeted drug combinations in different pheochromocytoma models including human tumor cultures. Secure Synology Webstorage NAS 2019. Deposited 7 May 2019. https://emds-nas.quickconnect.to/d/s/496975158882345550/sUrm8NYh5szKPD5NacKzaoXM18OrpRiV-TyKA5VWc5QY_. [DOI] [PMC free article] [PubMed]
- 59. Rump A, Benet-Pages A, Schubert S, Kuhlmann JD, Janavičius R, Macháčková E, Foretová L, Kleibl Z, Lhota F, Zemankova P, Betcheva-Krajcir E, Mackenroth L, Hackmann K, Lehmann J, Nissen A, DiDonato N, Opitz R, Thiele H, Kast K, Wimberger P, Holinski-Feder E, Emmert S, Schröck E, Klink B. Identification and functional testing of ERCC2 mutations in a multi-national cohort of patients with familial breast- and ovarian cancer. PLoS Genet. 2016;12(8):e1006248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Slinker BK. The statistics of synergism. J Mol Cell Cardiol. 1998;30(4):723–731. [DOI] [PubMed] [Google Scholar]
- 61. Maurer U, Preiss F, Brauns-Schubert P, Schlicher L, Charvet C. GSK-3: at the crossroads of cell death and survival. J Cell Sci. 2014;127(7):1369–1378. [DOI] [PubMed] [Google Scholar]
- 62. Pirola L, Bonnafous S, Johnston AM, Chaussade C, Portis F, Van Obberghen E. Phosphoinositide 3-kinase-mediated reduction of insulin receptor substrate-1/2 protein expression via different mechanisms contributes to the insulin-induced desensitization of its signaling pathways in L6 muscle cells. J Biol Chem. 2003;278(18):15641–15651. [DOI] [PubMed] [Google Scholar]
- 63. Aristizabal Prada ET, Weis C, Orth M, Lauseker M, Spöttl G, Maurer J, Grabowski P, Grossman A, Auernhammer CJ, Nölting S. GSK3α/β: a novel therapeutic target for neuroendocrine tumors. Neuroendocrinology. 2018;106(4):335–351. [DOI] [PubMed] [Google Scholar]
- 64. Timmers HJ, Kozupa A, Eisenhofer G, Raygada M, Adams KT, Solis D, Lenders JW, Pacak K. Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2007;92(3):779–786. [DOI] [PubMed] [Google Scholar]
- 65. Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM, Öberg K, Van Cutsem E, Yao JC; RADIANT-2 Study Group. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378(9808):2005–2012. [DOI] [PubMed] [Google Scholar]
- 66. Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EGE, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh D-Y, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fassnacht M, Weismann D, Ebert S, Adam P, Zink M, Beuschlein F, Hahner S, Allolio B. AKT is highly phosphorylated in pheochromocytomas but not in benign adrenocortical tumors. J Clin Endocrinol Metab. 2005;90(7):4366–4370. [DOI] [PubMed] [Google Scholar]
- 69. Briest F, Grabowski P. PI3K-AKT-mTOR-signaling and beyond: the complex network in gastroenteropancreatic neuroendocrine neoplasms. Theranostics. 2014;4(4):336–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pavel M. Translation of molecular pathways into clinical trials of neuroendocrine tumors. Neuroendocrinology. 2013;97(1):99–112. [DOI] [PubMed] [Google Scholar]
- 71. O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118(9):3065–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zitzmann K, Rüden J, Brand S, Göke B, Lichtl J, Spöttl G, Auernhammer CJ. Compensatory activation of Akt in response to mTOR and Raf inhibitors: a rationale for dual-targeted therapy approaches in neuroendocrine tumor disease. Cancer Lett. 2010;295(1):100–109. [DOI] [PubMed] [Google Scholar]
- 74. Svejda B, Kidd M, Kazberouk A, Lawrence B, Pfragner R, Modlin IM. Limitations in small intestinal neuroendocrine tumor therapy by mTor kinase inhibition reflect growth factor-mediated PI3K feedback loop activation via ERK1/2 and AKT. Cancer. 2011;117(18):4141–4154. [DOI] [PubMed] [Google Scholar]
- 75. Passacantilli I, Capurso G, Archibugi L, Calabretta S, Caldarola S, Loreni F, Delle Fave G, Sette C. Combined therapy with RAD001 e BEZ235 overcomes resistance of PET immortalized cell lines to mTOR inhibition. Oncotarget. 2014;5(14):5381–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nölting S, Rentsch J, Freitag H, Detjen K, Briest F, Möbs M, Weissmann V, Siegmund B, Auernhammer CJ, Aristizabal Prada ET, Lauseker M, Grossman A, Exner S, Fischer C, Grötzinger C, Schrader J, Grabowski P; GERMAN NET-Z study group. The selective PI3Kα inhibitor BYL719 as a novel therapeutic option for neuroendocrine tumors: Results from multiple cell line models. PLoS One. 2017;12(8):e0182852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2(10):769–776. [DOI] [PubMed] [Google Scholar]
- 78. Wada A. GSK-3 inhibitors and insulin receptor signaling in health, disease, and therapeutics. Front Biosci. 2009;14:1558–1570. [DOI] [PubMed] [Google Scholar]
- 79. Genther Williams SM, Kuznicki AM, Andrade P, Dolinski BM, Elbi C, O’Hagan RC, Toniatti C. Treatment with the PARP inhibitor, niraparib, sensitizes colorectal cancer cell lines to irinotecan regardless of MSI/MSS status. Cancer Cell Int. 2015;15(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kanjanapan Y, Lheureux S, Oza AM. Niraparib for the treatment of ovarian cancer. Expert Opin Pharmacother. 2017;18(6):631–640. [DOI] [PubMed] [Google Scholar]
- 81. Hagemann IS, Devarakonda S, Lockwood CM, Spencer DH, Guebert K, Bredemeyer AJ, Al-Kateb H, Nguyen TT, Duncavage EJ, Cottrell CE, Kulkarni S, Nagarajan R, Seibert K, Baggstrom M, Waqar SN, Pfeifer JD, Morgensztern D, Govindan R. Clinical next-generation sequencing in patients with non-small cell lung cancer. Cancer. 2015;121(4):631–639. [DOI] [PubMed] [Google Scholar]
- 82. Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR [published correction appears in Proc Natl Acad Sci USA. 2005;102(44):16119]. Proc Natl Acad Sci USA. 2005;102(24):8573–8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Johannessen CM, Johnson BW, Williams SM, Chan AW, Reczek EE, Lynch RC, Rioth MJ, McClatchey A, Ryeom S, Cichowski K. TORC1 is essential for NF1-associated malignancies. Curr Biol. 2008;18(1):56–62. [DOI] [PubMed] [Google Scholar]
- 84. Martin GA, Viskochil D, Bollag G, McCabe PC, Crosier WJ, Haubruck H, Conroy L, Clark R, O’Connell P, Cawthon RM, et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63(4):843–849. [DOI] [PubMed] [Google Scholar]
- 85. Welander J, Söderkvist P, Gimm O. The NF1 gene: a frequent mutational target in sporadic pheochromocytomas and beyond. Endocr Relat Cancer. 2013;20(4):C13–C17. [DOI] [PubMed] [Google Scholar]