Abstract
Objectives
Candida species are among the most important causes of hospital acquired blood borne infections, and with high rates of mortality and morbidity, these infections are still a major problem today. History of gastrointestinal surgery, administration of total parenteral nutrition and/or wide spectrum antibiotics and immune suppression following organ transplantations are considered serious risk factors for these infections. This study aimed to evaluate the patients from our general surgery department with diagnosed candidemia; by means of strain, treatment and prognosis.
Material and Methods
Patients with positive blood cultures for Candida species who were treated in the wards and Ege University Faculty of Medicine general surgery department of surgical intensive care units of our between 2012 and 2017 were retrospectively analyzed by means of strain, treatment and prognosis.
Results
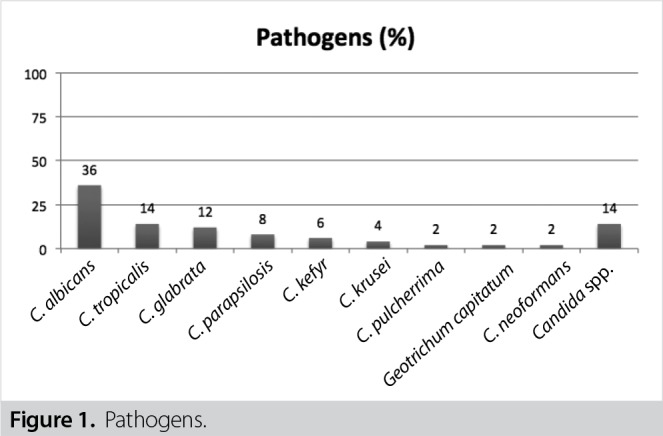
A total of 50 patients were enrolled in the study. Mean age was 58.96 years and 54% of the patients were female. There were nine patients with organ transplantation (four liver and five kidney transplantations), six with intestinal perforation and three with anastomotic leakage. Isolated strains were Candida albicans (36%; 18/50), Candida tropicalis (14%; 7/50), Candida glabrata (12%; 6/50), Candida parapsilosis (8%; 4/50), Candida kefyr (6%; 3/50), Candida krusei (4%; 2/50), Candida pulcherrima (2%; 1/50), Cryptococcus neoformans (2%, 1/50), Geotrichum capitatum (2%, 1/50), Candida spp. (unidentified, 14%; 7/50) with decreasing frequency. The highest antifungal sensitivity rates (> 90%) were measured for amphotericin B, voriconazole and echinocandins among all isolates. One-month mortality rate was 43.4% (20/46). Documented eradication was achieved among 24 of the 33 patients who had control blood culture samples (72.7%), and mean eradication time was 7.6 days. Echocardiography was performed in 14% (7/50) and ophthalmic examination in 8% (4/50).
Conclusion
Although C. albicans appears to be the dominant strain in patients with candidemia, frequencies of other strains are increasing. Early diagnosis and treatment of patients with candidemia is of vital importance due to high mortality and morbidity rates.
Keywords: Candidemia, prognosis, treatment, risk factors
Introduction
Candidemia demonstrated as an important part of invasive fungal infections and also a significant factor for the high mortality rates, prolonged length of hospital stay and high health-related costs, ranks between the fourth and seventh mostly seen disease among the blood stream infections in the United States and Europe (1). Candidemia incidence has been shown as 1.23 to 12.3 per 1000 admissions from our country which can be varied due to regional differences in terms of mortality rates, isolated pathogens, risk factors and antifungal susceptibility rates (2-5).
This study aimed to evaluate the pathogens, treatments and prognosis of the patients followed by the General Surgery Department of our hospital.
Patients and Methods
In our study, the patients with positive blood cultures for Candida who were followed up by the Ege University Faculty of Medicine, General Surgery Department (Inpatient Clinic/Intensive Care Unit/Transplantation Unit) between the years of 2012-2017 were evaluated in terms of pathogen, treatment and prognosis, retrospectively. At least one positive blood culture for Candida spp. was accepted as candidemia and only adult patients (≥ 18-year-old) were included into our study. Sociodemographic features, risk factors, blood culture results, 30-day (due to all causes) mortality rates and antifungal treatment regimens were screened via the patients’ files. Sabouraud dextrose agar (Acumedia; Michigan, USA) was used for the culture, and identification of the species was performed by conventional methods (Dalmou method) with micromorphologic features, ID32C (bioMerieux; France) with carbohydrate assimilation features and MALDI-TOFF MS with proteomic features. Antifungal susceptibilities were investigated via E-test method, and minimum inhibitor concentration levels were used according to the CLSI guideline (6,7).
Statistical Package for Social Sciences (SPSS) version 22.0 (IBM Corp.; Armonk, NY, USA) program was used to analyze our data. P value below 0.05 was accepted as statistically significant.
No ethics committee approval was sought due to the retrospective design of the study. Our study was performed in accordance to the ethical standards of the Helsinki Declaration which was accepted by the World Health Community in 1975 (revised in 2008).
Results
A total number of 50 patients (54% female) were recorded during the six-year period. Mean age was 58.96 ± 2.36 years. Comorbidities were recorded as nine organ transplantations (four liver transplantation, five renal transplantation), six intestinal perforations, four intraabdominal abscesses, and three anastomosis leakages (Table 1). Parenteral nutrition rate was 88% (44/50).
Table 1. Comorbidities.
| Comorbidities | Rates (n, %) |
| Organ transplantation | 9 (18%) |
| Renal transplantation | 5 (10%) |
| Liver transplantation | 4 (8%) |
| Intestinal perforation | 6 (12%) |
| Intraabdominal abscess | 4 (8%) |
| Anastomosis leakage | 3 (6%) |
Isolated pathogens from the blood cultures were Candida albicans(36%; 18/50), Candida tropicalis(14%; 7/50), Candida glabrata(12%; 6/50), Candida parapsilosis(8%; 4/50), Candida kefyr(6%; 3/50), Candida krusei(4%; 2/50), Candida pulcherrima(2%; 1/50), Cryptococcus neoformans(2%, 1/50), Geotrichum capitatum(2%, 1/50) , Candidaspp. (14%; 7/50), respectively (Figure 1).
Figure 1. Pathogens.
The highest sensitivity rate (> 90%) among all isolates was observed for amphotericin B, voriconazole, caspofungin and echinocandin. Fluconazole resistance was investigated among the antifungal susceptibility tested ones, and resistance rate was observed as 2/16 (12.5%) for both C. albicansand non albicans isolates. Fluconazole resistance was observed one out of three antifungal sensitivity tested C. glabrataisolates, which supports the fact that fluconazole resistance rate is increasing globally among them in recent years.
A total number of four patients were discharged or transported to another hospitals, and thus the rest of the patients’ all-cause mortality rate (for one month) was noted as 43.4% (40/46). Antifungal treatment was not started in nine patients and mortality was observed in five. Four patients, in whom antifungal treatment was not started, were discharged or transported, and hence their follow up cannot been done. In subgroup analysis of different treatment regimens, mortality (one-month) rates were recorded as (5/9; 55.6%) for fluconazole, (8/27; 29.6%) for anidulafungin, (1/3; 33.3%) for caspofungin, (1/1; 100%) and (0/1, 0%) for liposomal amphotericin B plus fluconazole, respectively. Mortality rate (one-month) was found as statistically significant for the anidulafungin treatment given and non-given group (8/27-12/19; p= 0.024).
Treatment was changed in five patients (anidulafungin treatment was changed in four and fluconazole treatment was changed in one patient), and among them, only one mortality was observed whose treatment was switched to liposomal amphotericin B from fluconazole.
Microbiologic eradication was noticed in 24/33 patients whose control cultures were performed with a mean duration of 7.6 days. Mortality rate (one-month) was found as statistically significant for the microbiologic responsive and nonresponsive group (4/24-7/9; p= 0.002).
Mean duration of treatment was noted as 17.9 ± 2.26 (min 1, max 58 days) in the treatment given patients. Echocardiography was performed in 14% (7/50) and ophthalmoscopic examination was done in 8% (4/50) during the follow up period of candidemia patients. Infective endocarditis was spotted in one out of seven patients via echocardiography, and none of the four patients had septic emboly in whom ophthalmoscopic examination was carried out.
Discussion
Although the mostly seen pathogen is C. albicansin candidemia patients, the incidence of non-albicansCandidaisolates has increased in recent years (1-3,13). Urethral catheterization, total parenteral nutrition, history of wide spectrum antibiotic usage, blood transfusion, central venous catheters are accepted as the important risk factors for candidemia (8). In our study, although the mostly detected pathogen was C. albicans(36%) in candidemia patients similar to the literature data, non-albicans Candidawas also seen with high numbers (50%). History of gastrointestinal surgery, total parenteral nutrition, organ transplantation and immunosupression were the main risk factors for candidemia in our study.
In recent years, fluconazole resistance is seen in high rates for non-albicans Candidaspp. like C. glabratabesides the natural resistant C. krusei, and Mencarini et al. have shown fluconazole resistance rate as 30% for C. glabratafrom Italy (9). In our study, although the numbers were low, we found fluconazole resistance as 33% similar to the literature thus, we believe that echinocandins should be preferred to fluconazole in candidemia patients with C. glabrata.
Karadag et al. have revealed a mortality rate as 30% for a total number of 89 candidemia patients from our country (10). Different mortality rates have been seen in the literature from our country like Kocak et al. who have confirmed a mortality rate as high as 58% for 38 candidemia patients (11). In our study, one-month mortality rate with all causes was spotted as 43.4% for 46 followed-up patients. Antifungal treatment was not started in nine patients, and mortality was observed as 100% in the rest of the four patients who were discharged or transported to other hospitals (5/5). These results also show that early diagnosis and treatment for candidemia patients are vitally important due to high mortality rates. In subgroup analysis, mortality rate (one-month) was found as statistically significant for the anidulafungin treatment given and non-given group (8/27-12/19; p= 0.024).
Five main subjects underlined to increase survival rates in candidemia patients are appropriate antifungal treatment, control blood culture follow-up, echocardiography control, ophthalmoscopic examination and removal of central venous catheter, respectively (12). Another important result of our study revealed that echocardiography and ophthalmoscopic examination were performed only in 14% (7/50) and 8% (4/50) of the candidemia patients.
The most important limitations of the study included its retrospective design and the fact that antifungal susceptibility test could not be performed in all samples.
Conclusion
In conclusion, appropriate antifungal treatment has a vital importance in candidemia patients due to high mortality rates despite the current diagnostic and therapeutic options. Although the mostly seen pathogen is C. albicansin candidemia patients, the incidence of non albicans Candidaisolates is increasing. Due to high resistance for fluconazole, we believe that echinocandins like anidulafungin are more appropriate choices of antifungal treatment rather than fluconazole especially for the hemodynamically unstable intensive care unit patients. In addition to this, echocardiography and ophthalmoscopic examination should be kept in mind for the follow-up of candidemia patients.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
Peer Review: Externally peer-reviewed.
Ethics Committee Approval: No ethics committee approval was sought due to the retrospective design of the study.
Informed Consent: Due to the retrospective design of the study, the informed consent was not obtained.
Author Contributions: Consept - U.Ö., M.I.T.; Design - U.Ö.; Supervision - M.I.T., Resource - D.Y.M., S.H.P., C.K., S.E., Materials - D.Y.M., S.H.P.; Data Collection and/or Processing - U.Ö., D.Y.M.; Analysis and Interpretation - U.Ö.; Literature Search - U.Ö.; Writing Manuscript - U.Ö.; Critical Reviews - M.I.T., D.Y.M.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Antinori S, Milazzo L, Sollima S, Galli M, Corbellino M. Candidemia and invasive candidiasis in adults: a narrative review. Eur J Intern Med. 2016;34:21–28. doi: 10.1016/j.ejim.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Kazak E, Akın H, Ener B, Sığırlı D, Özkan Ö, Gürcüoğlu E, et al. An investigation of Candida species isolated from blood cultures during 17 years in a university hospital. Mycoses. 2014;57:623–629. doi: 10.1111/myc.12209. [DOI] [PubMed] [Google Scholar]
- 3.Alp S, Arikan-Akdagli S, Gulmez D, Ascioglu S, Uzun O, Akova M. Epidemiology of candidaemia in a tertiary care university hospital: 10-year experience with 381 candidaemia episodes between 2001 and 2010. Mycoses. 2015;58:498–505. doi: 10.1111/myc.12349. [DOI] [PubMed] [Google Scholar]
- 4.Yeşilkaya A, Azap Ö, Aydın M, Akçil Ok M. Epidemiology, species distribution, clinical characteristics and mortality of candidaemia in a tertiary care university hospital in Turkey, 2007-2014. Mycoses. 2017;60:433–439. doi: 10.1111/myc.12618. [DOI] [PubMed] [Google Scholar]
- 5.Yapar N, Akan M, Avkan-Oguz V, Ergon CM, Hancer M, Doluca M. Risk factors, incidence and outcome of candidemia in a Turkish intensive care unit: a five-year retrospective cohort study. Anaesth Pain & Intensive Care. 2014;18:265–271. [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard-Third Edition. 2008. M27A3. [Google Scholar]
- 7.Cuenca-Estrella M, Verweij PE, Arendrup MC, Arikan-Akdagli S, Bille J, Donnelly JP, et al. ESCMID* *This guideline was presented in part at ECCMID 2011. European Society for Clinical Microbiology and Infectious Diseases. Guideline for the diagnosis and management of candida diseases 2012: diagnostic procedures. Clin Microbiol Infect. 2012;18 (Suppl 7):9–18. doi: 10.1111/1469-0691.12038. [DOI] [PubMed] [Google Scholar]
- 8.Yapar N, Pullukcu H, Avkan-Oguz V, Sayin-Kutlu S, Ertugrul B, Sacar S, et al. Evaluation of species distribution and risk factors of candidemia: a multicenter case-control study. Med Mycol. 2011;49:26–31. doi: 10.3109/13693786.2010.501344. [DOI] [PubMed] [Google Scholar]
- 9.Mencarini J, Mantengoli E, Tofani L, Riccobono E, Fornaini R, Bartalesi F, et al. Evaluation of candidemia and antifungal consumption in a large tertiary care Italian hospital over a 12-year period. Infection. 2018;46:469–476. doi: 10.1007/s15010-018-1139-z. [DOI] [PubMed] [Google Scholar]
- 10.Karadağ FY, Ergen P, Aydın Ö, Doğru A, Tanıdır B, Vahaboğlu MH. Evaluation of epidemiological characteristics and risk factors affecting mortality in patients with candidemia. Turk J Med Sci 2016: 20;46:1724–1728. doi: 10.3906/sag-1505-70. [DOI] [PubMed] [Google Scholar]
- 11.Koçak BY, Kuloğlu F, Doğan Çelik A, Akata F. Evaluation of epidemiological characteristics and risk factors of candidemia in adult patients in a tertiary-care hospital. Mikrobiyol Bul. 2011;45:489–503. [PubMed] [Google Scholar]
- 12.Murri R, Giovannenze F, Camici M, Torelli R, Ventura G, Scoppettuolo G, et al. Systematic clinical management of patients with candidemia improves survival. J Infect. 2018;77:145–150. doi: 10.1016/j.jinf.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Kostakoğlu U, Yılmaz G, Köksal İ. Fungal infections; species distribution and treatment response. FLORA. 2018;23:73–78. [Google Scholar]


