Abstract
Objective: The increasing prevalence of infertility every year is in line with the increasing need for in vitro fertilization (IVF) programs. Failure of oocyte maturation is an obstacle that often causes low success in IVF. According to several studies, controlled ovarian stimulation (COS) has been reported to increase the risk of granulosa-cell apoptosis associated with inhibition of oocyte maturation. This study was conducted to determine the effect of electroacupuncture (EA) on oocyte maturation; rate of fertilization; granulosa-cell apoptosis; and levels of growth-differentiation factor 9 (GDF9) and bone-morphogenetic protein 15 (BMP15) an IVF program.
Materials and Methods: A randomized controlled trial was conducted with 24 subjects who were in the IVF program. Subjects were randomly allocated into verum-EA (n = 12) and sham-EA groups (n = 12). Microscopic assessment of oocyte maturation and rate of fertilization was performed by embryologists, and examinations of granulosa-cell apoptosis index (Bax/Bcl-2 ratio), GDF9, and BMP15 were performed, using real-time quantitative polymerase chain reaction and messenger-RNA techniques.
Results: There were significant differences in oocyte maturation (P = 0.02) and fertilization rates (P = 0.03) between the verum-EA and sham-EA groups. There were differences in granulosa-cell apoptosis index between the verum-EA and sham-EA groups (P < 0.001). There were significant differences in Bax-protein expression (P = 0.04) and Bcl-2 (P = 0.03) granulosa cells between the verum-EA and sham-EA groups. There was no significant difference in GDF9 levels (P = 0.34) and BMP15 (p = 0.47) between the verum-EA and sham-EA groups.
Conclusions: EA can enhance oocyte maturation and fertilization rate, and reduce the granulosa-cell apoptosis index in an IVF program.
Keywords: Bax, Bcl-2, BMP15, GDF9, granulosa-cell apoptosis, oocyte maturation
Introduction
Controlled ovarian stimulation (COS) was introduced in the early 1980s as part of a therapeutic method in in vitro fertilization (IVF) to improve multifollicle development, thereby increasing the number of oocytes at the time of retrieval, which can then provide 1 or more embryos to be transferred. This would eventually increase the pregnancy rate.1 One factor that has an important influence on good embryo development is oocyte quality. The development and maturation of oocytes is potentially a key success factor for IVF.
The two main hormone therapies used in COS are analogue gonadotropin-releasing hormone (GnRH) and gonadotropin. In its development, the use of analogue GnRH on COS, which suppresses the pituitary gland and prevents the occurrence of a surge of luteinizing hormone (LH), was considered to reduce oocyte maturation.2,3
Studies have suggested that analogous GnRH acts on specific ovarian receptors that block gonadotropin actions, including follicular development and differentiation,4 and increases apoptosis in granulosa-cell DNA fragmentation in mice with hypofisectomy.5 Increased incidence of granulosa-cell apoptosis is associated with low numbers of oocytes retrieved, empty follicles, and low fertilization rates. Granulosa-cell apoptosis is also a sensitive indicator in estimating oocyte quality in patients undergoing IVF.3
The process of apoptosis in granulosa cells is activated through an intrinsic pathway and is influenced by several proteins and several other biomolecules. B-cell protein family lymphoma gene-2 (Bcl-2) is a protein that is known to have a large role in apoptosis. Members of the Bcl-2 protein family are divided into 2 groups based on their roles in apoptosis, namely the proapoptotic group Bcl-2–associated X (Bax) and the antiapoptosis group (Bcl-2). Apoptosis is influenced dominantly by the apoptotic index or the ratio of Bax/Bcl-2 found in granulosa cells.6,7
Administration of gonadotropin stimulates the formation of multifollicular development. However, this multifollicle development will occur in various amounts and functional activities, so that it contains oocytes at various maturation stages.8 Storage of maternal messenger RNA (mRNA) accumulated during folliculogenesis and exogenous gonadotropin administration accelerates nucleation maturation, which results in insufficient storage of mRNA, resulting in low oocyte and embryo-development quality.9
As a minimal risk therapy—and one that does not cause serious side-effects—in recent years, acupuncture has also been widely used as a complementary therapy in IVF programs. It is known that acupuncture can increase insulin-like growth factor–1 (IGF-1)10 and vascular endothelial growth factor (VEGF)11 in patients undergoing IVF. These factors are among the survival factors that play a role in reducing follicular apoptosis.12,13 In addition, acupuncture is also known to increase blood flow to the reproductive organs.14,15 Yet, research on acupuncture effects on granulosa and oocyte cells is limited. Therefore, this study was conducted to examine the effect of EA therapy on oocyte maturation index, rate of fertilization, granulosa-cell apoptosis index, and levels of growth-differentiation factor 9 (GDF9) and bone-morphogenetic protein 15 (BMP15) in an IVF program.
Materials and Methods
This study was a blinded randomized clinical trial, conducted from September to November 2018, at the Dr. Cipto Mangunkusumo Hospital, in Jakarta, Indonesia. The research was a collaborative study between the Department of Medical Acupuncture and the Department of Obstetrics and Gynecology, both in the Faculty of Medicine at Universitas Indonesia, of the Dr. Cipto Mangunkusumo Hospital, in Jakarta, Indonesia.
Subjects
Women who fit the inclusion criteria for this study were ages 30–39, with a body mass index (BMI) <29 kg/m2, who underwent COS antagonistic protocols, signed research-approval forms, and were willing to be in the study to completion. Exclusion criteria for this study were subjects with infections or swellings at the point locations and subjects with pacemakers.
Based on the calculation of the formula, the sample size needed for the verum-EA and the sham-EA groups—after adding the possibility of a drop of 10%—was 12 subjects for each group. The subjects were divided into the 2 groups, with a computer-generated table containing random numbers. The groups were a verum-EA group (subjects who received true manual acupuncture and EA interventions) and a sham-EA group (subjects who received sham manual acupuncture and sham-EA interventions).
Interventions
In the verum-EA group, the acupuncture points used were GV 20 (Baihui), MAIC 3 (Endocrine), CV 4 (Guanyuan), CV 3 (Zhongji), EX-CA 1 (Zigong), ST 36 (Zusanli), BL 57 (Chengsan), SP 6 (Sanyinjiao), and KI 3 (Taixi). Asepsis and antisepsis were carried out, then a plaster (small medical dressing) was applied to the place where the acupuncture needle was puncturing. For each patient, the connecting cable from the electrostimulator was connected to the acupuncture needle, then was activated by a continuous-wave, low-intensity current that was comfortable for the patient. A frequency of 2 Hz for 30 minutes was applied at CV 4, CV 3, and EX-CA 1. Thermal deep penetration (TDP) was applied 20 cm above CV 3, CV 4, EX-CA 1.
In the sham-EA group, the needle was inserted in the plaster pads at the same point locations but did not penetrate the patient's skin. The stimulator and TDP machines were not turned on.
Acupuncture therapy was carried out 6 times, 2 times per week, before COS and 4 times while each patient underwent a COS antagonistic protocol.
The oocyte retrieval was carried out by an obstetrician/gynecologist. Furthermore, follicular fluid was obtained as a source of granulosa cells. Granulosa cells were then sent to the INA-REPROMED research laboratory to check the mRNA expression levels of Bax, Bcl-2, GDF9 and BMP15. Oocyte maturation assessment was carried out microscopically by an embryologist. The oocyte maturation index was obtained by comparing the number of oocytes in the metaphase II stage, compared to the total number of oocytes retrieved when picking up oocytes. Fertilization rates—which were normal if 2 pronuclei were found in the cytoplasm—were assessed 18–19 hours after an intracytoplasmic sperm injection (ICSI). The rate of fertilization was obtained by comparing the amount of normal fertilization with the total number of mature oocytes retrieved after ICSI.
Ethics
This research was approved by the Medical Research Ethics Committee of the Universitas Indonesia, in Jakarta, Indonesia under No: 0867-8.UN2.F1 / ETIK / 2018 on August 27, 2018.
Statistical Analysis
Statistical analysis was performed using SPSS, version 20.0 (IBM Corp., New York, NY). A Student's t-test was applied when the variables were numerical data with normal distributions. A per-protocol analysis was used in this study.
Results
At the beginning of the study, data were collected from 28 subjects who were in the IVF program. Screening of research samples according to inclusion criteria and exclusion criteria was carried out, 1 subject was excluded because they were more than 39 years old and 3 subjects were excluded due to a BMI >29 kg/m2. Final data processing and analysis was carried out on 24 research subjects. No differences were found between the treatment group and the control group in the characteristics of the subject of this study, so the characteristics were homogeneous and feasible to compare (Table 1).
Table 1.
Demographic Characteristics
| Characteristics | Verum EA (n = 12) | Sham EA (n = 12) | P-value |
|---|---|---|---|
| Age (yr) | (35.00 ± 2,79) | (34.33 ± 3.72) | 0.62* |
| BMI (kg/m2) | (23.46 ± 3,03) | (23.41 ± 2.60) | 0.96* |
| Duration of infertility (yr) | (6.12 ± 3.64) | (4.91 ± 3.34) | 0.41* |
| AMH (ng/mL) | (1.73 ± 1.13) | (2.80 ± 2.03) | 0.13* |
| Causes of infertility | |||
| POR | 6 (50%) | 4 (33%) | |
| PCOS | 2 (17%) | 1 (8%) | |
| Tubal factor | 1 (8%) | 2 (17%) | |
| Ovarian cyst | 2 (17%) | 2 (17%) | |
| Endometrium factor | 0 (0%s) | 3 (25%) | |
| Sperm factor | 6 (50%) | 4 (33%) |
Unpaired T test.
EA, electroacupuncture; yr, years; BMI, body mass index; AMH, anti-Müllerian hormone; POR, poor ovarian response; PCOS, polycystic ovarian syndrome.
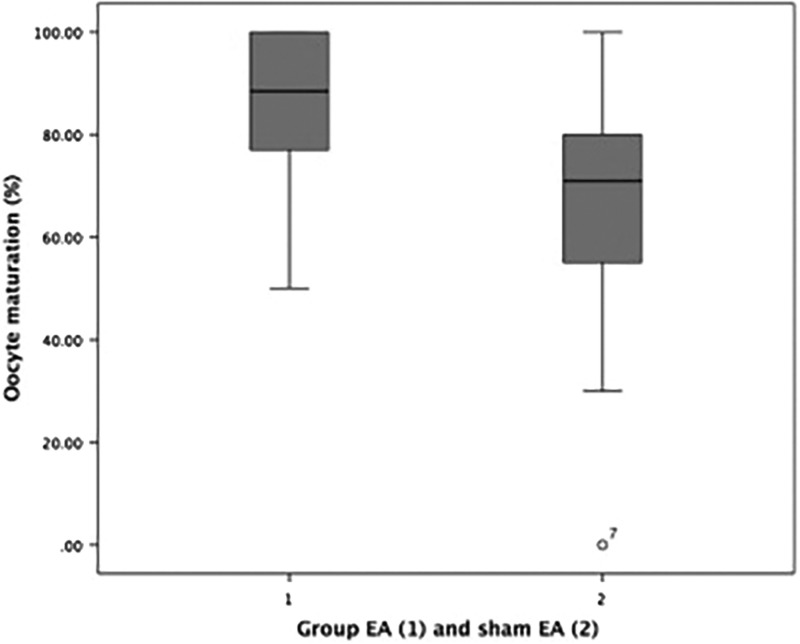
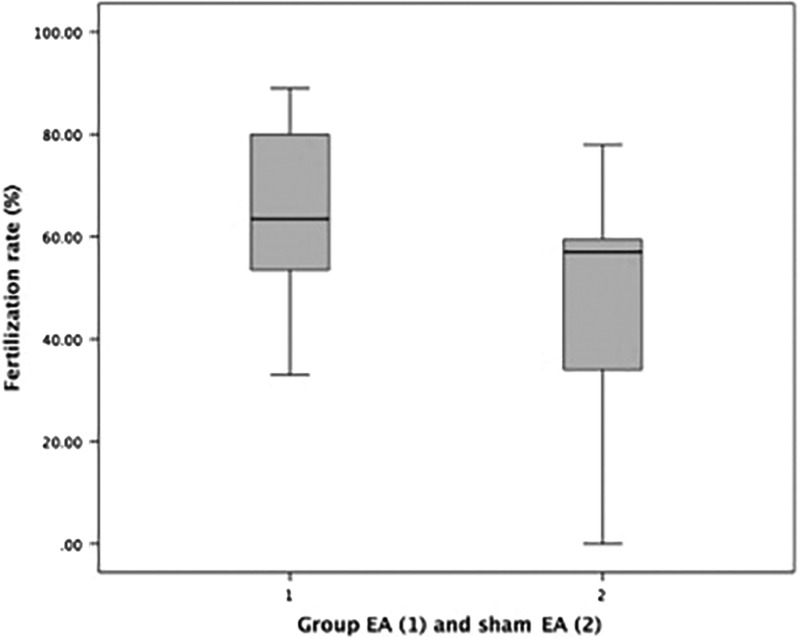
As can be seen in Table 2, there was a significant difference in the mean difference of the oocyte maturation index between EA group (mean 85.58 ± 16.68) and sham EA group (mean 65.17 ± 28.24), with a P-value is 0.02. There was also a significant difference in the rate of fertilization between the EA group (mean 64.83 ± 17.86) and the sham EA group (mean 48.83 ± 22.13) with a P-value is 0.03. The boxplot graphs can be seen in Figure 1 (oocyte maturation) and Figure 2 (fertilization rate).
Table 2.
Differences in Oocyte Maturation Index and Rate of Fertilization Between Verum-EA Group and Sham-EA Group
| Outcome | EA (n = 12) | Sham EA (n = 12) | P-value |
|---|---|---|---|
| Oocyte maturation (%) | 88.5 (50.0–100.0) | 71.0 (0–100.0) | 0.02** |
| Fertilization rate (%) | (64.83 ± 17.86) | (48.83 ± 22.13) | 0.03* |
Unpaired T test.
Mann-Whitney test.
EA, electroacupuncture.
FIG. 1.
Boxplot graph of oocyte maturation indices in verum electroacupuncture (EA) group and in sham-EA group.
FIG. 2.
Boxplot graph fertilization rates in verum electroacupuncture (EA) group and in sham-EA group.
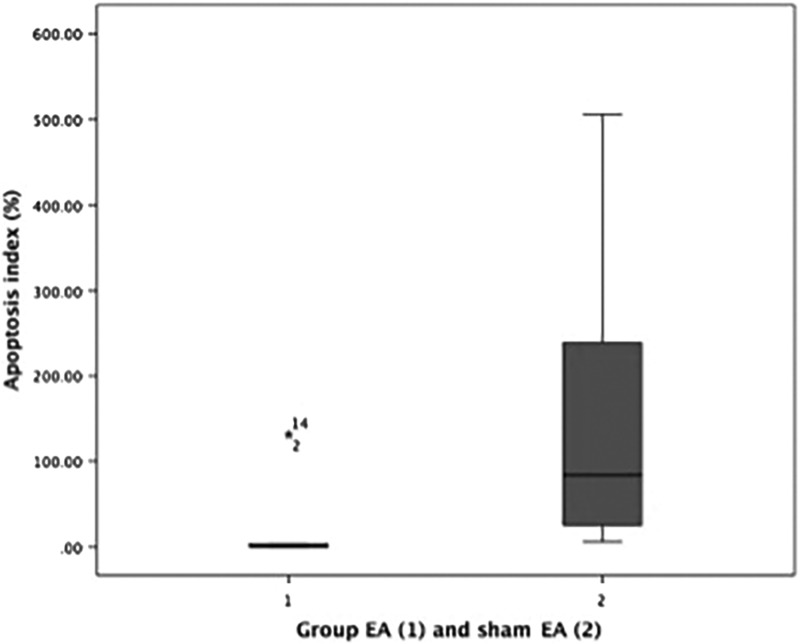
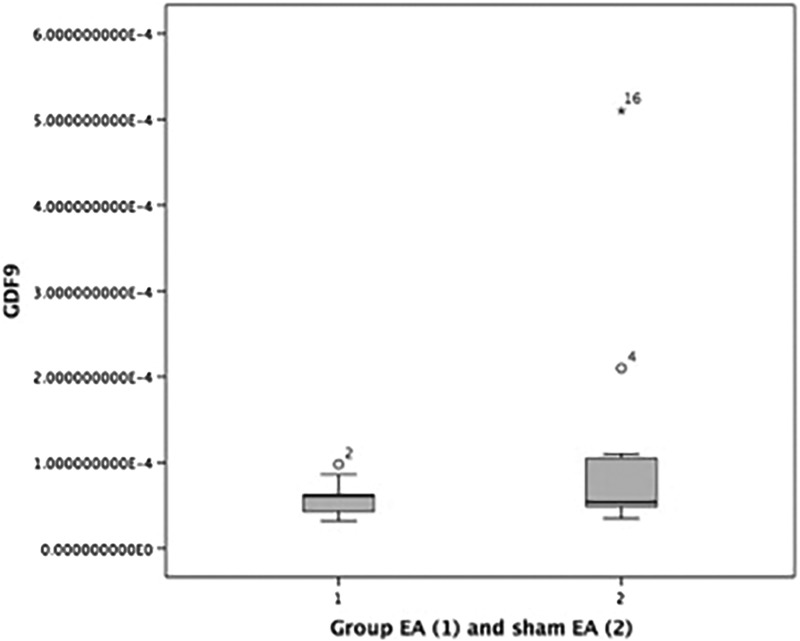
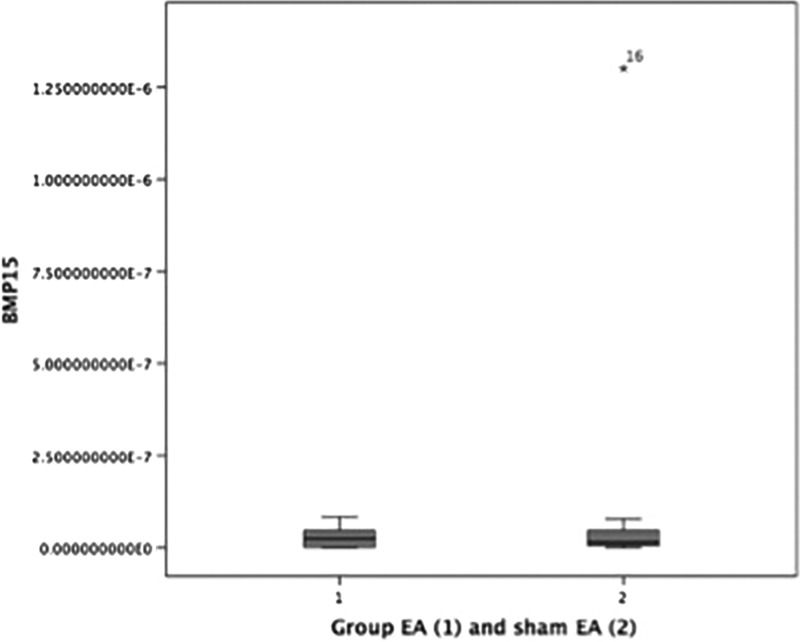
It can be seen the mean difference in Bax, Bcl-2, apoptosis index, GDF9 and BMP15 between the two groups in Table 3. For Bax, Bcl-2 and Apoptosis index there were significant differences between 2 two groups, with P-values are 0.047, 0.037, and <0.001 respectively. While the GDF9 and BMP15 were not significantly different (Table 3). The boxplot graphs can be seen in Figure 3 (apoptosis index), Figure 4 (GDF9), and Figure 5 (BMP15).
Table 3.
Differences in Apoptosis Index (Bax/Bcl-2 Ratio) Granulosa Cells, GDF9, and BMP15 Between Verum-EA and Sham-EA Groups
| Outcome | Verum EA (n = 12) | Sham EA (n = 12) | P-value |
|---|---|---|---|
| Bax | 2.05 × 10–6 (2.66 × 10–8 –2.80 × 10–4) | 16.95 × 10–6 (1.30 × 10–6–1.45 × 10–4) | 0.047** |
| Bcl-2 | 16.05 × 10–7 (3.75 × 10–8–8.92 × 10–5) | 11.10 × 10–7 (3.61 × 10–8–8.99 × 10–7) | 0.037** |
| Apoptosis index | 0.63 (1.23 × 10–2–132.00) | 83.95 (6.15 × 10–3–506.00) | < 0.001** |
| GDF9 ( × 10–5 ng/μL) | 6.10 (3.19–9.80) | 5.38 (3.46–50.6) | 0.34** |
| BMP15 ( × 10–8 ng/μL) | 2.40 (0.04–8.33) | 1.43 (0.04–130) | 0.47** |
Mann-Whitney test.
Bax, Bcl-2–associated X; Bcl-2, B-cell protein family lymphoma gene-2; GDF9, growth-differentiation factor 9; BMP 15, bone-morphogenetic protein 15; EA, electroacupuncture.
FIG. 3.
Boxplot graph apoptosis index in verum electroacupuncture (EA) group and in sham-EA group.
FIG. 4.
Boxplot graph GDF9 level in verum electroacupuncture (EA) group and in sham-EA group. GDF9, growth-differentiation factor 9.
FIG. 5.
Boxplot graph BMP15 level in verum electroacupuncture (EA) group and in sham EA group. BMP15, bone-morphogenetic protein 15.
Discussion
The quality of oocytes has an important role in embryonic development. Low-quality oocytes have a low potential to develop into good embryos, whereas high-quality oocytes have higher potential to develop into good embryos, thus, increasing the likelihood of a clinical pregnancy.16 The quality of oocytes can be assessed from during the meiotic stages, cytoplasmic competent development, fertilization, and developmental capabilities to determine whether or not an embryo will be good.17 The technique most often used to evaluate the quality of oocytes in vitro is meiotic maturation. Meiotic maturation assessment is a fast and noninvasive method that involves determining whether the oocytes can reach the metaphase II maturation stage; but, even so, this assessment does not provide information about the development of the cytoplasm and molecular maturation.18
The current study results showed that the oocyte maturation index in the verum-EA group was significantly higher than that index in the sham-EA group (P = 0.02). These results are parallel with previous studies by Cui et al.19 and Li et al.,20 who examined the effect of EA on the oocyte quality of patients with polycystic ovary syndrome (PCOS) undergoing IVF, and a study by Chen et al. in patients with poor ovarian response (POR) who also underwent IVF.21 All three studies showed improvements in oocyte quality.19–21 This was in accordance with previous research that suggested that the success of IVF programs was greater with acupuncture therapy, which had begun since the COS stage than only around embryo-transfer time.21 It was proven in this current study that complementary acupuncture therapy performed before and during COS could increase oocyte maturation significantly.
Fertilization rates in the verum-EA group were significantly higher than in the sham-EA group (P = 0.03); see Table 2. Fertilization itself a process in which there is a union between 2 parental gametes—namely, oocytes and spermatozoa.22 Many factors influence both success and failure of ICSI methods, such as oocyte quality, sperm quality, and ICSI procedure techniques. In this study, better oocyte maturation in the verum-EA group was an important factor in producing a higher rate of fertilization.
This study was the first research on the effect of acupuncture on the ratio of Bax/Bcl-2, GDF9, and BMP15-granulosa cells. The close relationship between granulosa cells and oocytes can make granulosa cells an oocyte-quality parameter. Apoptosis is one of the events in granulosa cells that can be an oocyte-quality parameter. Good-quality oocytes will have low granulosa-cell apoptosis in the follicles.
Bax protein levels, compared to Bcl-2 protein in cells, as well as Bcl-2 protein levels, compared to Bax protein levels determine the fates of cells; their existence will be eliminated or maintained through apoptotic events.6 Bax and Bcl-2 are 2 proteins that have a role in regulating apoptosis in cells activated by intrinsic pathways. According to Elmore, the ratio between the expression of Bax protein to Bcl-2 is one of the important things in determining cell fate and can be a marker of the occurrence of apoptosis in cells.6
The results of this current research showed that, in the granulosa cells of the verum-EA group, there was a lower incidence of apoptosis. This is in accordance with the literature that states that EA plays a role in reducing the incidence of apoptosis through the phosphatidylinositol 3-kinase (PI3K/AKT) pathway.23 The main signaling pathway of intracellular antiaptosis transduction is PI3K/AKT, which is initiated by survival factors such as hormones and growth factors. Granulosa cells have many survival factors, such as IGF-1 and follicle-stimulating hormone (FSH), both of which activate the PI3K/AKT pathway and regulate the transcription of the FOXO1 gene that plays a role in the proliferation, differentiation, and survival of granulosa cells. One of the growth factors is VEGF, which also plays a role in the proliferation and cytoprotection of antral follicles and granulosa cells through the PI3K / AKT and ERK/MEK pathways.13
In contrast to the results of previous studies, the results of this current study indicated that there were no significant differences in the levels of GDF9 and BMP15 between the 2 EA groups; but, the verum-EA group had a higher oocyte maturation rate, compared to the sham-EA group. In theory, normally GDF9 and BMP15 have an important role in follicular growth and oocytes, including oocyte maturation, but, certain circumstances can affect GDF9 and BMP15 levels. According to several studies, the GDF9 and BMP15 levels in primary, secondary, and tertiary follicles was significantly lower in patients with PCOS, compared with controls; thus, these levels might be associated with impaired follicular development in patients with PCOS.24,25 One theory states that there might be GDF9 or BMP15 gene mutations in patients with less than normal ovarian reserves, such as patients with POR, which can result in differences in the GDF9 or BMP15 levels, compared to women with normal ovarian reserves.26–28
Two previous studies, by Li et al.16 and Gode et al.,29 which examined GDF9 levels and BMP15 in women who underwent ICSI, had almost the same exclusion criteria including a history of poor response to ovarian stimulation, endometriosis, and PCOS. The exclusion of patients with POR and PCOS made the samples more homogeneous and reduced the conditions affecting subjects' levels of GDF9 and BMP15; thus these studies produced levels of GDF9 and BMP15 in their subjects that were in accordance with their increases in oocyte maturation. Compared to previous studies, this current study did not makes the diagnoses of POR and PCOS exclusion criteria. Thus, the current study reflects the situation in the field (an IVF clinic) better, where patients heterogeneous diagnoses. What is more, PCOS and POR are also the most-common infertility factors in patients undergoing IVF.
The point selections were chosen based on research by Cui et al.19 and Villahermosa et al,30 which improved oocyte quality, oocyte counts, and fertilization rates of patients undergoing IVF. These points included CV 4,CV 3, EX-CA 1, ST 36, SP 6, and KI 3. Of note, SP 6 and CV 4 were chosen because they can regulate hormone levels (estradiol, FSH, LH, GnRH) in the hypothalamic–pituitary–ovarian axis (HPOA).31 Endocrine point MA-IC 3 was chosen because of its location in the deepest indentation of the cavum concha and in the indentation of the intertragus, including the auricular area that is innervated by the auricular branch of the vagus. Stimulation of MA-IC3, thus, activates the vagus nerve, which is an important part of the parasympathetic nervous system.32 BL 57 was chosen because it corresponds to a dermatome as high as S1–S2 part of the pelvic nerve plexus that affects the parasympathetic/autonomic nervous system to the reproductive organs and is known to increase blood flow to the ovaries and uterus.14,33 GV 20 and ST 36 were chosen because they can increase antiapoptotic factors such as Akt, Bcl-2, and Bcl-xL, and can increase VEGF.34
The mechanism underlying EA therapy can improve oocyte maturation is not certain. However, it is known that acupuncture and EA therapy can inhibit pain perception by increasing several neurotransmitters, such as β-endorphins. The relationship between central endorphin and reproductive function involves a direct and indirect relationship to GnRH that affects LH and FSH.35 The effect of acupuncture on primary ovarian insufficiency (POI) indicates that acupuncture can modulate the HPOA by regulating FSH, LH, and estrogen.31,36
In addition to the hypothesis concerning the HPOA, modulation of autonomic-nerve function was also proposed as the reason acupuncture produces a therapeutic effect on reproductive disorders. A review of the showed that acupuncture stimulation in the sacral nerve region—that forms the pelvic and autonomic nerves that innervate the organs in the lower abdominal area—can increase vagal activity and suppress sympathetic activity.37 In 2 studies, EA in the lower abdomen increased ovarian blood flow.33,38 Other researchers found that acupuncture increased IGF-1 levels in the endometrium and follicular fluid, and also improved egg and embryo quality.10,39 Other studies showed that acupuncture regulated levels of VEGF, interleukin (IL)–15, and IL-18 in rats given COS.40 It is known that IGF-1 and VEGF are both survival factors that play a role in the antiapoptotic pathway, resulting in increased oocyte maturation and decreased granulosa-cell apoptosis.
Limitations of this study were: (1) granulosa cells originating from small-diameter follicles and large-diameter follicles, when oocytes were retrieved, were not analyzed separately; (2) patients had heterogeneous diagnoses of their infertility; (3) there are no data on whether the patients had repetitive IVF or if this was their first attempts; (4) there are no data on whether the patients used Chinese herbal medicine or not. Further research with larger sizes and other biomarker outcomes is needed.
Conclusions
Verum EA might increase the oocyte maturation index, the rate of fertilization, and decrease the apoptosis index (Bax/Bcl-2 ratio) in granulosa cells compared to sham EA in IVF programs.
Acknowledgments
The authors would like to show their gratitude to Dr. Andon Hestiantoro (Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Indonesia), who provided insight and expertise to assist the research greatly. The authors thank Drs. Hasan Mihardja, MD, PhD, and Adiningsih Srilestari, MD, PhD (Department of Medical Acupuncture, Faculty of Medicine, Universitas Indonesia) for their guidance and encouragement. The authors thank all the clinicians and staff members of the Division of Reproductive Endocrinology and Infertility (Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Indonesia) for providing the opportunity to do this research, and also the nurses for their kind help at the research site. Thanks are extended to Naylah Muna, a laboratory assistant, for her cooperation throughout the research period. Finally, the authors also thank Dwi Rachma Helianthi, MD (Department of Medical Acupuncture, Faculty of Medicine, Universitas Indonesia), for a valuable discussion and for feedback.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The authors received no financial support for the research and authorship involved with this article.
References
- 1. Cai Q, Wan F, Huang K, Zhang H. Does the number of oocytes retrieved influence pregnancy after fresh embryo transfer? PloS One. 2013;8(2):e56189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao S, Saito H, Wang X, Saito T, Kaneko T, Hiroi M. Effects of gonadotropin-releasing hormone agonist on the incidence of apoptosis in porcine and human granulosa cells. Gynecol Obstet Invest. 2000;49(1):52–56 [DOI] [PubMed] [Google Scholar]
- 3. Kaneko T, Saito H, Takahashi T, Ohta N, Saito T, Hiroi M. Effects of controlled ovarian hyperstimulation on oocyte quality in terms of the incidence of apoptotic granulosa cells. J Assist Reprod Genet. 2000;17(10):580–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knecht M, Katz MS, Catt KJ. Gonadotropin-releasing hormone inhibits cyclic nucleotide accumulation in cultured rat granulosa cells. J Biol Chem. 1981;256(1): 34–36 [PubMed] [Google Scholar]
- 5. Billig H, Furuta I, Hsueh AJ. Gonadotropin-releasing hormone directly induces apoptotic cell death in the rat ovary: Biochemical and in situ detection of deoxyribonucleic acid fragmentation in granulosa cells. Endocrinology. 1994;134(1):245–252 [DOI] [PubMed] [Google Scholar]
- 6. Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hadi RS. The mechanism of apoptosis in luteal cell regression [in Indonesian]. Pharma Medika. 2011;3(1):246–254 [Google Scholar]
- 8. Bosch E, Labarta E, Kolibianakis E, Rosen M, Meldrum D. Regimen of ovarian stimulation affects oocyte and therefore embryo quality. Fertil Steril. 2016;105(3):560–570 [DOI] [PubMed] [Google Scholar]
- 9. Mehlmann LM. Stops and starts in mammalian oocytes: Recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130(6):791–799 [DOI] [PubMed] [Google Scholar]
- 10. Zhu S, Liu J, Li C, Liu W, Fu Q, Wang F, Zhang Y, Huang Y. Impacts on the pregnancy outcome in the mice of controlled ovarian hyperstimulation treated with acupuncture at different time points [in Chinese]. Zhongguo Zhen Jiu. 2016;36(11):1181–1185 [DOI] [PubMed] [Google Scholar]
- 11. Dong H, Zhong Z, Chen W, Wu X, Zhang Q, Huang G, Yang W. Effect of acupuncture on endometrial angiogenesis and uterus dendritic cells in COH rats during peri-implantation period. Evid Based Complement Alternat Med. 2017;2017:3647080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58(1):44–50 [DOI] [PubMed] [Google Scholar]
- 13. Irusta G, Abramovich D, Parborell F, Tesone M. Direct survival role of vascular endothelial growth factor (VEGF) on rat ovarian follicular cells. Mol Cell Endocrinol. 2010;325(1–2):93–100 [DOI] [PubMed] [Google Scholar]
- 14. Stener-Victorin E, Waldenstrom U, Andersson SA, Wikland M. Reduction of blood flow impedance in the uterine arteries of infertile women with electro-acupuncture. Hum Reprod. 1996;11(6):1314–1317 [DOI] [PubMed] [Google Scholar]
- 15. Loonasultana S. Effects of Acupuncture Therapy on Blood Flow [in Indonesian]. Jakarta, Indonesia: Uterina Pasien Program Bayi Tabung Klinik Yasmin, FKUI-RSCM; 2009 [Google Scholar]
- 16. Li Y, Li RQ, Ou SB, et al. Increased GDF9 and BMP15 mRNA levels in cumulus granulosa cells correlate with oocyte maturation, fertilization, and embryo quality in humans. Reprod Biol Endocrinol. 2014;12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Combelles CM, Albertini DF. Assessment of oocyte quality following repeated gonadotropin stimulation in the mouse. Biol Reprod. 2003;68(3):812–821 [DOI] [PubMed] [Google Scholar]
- 18. Lindsay C. Oocyte Quality: Molecular Constituents Altered in the Oocyte Due to Various Environmental Factors (2016). Utah State University: Digital Commons@USU. All Graduate Theses and Dissertations. Online document at: https://pdfs.semanticscholar.org/2aa7/17ee93dc79e6c8697118a3a6404a42b587c1.pdf Accessed September17, 2019
- 19. Cui W, Li J, Sun W, Wen J. Effect of electroacupuncture on oocyte quality and pregnancy for patients with PCOS undergoing in vitro fertilization and embryo transfervitro fertilization and embryo transfer [in Chinese]. Zhongguo Zhen Jiu. 2011;31(8):687–691 [PubMed] [Google Scholar]
- 20. Li J, Cui W, Sun W, Zhang QY, Guan Q. Effect of electro-acupuncture on the spindle and oocytes quality in patients with PCOS [in Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35(3):304–309 [PubMed] [Google Scholar]
- 21. Qian Y, Xia XR, Ochin H, et al. Therapeutic effect of acupuncture on the outcomes of in vitro fertilization: A systematic review and meta-analysis. Arch Gynecol Obstet. 2017;295(3):543–558 [DOI] [PubMed] [Google Scholar]
- 22. Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium. 2014;55(1):24–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xue X, You Y, Tao J, et al. Electro-acupuncture at points of Zusanli and Quchi exerts anti-apoptotic effect through the modulation of PI3K/Akt signaling pathway. Neurosci Lett. 2014;558:14–19 [DOI] [PubMed] [Google Scholar]
- 24. Karagül Mİ, Aktaş S, Coşkun Yılmaz B, Yılmaz M, Orekici Temel G. GDF9 and BMP15 expressions and fine structure changes during folliculogenesis in polycystic ovary syndrome. Balkan Med J. 2018;35(1):43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wei LN, Huang R, Li LL, Fang C, Li Y, Liang XY. Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31(11):1483–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang TT, Wu YT, Dong MY, Sheng JZ, Leung PC, Huang HF. G546A polymorphism of growth differentiation factor–9 contributes to the poor outcome of ovarian stimulation in women with diminished ovarian reserve. Fertil Steril. 2010;94(6):2490–2492 [DOI] [PubMed] [Google Scholar]
- 27. Wang TT, Ke ZH, Song Y, et al. Identification of a mutation in GDF9 as a novel cause of diminished ovarian reserve in young women. Hum Reprod. 2013;28(9):2473–2481 [DOI] [PubMed] [Google Scholar]
- 28. Vanyan R, Dolgushina N, Donnikov A, Shubina E, Usman N, Kuznetsova M, Sukhikh G. Single nucleotide polymorphisms of BMP15 are associated with poor ovarian response in in vitro fertilization programs. JFIV Reprod Med Genet. 2015;3(4):159 [Google Scholar]
- 29. Gode F, Gulekli B, Dogan E, et al. Influence of follicular fluid GDF9 and BMP15 on embryo quality. Fertil Steril. 2011;95(7):2274-2278 [DOI] [PubMed] [Google Scholar]
- 30. Villahermosa DI, Santos LG, Nogueira MB, Vilarino FL, Barbosa CP. Influence of acupuncture on the outcomes of in vitro fertilisation when embryo implantation has failed: A prospective randomised controlled clinical trial. Acupunct Med. 2013;31(2):157–161 [DOI] [PubMed] [Google Scholar]
- 31. Johansson J, Redman L, Veldhuis PP, et al. Acupuncture for ovulation induction in polycystic ovary syndrome: A randomized controlled trial. Am J Physiol Endocrinol Metab. 2013;304(9):E934–E943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao YX, He W, Jing XH, et al. Transcutaneous auricular vagus nerve stimulation protects endotoxemic rat from lipopolysaccharide-induced inflammation. Evid Based Complement Alternat Med. 2012;2012:627023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stener-Victorin E, Kobayashi R, Watanabe O, Lundeberg T, Kurosawa M. Effect of electro-acupuncture stimulation of different frequencies and intensities on ovarian blood flow in anaesthetized rats with steroid-induced polycystic ovaries. Reprod Biol Endocrinol. 2004;2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chavez LM, Huang SS, MacDonald I, Lin JG, Lee YC, Chen YH. Mechanisms of acupuncture therapy in ischemic stroke rehabilitation: A literature review of basic studies. Int J Mol Sci. 2017;18(11):2270–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jo J, Lee YJ, Lee H. Effectiveness of acupuncture for primary ovarian insufficiency: A systematic review and meta-analysis. Evid Based Complement Alternat Med. 2015;2015:842180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang J, Cheng K, Qin Z, Wang Y, Zhai L, You M, Wu J. Effects of electroacupuncture at Guanyuan (CV 4) or Sanyinjiao (SP 6) on hypothalamus–pituitary–ovary axis and spatial learning and memory in female SAMP8 mice. J Tradit Chin Med. 2017;37(1):96–100 [DOI] [PubMed] [Google Scholar]
- 37. Stener-Victorin E, Fujisawa S, Kurosawa M. Ovarian blood flow responses to electroacupuncture stimulation depend on estrous cycle and on site and frequency of stimulation in anesthetized rats. J Appl Physiol. 2006;101(1):84–91 [DOI] [PubMed] [Google Scholar]
- 38. Kavoussi B, Ross BE. The neuroimmune basis of anti-inflammatory acupuncture. Integr Cancer Ther. 2007;6(3):251–257 [DOI] [PubMed] [Google Scholar]
- 39. Lian F, Chen C, Xiang S. Improvement of the oocyte quality with electroacupuncture in infertility patients of kidney deficiency pattern [in Chinese]. Zhongguo Zhen Jiu. 2015;35(2):109–113 [PubMed] [Google Scholar]
- 40. Spence DW, Kayumov L, Chen A, et al. Acupuncture increases nocturnal melatonin secretion and reduces insomnia and anxiety: A preliminary report. J Neuropsychiatry Clin Neurosci. 2004;16(1):19–28 [DOI] [PubMed] [Google Scholar]