Abstract
Purpose
The purpose of this study was to investigate the inflammatory response of cornea and conjunctiva to topically applied lipopolysaccharide (LPS) in mice with and without antibiotic (antibiotic cocktail, ABX) induced dysbiosis.
Methods
Dysbiosis was induced by oral antibiotics for 14 days in a group of conventional female C57BL/6J (B6) mice. 16S rRNA sequencing investigated microbiome composition. Intestinal microbiome differences were assessed using 16S rRNA sequencing of fecal pellet DNA. Blood was collected after euthanasia. CD86 expression in draining nodes was examined by flow cytometry. At day 15, a single dose of LPS or vehicle was topically applied to ABX and naïve mice. Corneal epithelium and conjunctiva were obtained after 4 hours and processed for gene expression analysis. A separate group of germ-free (GF) B6 mice was also topically challenged with LPS.
Results
Antibiotic treatment significantly decreased intestinal diversity and increased serum levels of LPS. This was accompanied by a significant increase in CD86+MHC II+CD11c+CD11b+ cells in draining nodes. Compared to vehicle, topically applied LPS increased IL-1β, TNF-α, and CXCL10 mRNA transcripts in cornea and IL-1β, TNF-α, and CXCL10 in the conjunctiva in conventional and antibiotic-treated groups. However, there was higher TNF-α, CXCL10, and IL-12 expression in the cornea of LPS-treated ABX mice compared to LPS-treated mice with intact microbiota. LPS stimulation on GF conjunctiva mirrored the results in ABX mice, although greater IL-12 and IFN-γ expression was observed in GF conjunctiva compared to conventional LPS-treated mice.
Conclusions
Acute depletion of commensals through antibiotics or germ-free environment worsens the inflammatory response to LPS.
Keywords: TLR4, toll-like receptors, dry eye, lipopolysaccharide, cornea, conjunctiva, inflammation, cytokines, dysbiosis, antibiotics
The ocular surface is an exposed mucosa and a component of the mucosal immune system. Similar to the intestinal lumen, the ocular surface is continuously exposed to a variety of external stresses, such as microbial pathogens, desiccation, pollution, and other environmental irritants, yet in healthy eyes homeostasis is maintained by a precisely modulated balance between immune tolerance and immunity.1,2 Despite being an exposed mucosa, 50% of swab cultures obtained from the conjunctiva are negative, and metagenomic analysis of the 16S ribosomal gene has found the ocular surface to have a microbiome with low abundance and diversity.3–5
Recent studies have demonstrated that alterations of the gut microbiota may lead to immune dysfunction in distal mucosal immune sites, such as skin, oral cavity, vagina, respiratory tract, and ocular surface.6–12 We have previously shown that germ-free C57BL/6J mice spontaneously develop Sjögren-like disease, which can be reversed by a fecal transplant of intestinal material from normal, conventionally housed C57BL/6J mice.12
Toll-like receptors (TLRs) are transmembrane molecules that play a fundamental role in the innate immune response. TLRs trigger host immune response following recognition of pathogen-associated molecule patterns (PAMPs). A number of TLRs, including TLR4, have been reported to be highly expressed on the ocular surface.13–17 Lipopolysaccharide (LPS), the major virulence factor of Gram-negative bacteria, specifically binds to TLR4 to initiate an innate immune response and stimulate production and release of proinflammatory cytokines and chemokines. We previously demonstrated that topical administration of LPS to the ocular surface of mice increased expression of inflammatory cytokines in both the cornea and conjunctiva.18 However, it is unknown whether similar ocular surface inflammatory responses occur in mice without commensal bacteria.
The purpose of this study was to investigate the influence of commensal bacteria on the inflammatory response to LPS topically applied to the ocular surface. We found that acute dysbiosis induced by a short course of oral antibiotics or a germ-free environment significantly increased the production of inflammatory mediators in the cornea and conjunctiva that are associated with dry eye in response to LPS, suggesting that commensal bacteria actively participate in regulating inflammation stimulated by microbial products on the ocular surface.
Materials and Methods
Mice
The research protocol was approved by the Baylor College of Medicine Institutional Animal Care and Use Committee, and it conformed to the standards in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Germ-free C57BL/6J mice were produced in the Gnotobiotic Rodent Facility at Baylor College of Medicine. Conventionally housed C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) for establishing breeding colonies. Germ-free mice received autoclaved LabDiet 5V0F, and conventional mice received irradiated LabDiet 5V5R (PMI Nutrition International, St. Louis, MO, USA). Both groups received water ad libitum, which was sterilized for the animals housed in germ-free conditions.
Female C57BL/6J mice with conventional (and complex) intestinal microbiota were used at 8 weeks of age. Because dry eye affects more women than men,19,20 only female C57BL/6J mice were used. A total of 12 naïve mice were used for antibiotic or normal water treatments, and tissues were collected from the same mouse whenever possible to mitigate their use (four for flow cytometry, eight for stool analysis, and six to seven to LPS measurement). The two conventional treatment groups (water and LPS) had 10 to 12 mice per group. Six female germ-free mice per treatment group were used for gene expression studies.
Mice were euthanized with an overdose of isoflurane in a desiccator, followed by cervical dislocation.
Oral Antibiotic Treatment
Mice were treated with a cocktail of broad-spectrum antibiotics [0.5 mg/mL ampicillin (Dava Pharmaceuticals, Fort Lee, NJ, USA), 0.5 mg/mL gentamicin (Life Technologies, Grand Island, NY, USA), 0.5 mg/mL metronidazole (Hospira, Lake Forest, IL, USA), 0.5 mg/mL neomycin (Sparhawk Lab, Lenexa, KS, USA), and 0.25 mg/mL vancomycin (Hospira)] dissolved in drinking water with 5 mg/mL artificial sweetener (Splenda; McNeil Nutritionals, Fort Washington, PA, USA), as previously described.5,21 Mice drank the antibiotic cocktail for 14 days. On the 15th day, they were left untreated for flow cytometry analysis or divided into the vehicle or LPS topical treatment groups.
Vancomycin and gentamycin have poor oral absorption, but have been used to treat intestinal infections, such as C. difficile and gut colonization with other bacteria, such as K. pneumoniae.22,23 Ampicillin, metronidazole, and neomycin are Food and Drug Administration approved for oral use.
LPS Measurement in Serum
Serum samples from antibiotic-treated and untreated mice (n = 6–7) were collected by cardiac puncture after euthanasia. LPS concentration in diluted sera was measured using a commercial chromogenic Limulus Amebocyte Assay according to the manufacturer's instructions (LAL; Pierce-Thermo Scientific, Rockford, IL, USA). The absorbance was read at 405 to 410 nm according to the manufacturer's instructions, and concentration of diluted samples was calculated according to the standard curve that was prepared at the same time.
DNA Extraction From Mouse Fecal Samples, 16S rRNA Gene Amplification, and Sequencing
Fecal pellets were collected in the morning of the 15th day, after 14 consecutive days on oral antibiotics. DNA for microbial sequence analysis was extracted from mouse fecal samples by bead-beating and modified extraction with Qiagen DNeasy Blood and Tissue kits as described previously.5 Bacterial 16S sequences spanning variable region V4 were amplified by PCR with primers F515/R806 and sequenced by Illumina MiSeq using our previously described protocol.24,25 Replicates were pooled and purified with Agencourt AMPure XP magnetic beads (Beckman Coulter, Brea, CA, USA). DNA samples were quantified using the QuantIt High Sensitivity DNA assay kit (Thermo Fisher Scientific, Waltham, MA, USA) and pooled at equimolar ratios. The quality of the pooled sample was evaluated with the Bioanalyzer High Sensitivity DNA Kit (Agilent, Santa Clara, CA, USA).
Microbial Community Analysis
Sequence data were processed using the MiSeq pipeline for mothur using software version 1.38.124,25 and the MiSeq SOP version 7 March 2018 (http://www.mothur.org/wiki/MiSeq_SOP; in the public domain), as described previously.2,7 Chimeric sequences were identified and removed using the mothur implementation of UCHIME. After classification with the mothur-formatted Ribosomal Database Project (version 16, February 2016) using the Bayesian classifier in mothur, sequences classified as Eukarya, Archaea, chloroplast, mitochondria, or unknown were removed. Sequences present only once in the data set were also removed. Sequences were clustered from a distance matrix into operational taxonomic units (OTUs) with 97% similarity using the average-neighbor algorithm in mothur. 1725 OTUs were identified across all samples with an average rarefaction depth of 26,749 reads per sample. Alpha and beta diversity analyses and visualization of microbiome communities were performed with R, utilizing the phyloseq package,24,25 and the ATIMA visualization toolkit developed by the Center for Metagenomics and Microbiome Research at Baylor College of Medicine (http://atima.jplab.net/; in the public domain). The Bray-Curtis dissimilarity matrix was used to describe differences in microbial community structure. Analysis using alternative dissimilarity measures, Jaccard and Sorensen, was performed with similar results (data not shown). SIMPER (Similarity Percentages) analyses in PAST (https://palaeo-electronica.org/2001_1/past/issue1_01.htm; in the public domain) was performed to identify taxa that contributed to microbial community differences between the experimental groups; significance was calculated using GraphPad Prism 7.0 software (GraphPad Software, Inc., San Diego, CA, USA) by multiple t-tests with FDR (false discovery rate) correction.
Topical LPS Challenge
Mice were treated topically (5 μL/eye) with ultrapure LPS from S. enterica serovar Minnesota mutant R595 (Invivogen, San Diego, CA, USA) dissolved in endotoxin-free water (Sigma-Aldrich Corp., St. Louis, MO, USA) at a dose of 1 μg/μL as previously described.18 Endotoxin-free water (5 μL/eye) was used a vehicle control. Mice were held in place for 1 minute to allow eye drops to distribute. After 4 hours, mice were euthanized for PCR or flow cytometry experiments.
RNA Isolation and Real-Time PCR
Corneal epithelium and conjunctiva were extracted to measure gene expression of inflammatory mediators via PCR. The corneal epithelium was scraped with a scalpel, and full-thickness conjunctiva was surgically excised. Tissues samples from cornea and conjunctiva were pooled separately from both eyes to give one cornea and one conjunctiva sample per mouse. RNA was extracted using the RNeasy Plus Micro Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. The concentration of isolated RNA was measured using a Nanodrop 2000 Spectrophotometer (Thermo Fisher Scientific). After RNA isolation, cDNA was synthesized using Ready-To-Go You-Prime First-Strand Beads (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) as previously reported.26,27 Real-time PCR was performed using specific MGB Taqman probes for interferon-gamma, IFN-γ (Ifng, Mm00801778), interleukin 1-beta, IL-1β (Il1b, Mm00434228), interleukin 6, IL-6 (Il6, Mm00446190), interleukin 12a, IL-12a (Il12a, Mm00434165), chemokine C-X-C ligand 10 CXCL10 (Cxcl10, Mm00445235), tumor necrosis factor alpha, TNF-α (Tnfa, Mm00443260) using Taqman Universal PCR Master Mix AmpErase UNG in a commercial thermocycling system (StepOnePlus Real-Time PCR System, Applied Biosystems, Grand Island, NY, USA), according to the manufacturer's recommendations. The hypoxanthine phosphoribosyltransferase 1 (HPRT1, Mm00446968) gene was used as an endogenous reference for each reaction. The results of quantitative PCR were analyzed by the delta-delta comparative Ct method. Fold differences in expression were calculated after comparing values for each gene to the vehicle-treated group.
Flow Cytometry Analysis
Cervical lymph nodes were surgically collected and single-cell suspensions were prepared. Briefly, nodes were collected in a Petri dish containing complete RPMI medium and smashed in between two layers of a nylon mesh that had been previously autoclaved. Cells were washed, and red blood cells were lysed using 5 mL Tris-buffered ammonium chloride solution at room temperature for approximately 10 minutes, followed by addition of the same volume of RPMI and two sequential washes. Cells were filtered through a 70-μm filter, made into a single-cell suspension, and counted using a hematocytometer and Trypan Blue dye. A total of 1 × 106 cells/tube were then stained with anti-CD16/32 (BD Pharmingen, San Diego, CA, USA) at 4°C for 10 minutes, followed by staining with anti-CD11c_FITC (clone HL3, BD Pharmingen), anti-CD11b_APC (clone M1/70; Ebiosciences, San Diego, CA, USA), MHC-II_PE (clone M5/114.15.2; BD Pharmingen), and CD86_Pacific blue (clone GL1; Biolegend, San Diego, CA, USA). Cells were resuspended in PBS/1% fetal bovine serum (FBS) containing 1 μL live/dead blue reconstituted fluorescent reactive dye (Thermo Fisher Scientific) and kept on ice until flow cytometry analysis was performed. A BD FACS CANTO II cytometer (Becton Dickinson, San Jose, CA, USA) was used, and data were analyzed using FlowJo software (version 10; TreeStar, Inc., Ashland, OR, USA). Single-cell preparations of splenocytes obtained from untreated mice were stained with the same antibodies and served as positive controls. Fluorescence minus one controls were performed to define the gating strategy. The gating strategy used in this study was as follows: Lymphocytes and monocytes were individually identified on the basis of forward scatter and side scatter properties, subsequently gated on the basis of forward scatter height versus forward scatter area (singlets 1), then gated on side scatter height versus side scatter area (singlets 2). Live/dead exclusion was performed. CD11c and MHC II were plotted and subsequently gated on MHC II versus CD11b. Mean fluorescence intensity of CD86 was then calculated. The experiment was repeated once with similar results.
Statistical Analysis
Sample size calculation was performed with StatMate Software version 2.0 (GraphPad Software, Inc.) based on preliminary data. Experimental data were averaged, and graphs were generated. Comparison of statistical data between two groups was analyzed using the Mann-Whitney U test. Kruskal-Wallis, followed by Tukey's comparison test, was used to analyze gene expression in three or more groups. P ≤ 0.05 was considered statistically significant. Statistical tests were performed using GraphPad Prism 7.0 software (GraphPad Software, Inc.). Experiments were repeated at least once, and representative experiments are shown.
Results
Oral Antibiotic Treatment for 14 Days Induced a Profound Change in Intestinal Microbiome Composition
There is a growing body of evidence that alterations in the intestinal microbiome, termed dysbiosis, are associated with autoimmune and inflammatory diseases, including dry eye.5,12,28,29 Oral antibiotics are well known to perturb the intestinal microbiome. We collected stools from mice at baseline (naïve) and after 14 days of treatment with a broad-spectrum oral antibiotic cocktail (ampicillin, gentamicin, metronidazole, neomycin, vancomycin) to investigate the impact in the intestinal microbiome.
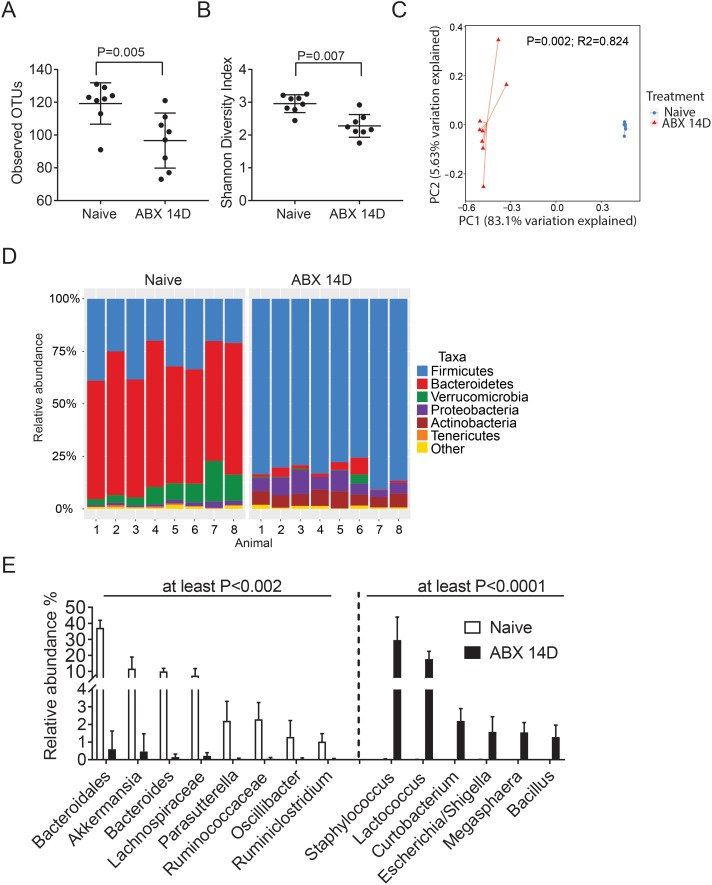
A significantly lower number of OTUs and significantly lower Shannon diversity index were observed after oral antibiotics for 14 days (Figs. 1A, 1B). Analysis of β diversity with the Bray-Curtis similarity measure revealed clustering of samples before and after oral antibiotics (P = 0.002, R2 = 0.824, Fig. 1C), demonstrating that the composition of the microbial communities was altered by antibiotic treatment. The relative abundance of the major bacterial phyla from the fecal samples of naïve mice shifted significantly after antibiotic treatment; Bacteroidetes taxa decreased in abundance, while there was an increase in the relative abundance of Proteobacteria and Firmicutes after antibiotics (Fig. 1D). The top genera responsible for these shifts were identified by SIMPER analysis and are depicted in Figure 1E. We observed that Bacteroidales, Akkermansia, Bacteroides, Lachnospiraceae, Parasutterella, Ruminococcaceae, Oscillibacter, and Ruminiclostridium all significantly decreased after antibiotic treatment (all P < 0.002) while Staphylococcus, Lactococcus, Curtobacterium, Escherichia/Shigella, Megasphaera, and Bacillus genera were significantly increased (all P < 0.0001).
Figure 1.
Antibiotic treatment induces a profound change in microbiome. C57BL/6J mice were treated with a cocktail of broad-spectrum antibiotics (ABX: ampicillin, gentamicin, metronidazole, neomycin, and vancomycin) dissolved in drinking water with artificial sweetener (Splenda) ad libitum. Stools were collected before treatment (naïve) and after 14 days (14D) of continuous oral cocktail and processed as described in Materials and Methods for 16S analysis. (A, B) Number of observed OTUs and Shannon Diversity Index scores (B) showing α diversity before (naïve) and after oral antibiotics (ABX) for 14 days (14D; n = 8). Mann-Whitney U test. (C) Principal coordinate analysis (PCoA) plot using the Bray-Curtis similarity measure. Each symbol represents an individual sample from naive and ABX-treated mice. (D) The relative abundance of major bacterial taxa before (naïve) and after oral antibiotics (ABX) for 14 days (14D). (E) Comparison of the significant relative abundance of different genera among groups before (naïve) and after oral antibiotics (ABX) for 14 days (14D). The dotted line divides significant genera that decreased (left) or increased (right) after treatment. For OTUs that do not have a genus classification, the family classification is given. Mann-Whitney U tests with false discovery rate correction. The P values shown in the graph were rounded to the largest value, as shown.
Oral antibiotic treatment can be associated with bacterial translocation and the appearance of bacterial products in the serum. To investigate this, blood was collected by cardiac puncture after euthanasia, and LPS concentration was measured in serum using a Limulus assay. We observed a 2-fold increase in LPS concentration in antibiotic-treated mice compared to conventional mice (0.275 ± 0.14 vs. 0.556 ± 0.2 [EU/mL], conventional versus antibiotic mice, P = 0.014, Mann-Whitney U test, n = 6–7/group).
These results indicate that the antibiotic treatment induced intestinal dysbiosis as expected,5,30,31 demonstrated by a significant decrease in commensal intestinal microbiota and increased LPS levels in serum.
Oral Antibiotics Modulate APC Number and Maturation Status in the Ocular Surface Draining Nodes
There is increasing evidence that the microbiome can modulate frequency and activation of antigen-presenting cells (APCs).12 Therefore, we investigated if dysbiosis could alter the APC phenotype in the ocular surface draining cervical lymph nodes. To accomplish this, we collected cervical nodes from mice that received either (1) normal water or (2) antibiotic cocktail for 14 days. Flow cytometry analysis showed the frequency of MHC II+CD11c+ cells increased in draining nodes after 14 days of antibiotic treatment (Fig. 2a), but the frequency of MHC II+CD11c+ CD11b+ cells was similar in both groups (Fig. 2b). Interestingly, there was an overall increase in CD86 median fluorescence intensity in MHC II+CD11c+CD11b+ cells in the antibiotic group compared to mice that drank normal water (Fig. 2c), indicating that acute ablation of microbiota increased maturation status of APCs in draining cervical lymph nodes.
Figure 2.
Antibiotic cocktail increases the frequency of antigen-presenting cells and their maturation status. (A) Single-cell preparations from lymph nodes were prepared and analyzed by flow cytometry. Lymphocytes were identified by forward and side scatter properties (SSC), single-cell gates were drawn, dead cells were excluded. CD11c+ cells were then plotted versus MHC II and further gated into MHC II and CD11b+ cells. (B) Representative histograms of CD11c+MHC II+CD11b+ showing CD86 fluorescence intensity. FMO, fluorescence minus one. (C) Accumulative data of flow cytometry analysis showing the frequency of CD11c+MHC II+ and median fluorescence intensity (MFI) of CD86 in cervical lymph nodes. Mean ± SEM, n = 4 mice/group, biological replicates from two independent experiments were averaged. Mann-Whitney U test.
These results indicate that the number and maturation status of APCs in ocular surface draining nodes are modulated by intestinal bacteria.
Lack of Commensal Bacteria Enhances Inflammatory Response to LPS on the Ocular Surface
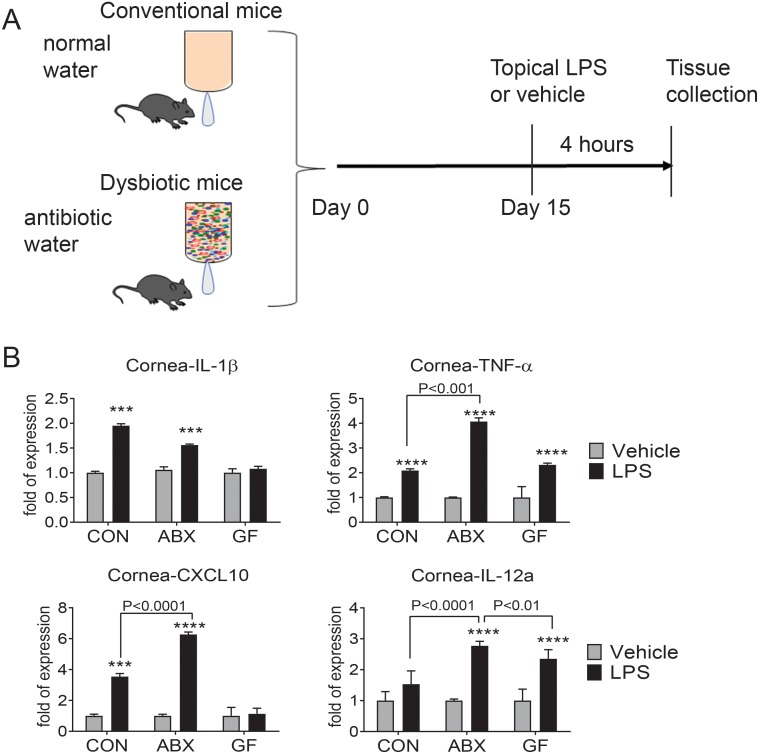
Corneal and conjunctival cells have TLRs that respond to danger-associated patterns and patterned associated signals. The TLR4 ligand LPS has been shown to stimulate an inflammatory response on the ocular surface.14,18,32 To investigate if depletion of the intestinal microbiome could modulate the inflammatory responses to danger signals in a distant organ (the eye surface), we used topical LPS as a surrogate bacterial danger signal. Mice that received antibiotics for 14 days were dosed with a single application of either LPS or endotoxin-free water and were compared to conventional mice that drank regular water (with intact diverse microbiome) and received similar topical treatment (Fig. 3A). Corneal epithelium and conjunctiva were collected 4 hours after topical treatment, and gene expression was analyzed by real-time PCR. For each comparison, the vehicle treatment group was set as the calibrator, and the fold response to LPS was compared among the groups with intact conventional microbiome or after oral antibiotics. We have previously reported that changes in mRNA levels correspond to increased protein expression after LPS stimulation.18
Figure 3.
Oral antibiotic treatment worsens the inflammatory response to LPS in the cornea. (A) Conventionally housed (CON) C57BL/6 mice were subjected to a cocktail of oral antibiotics (ABX) in drinking water for 14 days or drank normal water. On the 15th day, mouse eyes were treated topically with LPS or endotoxin-free water (vehicle) and mice were euthanized after 4 hours to analyze cytokine/chemokine expression. A separate group of germ-free (GF) mice also received LPS or vehicle. (B) Relative fold expression changes of IL-1β, tumor necrosis factor-α (TNF-α), CXCL10, and IL-12 mRNA in the cornea. Bar graphs show mean ± SEM of a representative experiment with four to six samples/group (the experiment was repeated once with similar results). *P < 0.05, **P < 0.01, ***P < 0.001, P < 0.0001 intragroup comparison vehicle versus LPS. Kruskal-Wallis followed by Tukey's comparison test.
In the corneal epithelium, LPS stimulation in conventional B6 and antibiotic-treated groups significantly increased IL-1β, TNF-α, and CXCL10 mRNA transcripts compared to the vehicle group (Fig. 3B). However, a greater fold increase in TNF-α and CXCL10 was observed in the antibiotic group that received topical LPS compared to the conventional LPS-treated group (Fig. 3B). A significant increase in IL-12 mRNA levels was observed only in the LPS + antibiotic-treated group (Fig. 3B). IL-6 and IFN-γ transcripts were not detected in any of the groups in the cornea. A similar analysis of gene expression was also performed in the conjunctiva (Fig. 4). Similar to the what occurred in the cornea, LPS stimulation induced a significant increase in IL-1β, TNF-α, and CXCL10 mRNA in the conjunctiva in the conventional and antibiotic groups compared to their vehicle-treated counterparts. LPS treatment induced greater increase of IL-1β, TNF-α, and CXCL10 in conventional mice with intact microbiome compared to antibiotic-treated mice. There was no change in IL-6 mRNA levels after LPS stimulation.
Figure 4.
Dysbiosis worsens the inflammatory response to LPS in the conjunctiva. Mice were treated as described in Figure 3. Relative fold expression changes of IL-1β, IL-6, TNF-α, IL-12, IFN-γ, and CXCL10 mRNA in the conjunctiva. Bar graphs show mean ± SEM of a representative experiment with four to six samples/group (the experiment was repeated once with similar results). *P < 0.05, **P < 0.01, ***P < 0.001, P < 0.0001 intragroup comparison vehicle versus LPS. Kruskal-Wallis followed by Tukey's comparison test.
Taken together, these results indicate that after oral antibiotics, there is an exaggerated inflammatory response at the cornea and conjunctiva after LPS challenge, indicating that commensal bacteria play a modulating role in TLR4 signaling-mediated immune response at the ocular surface.
Germ-Free Environment Recapitulates Response to Topical LPS Observed in Antibiotic-Treated Mice
Our results depicted in Figure 1 demonstrated that antibiotic treatment reduces and alters the diversity of the intestinal microbiome, but it did not completely sterilize the intestinal tract. Therefore, we asked if LPS stimulation of mice raised in a germ-free environment could also increase the ocular surface inflammatory response to topically applied LPS. To accomplish this, 8-week-old germ-free B6 mice were treated with topical LPS or vehicle immediately after exit from the gnotobiotic isolator. Similar to the experiment with conventional and antibiotic-treated mice, germ-free mice were euthanized 4 hours post stimulation and cornea and conjunctiva were collected, and gene expression was analyzed.
LPS treatment upregulated TNF-α and IL-12 mRNA transcripts in the cornea of germ-free mice (albeit not to the same level as LPS-stimulated conventional mice) but failed to upregulate IL-1β and CXCL10 compared to vehicle-treated animals (Fig. 3B).
In conjunctiva of germ-free mice, LPS stimulated significantly upregulated IL-1β, TNF-α, IL-12, IFN-γ, and CXCL10 transcripts compared to vehicle treatment. IL-12, CXCL10, and IFN-γ upregulation in conjunctiva was the greatest in germ-free mice compared to all LPS-treated groups.
These findings indicate that mice born and raised in a germ-free environment have augmented responses to LPS with the highest upregulation of IL-12, CXCL10, and IFN-γ in the conjunctiva in comparison with mice with conventional microbiome or antibiotic-induced dysbiosis.
Discussion
The ocular surface epithelium has TLR and NOD receptors18,32–34 and has been shown to respond to microbial and viral ligands by upregulating inflammatory markers such as IL-1β and TNF-α.18,33 Our results demonstrate that a 14-day course of antibiotics caused profound intestinal dysbiosis that significantly enhanced the ocular response to the microbial danger signal LPS, and markedly increased expression of TNF-α, CXCL10, and IL-12 genes in the cornea. Mice born and raised in a germ-free environment manifested an augmented response to the same challenge in the conjunctiva, which had increased levels of IL-12a, IFN-γ, and CXCL10 gene transcripts. These findings highlight that innate immune response in the cornea and conjunctiva is modulated by the microbiome and highlight differences between acute dysbiosis (a decrease in commensal bacteria with an increase of other potentially pathogenic microbes) that had more impact on the cornea and germ-free environment (absence of microbes) that had a greater effect on the conjunctiva.
The cocktail of five antibiotics in drinking water we used to induce intestinal dysbiosis in C57BL/6J mice caused profound changes in the intestinal microbiome composition, with a decrease in diversity, segregation in different community groups, and a decrease in Bacteroidetes and an increase in Proteobacteria taxa. There was a decrease in potentially protective genera such as Akkermansia and an increase in Escherichia/Shigella, gram-negative taxa with pathogenic potential.5,35–37 These results are in agreement with the literature about the effect of oral antibiotics on the intestinal microbiome.5,30,31
We have previously reported that antibiotic treatment alone is not sufficient to induce goblet cell loss5; however, germ-free mice spontaneously develop a dry eye phenotype, inclusive of an altered corneal barrier to a fluorescent tracer and conjunctival disease (goblet cell loss).12 Colonization of germ-free mice with fecal material from naïve mice reversed these changes, suggesting that bacteria themselves or their products are protective to the ocular surface. Response to LPS in the presence or absence of intestinal microbiota might be tissue-specific and even LPS-specific.38 For example, Escherichia coli LPS intraperitoneal injections in conventional mice induced more depressive behavioral signs than in germ-free mice,39 while periodontal injection of Porphromonos gingivalis LPS into the palatal gingival sulcus elicited an immune response of CD4+T cells, neutrophils. and production of cytokines only in conventional mice.40 However, LPS produced by Bacteroidetes, might be less inflammatory than LPS produced by Firmicutes and Proteobacteria genera.38
The presence of an ocular microbiome with resident commensal bacteria is still a matter of debate. While we are making progress identifying bacterial DNA in conjunctival swabs through next-generation sequencing in humans, it remains a challenge to identify a core ocular microbiome.5,41,42 Studies have shown that ocular commensals might modulate the immune response to ocular infections.11,43 Germ-free or antibiotic-treated Swiss Webster mice have a greater P. aeruginosa keratitis score and bacterial load. This effect was mediated through IL-1β and neutrophils.43 It has also been shown that Corynebacterium mastitidis, an ocular commensal isolated from the National Institutes of Health C57BL/6 mouse colony, can modulate Candida and P. aeruginosa keratitis through IL-17 secretion from gamma-delta T cells.11 C57BL/6 mice from The Jackson Laboratory (such as the ones we used in our studies) do not harbor cultivable bacteria in their conjunctival sac.11 In our pilot studies, when we tried culturing the conjunctiva of B6J mice, no bacterial cultures were observed, in agreement with the literature. 11 Therefore, it is possible that the observed effects on the cornea and conjunctiva inflammatory response after LPS are related to intestinal dysbiosis and not ocular dysbiosis in C57BL/6J mice.
We also observed an increased frequency of MHC II+CD11c+ cells and increased CD86 fluorescent intensity in MHC II+CD11c+CD11b+ cells in the eye-draining lymph nodes after 14 days of antibiotics. CD86 is constitutively expressed on several APCs, such as resident dendritic cells, Langerhans and B cells, and macrophages.44 CD86 is upregulated when APCs are activated. Antigen presentation and expression of costimulatory molecules, such as CD80 and CD86, are needed by T cells to proliferate and initiate cytokine production. It is unknown from the literature what is the minimum level of upregulation to be considered biologically relevant. Functional tests such as antigen-presenting assays can be performed to evaluate antigen presentation function after antibiotic-induced dysbiosis in the future, but they are beyond the scope of our study.
LPS stimulation of the ocular surface of dysbiotic mice modulated the gene expression of several inflammatory mediators, including IL-1β, TNF-α, CXCL10, IL-12, and IFN-γ transcripts. Regarding IL-1β, the fold change of LPS stimulation compared to the vehicle-treated samples seems to be largest in the conventional mice, and much less pronounced in the antibiotic mice, while not measurable in germ-free mice. This is in agreement with the literature, in which Kugadas and colleagues43 showed a microbiome dependency of IL-1β in the cornea. In the conjunctiva, a similar pattern was observed (greater response in conventional mice), and decreased response in either antibiotic-treated or germ-free mice, reinforcing the dependence of IL-1β on the microbiota.43
The fold increase in LPS-induced IL-12a mRNA is comparable in antibiotic-treated and germ-free mice in the cornea, while a greater IL-12 and IFN-γ mRNA response was observed in the conjunctiva of germ-free mice. We have shown that there is increased production of IL-12 in MHC II+CD11c+CD11b+ cells and macrophages in conjunctival APCs of germ-free C57BL/6J.12 The increased response of IL-12 in the absence of the microbiome is not restricted to germ-free C57BL/6J mice. CD25 knockout mice, which spontaneously develop a Sjögren syndrome-like disease, have increased frequency of IL-12–producing cells in their lacrimal glands, cervical lymph nodes, and conjunctiva when raised in a germ-free environment.29 This was accompanied by a greater frequency of CD4+IFN-γ+ cells (T helper 1) in the lacrimal glands of both germ-free C57BL/6 and CD25 knockout mice.12,29 Furthermore, IL-12 neutralization for 4 weeks in germ-free CD25 knockout mice decreased the dacryoadenitis score 4 weeks later, demonstrating the relationship between microbiota-dependent activation of APCs and their role in Sjögren syndrome.29 Taken together, our findings and the published literature suggest a modulation of Th-1 polarizing cytokine, IL-12, and IFN-γ-producing CD4+ T cells by the intestinal microbiota.
TNF-α is a pleiotropic cytokine produced by epithelial cells, macrophages, and dendritic cells. Compared to the vehicle, the fold changes of TNF-α transcripts after LPS stimulation are greater in antibiotic-treated mice compared to conventional mice and much smaller in the cornea in germ-free mice, while levels are comparable in the conjunctiva among the three groups. The literature suggests a very reciprocal relationship between TNF-α and the microbiome. It has been shown that TNF-α plasma levels are upregulated in the colitis model induced by trinitrobenzene and in aging, and that both dysbiosis and tissue damage are decreased in TNF-α knockout mice.45–47 Patients with Crohn's disease treated with anti-TNF-α have reported changes in microbiota composition.46,48 Either pharmacological or genetic modulation of TNF-α (or its receptors) improves lacrimal gland inflammation, dry eye signs, and decreases the production of cytokines in animal models of dry eye and Sjögren syndrome.49–52
CXCL10 is a potent chemokine produced by epithelial cells and fibroblasts that attracts Th-1 cells. We and others have shown upregulation of CXCL10 protein levels during desiccation.53–55 Our results showed that CXCL10 mRNA upregulation after LPS stimulation was the greatest after antibiotic treatment in the cornea, while the conventional conjunctiva had higher levels compared to antibiotic treatment, suggesting that the microbiome modulation of CXCL10 expression in the cornea after antibiotic secretion is greater than in the conjunctiva. Germ-free mice showed little upregulation in the cornea, while the increase was highest in all three groups in the conjunctiva. Bacterial ligands and cytokines such as TNF-α upregulate CXCL10 expression in epithelial cells and fibroblasts.56–58 Furthermore, administration of special diets with antioxidants or with VSL#3, a defined probiotic community, decreased the expression of CXCL10 in animal models of inflammatory bowel disease.59–62
Dry eye is a multifactorial disease in which there is production of innate inflammatory mediators by the ocular surface epithelium and resident immune cells, which can precede an adaptive response in which CD4+T cells are primed and activated in the draining nodes that home to the ocular surface and release cytokines that can cause goblet cell loss and corneal barrier disruption.27,63–65 Ocular surface desiccation or osmotic stress has been reported to serve as a stimulus triggering the production of cytokines and chemokines in dry eye.66–68 MyD88−/− or IL-1R−/− mice have been reported to be resistant to desiccation-induced dry eye.32,69,70 We have also shown that LPS stimulation of the ocular surface during experimental dry eye leads to further activation and greater production of cytokines compared to similar stimulation in nonstressed mice.18
Our findings further point to dysbiosis as a disruptor of ocular surface homeostasis after a pathogenic stimulus such as LPS. Further understanding of the mechanism(s) by which the microbiome modulates the ocular surface inflammatory response will pave the way for using the microbiome as potential new therapy for ocular surface diseases. One potential mechanism is through soluble anti-inflammatory mediators, such as short-chain fatty acids (such as butyrate, propionate, acetate) produced by commensal bacteria that have been found to modulate inflammation at remote sites (Hernandez et al. IOVS 2019;60:ARVO E-Abstract 2818).71 Another potential mechanism is the intestinal generation of regulatory T cells after fecal transplant of butyrate-producing bacteria.72 Proper identification of specific species, as well as strains of “good” bacteria will be necessary to advance the therapeutic potential of the microbiome.
Acknowledgments
Supported by National Institutes of Health (NIH) EY026893 (CSDP); Alkek Center for Metagenomics and Microbiome Research (CSDP); NIH EY-002520-36 (Core Grant for Vision Research Department of Ophthalmology); NIH Training Grant T32-AI053831 (FB); Biology of Inflammation Center (CSDP); Research to Prevent Blindness Stein Innovation Award (RAB); Research to Prevent Blindness (Department of Opthalmology), The Oshman Foundation, William Stamps Farish Fund, The Hamill Foundation, The Sid Richardson Foundation, and Baylor Cytometry and Cell Sorting Core (NIH NIAID P30AI036211, NCI P30CA125123, and NCRR S10RR024574). No foreigner funds were used for this project other than salary to CW (Scientific Research Foundation of Traditional Chinese Medicine of Zhejiang Province [2015ZB031] and Medicine and Health Science Technology: Project of Zhejiang Province [2015KYA113]).
Disclosure: C. Wang, None; L. Schaefer, None; F. Bian, None; Z. Yu, None; S.C. Pflugfelder, None; R.A. Britton, None; C.S. de Paiva, None
References
- 1.Guzman M, Sabbione F, Gabelloni ML, et al. Restoring conjunctival tolerance by topical nuclear factor-kappaB inhibitors reduces preservative-facilitated allergic conjunctivitis in mice. Invest Ophthalmol Vis Sci. 2014;55:6116–6126. doi: 10.1167/iovs.14-14075. [DOI] [PubMed] [Google Scholar]
- 2.Graham JE, Moore JE, Jiru X, et al. Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Invest Ophthalmol Vis Sci. 2007;48:5616–5623. doi: 10.1167/iovs.07-0588. [DOI] [PubMed] [Google Scholar]
- 3.Nolan J. Evaluation of conjunctival and nasal bacterial cultures before intra-ocular operations. Br J Ophthalmol. 1967;51:483–485. doi: 10.1136/bjo.51.7.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schabereiter-Gurtner C, Maca S, Rolleke S, et al. 16S rDNA-based identification of bacteria from conjunctival swabs by PCR and DGGE fingerprinting. Invest Ophthalmol Vis Sci. 2001;42:1164–1171. [PubMed] [Google Scholar]
- 5.de Paiva CS, Jones DB, Stern ME, et al. Altered mucosal microbiome diversity and disease severity in Sjogren syndrome. Sci Rep. 2016;6:23561–23571. doi: 10.1038/srep23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 7.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aagaard K, Petrosino J, Keitel W, et al. The Human Microbiome Project strategy for comprehensive sampling of the human microbiome and why it matters. FASEB J. 2013;27:1012–1022. doi: 10.1096/fj.12-220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szymula A, Rosenthal J, Szczerba BM, Bagavant H, Fu SM, Deshmukh US. T cell epitope mimicry between Sjogren's syndrome Antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin Immunol. 2014;152:1–9. doi: 10.1016/j.clim.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St Leger AJ, Desai JV, Drummond RA, et al. An ocular commensal protects against corneal infection by driving an interleukin-17 response from mucosal gammadelta T cells. Immunity. 2017;47:148–158. doi: 10.1016/j.immuni.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Zaheer M, Bian F, et al. Sjogren-like lacrimal keratoconjunctivitis in germ-free mice. Int J Mol Sci. 2018;19:E565. doi: 10.3390/ijms19020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redfern RL, McDermott AM. Toll-like receptors in ocular surface disease. Exp Eye Res. 2010;90:679–687. doi: 10.1016/j.exer.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redfern RL, Reins RY, McDermott AM. Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells. Exp Eye Res. 2011;92:209–220. doi: 10.1016/j.exer.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li DQ, Zhang L, Pflugfelder SC, et al. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways. J Allergy Clin Immunol. 2011;128:1318–1325. doi: 10.1016/j.jaci.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Filippo K, Dudeck A, Hasenberg M, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 17.Wu YY, Hsu CM, Chen PH, Fung CP, Chen LW. Toll-like receptor stimulation induces nondefensin protein expression and reverses antibiotic-induced gut defense impairment. Infect Immun. 2014;82:1994–2005. doi: 10.1128/IAI.01578-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmons KT, Xiao Y, Pflugfelder SC, de Paiva CS. Inflammatory response to lipopolysaccharide on the ocular surface in a murine dry eye model. Invest Ophthalmol Vis Sci. 2016;57:2443–2451. doi: 10.1167/iovs.15-18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao Y, Min K, Zhang Y, Su J, Greenwood M, Gronert K. Female-specific downregulation of tissue polymorphonuclear neutrophils drives impaired regulatory T cell and amplified effector T cell responses in autoimmune dry eye disease. J Immunol. 2015;195:3086–3099. doi: 10.4049/jimmunol.1500610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 21.Hill DA, Siracusa MC, Abt MC, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18:538–546. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao S, Kupfer Y, Pagala M, Chapnick E, Tessler S. Systemic absorption of oral vancomycin in patients with Clostridium difficile infection. Scand J Infect Dis. 2011;43:386–388. doi: 10.3109/00365548.2010.544671. [DOI] [PubMed] [Google Scholar]
- 23.Tascini C, Sbrana F, Flammini S, et al. Oral gentamicin gut decontamination for prevention of KPC-producing Klebsiella pneumoniae infections: relevance of concomitant systemic antibiotic therapy. Antimicrob Agents Chemother. 2014;58:1972–1976. doi: 10.1128/AAC.02283-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auchtung JM, Robinson CD, Britton RA. Cultivation of stable, reproducible microbial communities from different fecal donors using minibioreactor arrays (MBRAs) Microbiome. 2015;3:42. doi: 10.1186/s40168-015-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins J, Auchtung JM, Schaefer L, Eaton KA, Britton RA. Humanized microbiota mice as a model of recurrent Clostridium difficile disease. Microbiome. 2015;3:35. doi: 10.1186/s40168-015-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coursey TG, Gandhi NB, Volpe EA, Pflugfelder SC, de Paiva CS. Chemokine receptors CCR6 and CXCR3 are necessary for CD4(+) T cell mediated ocular surface disease in experimental dry eye disease. PLoS One. 2013;8:e78508. doi: 10.1371/journal.pone.0078508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikitakis N, Papaioannou W, Sakkas LI, Kousvelari E. The autoimmunity-oral microbiome connection. Oral Dis. 2017;23:828–839. doi: 10.1111/odi.12589. [DOI] [PubMed] [Google Scholar]
- 29.Zaheer M, Wang C, Bian F, et al. Protective role of commensal bacteria in Sjogren syndrome. J Autoimmun. 2018;93:45–56. doi: 10.1016/j.jaut.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grasa L, Abecia L, Forcen R, et al. Antibiotic-induced depletion of murine microbiota induces mild inflammation and changes in toll-like receptor patterns and intestinal motility. Microb Ecol. 2015;70:835–848. doi: 10.1007/s00248-015-0613-8. [DOI] [PubMed] [Google Scholar]
- 31.Kigerl KA, Hall JC, Wang L, Mo X, Yu Z, Popovich PG. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med. 2016. pp. 2603–2620. [DOI] [PMC free article] [PubMed]
- 32.Redfern RL, Patel N, Hanlon S, et al. Toll-like receptor expression and activation in mice with experimental dry eye. Invest Ophthalmol Vis Sci. 2013;54:1554–1563. doi: 10.1167/iovs.12-10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chi W, Hua X, Chen X, et al. Mitochondrial DNA oxidation induces imbalanced activity of NLRP3/NLRP6 inflammasomes by activation of caspase-8 and BRCC36 in dry eye. J Autoimmun. 2017;80:65–76. doi: 10.1016/j.jaut.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bian F, Xiao Y, Zaheer M, et al. Inhibition of NLRP3 inflammasome pathway by butyrate improves corneal wound healing in corneal alkali burn. Int J Mol Sci. 2017;18:E562. doi: 10.3390/ijms18030562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asquith M, Stauffer P, Davin S, Mitchell C, Lin MP, Rosenbaum JT. Perturbed mucosal immunity and dysbiosis accompany clinical disease in a rat model of spondyloarthritis. Arthritis Rheumatol. 2016;68:2151–2162. doi: 10.1002/art.39681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho SY, Kim J, Lee JH, et al. Modulation of gut microbiota and delayed immunosenescence as a result of syringaresinol consumption in middle-aged mice. Sci Rep. 2016;6:39026. doi: 10.1038/srep39026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen CH, Krych L, Nielsen DS, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55:2285–2294. doi: 10.1007/s00125-012-2564-7. [DOI] [PubMed] [Google Scholar]
- 38.d'Hennezel E, Abubucker S, Murphy LO, Cullen TW. Total lipopolysaccharide from the human gut microbiome silences toll-like receptor signaling. mSystems. 2017;2:e00046–17. doi: 10.1128/mSystems.00046-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campos AC, Rocha NP, Nicoli JR, Vieira LQ, Teixeira MM, Teixeira AL. Absence of gut microbiota influences lipopolysaccharide-induced behavioral changes in mice. Behav Brain Res. 2016;312:186–194. doi: 10.1016/j.bbr.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 40.Fukuhara D, Irie K, Uchida Y, et al. Impact of commensal flora on periodontal immune response to lipopolysaccharide. J Periodontol. 2018;89:1213–1220. doi: 10.1002/JPER.17-0567. [DOI] [PubMed] [Google Scholar]
- 41.Ozkan J, Willcox MD. The ocular microbiome: molecular characterization of a unique and low microbial environment. Curr Eye Res. 2019;44:685–694. doi: 10.1080/02713683.2019.1570526. [DOI] [PubMed] [Google Scholar]
- 42.Willcox MD. Characterization of the normal microbiota of the ocular surface. Exp Eye Res. 2013;117:99–105. doi: 10.1016/j.exer.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Kugadas A, Christiansen SH, Sankaranarayanan S, et al. Impact of microbiota on resistance to ocular Pseudomonas aeruginosa-induced keratitis. PLoS Pathog. 2016;12:e1005855. doi: 10.1371/journal.ppat.1005855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mir MA. Introduction to costimulation and costimulatory molecules. In: Mir MA, editor. Developing Costimulatory Molecules for Immunotherapy of Diseases. Academic Press; 2015. pp. 1–43. [Google Scholar]
- 45.Bodogai M, O'Connell J, Kim K, et al. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci Transl Med. 2018;10:eaat4271. doi: 10.1126/scitranslmed.aat4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones-Hall YL, Nakatsu CH. The intersection of TNF, IBD and the microbiome. Gut Microbes. 2016;7:58–62. doi: 10.1080/19490976.2015.1121364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thevaranjan N, Puchta A, Schulz C, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21:455–466. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Busquets D, Mas-de-Xaxars T, Lopez-Siles M, et al. Anti-tumour necrosis factor treatment with adalimumab induces changes in the microbiota of Crohn's disease. J Crohns Colitis. 2015;9:899–906. doi: 10.1093/ecco-jcc/jjv119. [DOI] [PubMed] [Google Scholar]
- 49.Choi W, Noh H, Yeo A, et al. The effect of TNF-alpha blocker HL036337 and its best concentration to inhibit dry eye inflammation. Kor J Ophthalmol. 2016;30:302–308. doi: 10.3341/kjo.2016.30.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji YW, Byun YJ, Choi W, et al. Neutralization of ocular surface TNF-alpha reduces ocular surface and lacrimal gland inflammation induced by in vivo dry eye. Invest Ophthalmol Vis Sci. 2013;54:7557–7566. doi: 10.1167/iovs.12-11515. [DOI] [PubMed] [Google Scholar]
- 51.Li Z, Choi W, Oh HJ, Yoon KC. Effectiveness of topical infliximab in a mouse model of experimental dry eye. Cornea. 2012;31(suppl 1):S25–S31. doi: 10.1097/ICO.0b013e31826a80ea. [DOI] [PubMed] [Google Scholar]
- 52.Trousdale MD, Zhu Z, Stevenson D, Schechter JE, Ritter T, Mircheff AK. Expression of TNF inhibitor gene in the lacrimal gland promotes recovery of tear production and tear stability and reduced immunopathology in rabbits with induced autoimmune dacryoadenitis. J Autoimmune Dis. 2005;2:6. doi: 10.1186/1740-2557-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao N, Yin J, Yoon GS, Mi QS, Yu FS. Dendritic cell-epithelium interplay is a determinant factor for corneal epithelial wound repair. Am J Pathol. 2011;179:2243–2253. doi: 10.1016/j.ajpath.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon KC, Park CS, You IC, et al. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010;51:643–650. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoon KC, de Paiva CS, Qi H, et al. Expression of th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 56.Hormannsperger G, Clavel T, Hoffmann M, et al. Posttranslational inhibition of proinflammatory chemokine secretion in intestinal epithelial cells: implications for specific IBD indications. J Clin Gastroenterol. 2010;44(suppl 1):S10–S15. doi: 10.1097/MCG.0b013e3181e102c1. [DOI] [PubMed] [Google Scholar]
- 57.Kimura K, Orita T, Nomi N, Fujitsu Y, Nishida T, Sonoda KH. Identification of common secreted factors in human corneal fibroblasts exposed to LPS, poly(I:C), or zymosan. Exp Eye Res. 2012;96:157–162. doi: 10.1016/j.exer.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 58.McInnis KA, Britain A, Lausch RN, Oakes JE. Synthesis of α-chemokines IP-10, I-TAC, and MIG are differentially regulated in human corneal keratocytes. Invest Ophthalmol Vis Sci. 2005;46:1668–1674. doi: 10.1167/iovs.04-1010. [DOI] [PubMed] [Google Scholar]
- 59.Toki S, Kagaya S, Shinohara M, et al. Lactobacillus rhamnosus GG and Lactobacillus casei suppress Escherichia coli-induced chemokine expression in intestinal epithelial cells. Int Arch Allergy Immunol. 2009;148:45–58. doi: 10.1159/000151505. [DOI] [PubMed] [Google Scholar]
- 60.Espley RV, Butts CA, Laing WA, et al. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J Nutr. 2014;144:146–154. doi: 10.3945/jn.113.182659. [DOI] [PubMed] [Google Scholar]
- 61.Mariman R, Tielen F, Koning F, Nagelkerken L. The probiotic mixture VSL#3 has differential effects on intestinal immune parameters in healthy female BALB/c and C57BL/6 mice. J Nutr. 2015;145:1354–1361. doi: 10.3945/jn.114.199729. [DOI] [PubMed] [Google Scholar]
- 62.Cremonesi E, Governa V, Garzon JFG, et al. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut. 2018;67:1984–1994. doi: 10.1136/gutjnl-2016-313498. [DOI] [PubMed] [Google Scholar]
- 63.de Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 64.de Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47:2847–2856. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- 65.Chauhan SK, El AJ, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 67.Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens. 2005;31:186–193. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- 68.Xiao Y, de Paiva CS, Yu Z, de Souza RG, Li DQ, Pflugfelder SC. Goblet cell-produced retinoic acid suppresses CD86 expression and IL-12 production in bone marrow-derived cells. Int Immunol. 2018;30:457–470. doi: 10.1093/intimm/dxy045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narayanan S, Corrales RM, Farley WJ, McDermott AM, Pflugfelder SC. Interleukin-1 receptor-1–deficient mice show attenuated production of ocular surface inflammatory cytokines in experimental dry eye. Cornea. 2008;27:811–817. doi: 10.1097/ICO.0b013e31816bf46c. [DOI] [PubMed] [Google Scholar]
- 70.Reins RY, Lema C, Courson J, Kunnen CME, Redfern RL. MyD88 deficiency protects against dry eye-induced damage. Invest Ophthalmol Vis Sci. 2018;59:2967–2976. doi: 10.1167/iovs.17-23397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Biragyn A, Ferrucci L. Gut dysbiosis: a potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 2018;19:e295–e304. doi: 10.1016/S1470-2045(18)30095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]