Abstract
We hypothesized that HIV-1 with dual-class but not single-class drug resistance mutations linked on the same viral genome, present in the virus population before initiation of antiretroviral therapy (ART), would be associated with failure of ART to suppress viremia. To test this hypothesis, we utilized an ultrasensitive single-genome sequencing assay that detects rare HIV-1 variants with linked drug resistance mutations (DRMs). A case (ART failure) control (nonfailure) study was designed to assess whether linkage of DRMs in pre-ART plasma samples was associated with treatment outcome in the nevirapine/tenofovir/emtricitabine arm of the AIDS Clinical Trials Group A5208/Optimal Combined Therapy After Nevirapine Exposure (OCTANE) Trial 1 among women who had received prior single-dose nevirapine. Ultrasensitive single-genome sequencing revealed a significant association between pre-ART HIV variants with DRMs to 2 drug classes linked on the same genome (dual class) and failure of combination ART with 3 drugs to suppress viremia. In contrast, linked, single-class DRMs were not associated with ART failure. We conclude that linked dual-class DRMs present before the initiation of ART are associated with ART failure, whereas linked single-class DRMs are not.
Keywords: AIDS/HIV
Keywords: Drug therapy
HIV-1 dual-class drug resistance mutations linked on the same genome, present prior to initiation of antiviral therapy, are associated with treatment failure.
Introduction
Despite the remarkable success of combination antiretroviral therapy (ART) in preventing morbidity and mortality from HIV-1 infection, the development of HIV-1 resistance to antiretroviral (ARV) drugs remains a threat to its continued effectiveness. Consequently, drug resistance testing is recommended as part of the standard of care in settings where resources are sufficient (1, 2). Recently, more sensitive technologies have been developed that can detect subpopulations of drug-resistant variants at frequencies of 1% or less in clinical samples, but the clinical significance of such low-frequency variants is controversial. Some analyses have shown that low-frequency drug resistance mutations (DRMs), especially those conferring resistance to non-nucleoside reverse transcriptase inhibitors (NNRTIs), are associated with virological failure after initiating combination ART–containing NNRTIs (3–11). In contrast, other studies have found no association with treatment outcomes when low-frequency resistance mutations are detected that confer resistance to an ARV drug used in the regimen (12–29). These conflicting results make interpretation of resistance results from newer, more sensitive resistance assays difficult (30).
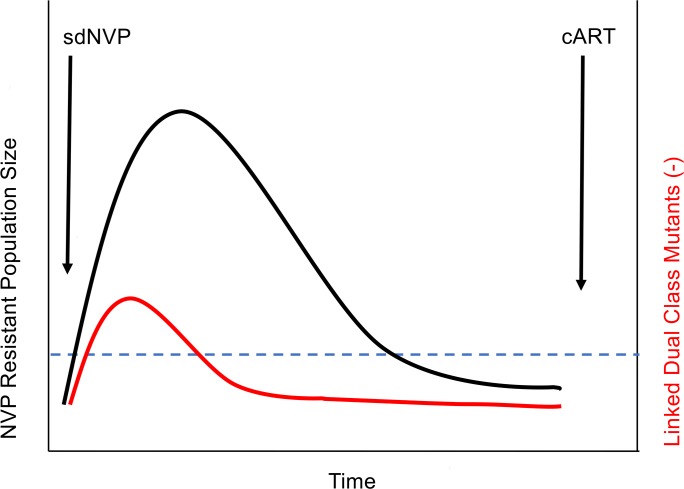
We hypothesized that the context in which low-frequency drug-resistant variants exist can help explain their variable impact on treatment outcome. Specifically, prior exposure to a single partially suppressive ARV, such as single-dose nevirapine (sdNVP), is known to select for outgrowth of NVP-resistant variants within the virus population (31, 32). We and others have shown an association between NVP-resistant variants arising from prior sdNVP and subsequent virological failure of initial NVP-based ART (16, 33, 34). In such a scenario, the expanded population of resistant variants would provide a genetic background on which additional mutations could occur stochastically, leading to dual-class resistance at the time of ART initiation. If such combinations of DRMs on the same viral genomes confer resistance to more than 1 drug in an ART regimen, the risk of ART failure would be increased (illustrated in Figure 1). In contrast, if low-frequency resistant variants arise stochastically (under no selection pressure), they would pose little to no risk of ART failure because the chance of DRMs to 2 drug classes becoming linked on the same genome would be much lower. An appropriate context to test our hypothesis about the association between the type of low-frequency drug-resistant variants and treatment outcome is the AIDS Clinical Trials Group (ACTG) 5208/Optimal Combined Therapy After Nevirapine Exposure (OCTANE) Trial 1 (35, 36), which assessed the response to NVP-containing ART (nevirapine/tenofovir/emtricitabine [NVP/TFV/FTC]) among women who had prior exposure to sdNVP 6–24 months before study entry and initiation of ART (ClinicalTrials.gov NCT00089505) (13, 33).
Figure 1. Hypothetical model explaining selection of linked mutations among women exposed to single-dose nevirapine.
The large replicating population of resistant variants selected by sdNVP is depicted in black. Linkage of dual-class resistance mutations, occurring at that time either stochastically or through recombination, is shown in red. The blue dashed line is the limit of detection of standard single-genome sequencing. cART, combination ART.
To test our hypotheses, we used a method called ultrasensitive single-genome sequencing (uSGS) that detects not only rare DRMs but also linkage of mutations on the same viral genome (37). The uSGS method uniquely tags each cDNA molecule and subsequently removes mutation and recombination artifacts arising from PCR and sequencing (33). Using this approach, the frequencies of linked and unlinked HIV-1 DRMs conferring resistance to the NVP/TFV/FTC regimen were determined in plasma samples obtained at study entry (following previous sdNVP exposure but before ART initiation) from women enrolled in the AIDS Clinical Trials Group 5208/OCTANE Trial 1 to assess their association with treatment outcome.
Results
Participants studied.
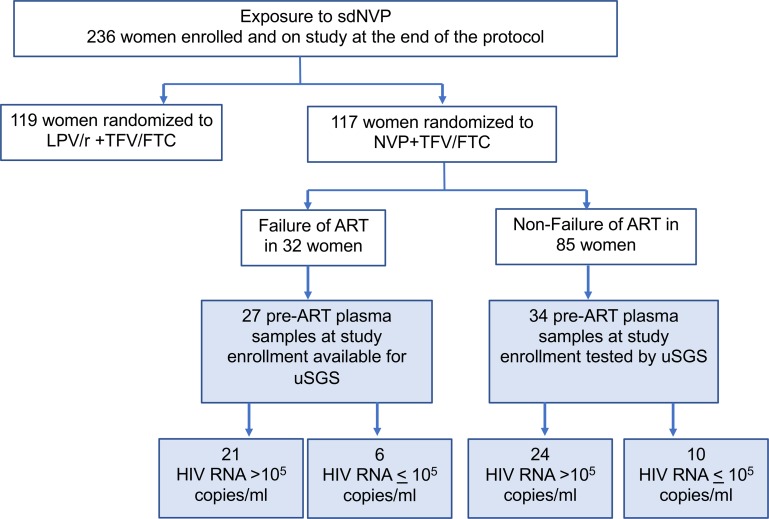
Our study encompassed stored pre-ART plasma samples obtained from AIDS Clinical Trials Group 5208 Trial 1, which included only women with prior exposure to sdNVP for prevention of mother-to-child transmission of HIV-1. In the NVP/TFV/FTC treatment arm (36), 32 women experienced ART failure, with failure defined as less than 1.0 log10 drop in plasma HIV-1 RNA copies/mL from baseline by week 12 or confirmed plasma HIV-1 RNA greater than 400 copies/mL at or after week 24. Pre-ART plasma samples from study entry were available from 27 of the 32 ART failure cases for uSGS testing (Figure 2). Of these samples, 21 (88%) had plasma HIV-1 RNA greater than 100,000 copies/mL. For comparison, pre-ART plasma samples from 34 women who did not experience ART failure (ART nonfailure controls), 24 (71%) of whom had plasma HIV-1 RNA greater than 100,000 copies/mL, were analyzed by uSGS (Figure 2). In total, we analyzed pre-ART plasma samples from 61 women from Trial 1.
Figure 2. CONSORT diagram and sample selection from the NVP arm of A5208/OCTANE Trial 1 in women with prior sdNVP exposure.
The blue-shaded boxes show plasma samples obtained from women at study entry randomized to the NVP/TFV/FTC arm and who were selected for analysis by ultrasensitive single-genome sequencing (uSGS); n = 61, 27 ART failures and 34 non-ART failures. Samples were selected based on availability and plasma HIV RNA above or below 100,000 copies/mL. Samples from a given participant with plasma HIV RNA above 100,000 copies/mL were selected when available to increase the depth of representation of the viral population.
Tabulation of uSGS results.
A total of 755,155 ultrasensitive single-genome sequences were obtained from the 61 pre-ART plasma samples. Overall, a median of 4531 ultrasensitive single-genome sequences (range 46–57,278) were obtained from each sample. The median number of ultrasensitive single-genome sequences obtained from participants who experienced ART failure was 6165 (46–57,278; first quartile [Q1], Q3: 3014, 18,350), not significantly different (Mann-Whitney P = 0.52) from the median number of ultrasensitive single-genome sequences obtained from nonfailure controls (4071; range 157–46,163; Q1, Q3: 2093, 13,977), indicating no significant sampling bias (Table 1).
Table 1. Tabulation of ultrasensitive single-genome sequences from pre-ART plasma samples from 61 participants of AIDS Clinical Trials Group A5208/OCTANE receiving sdNVP.
Frequency of DRMs.
All major NVP resistance mutations (100I, 101E, 103NS, 106AM, 181CIV, 188LCH, and 190AE) and major TFV and FTC resistance mutations (65R, 184IV) were assessed. Fifty-eight of the 61 pre-ART samples tested (95%) had 1 or more mutations conferring resistance to at least 1 of the drugs in the ART regimen (NVP/FTC/TFV) (Table 2). Three samples had no DRMs detected, 1 in the ART failure group and 2 in the nonfailure group, most likely because of the low number of sequences obtained (262, 157, and 358 ultrasensitive single-genome sequences, respectively). We compared the cumulative frequencies of resistance mutations to NVP, FTC, and TFV detected at study entry by treatment outcome (Table 2). NVP resistance mutations were detected pretreatment in 93% of women with treatment failure and 94% of nonfailures. Pretreatment FTC resistance mutations (largely M184I) were also evenly distributed between the 2 outcome groups: 21 of 27 (78%) in the treatment failure group and 25 of 34 (74%) in the nonfailure group. TFV resistance mutations were detected somewhat more frequently in the treatment failure group (14 of 27; 52%) than in the nonfailure group (13 of 34; 38%) but not significantly so (P = 0.38, Table 2). Overall, there were no significant differences in the proportions of women with NVP, FTC, and TFV resistance mutations or the frequencies of the resistant mutants between the 2 groups (P = 0.41, Table 2). In addition, the frequency of NVP resistance was not associated with failure in either group by logistic regression; P = 0.56; OR = 2.1 (95% CI: 0.16–27.82). The drug-resistant mutant “viral load” (mutant plasma HIV-1 RNA copies/mL) between those participants who experienced ART failure (median 1275 copies/mL) and those who did not (median 969 copies/mL) were similar, suggesting that neither the raw frequencies of drug-resistant mutants nor the mutant “viral load” explained the difference in treatment outcome (ART failure vs. nonfailure).
Table 2. Frequency of pretreatment resistance mutations detected categorized by treatment outcome.
Linkage of DRMs on the same viral genome.
Next, we analyzed the uSGS data from the pre-ART samples for the presence of linked DRMs (Figure 3 and Table 3). We identified linked DRMs in samples from 15 (26%) of the 58 women with mutations identified. Ten of the 15 samples had linkage of dual-class DRMs (linkage of an NVP resistance mutation with an FTC or TFV resistance mutation on the same genome), and 5 other samples had linkage of single-class DRMs, i.e., 2 or more NVP resistance mutations on the same genome. None of the 43 remaining samples had linked mutations.
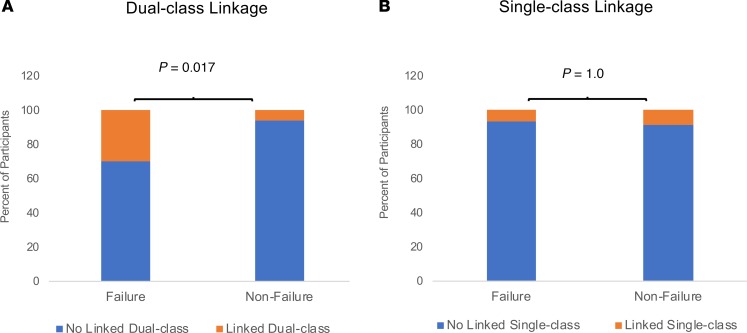
Figure 3. Association of dual-class but not single-class linked drug resistance mutations with treatment failure.
(A) Shown are the proportions of participants with linked dual-class resistant mutants in pretherapy plasma samples according to treatment outcome: 8/27 failure, 2/34 nonfailure. Linked dual-class resistance mutations were significantly associated with treatment failure: OR 6.7 (95% CI: 1.3–35.1); P = 0.013 (logistic regression). (B) The proportions of participants with linked single-class resistant mutants in pretherapy plasma samples according to treatment outcome were 2/27 failure and 3/34 nonfailure OR = 0.83 (95% CI: 0.13–5.3); P = 0.841 (logistic regression).
Table 3. Linked dual- and single-class resistance mutations detected in pre-ART samples from 8 donors with ART failure.
The presence before ART of linked dual-class DRMs was significantly associated with ART failure: 8 of 27 (30%) ART failures had pre-ART linked dual-class DRMs vs. 2 of 34 (6%) nonfailures (OR 6.7 [95% CI: 1.3–35.1]; P = 0.013) (Figure 3A). The association of dual-class linked mutations with failure remained significant after adjustment for the number of single genomes obtained per sample (P = 0.03) (Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.130118DS1), arguing against an influence of detection bias in the association. In addition, analyses of the ART failure and nonfailure groups by stratifying NVP DRM frequency into quartiles revealed that dual-class linked mutations were not associated with NVP DRM frequency (Supplemental Figure 1A), supporting the data in Table 2. Furthermore, the proportion failing treatment stratified by quartile of NVP DRM frequency was higher with than without dual-class linked mutations, indicating that dual-class resistance was associated with a higher risk of treatment failure independent of NVP DRM frequency (Supplemental Figure 1B).
Linked single-class DRMs were not significantly associated with treatment outcome: 2 of 27 (7%) ART failures had linked single-class DRMs vs. 3 of 34 (9%) nonfailures (OR = 0.83 [95% CI: 0.13—5.3]; P = 0.84) (Figure 3B). Linked single-class DRMs always consisted of 2 NVP resistance mutations, most frequently the 190A or 190E mutation linked to 103N or 101E. Less frequently, mutations associated with codon 103 were linked to mutants associated with codons 101 and 181 (Table 3). The K65R TFV resistance mutation was never found linked to 184I/V FTC resistance mutations (Table 3). In addition, 3 DRMs from 1 or 2 drug classes were never found on the same viral genome (Table 3).
Of the 43 pretreatment samples in which no linkage of DRMs was observed, 31 (72%) had DRMs to both NVP and FTC or NVP and TFV in the population, 10 in the ART failure group and 21 in the nonfailure outcome group. There was no association of unlinked mutations with ART failure (P = 0.649, calculated by χ2 test with Yates’s correction). To further investigate whether ART failure was associated with the pre-ART presence of unlinked dual-class DRMs, we analyzed all pre-ART samples for the presence of mutations to 2 drug classes in different sequences (i.e., unlinked mutations). In the ART failure group, there were 20/27 (74%) samples with unlinked mutations to 2 drug classes, and 25/34 (74%) in the nonfailure group (P = 1.0), indicating no association of unlinked dual-class resistance with treatment failure.
Validation of rare frequency of DRMs linked on the same viral genome.
The frequencies within the virus populations in pre-ART plasma samples of both single- and dual-class linked DRMs were extremely low, averaging just 0.01%, with a median of 0.007%, and ranging from 0.002% to 0.04% (Table 3). Although our bioinformatics pipeline was designed to ensure that technical artifacts were eliminated from the data set as reported previously (37), there remains a measurable assay error rate, attributable to the reverse transcription step. Because participants had prior exposure to sdNVP, resulting in high frequencies of resistant variants to this drug, we investigated whether background errors in the uSGS technique could have resulted in spurious detection of dual-class resistant sequences. We addressed this possibility by comparing the number of linked resistance mutations expected as the result of assay error on a template already containing an NVP resistance mutation to the observed number of linked mutations. In addition, because 184I was the most common mutation found in genomes with linked dual-class DRM genomes and is a very common RT error in vitro and in vivo, we determined the assay error rate to be 1.33 × 10–4 at this position (ATG to ATA) from the experiment shown in Table 3 of our previous study (37). This error rate was used to estimate the number of linked resistance mutations expected as the result of an assay error on a template already containing an NNRTI resistance mutation (Supplemental Table 2). The number of linked dual-class DRMs observed in the ART failure group was significantly different than expected (P < 0.000001; binomial test), indicating that the linked dual-class mutants detected were highly unlikely to have arisen from assay error (Supplemental Table 2).
Association between DRMs in pre-ART and DRMs at treatment failure.
Of the 8 participants experiencing treatment failure who had linked DRMs, 7 also had plasma population genotypes available at the time of ART failure (Table 3). Five of 7 (71%) (Table 3) had virus at treatment failure with 1 or 2 of the DRMs that were found to be linked in the pre-ART plasma. In the other 2 donors, the linked dual-class DRMs detected pretherapy were the dominant virus detected by population sequencing at treatment failure.
Donor T1F03 (Table 3) was one of a few participants who had plasma samples from both pre-ART and treatment failure available for uSGS. Plasma from this donor sampled at the time of failure was used to validate the bioinformatics linkage pipeline in an ART failure clinical sample before the beginning of the present study. uSGS revealed many genomes containing linked dual-class DRMs in a total of 2801 single-genome sequences, 130 of which were 181C/184I at the time of failure (Supplemental Table 3). Of note, pre-ART plasma uSGS detected 2 genomes with linked dual-class 65R/181C DRMs and 1 genome with 184I/103N (Table 3). These findings make a strong case for the occurrence of in vivo recombination between genomes found before ART (65R/181C and 184I/103N) resulting in 4.6% of the genomes at ART failure having 181C/184I mutations. However, the majority of the sequences at failure (2599 genomes) contained 181C/184V, indicating that once the M184V mutant appears, whether by mutation or recombination, it has a strong selective advantage over variants with M184I (38). This example illustrates continued evolution and recombination of virus populations with ART-imposed selection. This finding may help explain why there were inconsistent matches (2 of 8) between the linked dual-class resistant variants at pretherapy and the dominant virus population at treatment failure.
Discussion
Using powerful new uSGS technology, we have shown that, among women with prior exposure to a single partially suppressive ARV (sdNVP) to prevent mother-to-child HIV transmission, pretreatment linked dual-class resistant variants can be detected at frequencies as low as 0.002% of viral genomes. Detection of such rare linked mutations has not been reported previously. Indeed, such rare linked mutations could not have been detected using previously available next-generation sequencing (NGS) technology because of technological limitations, particularly high background error and PCR recombination rates. Importantly, we found that dual–drug class HIV-1 resistance mutations linked on the same viral genome were significantly associated with higher risk of failure of combination ART containing NVP (P = 0.013). In contrast, we found that 2 single-class NNTRI resistance mutations linked on the same genome or unlinked mutations were not associated with ART failure.
These findings provide proof of concept that the risk of ART failure is higher in individuals with pretreatment dual-class resistance mutations linked on the same viral genome, even if the dual mutants comprise only a very small fraction of the virus population. It is universally accepted that therapy with a single antiretroviral drug can rapidly fail because of the outgrowth of drug-resistant HIV-1 variants that exist in the virus population before therapy is started (39–41). Consequently, combination ART with 2 or 3 ARVs is needed to prevent the breakthrough of preexisting resistant variants (40). In the context of combination ART, variants resistant to a single drug are unlikely to result in treatment failure. However, a single variant that is resistant to 2 drugs in the 3-drug regimen is more likely to result in treatment failure, as was shown in the current study. These observations support the argument that these dual-class drug-resistant variants continue to replicate after ART initiation and evolve through many cycles of mutation and recombination, resulting in selection of the fittest variants for the conditions present at treatment failure.
We previously hypothesized that it is the context or clinical history in which these low-frequency DRMs are present that influences their effect on treatment outcomes (13). For example, as illustrated in Figure 1, when the replicating population size of a drug-resistant variant is large because of selection by prior drug treatment or by transmission of a resistant variant, there is a greater chance of additional mutations to occur stochastically, becoming linked on the same viral genome. We validated this concept here by finding linked DRMs conferring resistance to either single- or dual-class inhibitors in women in Trial 1 who had previously been exposed to sdNVP (i.e., positive selection for resistance); however, a limitation of this work is that sufficient numbers of comparable samples from women who had not received sdNVP were not available for testing. Validation of our hypothesis is also needed in an ARV drug–naive population in which low-frequency drug-resistant mutants likely arise but are also lost over time to stochastic processes. Results from the present study, however, imply that analysis of extremely large numbers of viral sequences, probably requiring impractically large blood volumes, would be required for such a study. Another limitation is that the study population is relatively small; thus, the CI for the OR for risk of treatment failure is broad. Finally, our results explain only a subset (30%) of virological failures. This result was not unexpected because it is likely that some participants had levels of linked DRMs below what we could detect, which nonetheless led to failure as well. In addition, other common causes of treatment failure, including medication nonadherence or suboptimal drug pharmacokinetics, could have contributed to an unfavorable outcome. Nevertheless, a significant association of treatment failure with linked dual-class mutations was revealed. In contrast, variants with linked single-class resistance to NVP were not associated with failure of an NVP-containing regimen likely because these variants would remain susceptible to both TFV and FTC, the 2 other components of the regimen. It is not clear from our study why some women had detectable dual-class linked mutations while others exhibited only single-class linked mutations or unlinked mutations. Possibilities include variation in the size or persistence of the replicating NVP-resistant virus population as a consequence of differences in NVP pharmacokinetics across women (31, 32, 42) or stochastic variation related to the occurrence of point mutations conferring resistance to TFV or FTC on the same genome that already encodes a NVP resistance mutation.
One or more of all the major NNRTI resistance mutations (100I, 101E, 103NS, 106AM, 181CIV, 188LCH, and 190AE) were identified at low frequency in pre-ART samples by uSGS in more than 93% of the women tested (all of whom previously took sdNVP). Similarly, and consistent with other studies (15), in about 70% of participants, uSGS revealed low-frequency variants having a single major nucleoside/nucleotide analog DRM (65R, 184IV) in pre-ART plasma samples. These findings are consistent with evolutionary theory that DRMs accumulate to a low level in replicating virus populations before the initiation of ART (39), thus making resistance ever-present despite counterselective pressure. The current study confirms that if many single genomes are analyzed, low-frequency unlinked DRMs can be found in most ARV-naive persons and are most often not associated with virological failure.
These new insights into the existence of rare linked and unlinked DRMs in ART-experienced individuals and their impact on treatment outcome have been enabled by uSGS technology that uses primer IDs to tag individual viral templates and a stringent bioinformatics pipeline that removes sequencing errors and PCR-related artifacts, including contaminants, recombinants, and template resampling (37). However, our study was limited to resistance to NVP-containing ART, which has since been replaced by more convenient, safer, and more effective ART regimens; the specific associations observed in the current study may not apply to newer combinations of ARVs that may require more than 1 mutation to confer resistance to each drug. Nevertheless, the principles derived from the current study are likely to apply to all regimens, including the presence of rare drug-resistant variants to most ARVs before therapy is started and the importance of such preexisting linked dual- or multi-class drug resistance to failure of combination ART. They also reinforce the principle that treatment history, along with resistance testing, is essential to guide the choice of ARV regimen. Of the 27 participants who failed ART in this study, only 5 had preexisting NVP resistance at levels (approximately more than 10% of genomes) that would have been detectable by standard clinical resistance assays.
Overall, our study shows that linked dual-class DRMs are significantly associated with ART failure, whereas low-frequency unlinked resistance to NVP or a single NRTI alone is not, helping explain why studies that detect resistance to a single ARV can draw different conclusions about the association between low-frequency drug-resistant variants and ART failure. We propose that it is not single low-frequency drug-resistant variants that are clinically relevant, but rather, rare dual-class resistant variants that predispose to ART failure.
Methods
Study design and sample testing.
The AIDS Clinical Trials Group A5208/OCTANE trials have been reported in detail by Lockman et al. (35, 36). In brief, A5208 enrolled African women who self-reported having received sdNVP during labor for prevention of mother-to-child transmission, 73% of whom had written documentation of sdNVP receipt (Figure 2 and ref. 33). Baseline plasma samples were collected, and the women were randomized to receive cART of either NVP/TFV/FTC or lopinavir/ritonavir/TFV/FTC.
The current study aimed to compare the number of single-genome sequences with linked single-and dual-class DRMs in entry plasma samples from the NVP/TFV/FTC treatment arm, including samples from women who did (cases) and did not meet protocol definitions of ART failure (controls). All the available pre-ART samples were tested from participants with ART failure. In addition, a similar number of pre-ART samples were tested from participants who did not experience ART failure. All samples were selected based on availability and plasma HIV-1 RNA no more than or more than 100,000 copies/mL, the latter to ensure deep representation of variants.
uSGS testing.
Viral RNA was extracted from baseline plasma samples, and cDNA libraries were generated with primer IDs for next-generation uSGS as previously described (37). Overall, the efficiency of cDNA synthesis ranges between 20% and 30% of the viral population. The proportions of samples with plasma HIV RNA more than 100,000 copies/mL were balanced between the failure and nonfailure groups for this study.
In brief, the primer IDs uniquely tag each cDNA molecule, which are then amplified with primers containing deoxyuridine residues. These residues are subsequently removed, leaving long, single-stranded overhangs available for efficient directional ligation of Illumina adapter sequences and library construction. Libraries were sequenced using paired-end MiSeq NGS technology, and raw sequencing reads were processed through an analytical pipeline where sequence reads from samples were separated according to their indexes and binned by common primer IDs (37). A bioinformatics pipeline was designed to ensure that technical artifacts were eliminated from the data set. A single genome sequence had to be derived from a set of common primer IDs that satisfied the “supermajorities rule” of greater than 80% consensus at each nucleotide position to be included in the data set. After generating the supermajority sequences from the alignments of reads sharing common primer IDs and applying the consensus cutoff model, the PCR/sequencing error rates were reduced to about 10–4, an error rate comparable to that of the reverse transcriptase enzyme step (37). This stringent bioinformatics program eliminated all sequences that had undergone PCR recombination, sequence or PCR errors, and where the primer IDs had been mutated (43). Hundreds to thousands of unique single-genome sequences covering amino acids 60–200 in the reverse transcriptase gene were generated from each sample, allowing for the detection of rare variants and characterization of the population genetics of the parent HIV-1 RNA in plasma.
Data analysis.
FASTQ files containing raw reads were exported for bioinformatics analyses. Paired-end reads were concatenated, and low-quality reads were removed using a program available at http://hannonlab.cshl.edu/fastx_toolkit with parameters set to base call qualities of at least 90% at Q20 or above (Q20–P90) and default for all other settings. The filtered FASTQ sequences were then converted to FASTA format, and the FASTA reads were sorted by indexes using programs available on the same website. Qualified primer IDs were identified and sorted as described previously (43). Reads with identical primer IDs were aligned and the percentage of consensus determined at each nucleotide. Individual primer ID sequence alignments with at least 80% identity at each nucleotide were included for subsequent analyses. The individual genomes were then analyzed for frequencies of the most common clinically significant NNRTI- and NRTI-resistant mutations to 1 or more of the inhibitors in the treatment arm of NVP/TFV/FTC, as determined by the Stanford HIV Drug Resistance Database (https://hivdb.stanford.edu/). The mutations included 65R, 100I, 101PE, 103NS, 106AM, 181CIV, 184IV, 188LCH, and 190ASE. Genomes containing more than 1 of these mutations were categorized as linked. Allele frequencies of linked and unlinked mutations were calculated across all consensus sequences generated from each plasma sample. Perl scripts used in the above analyses are available at the GitHub code repository at https://github.com/ShaoFred/MiSeq_consensus_builder.git The sequence data set was subjected to a bioinformatics test to determine cross contamination between patients where each sequence from a sample was compared to sequences from all other samples. All sequences are available on the National Cancer Institute HIV Dynamics and Replication Program’s website found at https://home.ncifcrf.gov/hivdrp/resources.html
Statistics.
To check for bias in the sampling, the Mann-Whitney U test was used to determine whether there were significant differences among median and interquartile numbers of ultrasensitive single-genome sequences obtained for each sample when comparing outcome groups. This test generated the P values for differences in median frequencies in each resistance category. In addition, logistic regression was used to investigate whether the frequency of NVP resistance was associated with failure in either group. Logistic regression tests were also used to compute P values when comparing associations between linkage of DRMs and failure as well as when adjusting for the number of genomes per sample in each outcome group. All summary statistics were performed using R statistical language, and P values of less than 0.05 were considered significant. When computing associations of unlinked mutations with ART failure, χ2 test was used. A binomial test was used to compare the observed frequency of linked dual-class DRMs to that expected from the measured assay error (ATG to ATA at reverse transcriptase codon 184).
Study approval.
All participants provided written informed consent to participate in the study. Testing of samples by uSGS was approved by the National Institutes of Health (NIH) Office of Human Subjects Protection (Clinical Trials Registration NCT00089505).
Author contributions
VFB conducted the experiments, acquired and analyzed the data, and wrote the manuscript. MJB, BL, and MDH calculated the statistics. WS and MJB analyzed the data, and EKH provided the reagents and conducted the population genotyping. JAM, RTS, SL, JSC, FS, and EH designed the A5208 OCTANE Trial research studies. MDH analyzed the data and wrote the manuscript. MFK and JMC contributed to the study design and wrote the manuscript. JWM designed the research studies and wrote the manuscript.
Supplementary Material
Acknowledgments
We thank the women who participated in the study because without their consent there would be no advancement of knowledge. We also thank the following members of the A5208 ACTG study team: Beth Zwickl, Cissy Kityo Mutuluuza, Christine Kaseba, Charles C. Maponga, Heather Watts, Daniel Kuritzkes, Thomas B. Campbell, Lynn Kidd-Freeman, Monica Carten, Jane Hitti, Mary Marovich, Peter N. Mugyenyi, Sandra Rwambuya, Ian M. Sanne, Beverly Putnam, Cheryl Marcus, Carolyn Wester, Robin DiFrancesco, Elias Halvas, Annie Beddison, Sandra Lehrman, Francesca Aweeka, Betty Dong, Peter Ndhleni Ziba, Michael S. Saag, William C. Holmes, Scott M. Hammer, and Robert T. Schooley.
This study would not have been possible without the 10 study sites in Africa and key personnel that implemented the study: Elizabeth Dangaiso, University of Zimbabwe-Parirenyatwa, Harare, Zimbabwe, CRS (Site 30313) Clinical Trial Unit (CTU) grant U01AI069436; Mohammed S. Rassool and Josephine Tsotsotetsi, WITS HIV Research Group, Johannesburg, South Africa (Site 11101), CTU grant U01 AI69463-03; KMRI/Walter Reed Project Clinical Research Center, Kericho, Kenya (Site 12501) CTU grant IAAY1AI8374; Charity Potani and Regina Mwausegha, UNC Project, Kamuzu Central Hospital, Lilongwe, Malawi (Site 12001), CTU grant 5 U01 AI069518; Fatima Laher and Reinet Hen-Boisen, Soweto, South Africa, ACTG CRS (Site 12301) CTU grant AI69453; Kipruto Kirwa and Agnes Nzioka, Moi University, Eldoret, Kenya, CRS (Site 12601) contract AACTG. 50.5208.07, the United States Military HIV Research Program; Margaret Chibowa and Jeffrey Stringer, Centre for Infectious Disease Research, Kalingalinga, Lusaka, Zambia (Site 12801), CTU grants 5U01AI069455-03 and 3U01AI32775-13S5; Kagiso Sebina, Kinuthia Mburu, and Tebogo Kakhu (Gaborone Unit), Banno Moorad (Molepolole Unit), Botswana (Site 12701), CTU grant 5U01AI069456-03; Cissy Kityo and Sandra Rwambuya, JCRC, Kampala, Uganda (Site 12401), CTU grant AI-069501; and Farida Amod, Umesh Lalloo, and Sandy Pillay, University of Natal, Durban, South Africa (Site 11201), CTU grant AI69426.
We thank Jason Rausch, Ann Wiegand, Andrew Musick, Sean Patro, William McManus, and Jon Spindler for helpful discussions and Sue Toms, Connie Kinna, and Valerie Turnquist for administrative support. We thank Allan Kane and Joe Meyer for help with the figures.
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the NIH under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701. Funding for this research was also provided by the National Cancer Institute’s Intramural Center for Cancer Research, which supports the HIV Dynamics and Replication Program, and Bench to Bedside grant B11-A-14 Kearney (principal investigator). JMC was a research professor of the American Cancer Society and was supported by contract I3XS110 through Leidos Biomedical Research, Inc. JWM receives support from Leidos Biomedical Research, Inc. (contract 12XS547) through the National Cancer Institute and from the National Institute of Allergy and Infectious Diseases of the NIH to the AIDS Clinical Trials Group under award numbers UM1AI068636 and UM1AI106701. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Version 1. 09/05/2019
In-Press Preview
Version 2. 10/03/2019
Electronic publication
Footnotes
Conflict of interest: RTS reports a consultant relationship with CytoDyn and Monogram Biosciences, as well as grant funds (to institution) from Gilead Sciences. JWM reports research grants from the NIH, Gilead Sciences, and Janssen Pharmaceuticals; personal fees from University of Pittsburgh, Gilead Sciences, Janssen Pharmaceuticals, Merck, AccelevirDx, and Xi’an Yufan Biotechnologies; and share options from Cocrystal Pharma, Inc.
Copyright: © 2019, American Society for Clinical Investigation.
Reference information: JCI Insight. 2019;4(19):e130118.https://doi.org/10.1172/jci.insight.130118.
Contributor Information
Valerie F. Boltz, Email: boltzv@mail.nih.gov.
Wei Shao, Email: wei.shao@nih.gov.
Michael J. Bale, Email: Michael.Bale@nih.gov.
Elias K. Halvas, Email: ekh2@pitt.edu.
Brian Luke, Email: Brian.luke@nih.gov.
James A. McIntyre, Email: mcintyre@pixie.co.za.
Robert T. Schooley, Email: rschooley@ucsd.edu.
Shahin Lockman, Email: shahin.lockman@gmail.com.
Judith S. Currier, Email: jscurrier@mednet.ucla.edu.
Fred Sawe, Email: Fredrick.Sawe@usamru-k.org.
Evelyn Hogg, Email: ehogg@s-3.com.
Michael D. Hughes, Email: mhughes@sdac.harvard.edu.
Mary F. Kearney, Email: kearneym@mail.nih.gov.
John M. Coffin, Email: john.coffin@tufts.edu.
John W. Mellors, Email: jwm1@pitt.edu.
References
- 1. European AIDS Clinical Society. EACS Guidelines Version 9.0. EACS website. http://www.eacsociety.org/files/guidelines_9.0 Accessed September 17, 2019.
- 2.Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK; Panel on Clinical Practices for Treatment of HIV. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med. 2002;137(5 Pt 2):381–433. doi: 10.7326/0003-4819-137-5_part_2-200209031-00001. [DOI] [PubMed] [Google Scholar]
- 3.Ávila-Ríos S, et al. Pretreatment HIV-drug resistance in Mexico and its impact on the effectiveness of first-line antiretroviral therapy: a nationally representative 2015 WHO survey. Lancet HIV. 2016;3(12):e579–e591. doi: 10.1016/S2352-3018(16)30119-9. [DOI] [PubMed] [Google Scholar]
- 4.Cozzi-Lepri A, et al. Low-frequency drug-resistant HIV-1 and risk of virological failure to first-line NNRTI-based ART: a multicohort European case-control study using centralized ultrasensitive 454 pyrosequencing. J Antimicrob Chemother. 2015;70(3):930–940. doi: 10.1093/jac/dku426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geretti AM, et al. Low-frequency K103N strengthens the impact of transmitted drug resistance on virologic responses to first-line efavirenz or nevirapine-based highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;52(5):569–573. doi: 10.1097/QAI.0b013e3181ba11e8. [DOI] [PubMed] [Google Scholar]
- 6.Goodman DD, et al. Low level of the K103N HIV-1 above a threshold is associated with virological failure in treatment-naive individuals undergoing efavirenz-containing therapy. AIDS. 2011;25(3):325–333. doi: 10.1097/QAD.0b013e3283427dcb. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JA, et al. Minority HIV-1 drug resistance mutations are present in antiretroviral treatment-naïve populations and associate with reduced treatment efficacy. PLoS Med. 2008;5(7):e158. doi: 10.1371/journal.pmed.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li JZ, et al. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure: a systematic review and pooled analysis. JAMA. 2011;305(13):1327–1335. doi: 10.1001/jama.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzner KJ, et al. Minority quasispecies of drug-resistant HIV-1 that lead to early therapy failure in treatment-naive and -adherent patients. Clin Infect Dis. 2009;48(2):239–247. doi: 10.1086/595703. [DOI] [PubMed] [Google Scholar]
- 10.Paredes R, et al. Pre-existing minority drug-resistant HIV-1 variants, adherence, and risk of antiretroviral treatment failure. J Infect Dis. 2010;201(5):662–671. doi: 10.1086/650543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simen BB, et al. Low-abundance drug-resistant viral variants in chronically HIV-infected, antiretroviral treatment-naive patients significantly impact treatment outcomes. J Infect Dis. 2009;199(5):693–701. doi: 10.1086/596736. [DOI] [PubMed] [Google Scholar]
- 12.Balduin M, Oette M, Däumer MP, Hoffmann D, Pfister HJ, Kaiser R. Prevalence of minor variants of HIV strains at reverse transcriptase position 103 in therapy-naïve patients and their impact on the virological failure. J Clin Virol. 2009;45(1):34–38. doi: 10.1016/j.jcv.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Boltz VF, et al. Low-frequency nevirapine (NVP)-resistant HIV-1 variants are not associated with failure of antiretroviral therapy in women without prior exposure to single-dose NVP. J Infect Dis. 2014;209(5):703–710. doi: 10.1093/infdis/jit635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casadellà M, et al. Clinical value of ultradeep HIV-1 genotyping and tropism testing in late presenters with advanced disease. AIDS. 2015;29(12):1493–1504. doi: 10.1097/QAD.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 15.Clutter DS, et al. Prevalence of Drug-Resistant Minority Variants in Untreated HIV-1-Infected Individuals With and Those Without Transmitted Drug Resistance Detected by Sanger Sequencing. J Infect Dis. 2017;216(3):387–391. doi: 10.1093/infdis/jix338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coovadia A, et al. Persistent minority K103N mutations among women exposed to single-dose nevirapine and virologic response to nonnucleoside reverse-transcriptase inhibitor-based therapy. Clin Infect Dis. 2009;48(4):462–472. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakobsen MR, et al. Transmission of HIV-1 drug-resistant variants: prevalence and effect on treatment outcome. Clin Infect Dis. 2010;50(4):566–573. doi: 10.1086/650001. [DOI] [PubMed] [Google Scholar]
- 18.Messiaen P, et al. Ultra-deep sequencing of HIV-1 reverse transcriptase before start of an NNRTI-based regimen in treatment-naive patients. Virology. 2012;426(1):7–11. doi: 10.1016/j.virol.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Metzner KJ, Allers K, Rauch P, Harrer T. Rapid selection of drug-resistant HIV-1 during the first months of suppressive ART in treatment-naive patients. AIDS. 2007;21(6):703–711. doi: 10.1097/QAD.0b013e3280121ac6. [DOI] [PubMed] [Google Scholar]
- 20.Metzner KJ, et al. Prevalence of key resistance mutations K65R, K103N, and M184V as minority HIV-1 variants in chronically HIV-1 infected, treatment-naïve patients. J Clin Virol. 2011;50(2):156–161. doi: 10.1016/j.jcv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Metzner KJ, et al. Limited clinical benefit of minority K103N and Y181C-variant detection in addition to routine genotypic resistance testing in antiretroviral therapy-naive patients. AIDS. 2014;28(15):2231–2239. doi: 10.1097/QAD.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 22.Mohamed S, Ravet S, Camus C, Khiri H, Olive D, Halfon P. Clinical and analytical relevance of NNRTIs minority mutations on viral failure in HIV-1 infected patients. J Med Virol. 2014;86(3):394–403. doi: 10.1002/jmv.23853. [DOI] [PubMed] [Google Scholar]
- 23.Mzingwane ML, Tiemessen CT, Richter KL, Mayaphi SH, Hunt G, Bowyer SM. Pre-treatment minority HIV-1 drug resistance mutations and long term virological outcomes: is prediction possible? Virol J. 2016;13(1):170. doi: 10.1186/s12985-016-0628-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neogi U, Sonnerborg A. Minor viral population with drug-resistant mutation and risk of persistent low-level viremia or ‘blips’ in HIV-1 subtype C. AIDS. 2014;28(17):2635–2636. doi: 10.1097/QAD.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 25.Nicot F, et al. Minority resistant HIV-1 variants and the response to first-line NNRTI therapy. J Clin Virol. 2015;62:20–24. doi: 10.1016/j.jcv.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Peuchant O, et al. Transmission of HIV-1 minority-resistant variants and response to first-line antiretroviral therapy. AIDS. 2008;22(12):1417–1423. doi: 10.1097/QAD.0b013e3283034953. [DOI] [PubMed] [Google Scholar]
- 27.Porter DP, et al. Emergent HIV-1 Drug Resistance Mutations Were Not Present at Low-Frequency at Baseline in Non-Nucleoside Reverse Transcriptase Inhibitor-Treated Subjects in the STaR Study. Viruses. 2015;7(12):6360–6370. doi: 10.3390/v7122943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Eygen V, et al. Deep sequencing analysis of HIV-1 reverse transcriptase at baseline and time of failure in patients receiving rilpivirine in the phase III studies ECHO and THRIVE. J Med Virol. 2016;88(5):798–806. doi: 10.1002/jmv.24395. [DOI] [PubMed] [Google Scholar]
- 29.Zoufaly A, et al. Virological failure after 1 year of first-line ART is not associated with HIV minority drug resistance in rural Cameroon. J Antimicrob Chemother. 2015;70(3):922–925. doi: 10.1093/jac/dku470. [DOI] [PubMed] [Google Scholar]
- 30.Wallis CL, Godfrey C, Fitzgibbon JE, Mellors JW. Key factors influencing the emergence of human immunodeficiency virus drug resistance in low- and middle-income countries. J Infect Dis. 2017;216(Suppl 9):S851–S856. doi: 10.1093/infdis/jix409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eshleman SH, et al. Comparison of nevirapine (NVP) resistance in Ugandan women 7 days vs. 6-8 weeks after single-dose nvp prophylaxis: HIVNET 012. AIDS Res Hum Retroviruses. 2004;20(6):595–599. doi: 10.1089/0889222041217518. [DOI] [PubMed] [Google Scholar]
- 32.Musoke P, et al. A phase I/II study of the safety and pharmacokinetics of nevirapine in HIV-1-infected pregnant Ugandan women and their neonates (HIVNET 006) AIDS. 1999;13(4):479–486. doi: 10.1097/00002030-199903110-00006. [DOI] [PubMed] [Google Scholar]
- 33.Boltz VF, et al. Role of low-frequency HIV-1 variants in failure of nevirapine-containing antiviral therapy in women previously exposed to single-dose nevirapine. Proc Natl Acad Sci USA. 2011;108(22):9202–9207. doi: 10.1073/pnas.1105688108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jourdain G, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351(3):229–240. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 35.Lockman S, et al. Nevirapine- versus lopinavir/ritonavir-based initial therapy for HIV-1 infection among women in Africa: a randomized trial. PLoS Med. 2012;9(6):e1001236. doi: 10.1371/journal.pmed.1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lockman S, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363(16):1499–1509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boltz VF, et al. Ultrasensitive single-genome sequencing: accurate, targeted, next generation sequencing of HIV-1 RNA. Retrovirology. 2016;13(1):87. doi: 10.1186/s12977-016-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuurman R, et al. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug-resistant virus populations in persons treated with lamivudine (3TC) J Infect Dis. 1995;171(6):1411–1419. doi: 10.1093/infdis/171.6.1411. [DOI] [PubMed] [Google Scholar]
- 39.Coffin JM. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267(5197):483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 40.Richman DD, et al. Nevirapine resistance mutations of human immunodeficiency virus type 1 selected during therapy. J Virol. 1994;68(3):1660–1666. doi: 10.1128/jvi.68.3.1660-1666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wainberg MA, et al. Enhanced fidelity of 3TC-selected mutant HIV-1 reverse transcriptase. Science. 1996;271(5253):1282–1285. doi: 10.1126/science.271.5253.1282. [DOI] [PubMed] [Google Scholar]
- 42.Jackson JB, et al. Identification of the K103N resistance mutation in Ugandan women receiving nevirapine to prevent HIV-1 vertical transmission. AIDS. 2000;14(11):F111–F115. doi: 10.1097/00002030-200007280-00001. [DOI] [PubMed] [Google Scholar]
- 43.Zhou S, Jones C, Mieczkowski P, Swanstrom R. Primer ID validates template sampling depth and greatly reduces the error rate of next-generation sequencing of HIV-1 genomic RNA populations. J Virol. 2015;89(16):8540–8555. doi: 10.1128/JVI.00522-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.