Abstract
A systematic review and meta-analysis was conducted to investigate the effects of yoga on glycemic control, lipid profiles, body composition and blood pressure in people in the pre-diabetic state. Studies on the effectiveness of yoga on population groups under high risk for diabetes, called prediabetic or suffering from metabolic syndromes were extracted from a thorough search of PubMed, Scopus, Cochrane Library, EBSCO and IndMED databases. Both Randomised Controlled Trial (RCT) and non-RCT studies were included in the systematic review and meta-analysis. Studies published between Jan 2002 and Dec 2018 were included. Studies were considered for evaluation if they investigated a yoga intervention to prevent T2DM, against a control group, while also reporting glycemic control and other health parameters of T2DM management. Summary effect sizes and 95% confidence intervals (CI) were calculated using the Comprehensive Meta-Analysis software in addition to publication bias. Of the 46,500 identified studies, 14 studies with 834 participants of whom were 50% women, were found to be eligible for inclusion in our systematic review. Our quantitative synthesis included 12 randomized control trials and 2 non-randomized control trials, with the follow-up period ranging from 4 to 52 weeks. Compared to controls, yoga intervention improved fasting blood glucose (FBG) [Standard Mean Difference (SMD -0.064 mg/dL (95% CI -0.201 to 0.074)]; low density lipoprotein (LDL) [SMD-0.090 mg/dL (95% CI -0.270 to 0.090)]; triglycerides [SMD -0.148 mg/dL (95% CI -0.285 to -0.012)]; total cholesterol [SMD -0.058 mg/dL (95% CI -0.220 to 0.104)] and systolic blood pressure [SMD -0.058 mm Hg (95% CI -0.168 to 0.053)]. This meta-analysis uncovered clinically improved effects of yoga intervention on glycemic control, lipid profiles and other parameters of T2DM management in prediabetic population. These results suggest that yoga intervention may be considered as a comprehensive and alternative approach to preventing T2DM. Further adequately powered, well designed RCTs are needed to support our findings and investigate the long-term effects of yoga in T2DM patients.
Introduction
Type II diabetes mellitus is a chronic metabolic disease characterized by persistent hyperglycemia because of a progressive condition in which the body becomes resistant to the typical effects of insulin or loses the ability to produce insulin [1]. The global prevalence of diabetes among adults above 18 years old has increased to 8.5% in 2014 from 4.7% in 1980. Approximately 422 million people were found to be living with diabetes in 2014 [2] and approximately 1.6 million deaths were directly caused by diabetes in 2016 [3].
The key risk factors often associated with the development and maintenance of Type 2 diabetes mellitus (T2DM) include sedentary lifestyle [4], or an unhealthy diet and psychological stress. Psychological stress is strongly associated with both the risk factors [5–7] and maintenance of the disease [8, 9]. In addition to the genetic background, the prediabetic state also contributes significantly to the development of T2DM [4, 10].
The critical components of diabetes management are medication, diet and physical activity/exercise [11]. However, many complementary and alternative practices have been used by people in both the prevention and treatment of diabetes [12, 13] such as yoga. Yoga originated in India over 5000 years ago as a form of traditional mind-body training [14, 15]. The efficacy of yoga has been studied in several chronic diseases, such as hypertension, asthma, chronic obstructive pulmonary disease and diabetes [16–18].
Previous studies have reported that the practice of yoga might reduce Insulin Resistance Syndrome, is an exclusive collection of risk factors for the development of T2DM and have shown promising results in improving signs, improving prognosis and reducing complications [16, 18–22]. Furthermore, studies showed the development of diabetes from the prediabetic state could be either delayed or ameliorated by consistent physical activity [23–26], healthy diet [24] and active stress management [27, 28].
Previous studies have highlighted that yoga could lessen fasting blood glucose (FBG) and glycosylated hemoglobin A1c (HbA1c) as well as reduce the lipid levels while improving the quality of life of T2DM patients [29–36]. However, these studies showed inconclusive results with wide variations in their sample size. A limitation of the study may include the inclusion of a few studies based on a non-randomized study design, not informed so could potentially impact the outcome [32, 33, 35]. Nevertheless, a few previously published systematic reviews have reported the efficacy of yoga on blood glucose levels, insulin sensitivity, oxidative stress, lipid profile, anthropometric measures, pulmonary measures, nerve conduction and quality of life for T2DM with promising results [37–40]. Raveendran and colleagues demonstrated the role of various yoga practices in the management of diabetes based on evidence from various clinical studies [41].
The promising benefits of yoga interventions for T2DM have also been recorded in a recent meta-analysis. Innes and Selfi demonstrated the impact of yoga among adults with T2DM to improve glycemic control, lipid levels and body composition measured by body weight and mass index from a systematic review of 25 controlled trials [42]. Cui and colleagues reported a meta-analysis of 12 randomized controlled trials and highlighted the progress in pooled weighted mean differences for fasting blood glucose and hemoglobin A1c. The authors of this particular study also measured the weighted effect size of yoga efficacy for postprandial blood glucose (PPBS), total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and triglycerides [11]. Kumar and colleagues documented beneficial effects of yoga as a complementary intervention to standard treatment in comparison to standard treatment on FBS for PPBS and HbA1C [43]. Thind and colleagues showed, in their meta-analysis that yoga T2DM participants were successful in improving their HbA1c, FBG and PPBG, in addition to significant improvements in lipid profile, blood pressure, body mass index, waist/hip ratio and cortisol level [44]. Because past meta-analysis has not been conducted on the effects yoga on prediabetic populations, we carried out a meta-analysis of RCT and non-RCT studies in the present study to determine the effectiveness of yoga in patients with high-risk T2DM. We hypothesize that yoga intervenes T2DM by two proposed mechanisms: downregulation of both the hypothalamic pituitary adrenal axis and the sympathetic nervous system [45–47].
Rationale
What is the issue and how will our study address this?
Studies published previously in the prediabetic population showed the effectiveness of yoga in reducing the risk of progression to diabetic state [48–50]. There are a few systematic reviews and meta-analyses that demonstrate the efficacy of yoga in T2DM. However, there is currently no systematic review and meta-analysis study that details the potential benefits of yoga in the prediabetic population. This is the first such study to show evidence that yoga intervention may impact the prediabetic state by assessing the glycemic control (HbA1c, fasting blood glucose (FBG) and postprandial glucose (PPBG) in both the intervention and control group conditions. This study also summarises the results of other markers of diabetes management including triglycerides, high-density lipoprotein, low-density lipoprotein, systolic and diastolic blood pressure, body composition and fasting cortisol, as influenced by yoga in prediabetic populations. The study provides further evidence that yoga intervention could be considered as an effective alternate treatment or lifestyle therapy for people who are under high risk of T2DM.
Review questions
Our systematic review and meta-analysis sought to clarify the association between yoga intervention and prediabetic state. We also compared the overall effect of the yoga intervention on glycemic control and other markers of diabetes management.
The questions for this review are as follows:
Does yoga delay or prevent the progression of diabetes in a prediabetic population?
What is the significance of yoga compared to exercise in a prediabetic population?
How much does the effect size of physiological outcomes vary between studies?
Methods
This systematic review and meta-analysis was registered in the PROSPERO (Registration number CRD 42018106657) database and followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [51]. This protocol has been published in Medicine Journal.
Search strategy
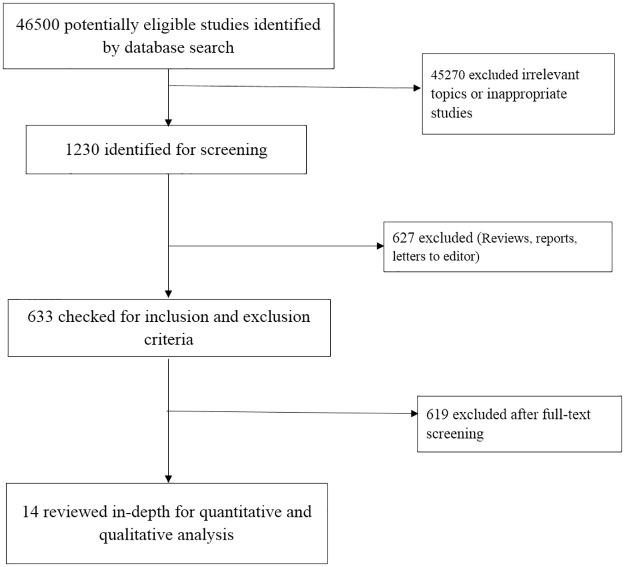
We searched 5 electronic bibliographic databases: PubMed, Scopus, Cochrane Library, EBSCO and IndMED using a combination of search terms for the effectiveness of yoga on people with high risk of diabetes or prediabetic population, or metabolic syndrome ((yoga, cardiovascular disease risk factors, prediabetes state, high risk for diabetes, metabolic syndrome, glucose, type II diabetes, exercise therapy and type 2 diabetes patients)) [52]. Two authors performed title and abstract screening and then accessed potential full-text eligible articles. The corresponding author performed a final assessment on eligible studies for inclusion, and the references of eligible studies were imported into an EndNote file to create an initial list of eligible studies and avoid duplication. Any discrepancies associated with the selection of the studies were resolved by mutual discussions involving a third reviewer. The entire selection process is illustrated in Fig 1.
Fig 1. A flowchart depicting the study selection and the search results.
Study selection criteria
The included studies had to meet the following (PICO) criteria
Inclusion criteria
Participants (P): Participants with prediabetic or designated as high risk for diabetes because of physiological measures.
Intervention (I): Inclusion of studies was dependent on the program following authentic or traditional yoga rules and techniques. Studies examining yoga intervention including at least one of asana, pranayama, meditation to promote T2DM management and comparing it with a control. Studies with randomised control trial’, randomised cross-over studies, cluster-randomised trials, or quasi-experimental design were included.
Control (C): Any control with other usual care or physical exercise or non-therapeutic intervention
Outcomes (O): Studies evaluating glycemic control as well as other measures of T2DM management such as HbA1c, blood pressure, fasting blood sugar, lipid profile (triglycerides, high and low-density lipoprotein (HDL and LDL) cholesterol), systolic blood pressure and diastolic blood pressure in both the intervention and control group conditions.
Exclusion criteria
We excluded study participants who were members of a specific age group, such as adolescents or geriatric age groups
Study participants were all in a transient state, such as pregnancy or menopause
Studies were excluded if the yoga intervention was modified to a dance program
Studies from conference proceedings, editorials, commentaries, book chapters and book reviews were excluded.
Data extraction and management
Two authors developed a data extraction form using MS Excel and independently evaluated the published studies with the predefined selection criteria for inclusion into the review. Data were extracted based on six categories: 1. Study information (first author, year of publication, country, a journal of publication, the study period); 2. Study design and methods (a type of research, details of randomised control trial, randomised cross-over studies and cluster-randomised trials or quasi-experimental design and the validity of confirmative diagnosis and method of data collection); 3. Study participants characteristics (condition, age, gender, race, sample size and sampling procedures); 4. Yoga intervention characteristics (Yoga type: asana, pranayama, meditation, components, frequency, duration); 5. Control intervention characteristics (Type: usual care or physical exercise or non-therapeutic intervention, frequency, duration); 6. Outcome measures (Primary and secondary outcome measures, assessment time points, blood pressure (systolic, diastolic); heart rate; respiratory rate; abdominal obesity (waist circumference, waist-hip ratio, index of central obesity); blood lipid levels (triglycerides, high and low-density lipoprotein (HDL and LDL) cholesterol); glycemic control (both the intervention and control group conditions, such as HbA1c, blood pressure, or fasting blood sugar).
Study outcomes
Primary outcome
The primary outcome was the measurement of glycemic control (HbA1c) fasting blood glucose (FBG) and postprandial glucose (PPBG).
Secondary outcomes
The secondary outcomes were other markers of diabetes management including triglycerides, high-density lipoprotein, low-density lipoprotein, systolic and diastolic blood pressure, body composition and fasting cortisol.
Assessment of risk of bias
Risk of bias of included studies was assessed using the Cochrane Risk of Bias Assessment tool. This tool assesses several items under seven categories such as random sequence generation, allocation concealment, blinding of participants and investigators, the blindness of outcome assessments, incomplete outcome data, selective outcome reporting, and other biases. Based on the assessment, the studies were evaluated as low, unclear, or high bias. The PRISMA checklist is given in S1 Table.
The Jadad scale was used to evaluate the quality of each study in which three domains in the scale cover randomization (0–2 points), blinding (0–2 points), and dropouts and withdrawals (0–1 point) [53]. A trial with a score of ≤ 2 indicates low quality whereas a score of ≥3 indicates high quality. Assessment of publication bias was performed using funnel plots generated by Comprehensive Meta-Analysis (CMA) 3.0 software.
Data synthesis
Meta-analysis-assessment of overall effect size
Meta-analyses were performed using CMA, and the effectiveness of yoga interventions on the glycemic status of the prediabetic population was computed. We combined effect sizes across included studies and reported pooled effect estimate as standardised mean difference (SMD) with their 95% Confidence Intervals (CI). Random-effects model of the meta-analysis was followed, and forest plots were generated to visually assess the pooled effect size of the study’s findings.
The outcome measures in this systematic review and meta-analysis were continuous. Negative SMDs indicated better performances of yoga interventions on the glycemic status and other markers of the prediabetic population or the beneficial effects of yoga for diabetes risk [43].
Assessment of heterogeneity
Heterogeneity in effect size was assessed using the Cochrane Q test and I2-statistic. Q statistics and the associated degree of freedom were estimated for each outcome. Q statistics provides a test of the null hypothesis that all studies in the proposed meta-analysis share a standard effect size. If all studies shared the same effect size, the expected value of Q would be equal to the degrees of freedom the number of studies minus 1. I2 statistics reflect the proportion of the observed variance that informs the difference in true effects sizes rather than the sampling error of the included studies. The tau-squared parameter indicates information of the heterogeneity between the effects for the test accuracy observed in different studies [54].
Publication bias
Publication bias of the included studies was assessed using Egger’s bias indicator test. Orwin’s [55] and classic fail-safe N test’ [56], ‘Begg and Mazumdar Rank correlation test’ [57], and ‘Duval and Tweedie’s Trim and Fill ‘calculations [58] were used to impute missing small studies with large effect size to be dispersed equally on either side of the overall effect, to provide a more accurate estimate of the likely publication bias
Funnel plot
The funnel plot is a plot of study size (standard error or precision) on the vertical axis versus a function of effect size (standard mean difference) on the horizontal axis. The included studies in the plot are distributed symmetrically about the combined effect size in the absence of publication bias. This plot was generated using Egger’s test [59]. Conversely, a higher concentration of studies is observed on one side of the mean than the other in the presence of bias.
Classic fail-safe N and Orwin fail-safe N
The critical reason for publication bias is that some non-significant studies are missing from this meta-analysis. The observed effect of yoga intervention will be invalidated if these missing studies are included in the analysis. If the missing number is relatively small, publication bias is likely. However, if these missing number of studies are large, the findings are interpreted as the intervention effect of yoga could be possibly inflated by the exclusion of some studies, is nevertheless not nil [60]. Both Classic and the Orwin fail-safe N reports the likelihood that studies are absent from the analysis and that these studies if included in the analysis, would shift the effect size toward the null [55].
Begg and Mazumdar rank correlation test
In general, extensive studies tend to be included in the quantitative synthesis or meta-analysis regardless of their yoga intervention and control group effects while small studies are more possible to be included when they display a comparatively sizeable intervention effect. Therefore, an inverse correlation between study size and effect size will tend to be observed. Begg and Mazumdar proposed that this kind of correlation can aid as a test for publication bias [61]. Therefore, the rank order correlation was computed (Kendall's tau b) between the intervention effect and the standard error (which is driven primarily by sample size).
Egger's test of the intercept
The Egger regression yields the degree of funnel plot asymmetry or the same bias by using precision -the inverse of the standard error to predict the standardised effect (effect size divided by the standard error). The Egger's Test of the Intercept was undertaken to gauge the relationship between test accuracy estimates and their precision [59].
Duval and Tweedie's Trim and Fill
Duval and Tweedie's Trim and Fill test imputes the missing studies of both yoga and control conditions are, add them to the analysis, and then recomputes the combined intervention effect. The 'Trim and Fill' method iteratively prunes the asymmetric studies from the right-hand side to localize the unbiased effect. Then this method completes by re-introducing the trimmed studies on the right as well as their imputed counterparts to the left of the mean intervention effect [62].
Subgroup analyses
Subgroup analyses were performed based on study, participant and outcome characteristics and methodological factors. We have investigated specific subgroup analyses according to differences in intervention and critical features of identified study participants such as glycemic control (both the intervention and control group conditions, such as HbA1c, blood pressure, or fasting blood sugar), blood lipid levels (low density lipoprotein (LDL), triglycerides, cholesterol), body composition (waist circumference and body weight) and blood pressure (systolic, diastolic).
Results
In total, 46,500 studies were identified by searching the bibliographic databases PubMed, Scopus, Cochrane Library, EBSCO and IndMED with search strings of keywords. Of these studies, 45270 studies were excluded because they were either irrelevant topics or inappropriate studies. Finally, 1230 articles were screened for further evaluation. A third reviewer monitored the review process to validate the complete study search and selection of most relevant studies for inclusion and monitored the review process. After removing the duplicates and abstract screening, 1216 articles were excluded, because the articles were either reviews, letters to the editor or case studies. This exclusion step resulted in 14 studies after careful manual screening. The reference lists of the existing narrative reviews and meta-analysis were checked and revealed no further relevant missed studies. Finally, 14 studies were eligible for inclusion in our systematic review. Also, some studies failed to mention the association between yoga and metabolic syndrome. Therefore, a final total of 14 studies were included in the systematic review. The study selection is depicted in Fig 1.
Of the 14 studies, six were from India, four from USA, two from China and one each from Sweden and Hong Kong. The total population included in this study was 834 participants from 14 studies. The gender details were available in 10 of the included 14 studies in the systematic analysis. In the total 14 studies, 285 males and 413 females were reported. Our quantitative synthesis demonstrated that 12 studies were randomised control trial, and two were non-randomized control trial. The follow-up period ranged from 4 to 52 weeks. The interventions were yoga practitioners, pre-diabetic, diabetic, obese and metabolic syndrome, as indicated in Table 1.
Table 1. Characteristics of the included studies.
| S.No | Author and Year | Country | Participation time | Yoga Asanas | Intervention | No of patients | Age and Sex | Control group | No of patients | Age and Sex | Follow-up period | Outcome measures | Study design | Mean and SD values |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Corey SM et al. 2014 [81] | USA | NA | Restorative yoga intervention | Yoga participants | 88 | 21–65 yrs | Stretching participants | 83 | 21–65 yrs | One year | Salivary cortisol and Psychosocial measurements | Two-arm RCT | NA |
| 2 | Hegde et al. 2013 [49] | India | 2007–2008 | 18 Yoga asanas | Pre-diabetics patients | 14 | 30 and 75 yrs: Male-6: Female-8 | Computer generator randomised list | 15 | 30 and 75 yrs Male-8: Female-7 | 3 months | Oxidative stress, glycemic status, Blood pressure, Anthropometry | RCT | Provided |
| 3 | Kanaya Am et al 2014 [72] | USA | 2009–2012 | Restorative yoga intervention | Patients | 88 | 21–65 yrs: F-65: M-23 | Stretching participants | 83 | 21–65 yrs F-59: M-24 | 48 weeks | fasting and 2-hour glucose, HbA1c, triglycerides, HDL Cholesterol | RCT | Provided |
| 4 | Keerthi GS et al. 2017 [66] | India | September 2013 to April 2016. | Asanas and pranayama, Meditation and Relaxation | Pre-diabetics and Diabetics | 124 | 18–45 yrs Prediabetics- M-69/F-55: Diabetics-M-64/F-60 | Healthy controls | 62 | M-34/F-28 | 12 weeks | Biochemical measures, Anthropometric measures, Bp, plasma glucose | RCT | Provided |
| 5 | Lau Caren et al. 2015 [83] | China | May 2010 -Jan 2011 | 57 Yoga Poses | Yoga Practitioners | 87 | 18 and Above M-34: F-53 | Non-Yoga practitioners | 88 | 18 and Above M-30: F-56 | 12 weeks | Metabolic risk factors, Mets z score, Glucose, BP, triglyceride, Smoking, Body weight | Non-RCT | Provided |
| 6 | McDermott KA et al. 2014 [69] | India | Oct-Nov 2004 | 8 types of Yoga asanas and chanting | Yoga Practitioners | 21 | Age -NA Male-9 Female-12 | Non-yoga practitioners, only physical exercises | 20 | Age -NA; Male-7 Female-13 | 8 weeks | Diabetic Risk Factors and Physiological Risk Factors | RCT | Provided |
| 7 | Netam et al. 2015 [67] | India | Dec 2011-Dec 2012 | Asanas and pranayama | overweight/obese individuals | 34 | M-21: F-13 | Pre-baseline | 34 | M-21: F-13 | one month | IL-6, 25-OH-vitamin D and diabetes risk factors | Non-RCT | Provided |
| 8 | Siu et al. 2015 [84] | Hongkong | Nov 2010 and Aug 2013 | 34 Yoga asanas | Metabolic syndrome | 84 | 30–80 M-23: F-75 | Computer generator randomised list | 98 | 30–80 M-24: F-60 | One year | Wait, BP, glucose, triglycerides, HDL-C, Heart rate | RCT | Provided |
| 9 | Sohl et al 2016 [71] | USA | June 2013 to January 2014 | 12 Yoga-asanas | Metabolic syndrome patients | 33 | M-17: F-16 | education only | 33 | M-16; F-17 | 12 weeks | Biometric and Patient-reported constructs | RCT | Provided |
| 10 | Supriya et al. 2017 [73] | China | NA | 18 Yoga-asanas | Metabolic syndrome patients associated with Diabetes & Cardiovascular diseases | 52 | M-17: F-35 | Computer generator randomised list | 45 | M-17: F-28 | One year | Blood glucose, triglycerides, HDL-C, Waist circumference | RCT | Provided |
| 11 | Tyagi A et al. 2014 [68] | India | Nov and Jan 2012 | Yoga teachers, Yoga therapists and Yoga active person | Yoga Practitioners | NA | 18 yrs to 55 yrs | Non-yoga practitioners | NA | 18 yrs to 55 yrs | 6 months | Anthropometric measures, BP measurement, metabolic measures | RCT | NA |
| 12 | Wolff M et al. 2013 [85] | Sweden | 1 May 2008 and 31 January 2010 | NA | three groups: through Yoga practitioner, Yoga at home, a control group who take mediation from a general practitioner | 56 | 20–80 yrs | Computer generator randomised list | 27 | 20–80 yrs | 12 weeks | Bp, glucose, HBA1c, Cholesterol | three-arm RCT | Provided |
| 13 | Yadhav et al. 2018 [70] | India | September 2013 to April 2016. | Asanas and pranayama, Meditation and Relaxation | Metabolic syndrome patients | 130 | 20–45 years Gender number details not provided | dietary intervention | 130 | 20–45 years Gender number details NA | 12 weeks | Weight, Height, Body mass, BP, Glucose, Insulin, HDL-C, Insulin, Triglyceride | RCT | Provided |
| 14 | Yang K et al. 2011 [86] | USA | NA | Asanas and pranayama | Patients | 23 | 45 and 65 years, M-2; F-21 | Pre-baseline | 23 | 45 and 65 years, M-2; F-21 | 3 months | Weight, Bp, Glucose, Insulin, Lipid panel | Randomised Control Trial | Provided |
Analysis of the overall effects of yoga intervention
Fourteen studies reported FBG as a primary outcome. The effects of yoga compared to control conditions were observed for the pooled SMD (glycemic control) of FBG was -0.064 mg/dL (95% CI -0.201 to 0.074); PPBG was 0.268 mg/dL (95% CI 0.006–0.530); and HbA1c was 0.021% (95% CI -0.164 to 0.205). Relative to controls, the subgroup analysis of other measures of T2DM management revealed effects of yoga compared to controls for lipid profile, body compositions and blood pressure. The efficacy of yoga compared to control for pooled SMD (lipid profile) of LDL was -0.090 mg/dL (95% CI -0.270 to 0.090); triglycerides was -0.148 mg/dL (95% CI -0.285 to -0.012); and total cholesterol was -0.058 mg/dL (95% CI -0.220 to 0.104). The pooled SMD (body composition) for waist circumference was 0.023 cm (95% CI -0.206 to 0.251) and body weight was 0.045 kg (95% CI -0.338 to 0.427). The mean SMD (blood pressure) for systolic blood pressure was -0.058 mm Hg (95% CI -0.168 to 0.053); and diastolic blood pressure was 0.010 mm Hg (95% CI -0.098 to 0.117).
Sensitivity analysis
In the included studies with a low risk of selection bias using random sequence generation, all studies were located. A significant effect of yoga in comparison to control group conditions was observed in pooled SMD of the primary outcome measure. In the case of allocation concealment, which is a source of bias, most of the studies except six generated high and unclear Risk of Bias assessment (ROB). This could imply that the results were not distinguishable from potential bias. In RCTs with low risk of detection bias (Blinding of Outcome Assessment), thirteen studies were located. In RCTs with a low risk of attrition bias (Incomplete Out-come Data), one study was identified. The risk of biases is shown in Table 2.
Table 2. Risk of bias assessment of the included studies.
| S.No | Author and Year | Random sequence generation (Selected bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (Attrition bias) | Selective reporting (reporting bias) | Other bias | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Corey SM et al. 2014 [81] | Low risk | unclear | High risk | High risk | High risk | Low risk | Unclear | 2 |
| 2 | Hegde et al. 2013 [49] | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | 4 |
| 3 | Kanaya Am et al. 2014 [72] | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | 3 |
| 4 | Keerthi GS et al. 2017 [66] | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | 3 |
| 5 | Lau Caren et al. 2015 [83] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | 3 |
| 6 | McDermott KA et al. 2014 [69] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | 4 |
| 7 | Netam et al. 2015 [67] | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | 3 |
| 8 | Siu et al. 2015 [84] | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | 3 |
| 9 | Sohl et al. 2016 [71] | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | 3 |
| 10 | Supriya et al. 2017 [73] | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | 3 |
| 11 | Tyagi A et al. 2014 [68] | Low risk | Unclear | Low risk | Low risk | Low risk | High risk | Unclear | 2 |
| 12 | Wolff M et al. 2013 [85] | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | 3 |
| 13 | Yadhav et al. 2018 [70] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | 4 |
| 14 | Yang K et al. 2011 [86] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | 4 |
Jadad Score:
Randomisation (0–2 points), blinding (0–2 points)
Dropouts and withdrawals (0–1 point)
Score ≤2 indicates low quality, whereas a score of ≥3 indicates high quality
Primary outcome -Glycemic control
The beneficial outcomes were noticed in fasting blood glucose, but not in postprandial glucose and glycosylated hemoglobin (HbA1c).
Fasting blood glucose
The meta-analysis revealed that yoga is beneficial in the control of blood sugar levels compared to control group conditions.
Does yoga intervention decrease fasting blood glucose level?
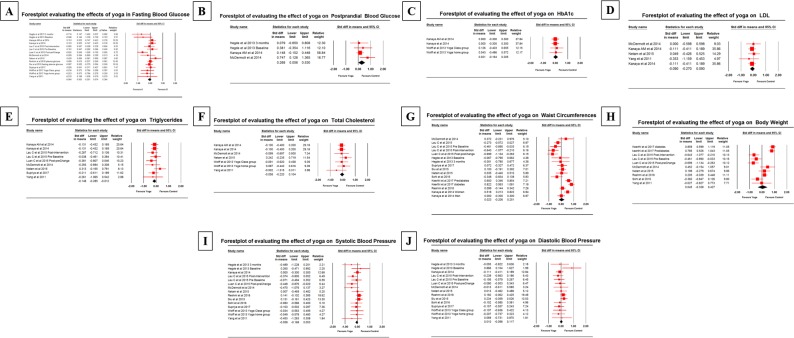
Fourteen studies provided the SMD values for the meta-analysis on the fasting blood glucose; the point estimate of effect size for random effects of SMD was -0.064 (95% CI -0.201 to 0.074) (Fig 2).
Fig 2. Forest plot of the effect of yoga on glycemic control, lipid profile, body composition and blood pressures in prediabetes.
A) fasting blood glucose, B) Postprandial blood glucose, C) Glycosylated hemoglobin (HbA1c), D) LDL, E) Triglycerides, F) Total Cholesterol (TC), G) Waist circumferences, H) Body weight, I) Systolic blood pressure (SBP) and J) Diastolic blood pressure (DBP). The pooled SMD data were calculated and analysed using CMA software (version 3.3.070, USA). The black diamond represents the pooled effect estimate of SMD of the included. The red square with line indicates the effect size of miRNA of the included studies with 95% confidence interval.
How much does the effect size of yoga efficacy differ across studies?
The Q statistics provide a test of the null hypothesis that all RCTs in the meta-analysis share a standard effect size. If all RCTs shared the same effect size, the expected value of Q would be equal to degrees of freedom. The Q value is 21.75 with 14 degrees of freedom and a corresponding P-value = 0.084. The I2 statistic indicates the proportions of the observed variance that reflects the difference in actual effect sizes rather than sampling error. Here I2 was 35%. T2 (tau2 is the variance of true effect sizes and tau2 = 0.024.
Postprandial blood glucose
Four studies provided the SMD values for the meta-analysis on the postprandial blood glucose; the point estimate for random effects ratio of SMD was 0.268 (95% 0.006 to 0.530) with the degrees of freedom (df) = 3, I2<7.756, P-value = 0.354 and tau2 = 0.007.
Glycosylated hemoglobin (HbA1c)
Four studies provided the SMD values for the meta-analysis on the HbA1c; the point estimate for random effects ratio of SMD was 0.021 (95% CI-0.164 to 0.205) with the degrees of freedom (df) = 3, I2<0.000, P-value = 0.978 and tau2 = 0.000.
Secondary outcomes
Lipid profile
Yoga intervention was successful in reducing all three measures of the lipid profile of yoga participants who exhibited lower LDL, triglycerides and total cholesterol compared to controls.
Low-density lipoprotein (LDL)
Five studies provided the SMD values for the meta-analysis on the low-density lipoprotein; the point estimate for random effects ratio of SMD was -0.090 (95% CI -0.270 to 0.090) with the degrees of freedom (df) = 4, I2<0.000, P-value = 0.930 and tau2 = 0.000.
Triglycerides
Nine studies provided the SMD values for the meta-analysis on the triglycerides; the point estimate for random effects ratio of SMD was -0.148 (95% CI -0.285 to -0.012) with the degrees of freedom (df) = 8, I2<0.000, P-value = 0.669 and tau2 = 0.000.
Total cholesterol
Seven studies provided the SMD values for the meta-analysis on the total cholesterol; the point estimate for fixed effects ratio of random effects ratio of SMD was -0.058 (95% CI -0.220 to 0.104) with the degrees of freedom (df) = 6, I2<0.000, P-value = 0.792 and tau2 = 0.000.
Body composition
On subgroup analysis, no effect on body composition was observed in the yoga intervention group as compared with the control conditions.
Waist circumference
Yoga intervention was less successful in decreasing waist circumference. Sixteen studies provided the SMD values for the meta-analysis on the waist circumference; the point estimate for random effects ratio of SMD was 0.023 (95% CI -0.206 to 0.251) with the degrees of freedom (df) = 15, I2<83.872, P-value = 0.000 and tau2 = 0.171.
Body weight
Ten studies provided the SMD values for the meta-analysis on the body weight; the point estimate for random effects of SMD 0.045 (95% CI -0.338 to 0.427) with the degrees of freedom (df) = 9, I2<89.974, P-value = 0.000 and tau2 = 0.328.
Blood pressure
The aggregated results suggested that yoga intervention was successful in reducing systolic blood pressure, but not diastolic, blood pressure.
Systolic blood pressure (mmHg)
Participants in yoga intervention showed a decrease in systolic blood pressure compared to controls. Fifteen studies provided the SMD values for the meta-analysis on the systolic blood pressure; the point estimate for random effects ratio of SMD-0.058 (95% CI -0.168 to 0.053) with the degrees of freedom (df) = 14, I2<3.352, P-value = 0.414 and tau2 = 0.002.
Diastolic blood pressure (mmHg)
Fourteen studies provided the SMD values for the meta-analysis on the diastolic blood pressure; the point estimate for random effects ratio of 0.010 (95% CI -0.098 to 0.117) with the degrees of freedom (df) = 14, I2<0.000, P-value = 0.485 and tau2 = 0.000. The effect size estimate of Glycemic control, lipid profile, body composition and blood pressure is shown in Table 3.
Table 3. Effect size estimate of outcome measures in prediabetes state (glycemic control, lipid profile, body composition and blood pressure.
| Outcomes | Measures | No of studies | Sample size | ES | 95% CI | I2 | Tau2 | Q | Z | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yoga | Control | Low | High | ||||||||
| Glycemic control | FBG | 15 | 722 | 720 | -0.037 | -0.14 | 0.067 | 35.654 | 0.024 | 21.757 | -0.908 |
| HbA1c | 4 | 232 | 220 | 0.021 | -0.164 | 0.205 | 0 | 0 | 0.196 | 0.218 | |
| PBG | 4 | 137 | 135 | 0.268 | 0.006 | 0.53 | 7.756 | 0.007 | 3.252 | 2.008 | |
| Lipid profile | LDL | 5 | 243 | 234 | -0.9 | -0.27 | 0.09 | 0 | 0 | 0.863 | -0.984 |
| Triglycerides | 9 | 424 | 408 | -0.148 | -0.285 | -0.012 | 0 | 0 | 5.801 | -2.133 | |
| Total cholesterol | 7 | 299 | 288 | -0.058 | -0.22 | 0.104 | 0 | 0 | 3.136 | -0.701 | |
| Body composition | WC | 16 | 1022 | 1021 | 0.023 | -0.206 | 0.251 | 83.872 | 0.171 | 93.005 | 0.194 |
| Body weight | 10 | 607 | 608 | 0.045 | -0.338 | 0.427 | 89.974 | 0.328 | 89.77 | 0.228 | |
| Blood pressure | SBP | 15 | 667 | 670 | -0.058 | -0.168 | 0.053 | 3.352 | 0.002 | 14.486 | -1.021 |
| DBP | 15 | 667 | 670 | 0.01 | -0.098 | 0.117 | 0 | 0 | 13.529 | 0.173 | |
FBG: Fasting Blood Glucose; ES: Effect Size; CI: Confidence Interval; PBG: Prandial Blood Glucose; LDL: Low Density Lipoprotein; WC: Waist Circumference; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure.
Publication bias
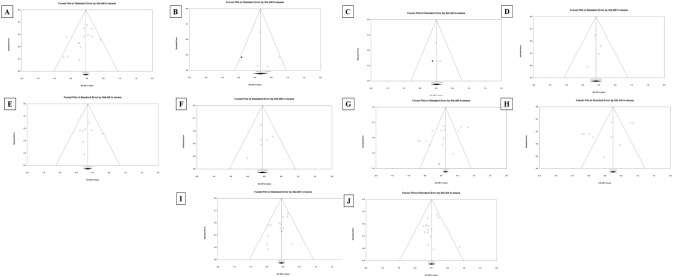
Funnel Plots for all outcome measures are represented in Fig 3. Because the intervention effect estimated from a biased collection of studies would tend to overestimate the exact intervention effect, researches must assess the likely extent of the bias and the impact of this goes on the conclusions. The detailed explanation of the publication bias among the included studies that examined fasting blood glucose is shown in Fig 2 and Table 4 shows the publication bias of remaining outcome measures.
Fig 3. Funnel plot of yoga intervention efficacy on glycemic control, lipid profile, body composition and blood pressure in prediabetes.
A) Fasting blood glucose (FBS), B) Postprandial blood glucose, C) Glycosylated hemoglobin (HbA1c), D) LDL, E) Triglycerides, F) Total Cholesterol (TC), G) Waist circumferences, H) Body weight, I) Systolic blood pressure (SBP), and J) Diastolic blood pressure (DBP).
Table 4. Publication bias of the included studies.
| Key variables | Outcome measures | Classic fail-safe N | Orwin fail-safe N | Begg and Mazumdar | Egger's regression | Duval and Tweedie | |||
|---|---|---|---|---|---|---|---|---|---|
| Z value | P value | SDM | Tau | Z value | P value | ||||
| Glycemic control | FBG | 1.419 | 0.155 | 0.132 | -0.291 | 1.575 | 0.115 | -3.65 | 0.132 |
| HbA1c | 0.313 | 0.754 | 0.02 | 0.8 | 1.358 | 0.174 | 0.723 | 0.005 | |
| PPBG | -0.942 | 0.346 | -0.09 | 0 | 0 | 1 | -0.07 | -0.09 | |
| Lipid profile | LDL | -0.942 | 0.346 | -0.09 | 0 | 0 | 1 | -0.07 | -0.09 |
| Triglycerides | -0.703 | 0.481 | -0.058 | -0.15 | 0.45 | 0.652 | -0.143 | -0.058 | |
| Total cholesterol | -2.103 | 0.035 | -0.148 | -0.114 | 0.417 | 0.676 | -0.219 | -0.148 | |
| Body composition | WC | -1.445 | 0.148 | -0.036 | -0.394 | 2.028 | 0.042 | -1.814 | -0.036 |
| Body weight | 2.108 | 0.035 | 0.248 | -0.088 | 0.357 | 0.72 | -5.014 | 0.248 | |
| Blood pressure | SBP | -1.656 | 0.097 | -0.053 | -0.285 | 1.484 | 0.137 | -1.749 | -0.053 |
| DBP | -0.02 | 0.983 | 0.009 | 0.171 | 0.89 | 0.373 | -0.439 | 0.009 | |
FBG: Fasting Blood Glucose; ES: Effect Size; CI: Confidence Interval; PPBG: Postprandial Blood Glucose; LDL: Low-Density Lipoprotein; WC: Waist Circumference; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure.
Classic fail-safe N
This meta-analysis incorporates data from 15 studies, which yield a z-value of -1.44545 and a corresponding 2-tailed p-value of 0.14833. Although the pooled result is not statistically significant, the Fail-Safe N -which addresses the concern that the observed significance may be spurious is not relevant.
Orwin fail-safe N
First, the mean STD difference in means in the added studies can be a value other than the nil value 0. Second, the criterion value is an effect size rather than a p-value. That is, the Orwin fail-safe N is the number of added studies that will shift the combined STD difference in means past a specified threshold from the starting point of 0.
Begg and Mazumdar rank correlation test
The Kendall's tau b is -0.39423, with a 1-tailed p-value of 0.02123 or a 2-tailed p-value of 0.04246.
Egger's test of the intercept
The Eggers bias indicator test predicted that the intercept (B0) is -1.81427, 95% confidence interval (-3.67106, 0.04253), with t = 2.11089, df = 13. The 1-tailed p-value (recommended) is 0.02736, and the 2-tailed p-value is 0.05472.
Duval and Tweedie's Trim and Fill
The test is in search of missing studies dependent on a fixed effect model and represents missing studies only to the left side of the mean intervention effect. Using these parameters, the method suggests that no studies are missing. Under the fixed effect model, the point estimate and 95% confidence interval for the combined studies is 0.13277 (0.04490, 0.22064). Using Trim and Fill the imputed point estimate is the same value above. Under the random effects model, the point estimate and 95% confidence interval for the combined studies is 0.02255(-0.20574, 0.25084). Using Trim and Fill the imputed point estimate is the same value as mentioned above.
Discussion
Summary of the key finding
We conducted as a systematic and meta-analysis to explore the association between yoga intervention and prediabetes state. This study was designed to address whether yoga delays or prevent the progression of diabetes in prediabetic population especially compared to exercise. The meta-analysis examined whether yoga improves glycemic control, lipid profile, body composition and blood pressure in prediabetes individuals. Fasting blood glucose, LDL, triglycerides, total cholesterol and systolic blood pressure were reduced by 0.064 mm Hg, 0.090 mg/dL, 0.148 mg/dL, 0.058 mg/dL, and 0.058 mmHg respectively.
Consistent with previous systematic review and meta-analysis
To our knowledge, no previous systematic review and meta-analysis have investigated the effect of yoga intervention on the prediabetic state. Therefore, our findings in the prediabetic population are consistent with studies that reported the effects of yoga practice on T2DM. Results on fasting plasma glucose (FPG) levels and lipid profiles are similar to studies that report a general effect of Yoga in diabetic patients [38, 63]. The findings of this systematic review on yoga on prediabetes included 12 RCTs and two non-RCTs and show that a yoga intervention could improve fasting blood glucose, lipid profiles and systolic blood pressure. More specifically, yoga intervention lowered lipid profiles such as LDL, TC, and triglycerides. Similarly, a previously published qualitative review on yoga for T2DM that included 4 RCTs and 21 non-RCTs concluded that yoga might improve glucose tolerance, lipid profiles (LDL, TC), anthropometric measures, and blood pressure in T2DM [64].
Consistent with the previous meta-analysis, Cui and colleagues demonstrated, in their meta-analysis with 12 RCTs with a total of 864 patients, that yoga could significantly decrease FBG, PPBG, HbA1c, TC and LDL levels [11]. Eun and Doi showed in a meta-analysis with 11 RCTs with a total of 993 participants that yoga intervention improves glycemic control and lipid profiles in T2DM [63]. Similar findings were observed in Thind and colleagues’ meta-analysis study on 23 studies investigating glycemic control, lipid profile, blood pressure and body mass index [44]. Kumar and colleagues showed similar observations with 17 studies focusing on three parameters of glycemic control [43]. Jayawardena and colleagues demonstrated that significant reduction in FBG, PPBG, HbA1c and BMI alter in 'Yoga group compared to the control group; however, they did not observe any significant difference between the two groups on lipid parameters, other body composition measures (WC and WHR) and Blood Pressure [65].
Applicability of evidence
The included studies in the meta-analysis were obtained from the currently available pool of research, in this field and included 12 RCTs and two non-RCTs. There were four studies from the USA, two studies from China, 1 study from Hongkong, six studies from India and 1 study from Sweden. The studies were drawn using the defined inclusion and exclusion criteria. This meta-analysis included studies with diverse age and ranged from 18 to 75 years old. The studies included 285 men and 413 women. The conclusive findings apply to diverse population and age groups.
Approximately 50% of studies [49, 66–70] and the first author from three studies that were conducted in other countries [71–73], were of Indian origin. Because yoga is centered part of Indian tradition and culture, the intervention would be more effective in this nation. Indeed, the implementation of yoga might be different India. For example, Kumar and colleagues revealed that Indian experimental studies had offered yoga intervention for six days a week in the majority of cases whereas studies performed outside countries delivered their intervention on two days per week [43]. Cramer and colleagues highlighted that the key reasons for the positive clinical outcomes and performance in Indian yoga studies could be that yoga interventions are more intense compared to non-Indian trials. They also added that the key reason for better performance and positive outcomes in Indian yoga studies could be the difference in skills of the yoga trainer trained and practising in India [74]. The findings from this systematic review and meta-analysis could apply to the clear majority of healthy as well as to non-diabetic people with a high risk for T2DM in clinical practice.
Quality of evidence
In this meta-analysis and subgroup analysis, we determined whether the outcome measures FBG, PPBG, HbA1c, TC, Triglycerides, LDL, waist circumferences, Body weight, SBP, and DBP from different studies exhibited clinical and statistical heterogeneity across the studies. Therefore, the estimated effect size of the standard mean difference between yoga interventions and control conditions for prediabetes state were calculated by using random-effects models in our analysis.
The quality assessment of these included studies was performed using the Jadad score and investigated key rigorous study parameters such as randomisation, blinding, allocation concealment, usage of multiple interventions and adjustment for confounders, dropouts and withdrawals. The included studies exhibited diverse parameters in this systematic review, indicating a moderate to high-quality level score [63, 75, 76]. Publication bias for ten different outcomes of T2DM management in prediabetes state was investigated using six different tools including inverted funnel plot, Orwin fail-safe N test, Classic fail-safe N test, Begg and Mazumdar Rank Correlation test, Harbords-Eggers test of intercept and Duval and Tweedie’s Trim and Fill method. Publication bias can compromise the integrity of any meta-analysis. Without assessing publication bias could be conveyed as null results or missing studies. Our findings showed that the publication bias was unlikely to occur in this meta-analysis. However, in two outcomes measures including body weight and total cholesterol, the number of missing studies would bring p-value >0.05 was 2, and thus these parameters might exhibit publication bias.
Strengths and limitations
This systematic review and meta-analysis are the first to explore the impact of a yoga intervention for the prediabetes state. This systematic review and meta-analysis were performed by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [40], and the protocol has been registered in the International prospective register of systematic reviews (PROSPERO). The included studies comprised of 12 RCTs (one three-arm RCT, two arm RCTs and 9 RCTs) and two non-RCTs. These studies investigated a diverse population with a wide range of age groups. In this systematic review and meta-analysis, clinical applicability and quality of evidence of the included studies were compared with ten different outcome measures between yoga and control conditions.
The interpretation of the effect size of glycemic control and other three key outcomes is limited by the non-availability and insufficient reporting of PPBG, HbA1c and other outcome measures. These issues might influence the inadequate definition of subgroups, substantial differences in interventions, control conditions, and outcome assessment [77, 78].
The follow-up duration in the RCT in most of the included studies ranged from 4 weeks to 1 year. The authors reported limited information about RCT study participant’s withdrawals and lost to follow. The outcome measures of the included studies were primarily calculated after follow-up. No reporting of intervention fidelity or inadequate reporting of baseline measures and demographic characteristics of participants (sample size) were observed. Two RCTs have reported three phases of yoga: Asanas, Pranayama and meditation. However, other information on yoga such as yoga postures, style of yoga (i.e. Vinyasa, Ashtanga, Iyengar, Kripalu, and Kundalini), intensity and duration of individual yoga asana or posture, type and duration of mediation were not reported in detail [44].
Future recommendations
Future studies must follow CONSORT statement for RCTs and TREND statement for non-RCTs to avoid inadequate reporting of sample size, randomization, allocation concealment, intention-to-treat analysis, and blinding of at least outcome assessors [44, 79, 80]. Thind and colleagues proposed that future studies should also report and record any adverse effects or injuries associated with yoga intervention [44].
Only a single study investigated the effects of yoga on oxidative stress [49], cortisol [81], inflammatory marker [67], or biometric measures [71], with only two studies available on anthropometric measures [66, 68]. All these parameters have been demonstrated to be important factors for cardiovascular (what?) and T2DM [74]. Therefore, future studies should consider evaluating these measures in their assessment. Future research focuses on yoga interventions with an emphasis on asanas, pranayama and meditation in addition to sathivka (healthy vegetarian diet) diet and yogic life-style [74, 82].
Conclusion
This meta-analysis of yoga for diabetes and associated factors revealed evidence for the potential clinically important effect of yoga on ameliorating diabetes factors such as fasting blood glucose, postprandial blood glucose, body weight, systolic blood, diastolic blood pressure, LDL, triglycerides, total cholesterol and HbA1c. However, well-designed RCTs are needed to examine the long-term efficacy of yoga intervention not only for the risk of T2DM but also metabolic syndrome and cardiovascular disease.
Supporting information
(DOC)
Abbreviations
- CI
Confidence Interval
- CMA
Comprehensive Meta-Analysis
- DBP
Diastolic Blood Pressure
- df
degrees of freedom
- FBG
Fasting Blood Glucose
- HbA1c
Hemoglobin A1c
- HDL
High Density Lipoprotein
- LDL
Low-Density Lipoprotein
- PICOS
Participants, Intervention, Control, Outcomes
- PPBS
Post-Prandial Blood Glucose
- PRISMA
Preferred Reporting Items for Systematic Review and Meta-Analysis
- PROSPERO
International prospective register of systematic reviews
- RCTs
Randomized Controlled Trials
- SBP
Systolic Blood Pressure
- SMD
Standardized Mean Difference
- T2DM
Type 2 diabetes mellitus
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
No funding support was received for this manuscript preparation.
References
- 1.care ADAJD. Diagnosis and classification of diabetes mellitus. 2014;37(Supplement 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 2.Organization WH. Global report on diabetes: World Health Organization; 2016.
- 3.Organization WH. Diabetes 2018 [cited 2019 19/01/2019]. https://www.who.int/news-room/fact-sheets/detail/diabetes.
- 4.Violi F, Targher G, Vestri A, Carnevale R, Averna M, Farcomeni A, et al. Effect of aspirin on renal disease progression in patients with type 2 diabetes: A multicenter, double-blind, placebo-controlled, randomized trial. The renaL disEase progression by aspirin in diabetic pAtients (LEDA) trial. Rationale and study design. 2017;189:120–7. [DOI] [PubMed] [Google Scholar]
- 5.Black PH JB, behavior, immunity. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. 2003;17(5):350–64. [DOI] [PubMed] [Google Scholar]
- 6.Heraclides A, Chandola T, Witte DR, Brunner EJ JDc. Psychosocial stress at work doubles the risk of type 2 diabetes in middle-aged women: evidence from the Whitehall II study. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali VJA. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? 2000;148(2):209–14. [DOI] [PubMed] [Google Scholar]
- 8.Delamater AM, Jacobson AM, Anderson B, Cox D, Fisher L, Lustman P, et al. Psychosocial therapies in diabetes: report of the Psychosocial Therapies Working Group. 2001;24(7):1286–92. [DOI] [PubMed] [Google Scholar]
- 9.Faulenbach M, Uthoff H, Schwegler K, Spinas G, Schmid C, Wiesli P JDM. Effect of psychological stress on glucose control in patients with type 2 diabetes. 2012;29(1):128–31. [DOI] [PubMed] [Google Scholar]
- 10.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, et al. Population-based incidence rates and risk factors for type 2 diabetes in white individuals: the Bruneck study. 2004;53(7):1782–9. [DOI] [PubMed] [Google Scholar]
- 11.Cui J, Yan JH, Yan LM, Pan L, Le JJ, Guo YZ JJodi. Effects of yoga in adults with type 2 diabetes mellitus: A meta-analysis. 2017;8(2):201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falci L, Shi Z, Greenlee H JPcd. Peer Reviewed: Multiple Chronic Conditions and Use of Complementary and Alternative Medicine Among US Adults: Results From the 2012 National Health Interview Survey. 2016;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medagama AB, Bandara R JNj. The use of complementary and alternative medicines (CAMs) in the treatment of diabetes mellitus: is continued use safe and effective? 2014;13(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mooventhan A, Nivethitha L JJob, therapies m. Evidence based effects of yoga practice on various health related problems of elderly people: A review. 2017;21(4):1028–32. [DOI] [PubMed] [Google Scholar]
- 15.Singh K JIJoADS. Effect of yoga on dental care: Pranayama techniques or rhythmic breathing exercises on the oral hygiene and gingival bleeding. 2017;3(3):91–5. [Google Scholar]
- 16.Chandler K. The emerging field of yoga therapy. 2001. [PubMed]
- 17.Liu X-C, Pan L, Hu Q, Dong W-P, Yan J-H, Dong L JJotd. Effects of yoga training in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. 2014;6(6):795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raub JA JTJoA, Medicine C. Psychophysiologic effects of Hatha Yoga on musculoskeletal and cardiopulmonary function: a literature review. 2002;8(6):797–812. [DOI] [PubMed] [Google Scholar]
- 19.Damodaran A, Malathi A, Patil N, Shah N, Marathe S JTJotAoPoI. Therapeutic potential of yoga practices in modifying cardiovascular risk profile in middle aged men and women. 2002;50(5):633–40. [PubMed] [Google Scholar]
- 20.Garfinkel M, Schumacher HR Jr JRDCoNA. Yoga. 2000;26(1):125–32. [DOI] [PubMed] [Google Scholar]
- 21.Pandya DP, Vyas VH, Vyas SH JCt. Mind-body therapy in the management and prevention of coronary disease. 1999;25(5):283–93. [DOI] [PubMed] [Google Scholar]
- 22.Sahay B, Sahay RK JJotIMA. Lifestyle modification in management of diabetes mellitus. 2002;100(3):178–80. [PubMed] [Google Scholar]
- 23.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. 2016;39(11):2065–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katula JA, Kirk JK, Pedley CF, Savoca MR, Effoe VS, Bell RA, et al. The Lifestyle Intervention for the Treatment of Diabetes study (LIFT Diabetes): design and baseline characteristics for a randomized translational trial to improve control of cardiovascular disease risk factors. 2017;53:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders AB. Effectiveness of a low dose behavior change intervention on physical activity maintenance following an exercise trial in pre-type II diabetics: Colorado State University. Libraries; 2017.
- 26.Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk CJ Aoim. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. 2013;159(8):543–51. [DOI] [PubMed] [Google Scholar]
- 27.Hardie EA, Critchley CR, Moore SM JAP. Prediabetes Subtypes: Patterns of risk, vulnerabilities, and intervention needs. 2015;50(6):455–63. [Google Scholar]
- 28.O’Dea A, Tierney M, McGuire BE, Newell J, Glynn LG, Gibson I, et al. Can the onset of type 2 diabetes be delayed by a group-based lifestyle intervention in women with prediabetes following gestational diabetes mellitus (GDM)? Findings from a randomized control mixed methods trial. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon L, Morrison EY, McGrowder DA, Young R, Garwood D, Zamora E, et al. Changes in clinical and metabolic parameters after exercise therapy in patients with type 2 diabetes. 2008;4(4):427–37. [Google Scholar]
- 30.Gordon LA, Morrison EY, McGrowder DA, Young R, Fraser YTP, Zamora EM, et al. Effect of exercise therapy on lipid profile and oxidative stress indicators in patients with type 2 diabetes. 2008;8(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jyotsna V, Dhawan A, Sreenivas V, Deepak K, Singla R JIjoe, metabolism. Completion report: Effect of Comprehensive Yogic Breathing program on type 2 diabetes: A randomized control trial. 2014;18(4):582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyizom T, Singh S, Singh K, Tandon O, Kumar R JIJoMR. Effect of pranayama & yoga-asana on cognitive brain functions in type 2 diabetes-P3 event related evoked potential (ERP). 2010;131(5). [PubMed] [Google Scholar]
- 33.Popli U, Subbe CP, Sunil K JAtih, medicine. The Role of Yoga as a Lifestyle Modification in Treatment of Diabetes Mellitus: Results of a Pilot Study. 2014;20(6):24–6. [PubMed] [Google Scholar]
- 34.Shantakumari N, Sequeira S JIhj. Effects of a yoga intervention on lipid profiles of diabetes patients with dyslipidemia. 2013;65(2):127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh S, Kyizom T, Singh K, Tandon O, Madhu S JIJoCB. Influence of pranayamas and yoga-asanas on serum insulin, blood glucose and lipid profile in type 2 diabetes. 2008;23(4):365–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skoro-Kondza L, Tai SS, Gadelrab R, Drincevic D, Greenhalgh T JBhsr. Community based yoga classes for type 2 diabetes: an exploratory randomised controlled trial. 2009;9(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander GK, Taylor AG, Innes KE, Kulbok P, Selfe TK JF, health c. Contextualizing the effects of yoga therapy on diabetes management: a review of the social determinants of physical activity. 2008;31(3):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aljasir B, Bryson M, Al-shehri B JE-BC, Medicine A. Yoga practice for the management of type II diabetes mellitus in adults: a systematic review. 2010;7(4):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Adhikari P, Jeganathan P JIJoAM, Yoga, 5. Biopsychosocial effects of Yoga in patients with diabetes: A focused review. 2011;11. [Google Scholar]
- 40.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raveendran AV, Deshpandae A, Joshi SR JE, Metabolism. Therapeutic Role of Yoga in Type 2 Diabetes. 2018;33(3):307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Innes KE, Selfe TK JJodr. Yoga for adults with type 2 diabetes: a systematic review of controlled trials. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar V, Jagannathan A, Philip M, Thulasi A, Angadi P, Raghuram N JCTiM. Role of yoga for patients with type II diabetes mellitus: a systematic review and meta-analysis. 2016;25:104–12. [DOI] [PubMed] [Google Scholar]
- 44.Thind H, Lantini R, Balletto BL, Donahue ML, Salmoirago-Blotcher E, Bock BC, et al. The effects of yoga among adults with type 2 diabetes: A systematic review and meta-analysis. 2017;105:116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Björntorp P, Holm G, Rosmond R JDm. Hypothalamic arousal, insulin resistance and type 2 diabetes mellitus. 1999;16(5):373–83. [DOI] [PubMed] [Google Scholar]
- 46.Ljung T, Holm G, Friberg P, Andersson B, Bengtsson BÅ, Svensson J, et al. The activity of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in relation to waist/hip circumference ratio in men. 2000;8(7):487–95. [DOI] [PubMed] [Google Scholar]
- 47.Tsigos C, Young RJ, White A JTJoCE, Metabolism. Diabetic neuropathy is associated with increased activity of the hypothalamic-pituitary-adrenal axis. 1993;76(3):554–8. [DOI] [PubMed] [Google Scholar]
- 48.Waryasz GR, McDermott AY JJotAaoNP. Exercise prescription and the patient with type 2 diabetes: a clinical approach to optimizing patient outcomes. 2010;22(4):217–27. [DOI] [PubMed] [Google Scholar]
- 49.Hegde SV, Adhikari P, Shetty S, Manjrekar P, D'Souza V JCtim. Effect of community-based yoga intervention on oxidative stress and glycemic parameters in prediabetes: a randomized controlled trial. 2013;21(6):571–6. [DOI] [PubMed] [Google Scholar]
- 50.Vaishali K, Kumar KV, Adhikari P, UnniKrishnan B JP, Geriatrics OTi. Effects of yoga-based program on glycosylated hemoglobin level serum lipid profile in community dwelling elderly subjects with chronic type 2 diabetes mellitus–a randomized controlled trial. 2012;30(1):22–30. [Google Scholar]
- 51.Moher D, Liberati A, Tetzlaff J, Altman DG JAoim. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 52.Ramamoorthi R, Gahreman D, Moss S, Skinner T JM. The effectiveness of yoga to prevent diabetes mellitus type 2: A protocol for systematic review and meta-analysis. 2019;98(3):e14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark HD, Wells GA, Huët C, McAlister FA, Salmi LR, Fergusson D, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. 1999;20(5):448–52. [DOI] [PubMed] [Google Scholar]
- 54.Hardy RJ, Thompson SG JSim. Detecting and describing heterogeneity in meta-analysis. 1998;17(8):841–56. [DOI] [PubMed] [Google Scholar]
- 55.Orwin RG JJoes. A fail-safe N for effect size in meta-analysis. 1983;8(2):157–9. [Google Scholar]
- 56.Rothstein HR JJoEC. Publication bias as a threat to the validity of meta-analytic results. 2008;4(1):61–81. [Google Scholar]
- 57.Begg CB, Mazumdar MJB. Operating characteristics of a rank correlation test for publication bias. 1994:1088–101. [PubMed] [Google Scholar]
- 58.Duval S, Tweedie RJ JotASA. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. 2000;95(449):89–98. [Google Scholar]
- 59.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenthal R. The file drawer problem and tolerance for null results. Psychological bulletin. 1979;86(3):638. [Google Scholar]
- 61.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994:1088–101. [PubMed] [Google Scholar]
- 62.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. [DOI] [PubMed] [Google Scholar]
- 63.Eun CS, Dol KS. Effects of yogic exercise on glycemic control and lipid profiles in Type 2 diabetes: A meta-analysis of randomized controlled trials. 2017. [Google Scholar]
- 64.Innes KE, Vincent HK JE-BC, Medicine A. The influence of yoga-based programs on risk profiles in adults with type 2 diabetes mellitus: a systematic review. 2007;4(4):469–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jayawardena R, Ranasinghe P, Chathuranga T, Atapattu PM, Misra AJD, Research MSC, et al. The benefits of yoga practice compared to physical exercise in the management of type 2 diabetes mellitus: a systematic review and meta-analysis. 2018. [DOI] [PubMed] [Google Scholar]
- 66.Keerthi GS, Pal P, Pal GK, Sahoo JP, Sridhar MG, Balachander J JJoc, et al. Effect of 12 Weeks of Yoga Therapy on Quality of Life and Indian Diabetes Risk Score in Normotensive Indian Young Adult Prediabetics and Diabetics: Randomized Control Trial. 2017;11(9):CC10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Netam R, Yadav RK, Khadgawat R, Sarvottam K, Yadav R JTIjomr. Interleukin-6, vitamin D & diabetes risk-factors modified by a short-term yoga-based lifestyle intervention in overweight/obese individuals. 2015;141(6):775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tyagi A, Cohen M, Reece J, Telles S JBc, medicine a. An explorative study of metabolic responses to mental stress and yoga practices in yoga practitioners, non-yoga practitioners and individuals with metabolic syndrome. 2014;14(1):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDermott KA, Rao MR, Nagarathna R, Murphy EJ, Burke A, Nagendra RH, et al. A yoga intervention for type 2 diabetes risk reduction: a pilot randomized controlled trial. 2014;14(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yadav R, Yadav RK, Khadgawat R, Pandey RM JTbm. Comparative efficacy of a 12 week yoga-based lifestyle intervention and dietary intervention on adipokines, inflammation, and oxidative stress in adults with metabolic syndrome: a randomized controlled trial. 2018. [DOI] [PubMed] [Google Scholar]
- 71.Sohl SJ, Wallston KA, Watkins K, Birdee GS JE-BC, Medicine A. Yoga for risk reduction of metabolic syndrome: Patient-reported outcomes from a randomized controlled pilot study. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kanaya AM, Araneta MRG, Pawlowsky SB, Barrett-Connor E, Grady D, Vittinghoff E, et al. Restorative yoga and metabolic risk factors: the Practicing Restorative Yoga vs. Stretching for the Metabolic Syndrome (PRYSMS) randomized trial. 2014;28(3):406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Supriya R, Yu AP, Lee PH, Lai CW, Cheng KK, Yau SY, et al. Yoga training modulates adipokines in adults with high-normal blood pressure and metabolic syndrome. 2018;28(3):1130–8. [DOI] [PubMed] [Google Scholar]
- 74.Cramer H, Lauche R, Langhorst J, Dobos G JCct. Are Indian yoga trials more likely to be positive than those from other countries? A systematic review of randomized controlled trials. 2015;41:269–72. [DOI] [PubMed] [Google Scholar]
- 75.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 76.Saffari M, Ghanizadeh G, Koenig HG JPcd. Health education via mobile text messaging for glycemic control in adults with type 2 diabetes: a systematic review and meta-analysis. 2014;8(4):275–85. [DOI] [PubMed] [Google Scholar]
- 77.Collaboration C. Cochrane handbook for systematic reviews of interventions: Cochrane Collaboration; 2008.
- 78.Higgins JP, Thompson SG, Deeks JJ, Altman DG JBBMJ. Measuring inconsistency in meta-analyses. 2003;327(7414):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schulz KF, Altman DG, Moher D JAoim. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. 2010;152(11):726–32. [DOI] [PubMed] [Google Scholar]
- 80.Des Jarlais DC, Lyles C, Crepaz N, health TGJAjop. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. 2004;94(3):361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corey SM, Epel E, Schembri M, Pawlowsky SB, Cole RJ, Araneta MRG, et al. Effect of restorative yoga vs. stretching on diurnal cortisol dynamics and psychosocial outcomes in individuals with the metabolic syndrome: the PRYSMS randomized controlled trial. 2014;49:260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ornish D, Brown SE, Billings J, Scherwitz L, Armstrong WT, Ports TA, et al. Can lifestyle changes reverse coronary heart disease?: The Lifestyle Heart Trial. 1990;336(8708):129–33. [DOI] [PubMed] [Google Scholar]
- 83.Lau C, Yu R, Woo J JPO. Effects of a 12-week hatha yoga intervention on metabolic risk and quality of life in Hong Kong Chinese adults with and without metabolic syndrome. 2015;10(6):e0130731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siu PM, Angus PY, Benzie IF, Woo J JD, syndrome m. Effects of 1-year yoga on cardiovascular risk factors in middle-aged and older adults with metabolic syndrome: a randomized trial. 2015;7(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolff M, Sundquist K, Lönn SL, Midlöv P JBcd. Impact of yoga on blood pressure and quality of life in patients with hypertension–a controlled trial in primary care, matched for systolic blood pressure. 2013;13(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang K, Bernardo LM, Sereika SM, Conroy MB, Balk J, Burke LE JE-BC, et al. Utilization of 3-month yoga program for adults at high risk for type 2 diabetes: a pilot study. 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.