Mindfulness-based therapy changes brain responses to drug cues and healthy natural rewards in chronic opioid users.
Abstract
Addiction neuroscience models posit that recurrent drug use increases reactivity to drug-related cues and blunts responsiveness to natural rewards, propelling a cycle of hedonic dysregulation that drives addictive behavior. Here, we assessed whether a cognitive intervention for addiction, Mindfulness-Oriented Recovery Enhancement (MORE), could restructure reward responsiveness from valuation of drug-related reward back to valuation of natural reward. Before and after 8 weeks of MORE or a support group control, prescription opioid users (N = 135) viewed opioid and natural reward cues while an electroencephalogram biomarker of target engagement was assessed. MORE was associated with decreased opioid cue-reactivity and enhanced capacity to regulate responses to opioid and natural reward cues. Increased positive affective responses to natural reward cues were associated with decreased craving and mediated MORE’s therapeutic effects on opioid misuse. This series of randomized experiments provide the first neurophysiological evidence that an integrative behavioral treatment can remediate hedonic dysregulation among chronic opioid users.
INTRODUCTION
Currently, the United States is in the midst of an opioid crisis. In 2015, 91.8 million U.S. adults used prescription opioids, and among these individuals, 16.7% reported an opioid use disorder (OUD) (1). The opioid crisis confronting the United States is thought to have emerged in large part from misuse of opioid analgesic medications prescribed for pain management. Chronic opioid use may render vulnerable individuals at risk for developing opioid misuse and OUD due to neuropsychopharmacological effects of opioids on reward processing and hedonic regulation in the brain (2).
Allostatic models have been advanced to explicate the downward spiral leading to opioid misuse and OUD (3, 4). These models posit that prolonged opioid use may shift hedonic set points in corticostriatal circuitry mediating reward and disrupt capacity to proactively regulate responses to emotional stimuli. Consequently, addiction involves a process of hedonic dysregulation, in which the motivation to obtain natural rewards is reorganized around seeking drug-associated reward and the desire to alleviate aversive states (e.g., stress and pain) (5). During the process of hedonic dysregulation, chronic use of drugs of abuse, including opioids, produces neurobiological alterations that increase the incentive salience of drug-related cues (6), resulting in heightened drug cue-reactivity and craving while, at the same time, decreasing sensitivity to natural reward derived from homeostatic goal attainment (7). The downward shift in salience of natural reward relative to drug reward may represent a crucial tipping point leading to the loss of control over drug use that is characteristic of addiction.
Hedonic dysregulation can be indexed by neurophysiological responses measured at the scalp via event-related potential (ERP) analysis of the electroencephalogram (EEG) and, specifically, by the late positive potential (LPP) of the EEG. Bottom-up processing of motivationally salient stimuli elicits a larger LPP than neutral stimuli that tends to reach maximum amplitude along the midline at centroparietal sites (Cz and Pz) between 400 and 800 ms after image onset (8). Simultaneous EEG and functional magnetic resonance imaging (fMRI) recordings demonstrate that the magnitude of the LPP during processing of motivationally salient stimuli is associated with blood oxygen level–dependent (BOLD) activity in emotion-processing structures like the amygdala, insula, and orbitofrontal cortex (9). Unlike earlier ERP components such as N200 and P300, thought to reflect cognitive control, novelty detection, and initial attentional orienting to an unexpected stimulus (10, 11), the LPP is theorized to reflect sustained, motivated attention to the emotional features of a stimulus (8). In that regard, LPP amplitude remains significantly larger for emotional stimuli than neutral stimuli to at least 1500 ms (12) and is robustly correlated with subjective ratings of arousal in response to emotional images (13). Furthermore, the LPP is subject to top-down regulation (14): that is, the proactive use of cognitive processes to increase (up-regulation) or decrease responses (down-regulation) to a motivationally salient stimulus. Attempts to consciously regulate responding to emotional stimuli result in amplitude changes in the LPP that reflect regulatory efficacy—with attentional deployment regulatory strategies operating as early as 700 to 900 ms and evaluative emotion regulation strategies operating as late as 1500 ms (14).
Individuals with substance use disorders exhibit a greater LPP to drug-related cues than to neutral cues and cues representing natural rewards (15). LPP indices of drug cue-reactivity predict increased craving (16) and reverse after a period of extended abstinence (17). Thus, the LPP represents an EEG biomarker of clinical target engagement in addiction treatment. Moreover, ERPs have been used to assess hedonic dysregulation among individuals with OUD who exhibit blunted ERPs to stimuli representing natural rewards relative to ERPs to drug cues (18). This decreased responsiveness to natural reward relative to drug reward significantly predicts future opioid consumption (19). Hence, efficacious addiction therapies that aim to down-regulate drug cue-reactivity and up-regulate responsiveness to natural rewards should modulate the LPP as evidence of target engagement.
Thus far, few studies have examined the effect of psychosocial therapies on neural indices of drug cue-reactivity; a recent review identified only four small randomized controlled trials (RCTs) and none in chronic prescription opioid users (20). Among psychosocial treatments, mindfulness-based interventions might be especially promising means of reducing drug cue-reactivity, given their established efficacy in reducing substance use and misuse (21). Neurocognitive models of mindfulness-centered regulation of addictive responses suggest that mindfulness-based interventions might decrease bottom-up cue-reactivity responses and improve top-down regulation of these responses (22). To date, these hypotheses have been tested in only two studies: a laboratory-based, within-subjects study, which found that a mindfulness induction reduced smoking cue-reactivity in the subgenual anterior cingulate cortex (23), and a quasi-experimental study, which found that a multiweek mindfulness-based intervention significantly reduced smoking cue-reactivity in the striatum (24). To our knowledge, the effects of mindfulness-based interventions on neurophysiological markers of drug cue-reactivity have not been assessed via randomized controlled pretest-posttest designs.
However, a mindfulness-based intervention for prescription opioid misuse that demonstrated efficacy in two stage 2 RCTs (25, 26), Mindfulness-Oriented Recovery Enhancement (MORE), was shown to significantly decrease opioid cue-reactivity, as manifested by decreased attentional bias (27) and cue-elicited craving responses (28). Similarly, MORE was shown to enhance autonomic (28) and electrocortical (29) responses to natural rewards among prescription opioid users, suggesting that this therapy may effectively target hedonic dysregulation in addiction. MORE is a cognitive training program that integrates skills designed to promote sustained attention to natural rewards with mindfulness and reappraisal techniques. Given its focus on orienting attention away from drug-related cues and toward healthful and socially affiliative objects and events, it is plausible that MORE may shift the relative salience of drug and natural rewards. Our restructuring reward hypothesis states that restructuring reward processing from valuation of drug-related reward back to valuation of natural rewards will reduce craving and addictive behavior (30). Thus, we expect that decreases in drug cue-reactivity and appetitive responses will be paralleled by improved capacity to down-regulate drug cue responses and up-regulate responsiveness to natural rewards.
Here, we methodically investigated the effects of MORE on bottom-up cue-reactivity and top-down regulation of responding to reward-related stimuli in the context of chronic prescription opioid use in an attempt to test the postulates of the restructuring reward hypothesis. To do so, we used experimental tasks in which participants viewed and regulated responses to stimuli representing drug and nondrug natural rewards. We conducted a series of four experiments across three samples of chronic prescription opioid users that in toto were designed to test the restructuring reward hypothesis vis-à-vis chronic use of prescription opioids. In experiment 1, chronic opioid users completed a blocked cue-reactivity task before and after being treated with MORE or an active support group (SG) control condition to determine whether MORE could decrease bottom-up reactivity to opioid cues. In experiment 2, chronic opioid users completed an event-related cue-reactivity task before and after MORE or an SG control to determine whether MORE could enhance down-regulation of opioid cue-reactivity versus passive viewing of opioid cues. In experiment 3, chronic opioid users completed an event-related cue-reactivity task before and after MORE or an SG control to determine whether MORE could enhance up-regulation of neurophysiological responses to naturally rewarding stimuli. In experiment 4, we examined data from a blocked cue-reactivity task implemented as a mechanistic aim from a clinical trial (NCT03298269) to determine whether MORE could enhance subjective emotional responses to naturally rewarding stimuli, and whether doing so was associated with decreases in craving and clinical improvements in opioid misuse behavior.
In 135 chronic opioid users participating across four experiments, we found neurophysiological and behavioral patterns indicating that MORE may reduce opioid cue-reactivity by increasing top-down regulatory control and boosting responsiveness to natural rewards, effects that were associated with reduced addictive behaviors and in line with the restructuring reward hypothesis. We therefore suggest that targeting reward system function with integrative behavioral treatments and other cognitive training interventions may provide the learning signal needed to restore adaptive hedonic regulation and, ultimately, to reverse addiction.
RESULTS
We collected data in four experiments in which chronic opioid users (average duration of opioid use, 10.13 ± 7.17 years; see Table 1 for other demographic and clinical characteristics) performed different versions of a cue-reactivity task (see Materials and Methods). On each trial, participants (N = 135) saw an emotionally salient (i.e., opioid- or natural reward–related) image or neutral image and then were asked to merely view the image or (in the case of an emotionally salient image) to regulate their response to the image. During these tasks, EEG was recorded (see Materials and Methods). The experiments differed with regard to their design parameters (blocked versus event-related design) and their focus on a particular image type (opioid versus natural reward) and whether the regulation condition was central to the subhypothesis under question. On regulation trials in response to opioid-related images, participants were instructed to down-regulate their response to the image by adopting a nonreactive attentional stance toward the image while maintaining meta-awareness of their thoughts, emotions, and body sensations. These instructions parallel those used in other laboratory-based research of the effects of state mindfulness on drug cue-reactivity (23). On regulation trials in response to natural reward–related images, participants were instructed to up-regulate their response to the image by savoring the pleasant, beautiful, or meaningful aspects of the image and immersing themselves in the positive emotions and pleasurable body sensations elicited by the image. These instructions parallel those used in other studies of MORE (24).
Table 1. Demographic and clinical characteristics of the chronic opioid using samples (N = 135).
| Measure | Sample 1 (n = 40) | Sample 2 (n = 31) | Sample 3 (n = 64) |
| Age (mean ± SD) | 55.4 (11.1) | 57.8 (11.3) | 56.7 (10.9) |
| Female, n (%) | 23 (57.5) | 4 (12.9) | 42 (65.6) |
| Race, n (%) | |||
| White or Caucasian | 36 (90.0) | 25 (80.6) | 53 (82.8) |
| Hispanic or Latino | 1 (2.5) | 1 (3.2) | 3 (4.7) |
| Black or African American | 0 | 1 (3.2) | 3 (4.7) |
| Asian | 0 | 1 (3.2) | 0 |
| American Indian/Native Alaskan | 0 | 2 (6.5) | 0 |
| Native Hawaiian/Pacific Islander | 1 (2.5) | 0 | 1 (1.6) |
| Other | 0 | 0 | 1 (1.6) |
| Not reported | 2 (5) | 1 (3.2) | 3 (4.7) |
| Primary pain location, n (%) | |||
| Back pain | 22 (55.0) | 19 (61.3) | 30 (46.9) |
| Hip/leg/foot pain | 3 (7.5) | 2 (6.4) | 8 (12.5) |
| Joint pain | 3 (7.5) | 5 (16.1) | 6 (9.4) |
| Neck/shoulder pain | 6 (15.0) | 1 (3.2) | 4 (6.3) |
| Other pain location | 6 (15.0) | 4 (12.9) | 16 (25.0) |
| Average pain severity (0–10) | 5.2 (1.5) | 5.26 (1.7) | 5.13 (1.5) |
| Primary opioid type, n (%) | |||
| Hydrocodone | 10 (25.0) | 8 (25.8) | 20 (31.3) |
| Oxycodone | 8 (20.0) | 9 (29.0) | 19 (29.7) |
| Tramadol | 10 (25.0) | 10 (32.3) | 10 (15.6) |
| Morphine | 4 (10.0) | 3 (9.7) | 8 (12.5) |
| Methadone or buprenorphine | 8 (20.0) | 1 (3.2) | 2 (3.1) |
| Fentanyl | 0 | 0 | 2 (3.1) |
| Other | 0 | 0 | 2 (4.7) |
| Duration of opioid use in years (mean ± SD) |
10.3 (8.5) | 8.3 (5.7) | 10.9 (7.1) |
| Morphine equivalent daily dose (mean ± SD) |
84.1 (102.4) | 62.3 (131.5) | 71.4 (96.6) |
Verification of successful opioid cue-reactivity at pretreatment via neurophysiological responses
As a prerequisite, we assessed the quality and validity of our experimental cue-reactivity design, irrespective of the effects of treatment. At pretreatment, opioid cues elicited significantly greater centroparietal LPP than neutral cues (t39 = 4.28, P < 0.001), indicating the presence of opioid cue-reactivity in this chronic opioid using sample. However, at pretreatment, LPPs did not significantly differ by group assignment (F1,38 = 0.02, P = 0.89, ηpartial2 < 0.01), indicating that randomization was successful in generating groups that were equivalent at baseline.
Treatment effects on neurophysiological responses to opioid cues
Experiment 1 assessed the effects of treatment on LPP indices of opioid cue-reactivity relative to reactivity to neutral cues (Fig. 1). In repeated-measures analysis of variance (RM-ANOVA) of signal-averaged LPP data, the Group × Time × Stimulus Type interaction indicated significant effects of MORE versus the active SG control condition on LPP opioid cue-reactivity. Relative to the SG control, MORE was associated with significantly greater decreases in LPP response to opioid cues (relative to neutral cues) from pretreatment to posttreatment (F1,37 = 4.89, P = 0.033, ηpartial2 = 0.12). Within-group comparisons indicated that for individuals in the SG, posttreatment LPP activations remained significantly higher in response to opioid cues compared to the neutral cues, suggesting that participants in the SG continued to exhibit opioid cue-reactivity (t20 = 5.93, P < 0.001). In contrast, for individuals in the MORE group, there were no significant differences in posttreatment LPP activations between opioid and neutral cue conditions (t18 = 0.30, P = 0.77).
Fig. 1. Experiment 1: Treatment effects on centroparietal LPP during opioid cue-reactivity.
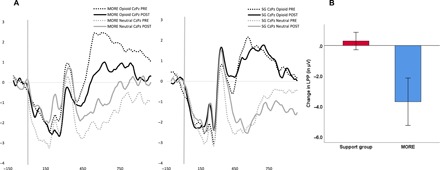
(A) Experiment 1: Treatment by time effects on centroparietal LPP during opioid cue-reactivity. (B) Experiment 1: Change in centroparietal LPP (in μV) index of opioid cue-reactivity (opioid cue − neutral cue) from pretreatment to posttreatment (n = 40).
Treatment effects on the down-regulation of neurophysiological responses to opioid cues
Experiment 2 assessed the effects of treatment on the capacity to down-regulate the LPP response to opioid cues (Fig. 2). In RM-ANOVA of signal-averaged LPP data, the Group × Time × Condition interaction indicated significant effects of MORE versus the active SG control condition on LPP responses (regulate < view) to opioid cues. Relative to those in the SG, participants who were treated with MORE exhibited significantly greater decreases in the LPP response to opioid cues during regulation (regulate < view) from pretreatment to posttreatment (F1,21 = 7.22, P = 0.014, ηpartial2 = 0.26).
Fig. 2. Experiment 2: Treatment effects on centroparietal LPP during down-regulation of opioid cue-reactivity.
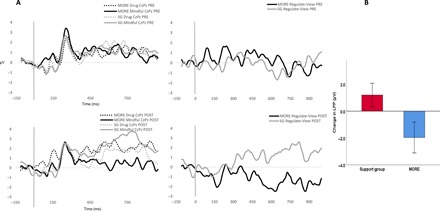
(A) Experiment 2: Treatment by time effects on centroparietal LPP during down-regulation of opioid cue-reactivity. (B) Experiment 2: Change in centroparietal LPP (in μV) index of regulation of opioid cue-reactivity (regulate-view) from pretreatment to posttreatment (n = 24).
Treatment effects on the up-regulation of neurophysiological responses to natural reward cues
Experiment 3 assessed the effects of treatment on the capacity to up-regulate the LPP response to natural reward cues (Fig. 3). In RM-ANOVA of signal-averaged LPP data, the Group × Time × Condition interaction indicated significant effects of MORE versus the active SG control condition on LPP responses (regulate > view) to natural reward cues. Relative to those in the SG, participants who were treated with MORE exhibited significantly greater increases in the LPP response to natural reward cues during regulation (regulate > view) from pretreatment to posttreatment (F1,26 = 4.79, P = 0.038, ηpartial2 = 0.16).
Fig. 3. Experiment 3: Treatment effects on centroparietal LPP during up-regulation of natural reward.
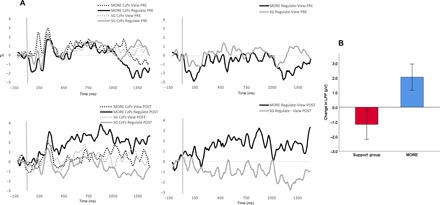
(A) Experiment 3. Treatment by time effects on centroparietal LPP during up-regulation of natural reward. (B) Experiment 3: Change in LPP (in μV) index of regulation of natural reward cue-reactivity (regulate − view) from pretreatment to posttreatment (n = 29).
Regulatory effects on relative responsiveness to drug and natural rewards
To examine the effects of regulation on relative responsiveness to drug and natural rewards, we computed a relative responsiveness measure by subtracting LPP regulatory response to opioid cues from LPP regulatory response to natural reward cues (regulate − view difference scores). Comparable measures of differential responsiveness to drug and natural reward images have been used in previous LPP research in populations with substance use disorders (17). A similar contrast, in which drug approach behavior is assessed in the context of a contemporaneously available natural reward, is used in drug choice paradigms in both humans and animals (31). This contrast was recently shown to predict opioid misuse following behavioral treatment (32) and parallels a key diagnostic criterion for substance use disorder: compulsive use of the drug of choice to the exclusion of other rewarding activities. When compared to those in the SG, participants treated with MORE exhibited significantly greater increases in the LPP measure of relative responsiveness to natural reward relative to drug reward from pretreatment to posttreatment (F1,21 = 10.00, P = 0.005, ηpartial2 = 0.32).
Treatment effects on the regulation of affective and craving responses to natural reward cues
Experiment 4 evaluated affect ratings in response to natural reward cues collected from a sample opioid-treated chronic pain patients participating in a stage 2 RCT of MORE. In RM-ANOVA, the Group × Time interaction indicated significant effects of MORE versus an SG control condition in affect ratings. Relative to those in the SG, participants who were treated with MORE exhibited significantly greater positive affective response to natural reward cues from pretreatment to posttreatment (F1,62 = 6.00, P = 0.017, ηpartial2 = 0.09) (Fig. 4A). However, this increase in positive affective response to natural reward cues did not significantly differ on regulate versus view trials (F1,62 = 1.52, P = 0.22, ηpartial2 = 0.02).
Fig. 4. Experiment 4: Treatment effects on positive affect and craving ratings across view and regulate conditions of the natural reward cue-reactivity task.
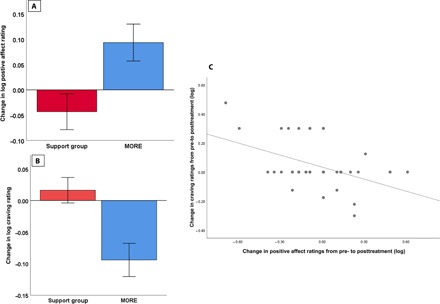
(A) Experiment 4: Change in positive affect ratings (log transformed) across view and regulate conditions of the natural reward cue-reactivity task from pretreatment to posttreatment (n = 64). (B) Experiment 4: Change in craving ratings (log-transformed) during up-regulation of natural reward cue-reactivity (regulate − view) from pretreatment to posttreatment (n = 64). (C) Experiment 4: Associations between treatment-related changes in positive affect reactivity and decreases in craving during up-regulation of responding to natural reward (n = 64). Note that several cases had identical change scores and are therefore represented by a single data point in the scatterplot.
As a secondary outcome of our fourth experiment, we examined the effects of treatment on opioid craving ratings in response to natural reward cues (Fig. 4B). In RM-ANOVA, the Group × Time × Condition interaction indicated that, relative to those in the SG, participants who were treated with MORE exhibited significantly greater decreases in craving from pretreatment to posttreatment during up-regulation of response to natural reward cues from pretreatment to posttreatment (F1,62 = 11.09, P = 0.001, ηpartial2 = 0.15). However, the craving-reducing effect of MORE was only evident during regulation but not during view trials (F1,62 = 1.09, P = 0.30, ηpartial2 = 0.02). Decreases in craving response were associated with increases in positive affective response to natural reward cues from pretreatment to posttreatment (r = −0.41, P = 0.001) (Fig. 4C).
Increases in natural reward responsiveness mediate the effect of MORE on reduced opioid misuse
Also in experiment 4, path analysis revealed that the effect of MORE on decreases in opioid misuse scores was mediated by increases in natural reward responsiveness from pretreatment to posttreatment [B = 1.28; SE = 0.62; P = 0.04; 95% confidence interval (CI), 0.29 to 2.79] (Fig. 5). The overall model explained 30% of the variance in changes in opioid misuse scores measured by the Current Opioid Misuse Measure [COMM; (33)].
Fig. 5. Path analysis demonstrating that the effect of MORE on reducing opioid misuse by 3-month follow-up is mediated by increased positive affective reactivity to natural reward cues.
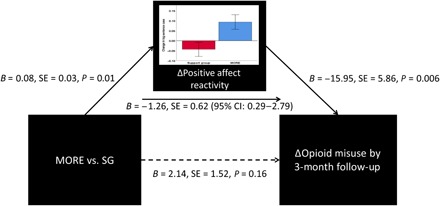
These findings, when combined with the aforementioned craving-reducing effects of up-regulation of responding to natural reward cues, provide direct support for the restructuring reward hypothesis.
Mindfulness practice and restructuring reward
To determine whether more intensive practice of mindfulness-related skills was associated with greater restructuring of neurophysiological and subjective reward processes, we examined correlations between the duration of mindfulness practice and changes in reward-related processes among participants treated with MORE. Total number of minutes of mindfulness practice was associated with decreases in LPP opioid cue-reactivity (r = −0.73, experiment 1), minutes of mindful breathing practice were associated with increases in the capacity to regulate LPP responses to natural reward relative to drug reward (r = 0.63, experiments 2 and 3), and minutes of savoring practice were associated with reductions in craving during up-regulation of responding to reward cues (r = −0.49, experiment 4).
DISCUSSION
For decades, hedonic dysregulation in brain reward circuitry has been considered a core mechanism of addictive behavior (5); therefore, successful addiction treatments should restructure reward processes by decreasing reactivity to drug cues while increasing reactivity to naturally rewarding objects and events in the social environment. However, to date, no treatment approach has demonstrated both of these effects via neurophysiology in a single, randomized controlled study. In the present investigation, we used several variations of a cue-reactivity task to investigate the effects of a mindfulness-based treatment, MORE, on hedonic regulation of responses to drug and natural reward–related cues among chronic prescription opioid users. Here, across four experiments and 135 participants, we reveal neurophysiological and psychological evidence in support of the restructuring reward hypothesis, suggesting that modulating hedonic responses to drug-related and natural rewards may have a therapeutic impact on addiction.
Before treatment, chronic prescription opioid users in the present study exhibited opioid cue-reactivity, evidenced by heightened LPP responses to opioid-related images relative to neutral images. Compared to opioid users in an active control condition, opioid users treated with MORE evidenced significantly reduced opioid cue-reactivity, as revealed by the LPP—an index of attention to emotional information (8). Following 8 weeks of treatment, individuals participating in the control condition continued to show significant LPP opioid cue-reactivity, whereas participants who received the MORE intervention did not exhibit significantly different LPP responses to opioid and neutral images. This attenuated opioid cue-reactivity following treatment with MORE suggests that MORE might decouple associations between conditioned drug cues and their reward value via bottom-up mechanisms. Although MORE significantly modulated the LPP, a known EEG biomarker of clinical target engagement in addiction (15–17), in the present study, we were unable to ascertain whether the observed neurophysiological changes were associated with changes in opioid misuse behaviors.
Furthermore, when participants were instructed to consciously down-regulate opioid cue-reactivity, MORE augmented this capacity, as evidenced by significantly greater attenuations in the LPP following 8 weeks of treatment with MORE than the active control condition. Regulatory instructions in this study corresponded to typical mindfulness training instructions to adopt a state of meta-awareness in which one monitors and accepts thoughts, emotions, and body sensations as they arise without attempting to suppress or sustain them. To control for demand characteristics, regulatory instructions were kept constant across both treatment conditions at both assessment time points, allowing us to isolate the effects of mindfulness training through the MORE intervention from any potential instruction effects. The observed LPP modulations suggest that MORE facilitates top-down, mindfulness-centered regulation of cue-reactivity, a finding that supports prominent neurocognitive models of mindfulness as a treatment for addiction (22). To our knowledge, this study provides the first evidence from an experiment using a randomized, controlled pretest-posttest design that a mindfulness-based intervention can significantly reduce neural indices of drug cue-reactivity.
In parallel with the observed effects on reactivity to drug-related reward stimuli, we obtained neurophysiological evidence that MORE strengthens the capacity to consciously up-regulate responsiveness to natural reward stimuli. At pretreatment, when participants in both conditions were provided the regulatory instruction to savor the pleasant sensory features and positive affective meaning associated with images of natural rewards (e.g., social affiliation, natural beauty, and athletic victories), they were unable to do so, evidenced by little change in LPP response from simply viewing the images. Of note, in previous research, opioid misusing chronic pain patients evidenced an inability to consciously up-regulate responsiveness to natural reward stimuli, as indicated by blunted autonomic responses during savoring (33). However, among healthy controls, focusing attention on the reward value of a stimulus can enhance the LPP in response to that stimulus (34), indicating that such up-regulation is possible. Following 8 weeks of MORE, which involves daily practice of mindful savoring, participants were able to significantly up-regulate the LPP to natural reward cues when savoring relative to passively viewing the images. Because previous research found that MORE was associated with significantly increased LPP reactivity during viewing of natural reward images relative to neutral images (29), we did not opt to replicate this experiment here. Instead, the present randomized controlled experiment extends these findings by suggesting that, in addition to increasing bottom-up reactivity to natural rewards, MORE may strengthen top-down regulatory capacity to consciously amplify natural reward responses—congruent with fMRI findings from a previous quasi-experimental evaluation of MORE (24).
MORE was associated with increased capacity to regulate responsiveness to natural reward images relative to drug-related images. This neurophysiological finding parallels cardiac autonomic evidence from a randomized controlled study of chronic opioid users indicating that MORE shifts relative responsiveness from drug cues to natural reward cues, a shift that predicts decreased opioid misuse following treatment (35). Similarly, among individuals with cocaine use disorder (who exhibit heightened LPP to drug images relative to natural reward images at baseline), longer periods of abstinence are associated with a reversal of this relative responsiveness measure such that motivated attention is allocated more toward natural reward cues than drug cues—a neurophysiological change correlated with decreased craving (17). Furthermore, greater responsiveness to drug cues relative to natural reward cues predicts relapse among individuals with OUD (19). In the context of these previous findings, evidence from the present study that MORE is associated with increased LPP in response to natural reward cues relative to drug cues may have important implications for addiction treatment outcomes among chronic opioid users.
In that regard, for our final experiment in this study, we analyzed data from a mechanistic probe of natural reward responsiveness included in recently completed stage 2 RCT of MORE. In this trial, MORE was shown to significantly reduce opioid misuse risk by 3 months following the end of treatment (26). Here, using a variant of the cue-reactivity task described above, we found evidence that MORE significantly increased positive affective reactivity to natural reward cues from pretreatment to posttreatment, irrespective of any conscious regulatory attempts. In contrast, when participants treated with MORE engaged in conscious up-regulation of responding to natural reward cues by savoring the pleasant aspects of the cue, these regulatory efforts significantly decreased opioid craving from baseline levels—decreases that were associated with increased positive affect reactivity over the course of treatment. In complementary fashion, increases in positive affective reactivity to natural reward cues mediated the effect of MORE on decreasing opioid misuse by 3-month follow-up. Together with the neurophysiological findings described above, study results provide support for the restructuring reward hypothesis, suggesting that shifting valuation from drug-related reward back to valuation of natural reward decreases craving and addictive behavior.
MORE is a sequenced treatment designed to modify associative learning mechanisms hijacked during the allostatic process of addiction by strengthening top-down cognitive control to restructure bottom-up reward learning from valuation of drug reward back to valuation of natural reward. In MORE, patients initially practice mindfulness to build capacity for attention regulation and meta-awareness. Later, mindfulness skills are used to synergize more elaborate therapeutic techniques designed to restructure valuation processes underpinning addiction. In that regard, MORE provides instruction in using mindfulness to disengage attention from addiction-related interoceptive and exteroceptive cues and then shift and sustain attention on the pleasant sensory features of healthful experiences. Mindfulness is also used to metacognitively reflect on positive emotions or higher-order meaning arising in response to rewarding stimuli. This “mindful savoring” technique is intended to broaden and deepen the array of pleasurable sensations derived from the savored experience. Restructuring of reward processing in MORE may arise from restoration of a frontostriatal feedback loop involved in executive control of motivated behavior and reward learning (22). In this regard, dopaminergic and opioidergic systems implicated in salience attribution and hedonic responses are known to be dysregulated in the context of addiction and chronic pain (4). It is possible that MORE would facilitate the functional recovery of these mechanisms. Additional neuroimaging research would now be indicated to localize the neural generators of the observed shifts in reward responsiveness.
Despite its strengths, this study had several limitations. First, this investigation was a mechanistic study—not a clinical trial powered to detect changes in treatment outcomes. However, two stage 2 RCTs demonstrated the efficacy of MORE for reducing opioid misuse among opioid-treated chronic pain patients (25, 26), and a stage 3 RCT of MORE is currently underway. Relatedly, it is unknown whether the observed modulations in LPP responses reflect direct changes in clinical outcomes, in the therapeutic mechanisms that lead to them, or indirect effects that might not be associated with behavioral improvements. As a plausible alternative interpretation of the current study findings, it is possible that the observed modulations of LPP function were due to reductions in opioid intake over the course of the study. Reduction in opioid intake, in turn, might improve the function of the mesolimbic reward system. To account for this possibility, we controlled for posttreatment opioid dose in our ERP analyses, suggesting that the observed effects on reward processing are at least partially independent from the neuropharmacological effects of opioid consumption. In addition, the study was limited by the moderate amount of noise in the ERP data from experiments 2 and 3 due to the modest sample size and the fact that we used relatively few trials per condition. We chose to present a limited number of trials during these two experiments to reduce participant burden because the participants in these experiments—military veterans with opioid-treated chronic pain—were highly vulnerable and therefore unable/unwilling to sit still for a long experimental protocol. Psychometric analyses of the LPP indicate that 12 trials per condition are needed to obtain stable difference waves (36); thus, our experiments were designed to generate LPPs with adequate internal consistency, and despite the noise, we identified a significant signal associated with participation in the MORE intervention. In the future, fully powered replication studies with larger sample sizes and a greater number of trials per condition could generate more stable, reliable estimates of the effects of MORE on LPP responses. The present EEG analysis was also limited in its spatial resolution. Future studies could use high-density EEG arrays (128 or 256 electrodes) to allow better localization of the observed neurophysiological effects of MORE. Last, MORE integrates mindfulness training with reappraisal and savoring techniques, and any of these techniques, separately or in synergy, might restructure reward responsiveness. Future trials could use dismantling designs to parse neural effects of intervention components.
In summary, the present series of experiments suggests that MORE may remediate hedonic dysregulation among chronic opioid users by increasing responsiveness to natural reward while simultaneously decreasing reactivity to drug-related reward. Restructuring reward processing through cognitive training interventions like MORE may modulate core addiction mechanisms by reversing the shift in salience of natural reward relative to drug reward, providing a novel therapeutic target and representing a crucial tipping point to halt the ongoing opioid crisis.
MATERIALS AND METHODS
Participants
Participants (N = 135) were recruited from primary care and pain clinics in Salt Lake City, UT through electronic health record review, opt-out letters, flyers, and radio advertisements. Advertisements recruited individuals who suffered from, and were prescribed medicine for, chronic pain to participate in a study investigating ways to better address problems with chronic pain and prescription pain medication. Participants met study inclusion criteria if they reported chronic noncancer pain on more days than not and had taken opioid analgesics daily or nearly every day for at least the past 90 days. In the combined sample (see Table 1 for demographic and clinical characteristics), participant average duration of opioid use was 10.13 ± 7.17 years, and at baseline, the mean score on the COMM was 11.64 ± 7.68; thus, on average, this was an opioid misusing sample, insofar as participants exhibited mean COMM scores that surpassed a clinically validated cutpoint indicating opioid misuse (COMM ≥ 9) (37). Participants were excluded if they had engaged in previous mindfulness-based intervention or were actively suicidal or psychotic as determined by the Mini-International Neuropsychiatric Interview 6.0 (38). Research procedures were approved by the University of Utah Institutional Review Board. Participants gave their written informed consent, could withdraw from the study at any time, and were financially compensated.
Experiment 1 evaluated EEG data from a mechanistic study of civilian chronic prescription opioid users (n = 40). Experiments 2 and 3 evaluated EEG data from a mechanistic study of U.S. military veterans who were chronic prescription opioid users (n = 31). In this study, several participants were missing pre- or posttreatment data due to hardware problems or inability to continue the assessment due to chronic pain; consequently, the analysis n for experiment 2 was 24, whereas the analysis n for experiment 3 was 29. Experiment 4 evaluated affect and craving ratings from a cue-reactivity task deployed as part of a mechanistic aim from a recently completed stage 2 RCT of civilian chronic prescription opioid users. Individuals with data from this mechanistic task (n = 64) were included in the present investigation.
Experimental design
All four experiments used a randomized controlled pretest-posttest design. At pre- and posttreatment assessments, participants completed a laboratory-based cue-reactivity task (described below). In experiments 1 to 3, EEG was recorded during the task. In experiment 4, self-reported affect and craving ratings were obtained during the task. In experiment 4, participants were also assessed with the COMM (38), a validated measure of opioid misuse risk, at pretreatment and again at 3-month follow-up. Across all four experiments, following the pretreatment assessment, participants were randomly assigned by a project staff member who was uninvolved with assessment or treatment to an 8-week MORE group or SG. Random assignment occurred via a computerized random number table generated by a researcher who was uninvolved in assessment, treatment, or enrollment using simple randomization in blocks of varying sizes (two to four) to preserve unpredictability of allocation. The allocation list was stored in a protected file inaccessible to project staff involved in assessment or treatment. Assessments were conducted by project staff blinded to group assignment (which remained concealed throughout the study). Before each assessment, participants were reminded to not disclose their group assignment to study staff to ensure blinding of study personnel.
Study interventions
Participants were randomly assigned to receive eight weekly sessions of MORE or eight weekly sessions of a therapist-led SG control condition. MORE sessions involved mindfulness training to cultivate self-awareness, cognitive control, and self-transcendence; reappraisal training to facilitate emotion regulation; and training in savoring pleasant events and emotions to enhance natural reward processing and positive affectivity. Per the MORE treatment manual (39), session topics focused on applying mindfulness, reappraisal, and savoring skills to reduce addictive tendencies toward opioids (including opioid cue-reactivity) and promote responsiveness to natural rewards. Mindfulness skills involved mindful breathing and body scan techniques. In MORE, participants were instructed to cultivate mindful awareness of automaticity in the context of opioid use and to shift attention to the sensations of breathing as a means of interrupting automatic, habitual, and compulsive use of opioids. When craving arises during this process, participants were instructed to use mindfulness to deconstruct it into its cognitive, emotional, and sensorial components while metacognitively monitoring these mental phenomena from a psychological distance. Next, participants were instructed to reappraise the meaning of the craving by contemplating the consequences of indulging the craving versus remaining abstinent. Last, participants were instructed to use mindfulness to shift attention away from drug cues and cravings and toward the pleasurable features of naturally rewarding, salutary objects and events as a means of savoring the positive emotions and sensations that arise in response to pleasant life experiences. Participants were also asked to engage in daily 15-min mindfulness, reappraisal, and savoring practice sessions at home guided by an audio recording. In addition, participants were asked to engage in 3 min of mindful breathing before making a decision about whether to take their next opioid dose. This exercise was intended to increase awareness and self-regulation of opioid cue-reactivity. Group sessions were 2 hours long and led by a master’s-level clinical social worker.
The manualized active SG control condition in this study consisted of eight weekly, 2-hour SG sessions, in which a master’s-level social worker facilitated emotional expression and discussion of topics pertinent to chronic pain and opioid use/misuse. This Rogerian, client-centered SG format was based on the evidence-based matrix model intensive outpatient treatment manual and validated as a control condition in a stage 2 RCT of MORE (25). SG participants were asked to engage in 15 min of journaling a day on chronic pain–related themes. To prevent treatment diffusion, participants in the SG condition were instructed to not engage in mindfulness training during the course of the study. A licensed clinician with over 15 years of experience conducted clinical supervision and reviewed session audio recordings to monitor therapist adherence to the MORE and SG treatment manuals and maintain intervention fidelity.
Assessment of opioid cue-reactivity
In experiments 1 and 2, while EEG was recorded (see the “Electrophysiological recordings” section below), participants were presented with opioid and neutral cues. On each trial, participants were first shown a fixation cross for 500 ms, followed by 250- to 500-ms jittered blank screen and then an image and instruction label for 6000 ms. In experiment 1, two opioid cue blocks and one neutral cue block each composed of 40 trials were presented in counterbalanced order, with neutral blocks presented in between opioid blocks, to maximize sensitivity to detect the effects on cue-reactivity. Before formal analyses to test our primary hypothesis in experiment 1 that MORE could affect opioid cue-reactivity, we first tested whether the effects of MORE on the LPP differed on view versus regulate blocks. Treatment groups differed by time on LPP response to neutral versus both opioid cue conditions (P = 0.03), but view and regulate strategies did not significantly differ from one another (P = 0.96). Therefore, our primary analysis in experiment 1 collapsed across all opioid trials to focus on the Group (MORE versus SG) × Time (Pretreatment versus Posttreatment) × Stimulus (Neutral versus Opioid) interaction. In experiment 2, 48 opioid trials were presented in a randomized event-related design to maximize sensitivity to detect the effects of conscious down-regulation (regulate < view) of neurophysiological responses to opioid cues on a trial-by-trial basis. In experiment 2, significant differences between view and regulate strategies were observed and reported above.
Participants were instructed to view or regulate responses to the stimuli. On view trials, participants were instructed to simply attend to images of opioid pills and pill bottles validated in previous studies (27) or neutral images (e.g., household objects) whose basic visual properties were matched to the opioid cues. At baseline, opioid images were rated to be significantly more arousing than neutral images (P = 0.02), providing a manipulation check and demonstrating the validity of the stimuli used in our experimental paradigm. On regulation trials, participants were instructed to observe the image while maintaining nonreactive, nonjudgmental awareness of their thoughts, emotions, and body sensations—using a mindfulness strategy to down-regulate their response to the opioid image. In a training session before EEG assessment, participants practiced this regulatory strategy and described their experience to a trained research assistant to ensure comprehension of the instructions. EEG assessment did not commence until participants could accurately describe implementation of regulatory instruction.
Assessment of natural reward responsiveness
In experiments 3 and 4, participants were presented with images representing natural rewards. On each trial, participants were first shown a fixation cross for 500 ms, followed by 250- to 500-ms jittered blank screen and then an image and instruction label for 6000 ms. In experiment 3, 32 natural reward trials were presented in a randomized event-related design to maximize sensitivity to detect the effects of conscious up-regulation (regulate > view) of neurophysiological responses to natural reward cues on a trial-by-trial basis. In experiment 4, two natural reward cue blocks (regulate versus view) each composed of 40 trials were presented in counterbalanced order.
Participants were instructed to view or regulate responses to stimuli. On view trials, participants were instructed to simply attend to images of naturally rewarding stimuli (e.g., social affiliation, natural beauty, and athletic victories) validated in previous studies (33). On regulate trials, to approximate mindful savoring techniques and conform with typical “increase positive” instructions on emotion regulation tasks (24), participants were instructed to imagine experiencing the positive event occurring in the image and to focus on enjoyable aspects of the image and their own positive emotional response to the image. As above, in a training session before EEG assessment, participants practiced this regulatory strategy and described their experience to a trained research assistant to ensure comprehension of the instructions. EEG assessment did not commence until participants could accurately describe implementation of regulatory instruction.
In experiment 4, at baseline and then after each task block, participants rated their current positive affective state from 1 (not positive at all) to 4 (extremely positive), as well as their current opioid craving from 1 (no craving) to 4 (extreme craving). Self-reported affect and craving ratings were skewed and subsequently log-transformed before analysis. Reactivity scores were computed by subtracting self-reported affect and craving ratings at baseline from ratings following task blocks. These reactivity scores were entered into RM-ANOVA models for analysis.
Electrophysiological recordings
EEGs were continuously recorded using a 32-channel active sensor cap with Ag/AgCl electrodes (actiCap GmbH, Herrsching, Germany). In addition, electro-oculograms (EOG) of vertical eye movements were recorded using actiCHamp sensors (Brain Products GmbH, Gilching, Germany). All recordings were collected using an actiCHamp amplifier and BrainVision Recorder software (version 1.21.0004, Brain Products GmbH, Germany). Data were acquired at a sampling rate of 500 Hz, a resolution of 0.489 μV, and a hardware amplification cutoff of 140 Hz.
Electrophysiological preprocessing
Data from the Cz and Pz midline electrode sites were averaged to compute the centroparietal LPP. Offline processing rereferenced EEG channels with a TP11 mastoid reference/TP9 linked ear clips in BrainVision Analyzer 2.1.2.327 (Brain Products GmbH, Germany). Next, a low cutoff filter of 0.1 Hz, a high cutoff filter of 40 Hz, and a notch filter of 60 Hz were applied offline. Independent component analysis (ICA), specifically a fast ICA restricted algorithm, was executed to semiautomatically remove ocular artifacts from EEG channel recordings. Tasks were segmented and then blocked by trial type for further processing. Artifact rejection was semiautomatic with parameters set as follows: maximal allowed voltage step of 50 μV/ms; maximal allowed absolute difference of values in intervals: 200 μV with a 200-ms interval length; lowest allowed activity in intervals: 0.5 μV with an interval length of 100 ms. All intervals contaminated with muscle, ocular, or non-neuronal electrical activity were marked 200 ms before and after stimulus presentation and removed. Program-flagged artifacts were inspected manually for artifacts. Nine percent of trials were rejected for artifacts in experiment 1, whereas 19% of trials in experiment 2 and 24% of trials in experiment 3 were rejected for artifacts.
ERP grand average waveforms were generated separately for each condition, referenced to a 150-ms prestimulus baseline preceding image onset. To isolate LPP maxima, we examined the morphology of the waveforms in the present study and followed conventions from previous research to define the LPP time window. In experiment 1, we examined the LPP from 400- to 800-ms poststimulus presentation to capture the maximal LPP peak known to be modulated during reactivity to emotionally salient cues (8, 13). In experiment 2, we examined the LPP from 400 to 1000 ms to capture the effects of attention regulation via mindfulness; attention regulation strategies are known to operate between 700 and 900 ms (14). In experiment 3, we examined the LPP from 400 to 1500 ms to capture the effects of evaluative emotion regulation via savoring; evaluative emotion regulation strategies are known to operate as late as 1500 ms (14). The LPP was scored by computing mean activity in microvolts in these windows on each trial. Statistical analyses were conducted on signal-averaged LPP data.
Statistical analysis
Effects of treatment on the signal-averaged LPP response and subjective responses were examined using RM-ANOVA. To ensure the success of our randomization procedure, we first tested whether there were group differences at pretreatment in LPP response. No significant between-groups differences in pretreatment LPP response were observed in experiment 1 (F1,38 = 0.02, P = 0.89, ηpartial2 < 0.01), experiment 2 (F1,22 = 0.06, P = 0.81, ηpartial2 < 0.01), or experiment 3 (F1,27 = 1.29, P = 0.27, ηpartial2 = 0.05).
We performed a power analysis under a range of possible effect sizes and RM correlations. For instance, assuming a small-medium effect size (Cohen’s f = .25) and a medium RM correlation (r = 0.35), power for the signal-averaged LPP analysis is 0.80 with n = 43. With a Cohen’s f = 0.30 and a large RM correlation (r = 0.50), statistical power is 0.83 with n = 26. In contrast, assuming a medium-large effect size (Cohen’s f = 0.35) and a medium RM correlation (r = 0.35), statistical power is 0.82 with n = 24. This latter effect size estimate was based on effect sizes observed in three published studies of the effects of MORE on cardiac autonomic reactivity (ηpartial2 = 0.18) (28), LPP responses (ηpartial2 = 0.17), and BOLD responses in striatal reward circuitry (Cohen’s d = 2.13) (29) to natural reward cues. Our actual observed effect size in experiment 1 (Cohen’s f = 0.37) was close to the latter assumption, yielding an achieved power of 0.95. For hypothesis testing, in experiment 1, we assessed the interaction of Group (MORE versus SG) with Time (Pretreatment versus Posttreatment) and Stimulus Type (Opioid versus Neutral) on LPP response. In experiments 2 and 3, we assessed the interaction of Group (MORE versus SG) with Time (Pretreatment versus Posttreatment) and Condition (View versus Regulate) on LPP response. When appropriate, Greenhouse-Geisser corrected values were used, and significant (P < 0.05) main effects and interactions were interrogated with Bonferroni-adjusted planned post hoc tests. RM-ANOVA models of LPP response included posttreatment opioid dose (in average daily morphine equivalents) to control for the pharmacological impact of opioid exposure on electrocortical responses. In a separate series of sensitivity analyses, we controlled for pain severity, and effects of MORE on LPP responses in experiments 1 to 3 remained statistically significant.
Because of the small sample size in experiments 2 and 3, we used multilevel modeling (MLM) of trial-level LPP data as an additional sensitivity analysis for these two experiments. Given its capacity to partition sources of variance from the error term, MLM increases power to detect fixed effects without averaging across trials to minimize noise, and thus, this analytic technique is seen as well suited for application to ERP research (40). MLM models included random intercepts and no random slopes. Change in model fit statistics (i.e., −2LL) was used to select the optimal covariance structure for repeated effects (e.g., AR1 versus diagonal) as well as for our overall model building approach. We began parsimoniously by first examining an unconditional growth model and then adding fixed effects and evaluating model fit with likelihood ratio tests. Satterthwaite approximations estimated degrees of freedom. In experiment 2, the significant likelihood ratio test (χ2diff = 17.87, dfdiff = 6, P = 0.006) indicated that the fully parameterized model should be retained over the unconditional growth model, and the Group × Time × Condition interaction was significant in trial-level MLM analyses (F1,1473.29 = 5.49, P = 0.019). In experiment 3, the significant likelihood ratio test (χ2diff = 22.04, dfdiff = 6, P = 0.001) indicated that the fully parameterized model should be retained over the unconditional growth model, and the significant likelihood ratio and the Group × Time × Condition interaction were also significant in trial-level MLM analyses (F1,1034.53 = 6.25, P = 0.013).
We then computed a measure of relative responsiveness to natural reward versus drug reward by subtracting LPP regulatory response to opioid cues from LPP regulatory response to natural reward cues (regulate − view difference scores) and assessed the Group × Time interaction on this relative responsiveness measure. This analytic approach has been used in previous LPP studies of individuals with substance use disorders (17). Our statistical plan for this approach was modeled from a previous study of MORE in which we identified a significant Group × Time interaction on an autonomic measure of relative responsiveness to natural reward versus drug reward (35).
In experiment 4, we assessed the interaction of Group (MORE versus SG) with Time (Pretreatment versus Posttreatment) and Condition (View versus Regulate) on log-transformed positive affect and craving reactivity scores (task block ratings − baseline ratings). Last, in experiment 4, we conducted a path analysis with bootstrapping in PROCESS 2.16.1 software to evaluate pre-post changes in subjective natural reward responsiveness as a mediator of treatment effects (MORE versus SG) on changes in opioid misuse (as measured by the COMM) from pretreatment to 3-month follow-up, with significant mediation indicated by the 95% bias-corrected confidence intervals not spanning zero.
Acknowledgments
Funding: Data analysis and manuscript preparation were supported by grant numbers R01DA042033 and R61AT009296 from the NIH (principal investigator: E.L.G.), W81XWH-15-PRMRP-CTA from the Department of Defense (principal investigator: E.L.G.), and a grant from the Fahs Beck Fund for Research and Experimentation (principal investigator: E.L.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Author contributions: E.L.G. conceptualized the study, spearheaded manuscript preparation, and performed statistical analyses. R.M.A. processed the neurophysiology data and computed ERP grand averages. A.W.H. processed mindfulness practice data and analyzed associations between practice and reward-related processes. E.L.G., B.F., R.M.A., J.-K.Z., and A.W.H. assisted in interpretation of the data, helped in the literature review, and participated in the drafting of the manuscript. All authors have approved the final article. Competing interests: E.L.G. is the Director of the Center on Mindfulness and Integrative Health Intervention Development. The Center provides MORE, mindfulness-based therapy, and cognitive behavioral therapy in the context of research trials for no cost to research participants; however, E.L.G. has received honoraria and payment for delivering seminars, lectures, and teaching engagements (related to training clinicians in MORE and mindfulness) sponsored by institutions of higher education, government agencies, academic teaching hospitals, and medical centers. E.L.G. also receives royalties from the sale of books related to MORE. J.-K.Z. is a consultant of, and received consultant fees from, Alkermes Inc. for work unrelated to the content of the manuscript. The other authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper. Additional data related to this paper may be requested from the authors.
REFERENCES AND NOTES
- 1.Han B., Compton W. M., Blanco C., Crane E., Lee J., Jones C. M., Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann. Intern. Med. 167, 293–301 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Volkow N. D., Koob G. F., McLellan A. T., Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med. 374, 363–371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garland E. L., Froeliger B., Zeidan F., Partin K., Howard M. O., The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neurosci. Biobehav. Rev. 37, 2597–2607 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elman I., Borsook D., Common brain mechanisms of chronic pain and addiction. Neuron 89, 11–36 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Koob G. F., Le Moal M., Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24, 97–129 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Robinson T. E., Berridge K. C., Review. The incentive sensitization theory of addiction: Some current issues. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3137–3146 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koob G. F., Le Moal M., Addiction and the brain antireward system. Annu. Rev. Psychol. 59, 29–53 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Schupp H. T., Cuthbert B. N., Bradley M. M., Cacioppo J. T., Ito T., Lang P. J., Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology 37, 257–261 (2000). [PubMed] [Google Scholar]
- 9.Liu Y., Huang H., McGinnis-Deweese M., Keil A., Ding M., Neural substrate of the late positive potential in emotional processing. J. Neurosci. 32, 14563–14572 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folstein J. R., Van Petten C., Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 45, 152–170 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson R., A triarchic model of P300 amplitude. Psychophysiology 23, 367–384 (1986). [DOI] [PubMed] [Google Scholar]
- 12.Weinberg A., Hajcak G., Beyond good and evil: The time-course of neural activity elicited by specific picture content. Emotion 10, 767–782 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Cuthbert B. N., Schupp H. T., Bradley M. M., Birbaumer N., Lang P. J., Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biol. Psychol. 52, 95–111 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Thiruchselvam R., Blechert J., Sheppes G., Rydstrom A., Gross J. J., The temporal dynamics of emotion regulation: An EEG study of distraction and reappraisal. Biol. Psychol. 87, 84–92 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Littel M., Euser A. S., Munafò M. R., Franken I. H., Electrophysiological indices of biased cognitive processing of substance-related cues: A meta-analysis. Neurosci. Biobehav. Rev. 36, 1803–1816 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Franken I. H., Dietvorst R. C., Hesselmans M., Franzek E. J., van de Wetering B. J., Van Strien J. W., Cocaine craving is associated with electrophysiological brain responses to cocaine-related stimuli. Addict. Biol. 13, 386–392 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Parvaz M. A., Moeller S. J., Malaker P., Sinha R., Alia-Klein N., Goldstein R. Z., Abstinence reverses EEG-indexed attention bias between drug-related and pleasant stimuli in cocaine-addicted individuals. J. Psychiatry Neurosci. 42, 78–86 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lubman D. I., Allen N. B., Peters L. A., Deakin J. F., Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. J. Psychopharmacol. 22, 836–842 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Lubman D. I., Yücel M., Kettle J. W., Scaffidi A., Mackenzie T., Simmons J. G., Allen N. B., Responsiveness to drug cues and natural rewards in opiate addiction: Associations with later heroin use. Arch. Gen. Psychiatry 66, 205–212 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Courtney K. E., Schacht J. P., Hutchison K., Roche D. J. O., Ray L. A., Neural substrates of cue reactivity: Association with treatment outcomes and relapse. Addict. Biol. 21, 3–22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W., Howard M. O., Garland E. L., McGovern P., Lazar M., Mindfulness treatment for substance misuse: A systematic review and meta-analysis. J. Subst. Abuse Treat. 75, 62–96 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Garland E., Froeliger B., Howard M. O., Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Front. Psychiatry 4, 173 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westbrook C., Creswell J. D., Tabibnia G., Julson E., Kober H., Tindle H. A., Mindful attention reduces neural and self-reported cue-induced craving in smokers. Soc. Cogn. Affect. Neurosci. 8, 73–84 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Froeliger B., Mathew A. R., McConnell P. A., Eichberg C., Saladin M. E., Carpenter M. J., Garland E. L., Restructuring reward mechanisms in nicotine addiction: A pilot fMRI study of Mindfulness-Oriented Recovery Enhancement for cigarette smokers. Evid. Based Complement. Alternat. Med. 2017, e7018014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garland E. L., Manusov E. G., Froeliger B., Kelly A., Williams J. M., Howard M. O., Mindfulness-Oriented Recovery Enhancement for chronic pain and prescription opioid misuse: Results from an early-stage randomized controlled trial. J. Consult. Clin. Psychol. 82, 448–459 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garland E. L., Hanley A. W., Riquino M. R., Reese S. E., Baker A. K., Bryan M. A., Salas K., Yack B., Bedford C. E., Atchley R. M., Nakamura Y., Froeliger B., Howard M. O., Mindfulness-Oriented Recovery Enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial. J. Consult. Clin. Psychol. 87, 927–940 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garland E. L., Baker A. K., Howard M. O., Mindfulness-Oriented Recovery Enhancement reduces opioid attentional bias among prescription opioid-treated chronic pain patients. J. Soc. Soc. Work Res. , 311–318 (2017). [Google Scholar]
- 28.Garland E. L., Froeliger B., Howard M. O., Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology 231, 3229–3238 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garland E. L., Froeliger B., Howard M. O., Neurophysiological evidence for remediation of reward processing deficits in chronic pain and opioid misuse following treatment with Mindfulness-Oriented Recovery Enhancement: Exploratory ERP findings from a pilot RCT. J. Behav. Med. 38, 327–336 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garland E. L., Restructuring reward processing with Mindfulness-Oriented Recovery Enhancement: Novel therapeutic mechanisms to remediate hedonic dysregulation in addiction, stress, and pain. Ann. N. Y. Acad. Sci. 1373, 25–37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moeller S. J., Stoops W. W., Cocaine choice procedures in animals, humans, and treatment-seekers: Can we bridge the divide? Pharmacol. Biochem. Behav. 138, 133–141 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moeller S. J., Hanley A. W., Garland E. L., Behavioral preference for viewing drug v. pleasant images predicts current and future opioid misuse among chronic pain patients. Psychol. Med. 2019, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garland E. L., Bryan C. J., Nakamura Y., Froeliger B., Howard M. O., Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology 234, 621–629 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langeslag S. J. E., van Strien J. W., Up-regulation of emotional responses to reward-predicting stimuli: An ERP study. Biol. Psychol. 94, 228–233 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Garland E. L., Howard M. O., Zubieta J.-K., Froeliger B., Restructuring hedonic dysregulation in chronic pain and prescription opioid misuse: Effects of Mindfulness-Oriented Recovery Enhancement on responsiveness to drug cues and natural rewards. Psychother. Psychosom. 86, 111–112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran T. P., Jendrusina A. A., Moser J. S., The psychometric properties of the late positive potential during emotion processing and regulation. Brain Res. 1516, 66–75 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Butler S. F., Budman S. H., Fernandez K. C., Houle B., Benoit C., Katz N., Jamison R. N., Development and validation of the Current Opioid Misuse Measure. Pain 130, 144–156 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheehan D. V., Lecrubier Y., Sheehan K. H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G. C., The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 59 (Suppl 20), 22–33 (1998). [PubMed] [Google Scholar]
- 39.E. L. Garland, Mindfulness-Oriented Recovery Enhancement for Addiction, Stress, and Pain (NASW Press, 2013). [Google Scholar]
- 40.Volpert-Esmond H. I., Merkle E. C., Levsen M. P., Ito T. A., Bartholow B. D., Using trial-level data and multilevel modeling to investigate within-task change in event-related potentials. Psychophysiology 55, e13044 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


