Maternal behavior affects epigenetic tuning of the oxytocin receptor in human infants.
Abstract
The contribution of nature versus nurture to the development of human behavior has been debated for centuries. Here, we offer a piece to this complex puzzle by identifying the human endogenous oxytocin system—known for its critical role in mammalian sociality—as a system sensitive to its early environment and subject to epigenetic change. Recent animal work suggests that early parental care is associated with changes in DNA methylation of conserved regulatory sites within the oxytocin receptor gene (OXTRm). Through dyadic modeling of behavior and OXTRm status across the first year and a half of life, we translated these findings to 101 human mother-infant dyads. We show that OXTRm is dynamic in infancy and its change is predicted by maternal engagement and reflective of behavioral temperament. We provide evidence for an early window of environmental epigenetic regulation of the oxytocin system, facilitating the emergence of individual differences in human behavior.
INTRODUCTION
The contribution of nature versus nurture to the development of human behavior has been debated for centuries by philosophers, biologists, and psychologists. Through an increased understanding of epigenetic processes, it is now possible to precisely investigate how nature and nurture might interact and influence behavioral phenotypes. Here, we present the first evidence for these epigenetic processes during the earliest period of postnatal development: human infancy. Specifically, we find that early experience in maternal care predicts epigenetic change within the infant oxytocin system, levels of which are in turn reflective of infant temperament. Oxytocin is a crucial regulator of human social behavior. Individual differences within the oxytocin system—assessed through hormonal levels, epigenetic modification, or genetic variation—have been linked to differential sensitivity to social cues, prosocial behaviors, and stress responsiveness in adults (1–4). Despite the important impact of these differences on typical and atypical social functioning, very little is known about the development of the endogenous oxytocin system. Infancy marks one of the most dynamic, environmentally malleable stages of postnatal life, and a point when many physiological systems are established and tuned (5, 6). Early caregiving experience in particular can exert profound effects on offspring physiology, neural development, and behavior (7–11). The combination of plasticity and sensitivity makes infancy an informative time to investigate the development of the endogenous oxytocin system, which has the capacity to aid establishment of early social perceptual and cognitive processes as well as set trajectories for the emergence of complex social behaviors.
We investigated variability and plasticity of the oxytocin system in infancy by examining epigenetic modification of the oxytocin receptor gene (OXTR) during the first year and a half of human infancy. OXTR DNA methylation (OXTRm) is variable in humans, associated with individual differences in adult and infant brain responses to social information, and higher levels have been associated with reduced expression of OXTR (4, 12–14). Our team has demonstrated in the prairie vole that low levels of early care lead to de novo DNA methylation at regulatory sites in Oxtr in both the brain and the blood, as well as down-regulation of oxytocin receptor in the brain (15). Crucially, a site at which Oxtr methylation is affected in prairie voles is directly conserved in human OXTR (fig. S1), providing a promising opportunity to test a similar question in humans. It remains unknown whether OXTRm is a stable marker in individuals or whether it is sensitive to changes in the environment. Moreover, in congruence with growth curves of other biological systems such as brain development (5), it is possible that OXTRm is more dynamic in infancy than in adulthood.
In the current experiment, we examined the hypothesis that early nurture might critically affect development of the oxytocin system in human infancy. We considered the mother-infant dyad as a dynamic, biobehavioral system by analyzing behavior and OXTRm from both mother and infant. A cohort of 101 infants and their mothers were followed from 5 to 18 months of age. At 5 months, maternal and infant behavioral engagement was assessed during a free-play interaction. At 18 months, we examined infant behavioral temperament. We assessed OXTRm at both visits. Because of the dynamic nature of infancy and previous work suggesting high sensitivity to the early environment, we hypothesized that OXTRm would display greater change across visits in infants than in mothers and this change would be sensitive to and predicted by quality of maternal engagement. In addition, we tested the hypothesis that infant OXTRm levels at 18 months would be associated with their behavioral temperament, positing OXTRm as a promising epigenetic mark reflective of variability in early behavioral traits.
RESULTS
OXTRm is correlated between mothers and infants and between visits
A total of 101 mother-infant dyads of Western European ancestry participated in this study (see Materials and Methods). We analyzed saliva-derived DNA methylation at CpG site −924, a conserved site located in the promoter region of OXTR (fig. S1). DNA methylation in saliva at this site has been associated with individual differences in infant brain response to emotional faces at 7 months (14). In addition, DNA methylation derived from saliva is highly correlated with methylation derived from blood at this site (14). Average infant OXTRm was 61.81% at the 5-month visit (SD = 3.97; n = 100) and 61.71% at the 18-month visit (SD = 5.38; n = 82). Average maternal methylation was 65.86% at the 5-month visit (SD = 6.35; n = 98) and 66.63% at the 18-month visit (SD = 6.33; n = 93) (fig. S2A and table S1). Correlations were conducted between OXTRm and potentially confounding factors, including infant and maternal age, parity, maternal education, and breastfeeding experience. None of these factors associated with infant or maternal OXTRm at either visit (all P values are >0.05) (table S2). Maternal and infant OXTRm levels were positively correlated with each other to a similar extent at both visits [5 months: r(97) = 0.293, P = 0.004; 18 months: r(79) = 0.320, P = 0.004; Fisher’s r to z = −0.19, P = 0.849] (fig. S3).
We tested the stability of OXTRm over time by comparing methylation levels from the 5-month visit to levels at the 18-month visit, occurring roughly 13 months apart (Mduration = 13.41 months; SD = 0.57). Methylation levels positively correlated between visits in both infants and mothers [infants: r(81) = 0.774, P < 0.001; mothers: r(90) = 0.960, P < 0.001] (fig. S2B), although maternal OXTRm correlated most strongly over time (Fisher’s r to z = −5.87, P < 0.001). The difference in the strength of these correlations suggests that there may be more room for OXTRm to change in infancy than in adulthood.
OXTRm is dynamic in infancy but relatively stable in early motherhood
To test the hypothesis that OXTRm is more dynamic in infants than in mothers, we used a latent multigroup path analysis to compare the 5- and 18-month DNA methylation distributions (Ωnyx software, open source, onyx.brandmaier.de). This method allowed us to explore differences in distribution averages and variances. In addition, the use of latent variable modeling allowed for the separation of measurement error from meaningful variation (see Materials and Methods). In infants, OXTRm variance significantly increased from 5 to 18 months [χ2(1) = 4.04, P = 0.0445, Cramer’s V = 0.22] (fig. S4), although the group averages of OXTRm were not significantly different between the two visits [χ2(1) = 1.60 × 10−4, P = 0.990, Cramer’s V = 0.001]. In mothers, the 5- and 18-month distributions did not significantly differ, neither by variance [χ2(1) = 0.16, P = 0.687, Cramer’s V = 0.04] nor by mean [χ2(1) = 0.25, P = 0.614, Cramer’s V = 0.05] (fig. S5). Overall, these analyses provide statistical evidence that OXTRm changes from 5 to 18 months in infancy. The group average of methylation remained stable, demonstrating that infants did not simply increase or decrease as a group. Instead, methylation change was indexed by a wider range of change in both directions, such that an equal number of infants increase or decrease in DNA methylation over time. In contrast, mothers’ OXTRm remained stable across the two visits in variance and mean, providing evidence that methylation of OXTR remains relatively constant in early motherhood.
To further investigate change in OXTRm from 5 to 18 months, we calculated difference scores by subtracting 18-month methylation levels from 5-month methylation levels. In this case, positive values indicate an increase in OXTRm over time, while negative values indicate a decrease in methylation (Fig. 1). Although average change in infants and mothers was below 1% (0.11 and 0.48%, respectively), the range of change was nearly double in infants (17.60% compared to 9.70% in mothers) (table S1). We again used a multigroup path analysis to statistically test whether the distributions of change differed between infants and mothers. The variance of infant methylation change was significantly greater than that of mothers [χ2(1) = 34.58, P < 0.001, Cramer’s V = 0.45], but mean change did not differ [χ2(1) = 0.80, P = 0.37, Cramer’s V = 0.07]. We repeated this analysis using a latent difference score model (see fig. S6). Although there was a trend for unequal variance [χ2(1) = 2.90, P = 0.088, Cramer’s V = 0.13], a larger sample size is necessary for appropriate power to assess this model.
Fig. 1. Infant OXTRm changes across infancy.
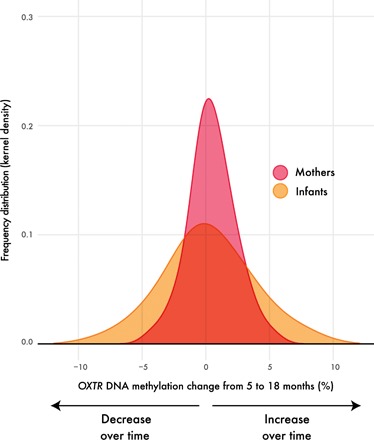
Plotted are the frequency distributions of OXTRm change scores for mothers (magenta) and infants (orange). Change scores were calculated by subtracting methylation levels at the 5-month visit from methylation levels at the 18-month visit. Scores above zero indicate an increase in OXTRm over time, while scores below zero represent a decrease. Infants display nearly double the amount of change (range = 17.60; P = 0.04) than that displayed in mothers (range = 9.70; P = 0.61).
Maternal engagement at 5 months predicts infant OXTRm change over time
We have demonstrated that infants’ DNA methylation levels at a regulatory site in OXTR changed from the 5- to 18-month visit, while mothers’ remained stable. Given evidence from animal literature (15), we next aimed to explore whether maternal behavior might affect this change. Maternal and infant engagement was coded during a free-play interaction at the 5-month visit. To assess whether behavioral engagement predicted change in OXTRm, we created an actor-partner interdependence model (16) through path analysis in Ωnyx software. This analysis capitalizes on the idea of the mother and infant as a dyadic system, such that each actor (mother and infant) can have an impact on themselves and their partner. Specifically, we considered the potential impacts of, and relationship between, maternal and infant engagement on maternal and infant OXTRm change (Fig. 2A). Latent variable estimates were again used to account for replicate variability and to separate meaningful variation from measurement error. Because we specifically tested the impact of mothers and infants on each other, we ensured that only dyads who provided engagement scores and methylation values at both visits were included in the analysis (ndyads = 74). The full model demonstrated strong fit, suggesting that as a full dyadic system, maternal and infant behavioral engagement predicted OXTRm change: χ2(24) = 8.814, P = 0.998, root mean square error of approximation (RMSEA) = 0.0, confirmatory fit index (CFI) = 1, and Tucker-Lewis index (TLI) = 1 (note that infant gender did not significantly influence the full model, χ2(20) = 17.10, P = 0.65, and was therefore not included in subsequent analyses). To understand which paths drove these effects, we used likelihood-ratio tests based on path removal and concluded with a reduced model with similarly strong fit statistics [χ2(20) = 12.072, P = 0.914, RMSEA = 0.0, CFI = 1, and TLI = 1] that did not significantly differ from the full model [χ2(4) = 3.26, P = 0.516]. In line with animal literature, we found that the mother’s behavior affected the infant, such that maternal engagement predicted a reduction in infant OXTRm from 5 to 18 months (β = −0.30) and this accounted for covariance between infant and maternal engagement (Fig. 2B). Note that the effect of maternal engagement on infant methylation change remained significant on its own: χ2(1) = 6.76, β = −0.30, P = 0.009, Cramer’s V = 0.30 (Fig. 3A). In summary, we find a specific association with the quality of maternal behavior at 5 months and the reduction of infants’ OXTRm from 5 to 18 months. We do not see a significant effect of maternal behavior on her own OXTRm change nor do we see a significant effect of infant behavior on infant or maternal OXTRm change.
Fig. 2. Maternal engagement predicts a reduction in infant OXTRm over time.
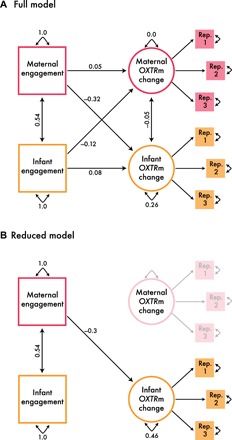
(A) Full model. An actor-partner interdependence model was used to test the effect of maternal and infant behavioral engagement on maternal and infant OXTRm change. To separate meaningful variation from measurement error, we created latent variables for the methylation data derived from values from three technical replicates (labeled Rep. 1 to Rep. 3 in the figure) (RMSEA = 0.0, CFI = 1, and TLI = 1). Single-headed linear arrows are labeled with standardized β coefficients, indicating directionality. Covariances are represented by double-headed linear arrows and indicate no directionality. Double-headed, curved error paths pointing to each respective variable represent the residual variance within each variable not accounted for by the model paths. (B) Reduced model. Path removal was used to determine the driving paths of the model (RMSEA = 0.0, CFI = 1, and TLI = 1). This model does not significantly differ from the full model (P = 0.516). Even when taking into account the positive covariance between infant and maternal engagement at 5 months, only maternal engagement predicts infant OXTRm change (reduction) from 5 to 18 months (β = −0.30).
Fig. 3. Maternal engagement fine-tunes infant OXTRm from 5 to 18 months, which in turn associates with overt infant temperament at 18 months.
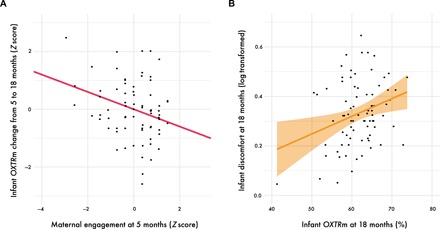
(A) Plotted is the association between maternal engagement at 5 months (x axis) and infant OXTRm change from 5 to 18 months (y axis). The slope of the regression line is the predicted β value derived from the actor-partner interdependence model (β = −0.30, P = 0.009). Note that engagement and methylation scores were standardized for this analysis (Z score transformed). (B) Scatterplot visualizing the association between infant OXTRm at 18 months and temperamental discomfort (log transformed) as measured by the Early Childhood Behavior Questionnaire (ECBQ) (17), r(80) = 0.293, P = 0.008. The discomfort subscale of the ECBQ measures the amount of negative affect one’s infant feels toward certain sensory qualities of stimulation, such as complexity, rate, and intensity of sounds, light, and textures. Shaded orange bar represents the 95% confidence interval.
To detect and account for factors that may have influenced behavioral engagement, we looked for associations between maternal questionnaire variables (social support quantity and quality, parental sense of competence, and postnatal depression) and behavioral engagement coded during free play. These factors did not significantly associate with maternal or infant behavioral engagement (all P values are >0.05).
OXTRm at 18 months is reflective of infant temperament
One final aim was to assess whether OXTRm might be reflective of individual variability in early behavioral temperament. We assessed whether OXTRm at 18 months was associated with infant temperament by analyzing subscales from the parent-reported Early Childhood Behavior Questionnaire (ECBQ) at 18 months. Infant temperamental discomfort was positively associated with OXTRm [r(80) = 0.293, P = 0.008; survives Bonferroni correction at adjusted P < 0.0083] (Fig. 3B). The discomfort subscale of the ECBQ measures the amount of negative affect one’s infant feels toward certain sensory qualities of stimulation, such as complexity, rate, and intensity of sounds, light, and textures (17). Items on this subscale include questions that assess how often the child is disturbed by scratchy sounds, materials, tight-fitting clothes, tags, bright lights, and odors. These findings suggest that OXTRm may be an epigenetic mark associated with behavioral temperament traits early in ontogeny.
DISCUSSION
We provide the first evidence that OXTRm in infancy is dynamic, associated with early maternal experience, and reflective of individual differences in behavioral temperament. Notably, we find that maternal OXTRm did not change from the 5- to 18-month visit, suggesting stability of this epigenetic mark in early motherhood. In contrast, infancy may provide a sensitive period in development during which the oxytocin system is dynamic, plastic, and sensitive to the social environment. Using a latent actor-partner interdependence model, we observed that quality of maternal engagement at 5 months significantly predicted infants’ change in methylation from 5 to 18 months. Specifically, increased quality of engagement displayed by mothers predicted a reduction in OXTRm in their infants seen more than 1 year later. As reduced promoter OXTRm has been associated with increased expression of the oxytocin receptor gene in human and animal models (13, 15), this study suggests that higher maternal engagement may have the potential to up-regulate the oxytocin system in human offspring. This work is directly congruent with work in animal models (15). We therefore propose OXTRm as a mechanism by which early social experience can affect infant development.
We also found that infant OXTRm at 18 months was associated with temperamental discomfort at the same age. Specifically, our results showed that increased levels of OXTRm are associated with increased discomfort to aversive sounds, strong odors, bright lights, and tight or ill-fitting clothes. Recent studies indicate that atypical sensory processing is linked to reduced language abilities, joint attention, and social play behavior (18–20). Discomfort toward touch and loud voices early on might lead the infant to be less responsive or even withdraw from social interactions (21). In more extreme cases, atypical sensory processing is the key feature of sensory processing disorder, subtypes of which are found in attention deficit hyperactivity disorder and autism spectrum disorder (22–24). Our results may suggest that training focusing on increasing social engagement between parent and infant might be one possible intervention strategy to reduce discomfort in early development (25).
While the focus of this study is on the development of the oxytocin system, it is important to note that oxytocin is just one system of many that may be affected by early experience in parental care. For example, there is a growing literature demonstrating the impact of early caregiving on the epigenetic modification of the glucocorticoid receptor gene of offspring. Much of this work has been conducted in rodents, but more recent work exists translating these findings to humans (11, 26–28). The current study complements and extends this work by identifying the human oxytocin system as another system epigenetically sensitive to maternal behavior. However, the current study critically sets itself apart by considering DNA methylation across infancy and motherhood, thus having the capacity to explore change over time. Future work using similar dyadic modeling to examine stress-related genes, other “social” genes such as the vasopressin receptor gene, as well as “control” genes that are not expected to change, will be informative, as the likely far-reaching impact of maternal behavior on the development of the human epigenome is further elucidated.
One final point warranting discussion is the use of saliva to isolate DNA and derive methylation values. Working with human infants required us to use a noninvasive, peripheral tissue for methylation analysis. In previous work, we performed critical experiments to establish the association between blood-derived and brain-derived DNA methylation at our CpG site of interest in humans (12) and animals (15). Moreover, we have recently reported a strong correlation of methylation at this site derived from blood and saliva within the same human adults (14). As the use of heterogeneous tissue becomes commonplace in behavioral and neuroimaging epigenetic research, determining whether there are specific cell types that are more reflective of epigenetic change (perhaps due to differences in cell turnover or cell lineage) will be an important next step. This is especially important for the interpretation of epigenetic change over time as examined in the current study. For example, it is currently unknown whether and how salivary cell composition changes across the human life span.
CONCLUSION
We provide the first evidence that the endogenous oxytocin system—known for its essential role in processes crucial for human social interaction and the propagation of our species (29)—is malleable and can be shaped by the early caregiving environment. Specifically, we identify OXTRm as a marker to study the impact of the environment on the infant epigenome. This finding has substantial implications for how we view the developing oxytocin system. Early in ontogeny, successful interactions with caregivers are crucial as infants begin to navigate the social world. Children come to depend on these early, foundational interactions, which ultimately facilitate their lifetime capacity to affiliate and engage with others. We demonstrate one potential mechanism by which early experience epigenetically establishes and shapes trajectories of human development.
MATERIALS AND METHODS
Experimental design
The current study investigated change in OXTRm over the first 2 years of life in infants and their mothers. In addition, we explored the potential impact of maternal and infant behavioral engagement on epigenetic change during a coded free-play session. We further investigated the association between OXTRm and infant temperament at 18 months using the ECBQ (17). A total of 101 mother-infant dyads participated in this study when infants were 5 months old (M = 147.62 days; SD = 14.57) and 18 months old (M = 556.60 days; SD = 13.29). Mothers were about 31 years old at the 5-month visit (M = 31.23; SD = 4.49) (table S1). All dyads were of Western European ancestry with no reported family history of neurodevelopmental or mental health disorders. Infants were born at standard gestational age (over 38 weeks) and were of normal birth weight (over 2500 g). Written informed consent was provided by parents before participation in this study, and families were compensated with travel money, an infant T-shirt, a photograph of the infant, and a toy at each visit. Procedures were approved by the Leipzig University Medical School Ethics Committee and were conducted in accordance with the Declaration of Helsinki.
Five-month procedure
Upon arrival mother-infant dyads settled in and familiarized with the experimenter and laboratory space for about 10 to 15 min, during which time, the experimenter carefully went over the study with the mother. Following familiarization, the experimenter left the testing room, and dyads underwent a 5-min video-recorded free-play interaction (see “Five-month free-play analysis” section below). Saliva was collected from both mother and infant using kits from DNA Genotek (Ottawa, Canada). Infant saliva was obtained with assisted collection sponges (CS-2) that were cut and dropped into storage kits (OG-250). Mothers provided passive drool in collection tubes (OG-500). After saliva collection, the experimenter quietly played with the infant, while the mother filled out four brief questionnaires in the following order: an in-house breastfeeding questionnaire, which obtained information regarding feeding frequency and exclusive breastfeeding duration, maternal demographics such as age, education, parity, and delivery method of the infant (30); the short form of the Social Support Questionnaire (SSQ6) (31), in which mothers documented how much social support they had and how satisfied they were with the support; the Parenting Sense of Competence Scale (32), which indexed how mothers felt about the quality of their parenting skills; and last, the Edinburgh Postnatal Depression Scale (33) was administered to detect potential signs and symptomatology of postpartum depression (see table S3 for descriptives).
Five-month free-play analysis
At the 5-month visit, mother-infant dyads underwent a natural free-play interaction, described in detail elsewhere (34). Briefly, mothers were instructed to play with their infants as they naturally did at home. Infants were laid on their backs on a designated blanket, and two cameras were set up to record the behavior and facial expressions of the infant and mother, respectively. Mothers were aware of the location of the cameras and were asked to remain in view. The same five objects were provided for dyads to interact with if they chose to do so (four assorted toys and one infant play book). No further instructions were given in an attempt to keep the interaction as natural as possible. Once assembled, the experimenter left the room, and the dyads were left alone for 5 min.
To code the interactions, the two videos were time aligned and merged using the open-source software, Kdenlive (https://kdenlive.org). One hundred percent of interactions were coded by a trained primary coder using an in-house coding scheme (34). This scheme consisted of infant and maternal factors scored on a one to five scale as well as factors coded for duration using INTERACT software (Mangold International, Arnstorf, Germany). Maternal variables included (i) how talkative was the mother, (ii) how close was the mother to the infant (proximity), (iii) how attentive and engaged was the mother to the infant’s needs, (iv) to what extent did the mother display a positive mood, and (v) the duration of passive and active touch. We defined passive touch as unintentional contact, whereas active touch implied that the mother reached out to the infant (goal oriented). Infant variables included (i) how attentive and engaged was the infant toward the mother, (ii) to what extent did the infant display a positive mood, (iii) the duration of laughter (a combination of smile and vocalization), and (iv) the duration of smiles (without vocalizations) (table S4). A second trained coder scored a randomly selected subset of videos (25%) to test reliability of the primary coder. Inter-rater reliability was very high (Chronbach’s α = 0.855). All scores were z-transformed, and distributions were visually inspected. Maternal and infant engagement composite scores were created by averaging the three highest-loading variables in a factor analysis (see “Statistical analysis” section for additional information). Maternal engagement score included talkativeness, proximity, and attention. Infant engagement included laughter, attention, and positive mood. The mean item complexity for both engagement scores was equal to one, verifying that the individual items successfully loaded onto their respective engagement factors.
Eighteen-month procedure
Two weeks before their scheduled visit, mothers were mailed the ECBQ (17), a 201-item questionnaire designed to assess three dimensions of temperament in toddlers from 18 to 36 months: Surgency, Negative Affectivity, and Effortful Control. Our analysis focused on the Negative Affectivity domain, which includes the subscales Discomfort, Fear, Sadness, Shyness, Soothability (reverse coded), and Frustration. Previous studies have observed that Negative Affectivity domain subscales reported in the second year of life are higher in children later diagnosed with autism (35–37). These subscales may therefore be most sensitive in reporting variability in sociodevelopmental trajectories. Note that subscales on the ECBQ were checked for normality. In the event that distributions were negatively skewed, scores were subjected to log transformation. Mothers returned the completed questionnaire at the visit, during which time another saliva sample was collected using the same methodology as in the 5-month visit. Saliva samples were stored at room temperature until analysis.
Epigenotyping
Before DNA isolation, collection kits were incubated at 50°C for 1 hour. Infant sample liquid was released from sponges using the DNA Recovery from Samples Collected with Sponges Protocol from DNA Genotek. DNA isolation procedures followed the Manual Purification Protocol from DNA Genotek. DNA was stored in hydration solution from QIAGEN (Hilden, Germany) and quantitated using NanoDrop. Note that all samples from this experiment were processed simultaneously (infants and mothers, both visits). Two hundred nanograms of isolated DNA was subjected to bisulfite conversion using MECOV50 Kits (Thermo Fisher Scientific, Waltham, MA). Rather than full randomization, sample locations within 96-well plates were pseudorandomized by “family.” Specifically, every four wells contained all samples from one mother-infant dyad (5 months, infant and mom; 18 months, infant and mom). Locations of families on plates were randomized using a random number generator. In addition, the location order of visit and family member (infant or mother) within the four-well family was randomized. As one of our major goals was to compare infant to mother and the 5-month visit to the 18-month visit, it was crucial that families were treated as similarly as possible. Their close proximity on the plate ensured this.
Following bisulfite conversion, the remaining procedures were conducted in triplicate. Forty nanograms of bisulfite-converted DNA was subjected to amplification using polymerase chain reaction (PCR) using PyroMark PCR Kits (QIAGEN, Hilden, Germany) and 0.2 μM primers [TSL101F, 5′-TTGAGTTTTGGATTTAGATAATTAAGGATT-3′ (forward); TSL101R, 5′-biotin-AATAAAATACCTCCCACTCCTTATTCCTAA-3′ (reverse)]. Each PCR plate contained methylation standards (0, 50, and 100% methylated) and negative controls from bisulfite conversion and PCR. Thermocycling was performed as follows: Steps (i) 95°C for 15 min; (ii) 50 cycles at 94°C for 30 s, 56°C for 30 s, and 72°C for 30 s; (iii) 72°C for 10 s; and (iv) 4°C until analysis. A 116–base pair region was amplified on the coding strand of OXTR containing CpG-924 (Genome Reference Consortium Human Build 38, chromosome 3: 8,769,044 to 8,769,159) and confirmed by agarose gel electrophoresis. DNA methylation level for each sample was assessed using pyrosequencing (PyroMark Q24, QIAGEN) (sequencing primer: TSL101S, 5′-AGAAGTTATTTTATAATTTTT-3′) [note that our PCR and sequencing primers cover another CpG site (−934), which has been reported on in human adult studies. Although our decision to exclusively consider site −924 was hypothesis-driven on the basis of human infant work and our specific interest in translating prairie vole work, we have provided statistics on site −934 at a reviewer’s request (see the Supplementary Materials)]. Mean deviation within replicates averaged ±1.796%. For main change analyses, replicate variability was accounted for by creating latent methylation scores composed of the three replicate values. For visualization purposes and general descriptives, reported epigenotypes are the average of the three replicate values.
Statistical analysis
Creation of maternal and infant engagement scores
A video-recorded free-play interaction was coded for several aspects of maternal and infant behavior. Upon inspection, it was found that duration of touch and infant smiles were negatively skewed, such that a great majority of dyads showed little to no physical contact and a great majority of infants rarely smiled in the absence of a vocalization (active touch: Zskewness = 9.83, P < 0.001; passive touch: Zskewness = 10.37, P < 0.001; infant smiles: Zskewness = 7.46, P < 0.001). As the coding scheme already contained the scaled measures of infant positive mood, maternal proximity, and the duration of infant laughter (smiles and vocalizations), we decided to drop duration of touch and infant smiles from further analyses. Remaining variables were subjected to a factor analysis within RStudio (open source, www.rstudio.com) using a promax rotation. Analyses were conducted for maternal and infant variables separately to identify item scores with the highest loadings. With this, composite scores of maternal and infant engagement were created by averaging the three variables that loaded highest onto the factors (all standardized loadings above 0.3). Note that because of our relatively low sample size for the construction of structural equation models, these composite scores, rather than individual variable loadings, were used in all reported analyses to reduce model complexity.
Multigroup path analyses
DNA methylation analyses were performed in triplicate, such that three technical replicates were generated for every sample. To account for variability between replicates and to better test differences between methylation distributions, we used latent multigroup path analysis. Methylation distributions were estimated using the methylation values generated from three technical replicates from each individual (average mean deviation was below 2%). The use of latent variables is a more conservative approach than using methylation averages, as it is more sensitive to potential methylation change by separating measurement error from meaningful variation. For each analysis, a null model was created in which the variance and means of both distributions were constrained to be equal to each other (i.e., infant methylation at 5 months was equal to infant methylation at 18 months in mean and variance). We then created hypothesized models in which means were constrained and variance was set to be free. We then assessed change in OXTR methylation over time by comparing the null model to hypothesized models (see Results and figs. S4 and S5).
Supplementary Material
Acknowledgments
We are grateful to all families who participated in this study as well as C. Boettcher, J. Tippmann, M. Missana, and D. Goedikmeier for assistance with the infant data collection at the Max Planck Institute for Human Cognitive and Brain Sciences. We also thank T. Wilson for providing valuable feedback on this manuscript. Funding: This research was supported by the Max Planck Society (T.G.), the University of Virginia and the NSF grant 1729289, “Epigenetic influences on the early development of social brain functions” (to T.G. and J.J.C.), and an International Max Planck Research School (IMPRS NeuroCom) scholarship, an NIH NRSA T32 Research Training in Neuroendocrinology Fellowship, and a Hartwell Biomedical Research Fellowship (to K.M.K.). Author contributions: K.M.K., T.G., and J.J.C. conceptualized and designed the study. K.M.K. performed the behavioral experiments, saliva collection, and coding of the behavioral data. T.S.L. and J.J.C. provided reagents, laboratory resources, and instruments necessary for molecular analyses. K.M.K. and T.S.L. performed the molecular analyses. K.M.K. and R.G.M. created the statistical models and analyzed the data. K.M.K. visualized the data. T.G. and J.J.C. provided supervision and oversight. K.M.K., T.G., and J.J.C. wrote the manuscript. R.G.M. provided comments and edits of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors and will be made available upon request on the Open Science Framework (osf.io).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/10/eaay0680/DC1
Supplementary Results
Fig. S1. Regulatory CpG site −924 in the promoter region of human OXTR is directly conserved in prairie vole Oxtr.
Fig. S2. Infant and maternal OXTRm from 5 to 18 months.
Fig. S3. Infant and maternal OXTRm is correlated at both visits.
Fig. S4. The variance in infant OXTRm increases from 5 to 18 months.
Fig. S5. Maternal OXTRm does not change from 5 to 18 months.
Fig. S6. Exploratory latent difference score model.
Table S1. Infant and maternal OXTR methylation at 5- and 18-month visits.
Table S2. Table displaying Pearson’s r coefficients for each OXTR methylation measurement and demographic variable.
Table S3. Mother-reported questionnaire descriptives and demographics.
Table S4. Descriptives for each subscale of the free-play analysis coding scheme.
REFERENCES AND NOTES
- 1.Marsh A. A., Yu H. H., Pine D. S., Blair R. J., Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology 209, 225–232 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Grewen K. M., Light K. C., Plasma oxytocin is related to lower cardiovascular and sympathetic reactivity to stress. Biol. Psychol. 87, 340–349 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen F. S., Kumsta R., von Dawans B., Monakhov M., Ebstein R. P., Heinrichs M., Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc. Natl. Acad. Sci. U.S.A. 108, 19937–19942 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puglia M. H., Lillard T. S., Morris J. P., Connelly J. J., Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc. Natl. Acad. Sci. U.S.A. 112, 3308–3313 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson M. H., Functional brain development in humans. Nat. Rev. Neurosci. 2, 475–483 (2001). [DOI] [PubMed] [Google Scholar]
- 6.de Weerth C., Zijl R. H., Buitelaar J. K., Development of cortisol circadian rhythm in infancy. Early Hum. Dev. 73, 39–52 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Callaghan B. L., Tottenham N., The neuro-environmental loop of plasticity: A cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology 41, 163–176 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champagne F. A., Curley J. P., Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci. Biobehav. Rev. 33, 593–600 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Ainsworth M. D., Object relations, dependency, and attachment: A theoretical review of the infant-mother relationship. Child Dev. 40, 969–1025 (1969). [PubMed] [Google Scholar]
- 10.Francis D. D., Meaney M. J., Maternal care and the development of stress responses. Curr. Opin. Neurobiol. 9, 128–134 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Weaver I. C. G., Cervoni N., Champagne F. A., D’Alessio A. C., Sharma S., Jr S., Dymov S., Szyf M., Meaney M. J., Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Gregory S. G., Connelly J. J., Towers A. J., Johnson J., Biscocho D., Markunas C. A., Lintas C., Abramson R. K., Wright H. H., Ellis P., Langford C. F., Worley G., Delong G. R., Murphy S. K., Cuccaro M. L., Persico A., Pericak-Vance M. A., Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 7, 62 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusui C., Kimura T., Ogita K., Nakamura H., Matsumura Y., Koyama M., Azuma C., Murata Y., DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochem. Biophsys. Res. Commun. 289, 681–686 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Krol K. M., Puglia M. H., Morris J. P., Connelly J. J., Grossmann T., Epigenetic modification of the oxytocin receptor gene is associated with emotion processing in the infant brain. Dev. Cogn. Neurosci. 37, 100648 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkeybile A. M., Carter C. S., Wroblewski K. L., Puglia M. H., Kenkel W. M., Lillard T. S., Karaoli T., Gregory S. G., Mohammadi N., Epstein L., Bales K. L., Connelly J. J., Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology 99, 128–136 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook W. L., Kenny D. A., The actor–partner interdependence model: A model of bidirectional effects in developmental studies. Int. J. Behav. Dev. 29, 101–109 (2005). [Google Scholar]
- 17.Putnam S. P., Gartstein M. A., Rothbart M. K., Measurement of fine-grained aspects of toddler temperament: The Early Childhood Behavior Questionnaire. Infant Behav. Dev. 29, 386–401 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson L. R., Patten E., Baranek G. T., Poe M., Boyd B. A., Freuler A., Lorenzi J., Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disabilities. J. Speech Lang. Hear. Res. 54, 1562–1576 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhaneck H. M., Britner P. A., A Preliminary investigation of the relationship between sensory processing and social play in autism spectrum disorder. Otjr-Occup. Part. Heal. 33, 159–167 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Baranek G. T., Watson L. R., Boyd B. A., Poe M. D., David F. J., McGuire L., Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Dev. Psychopathol. 25, 307–320 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thye M. D., Bednarz H. M., Herringshaw A. J., Sartin E. B., Kana R. K., The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev. Cogn. Neurosci. 29, 151–167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghanizadeh A., Sensory processing problems in children with ADHD, a systematic review. Psychiatry Investig. 8, 89–94 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konstantareas M. M., Stewart K., Affect regulation and temperament in children with autism spectrum disorder. J. Autism Dev. Disord. 36, 143–154 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Salley B., Miller A., Bell M. A., Associations between temperament and social responsiveness in young children. Infant Child Dev. 22, 270–288 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green J., Charman T., Pickles A., Wan M. W., Elsabbagh M., Slonims V., Taylor C., McNally J., Booth R., Gliga T., Jones E. J. H., Harrop C., Bedford R., Johnson M. H., Team B., Parent-mediated intervention versus no intervention for infants at high risk of autism: A parallel, single-blind, randomised trial. Lancet Psychiat. 2, 133–140 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fish E. W., Shahrokh D., Bagot R., Caldji C., Bredy T., Szyf M., Meaney M. J., Epigenetic programming of stress responses through variations in maternal care. Ann. N. Y. Acad. Sci. 1036, 167–180 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Meaney M. J., Epigenetics and the biological definition of gene x environment interactions. Child Dev. 81, 41–79 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Lester B. M., Conradt E., LaGasse L. L., Tronick E. Z., Padbury J. F., Marsit C. J., Epigenetic programming by maternal behavior in the human infant. Pediatrics 142, e20171890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H. J., Macbeth A. H., Pagani J. H., Young W. S., Oxytocin: The great facilitator of life. Prog. Neurobiol. 88, 127–151 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krol K. M., Kamboj S. K., Curran H. V., Grossmann T., Breastfeeding experience differentially impacts recognition of happiness and anger in mothers. Sci. Rep. 4, 7006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarason I. G., Sarason B. R., Shearin E. N., Pierce E. N., Pierce G. R., A brief measure of social support: Practical and theoretical implications. J. Soc. Pers. Relat. 4, 497–510 (1987). [Google Scholar]
- 32.Johnston C., Mash E. J., A measure of parenting satisfaction and efficacy. J. Clin. Child Psychol. 18, 167–175 (1989). [Google Scholar]
- 33.Cox J. L., Holden J. M., Sagovsky R., Detection of postnatal depression. Br. J. Psychiatry 150, 782–786 (1987). [DOI] [PubMed] [Google Scholar]
- 34.Grossmann T., Missana M., Krol K. M., The neurodevelopmental precursors of altruistic behavior in infancy. PLOS Biol. 16, e2005281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clifford S. M., Hudry K., Elsabbagh M., Charman T., Johnson M. H., Team B., Temperament in the first 2 years of life in infants at high-risk for autism spectrum disorders. J. Autism. Dev. Dis. 43, 673–686 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Garon N., Bryson S., Zwaigenbaum L., Smith I. M., Brian J., Roberts W., Szatmari P., Temperament and its relationship to autistic symptoms in a high-risk infant sib cohort. J. Abnorm. Child Psychol. 37, 59–78 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Zwaigenbaum L., Bryson S., Rogers T., Roberts W., Brian J., Szatmari P., Behavioral manifestations of autism in the first year of life. Int. J. Dev. Neurosci. 23, 143–152 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/10/eaay0680/DC1
Supplementary Results
Fig. S1. Regulatory CpG site −924 in the promoter region of human OXTR is directly conserved in prairie vole Oxtr.
Fig. S2. Infant and maternal OXTRm from 5 to 18 months.
Fig. S3. Infant and maternal OXTRm is correlated at both visits.
Fig. S4. The variance in infant OXTRm increases from 5 to 18 months.
Fig. S5. Maternal OXTRm does not change from 5 to 18 months.
Fig. S6. Exploratory latent difference score model.
Table S1. Infant and maternal OXTR methylation at 5- and 18-month visits.
Table S2. Table displaying Pearson’s r coefficients for each OXTR methylation measurement and demographic variable.
Table S3. Mother-reported questionnaire descriptives and demographics.
Table S4. Descriptives for each subscale of the free-play analysis coding scheme.


