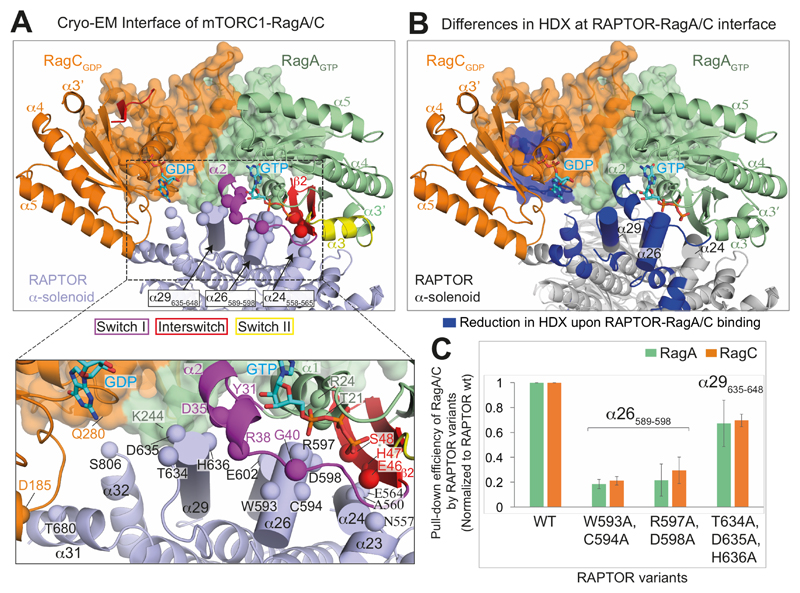
Fig. 3. Interface between RAPTOR and the RagA/C complex.
(A) Close-up views of RagA/C binding to the RAPTOR subunit of mTORC1. The CRDs are shown as transparent surfaces. RAPTOR helices contacting switch I and interswitch of RagA are shown as cylinders. Spheres mark RAPTOR/RagA interface residues.
(B) View of the interface, illustrating regions with a decrease in HDX (blue) upon formation of the RagA/C/RAPTOR complex.
(C) Mutational analysis of the binding interface. Strep-tagged wild-type RAPTOR (WT) and three different RAPTOR mutants (WC(593,594)AA, RD(597,598)AA and TDH(634-636)AAA) were assayed for their ability to pull-down RagA-Q66LGTP/RagC-T90NGDP in vitro. The pull-down efficiencies of RAPTOR mutants were normalized to WT RAPTOR. Values are means from three independent experiments, and error bars show standard deviations.