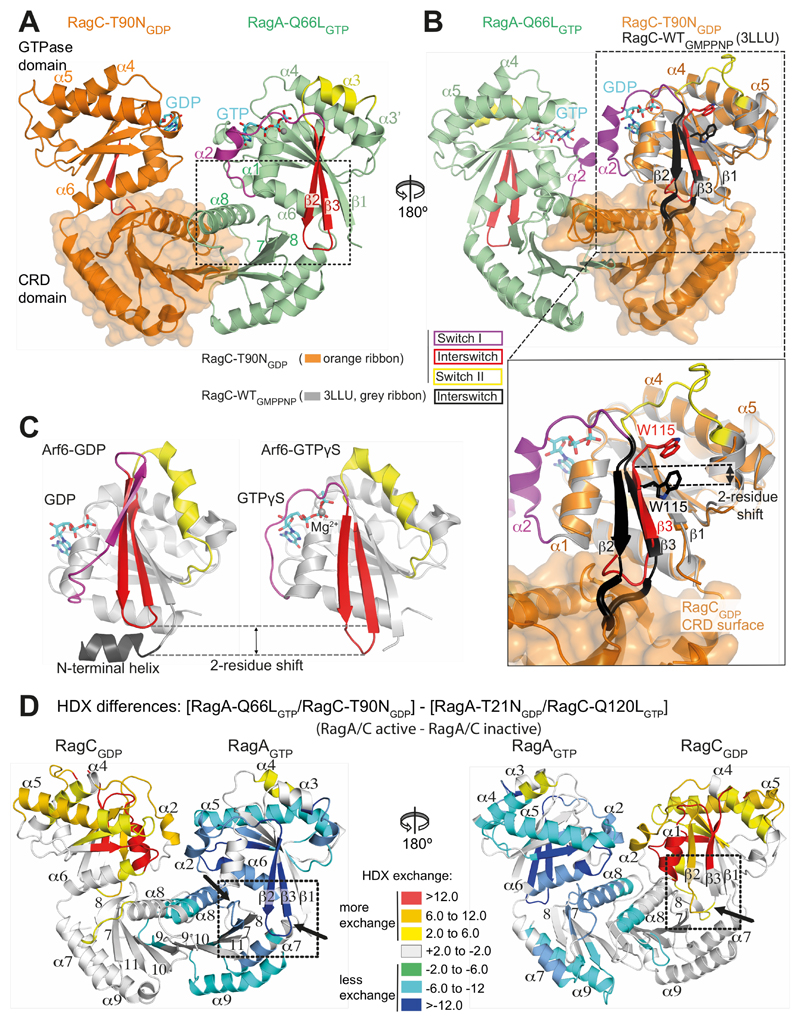
Fig. 5. Structural basis for communicating nucleotide binding within the RagA/C heterodimer.
(A) Both switch I and the interswitch make nucleotide-state-dependent direct contacts with the CRD.
(B) Superposition of GTP-bound RagC (PDB ID 3LLU, in gray) on the GDP-bound RagC (from our RagA-Q66LGTP/RagC-T90NGDP complex). The interswitch of GTP-bound RagC is black and GDP-bound RagC is red. The W115 position illustrates a two-residue shift in strand β3 (relative to strand β1). The β2/β3 loop toggles between a retracted conformation in the GDP state and an extended conformation in the GTP state that would clash with the CRD, if there were no conformational changes.
(C) The structures of Arf6 bound to either GDP (PDB ID 1E0S (45)) or GTPγs (PDB ID 2J5X (46)), with switches colored as in (A). The interswitch toggle couples nucleotide binding with membrane binding by the N-terminal helix.
(D) Differences in HDX between the active (RagAGTP/RagCGDP) and inactive (RagAGDP/RagCGTP) states, illustrating changes in the CRDs, in addition to the expected changes in the GTPase domains. In RagCGDP, disordered regions in the switches have been modelled to illustrate all of the HDX changes.