Abstract
A set of frontoparietal brain regions - the multiple-demand (MD) system [1, 2] - has been linked to fluid intelligence in brain imaging [3, 4] and in studies of patients with brain damage [5–7].For example, the amount of damage to frontal or parietal, but not temporal, cortices predicts fluid intelligence deficit [5]. However, frontal and parietal lobes are structurally [8] and functionally [9, 10] heterogeneous. They contain domain-general regions that respond across diverse tasks [11, 12], but also specialized regions that respond selectively during language processing [13]. Since language may be critical for complex thought [14–24, cf. 25–26], intelligence loss following damage to frontoparietal cortex could have important contributions from damage to language-selective regions. To evaluate the relative contributions of MD vs. language-selective regions, we employed large fMRI datasets to construct probabilistic maps of the two systems. We used these maps to weigh the volume of lesion (in each of 80 patients) falling within each system. MD-weighted, but not language-weighted, lesion volumes predicted fluid intelligence deficit (with the opposite pattern observed for verbal fluency), suggesting that fluid intelligence is specifically tied to the MD system, and undermining claims that language is at the core of complex thought.
Keywords: Fluid intelligence, language, cognitive control, frontal, parietal, multiple-demand, neuropsychology, brain injury
Humans are unique in the animal kingdom in that they possess a highly sophisticated communication system that can be used to exchange complex ideas. Humans are also vastly more intelligent than even our closest primate relatives [27–30]. Some have therefore argued that language is the foundation of complex thought, including our abilities for hierarchical structured thought, our ability to reason flexibly about novel problems, and our ability for future-oriented thought and planning [14–24, cf. 25–26]. Following brain damage, loss of fluid intelligence has long been linked to lesions of the frontal lobes [6, 7] – which do house an important component of the language system [31]. However, the frontal lobes are highly structurally [8] and functionally [9] heterogeneous. In particular, they contain not only language-selective brain regions [13, 32] but also highly domain-general regions of the multiple demand (MD) system [11, 12, 33]. The MD system is an extensive bilateral fronto-parietal network of brain regions active during diverse demanding tasks [11, 12, 34–38], and has been linked to such important constructs as cognitive control [e.g. 39, 40, 41], working memory [38], attention [2, 42], and goal-directed behaviour [1, 43]. Consequently, this system has been argued to underlie the human ability for flexible thought and problem solving – the core ingredients of fluid intelligence [1]. Some have even hypothesized that it is specifically the expansion of the MD system in humans that endowed us with our unique cognitive capacities [44].
However, given that a) MD regions and language-selective regions lie side-by-side on the lateral surface of frontal cortex [9], and b) the precise locations of these sets of regions are highly variable across individual brains [9], it is difficult to interpret findings that link frontal lobe damage to loss of fluid intelligence. A similar picture obtains in the parietal cortex, which also houses both MD and language regions [1, 45] and whose damage has also been linked to intelligence loss [5]. Thus, the relative contributions of the domain-general regions of the MD system and adjacent language-selective regions are unclear. We here attempt to disentangle the contributions of these two systems by combining data from 80 patients with focal brain lesions with large fMRI datasets from healthy participants.
The eighty patients in our study had chronic, focal, adult-onset brain lesions. Patients were chosen so that lesions were confined to either frontal or posterior (occipital, temporal, parietal) lobes. Each patient’s lesion was weighted with respect to a) a probabilistic fMRI activation overlap map (from 63 healthy participants) for a contrast targeting the MD system [12, 46], and b) a probabilistic activation overlap map (from 220 healthy participants) for a contrast targeting the high-level language processing system [45]. For the MD system map, we used data from a spatial working memory task which reliably activates the frontoparietal MD network [12]. For the language system map, we used data from a language task in which participants read sentences vs. lists of pseudowords. The sentence > pseudoword-list contrast robustly and reliably activates the fronto-temporo-parietal language system [45, 47]. For each contrast, the individual fMRI participants’ maps were thresholded and overlaid in template space to create probabilistic activation overlap maps. In these maps, each voxel contains information on how many participants show an effect at the specified (p<0.001) threshold. Thus for any given voxel we can calculate the probability that it falls within the MD system vs. within the language system.
For each patient, we estimated the deficit in fluid intelligence resulting from their lesion (i.e., their postmorbid change in fluid intelligence), by comparing current functioning to an estimate of premorbid function. We measured current fluid intelligence using two well-established tests [48,49], and estimated premorbid scores on each of these tests based on a multiple regression, derived from healthy controls, predicting fluid intelligence score from age and crystalized intelligence [50, 51], as in our previous work [5]. (Using only one of the tests [48] to assess current function, and comparing current scores to estimated premorbid scores in the same way, produced a similar pattern of results.)
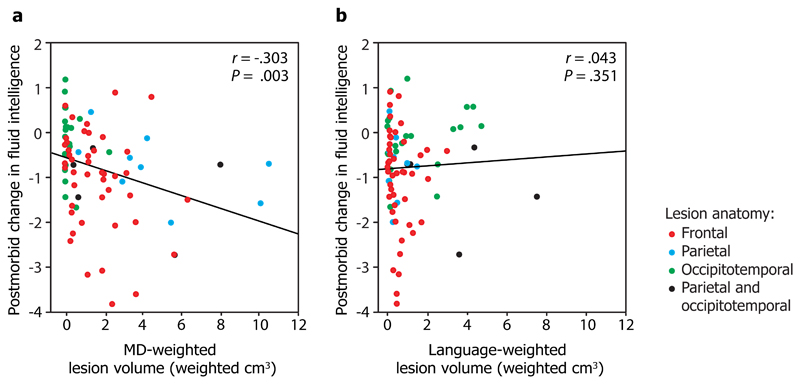
We then weighted each patient’s lesion against the probabilistic activation maps for the MD and language system to examine i) the relationship between the MD-weighted lesions and postmorbid change in fluid intelligence, and ii) the relationship between the language-weighted lesions and postmorbid change in fluid intelligence. The key result is shown in Fig. 1: MD-weighted, but not language-weighted, lesions predicted fluid intelligence deficit (MD: Pearson’s r = -0.304, p = 0.003, all p-values one-tailed; language: r = 0.043, p = 0.351). Moreover, MD-weighted lesion volume predicted fluid intelligence deficit after language-weighted lesion volume was partialled out (r = -0.303, p = 0.003), whereas the converse partial correlation was not significant (r = 0.031, p = 0.393). This suggests that MD lesion volume is a better predictor of fluid intelligence deficit than language lesion volume, and that after lesions to the MD system are taken into account, no further fluid intelligence deficit is accounted for by the extent to which the lesion affects language regions.
Figure 1. Correlation of a) MD-weighted lesion volume, and b) language-weighted lesion volume with postmorbid change in fluid intelligence.
For each patient (N=80), lesion volume was weighted for the extent of damage to the MD and language systems using probabilistic maps which indicate the likelihood that each voxel belongs to the MD and language systems in healthy participants. We estimated postmorbid change in fluid intelligence by comparing current function to estimated premorbid function (postmorbid minus premorbid: a negative score indicates a deficit). Point colour indicates lesion anatomy: frontal (red), parietal (blue), occipitotemporal (green), or parietal and occipitotemporal (black). r is Pearson’s correlation coefficient, P is the corresponding one-tailed P-value of the correlation. r, P, and fit lines are shown for the whole group. The extent to which lesions affect the MD, but not language, system predicts fluid intelligence deficit.
To evaluate whether this effect obtains specifically in the frontal lobe, which has historically been at the core of the debates about human intelligence, we carried out a further analysis restricted to patients with frontal lesions only (n=44). Here again, MD-weighted lesion volume predicted behavioural deficit (r = -0.258, p = 0.046), whereas language-weighted lesion volume did not (r = -0.087, p = 0.287) (red points in Fig. 1, see also Supplementary Figure 1). The result was the same if we instead restricted the analysis to patients with lesions affecting the left hemisphere (n=46): MD-weighted lesion volume predicted behavioural deficit (r = -0.267, p = 0.036) whereas language-weighted volume did not (r = 0.152, p = 0.156) (Supplementary Figure 2).
In two further analyses, we asked whether the results were robust to the details of how the MD and language maps were derived. First, we re-ran the analysis deriving the MD probabilistic map from the composite map of [12], in which the value at each voxel corresponds to the average t-value for the contrast of hard > easy across seven cognitively demanding tasks (thresholded at t > 0). Second, we derived a more restricted probabilistic map for the language system. For this, we masked our original map (derived from the contrast of sentences > pseudowords) with the equivalent map derived from the contrast of reading sentences > passive viewing of a fixation cross in the same 220 participants. Voxels were masked out of the restricted probabilistic language map if they did not show activation for sentences > passive viewing in at least 9/220 participants (individual sentences > passive viewing maps thresholded at p<0.001 uncorrected). This masking procedure removed default mode network activity from the language map. The result did not change: MD-weighted lesion volume predicted fluid intelligence deficit (r = - 0.341, p = 0.001) whereas language-weighted lesion volume did not (r = 0.097, p = 0.196) (Supplementary Figure 3).
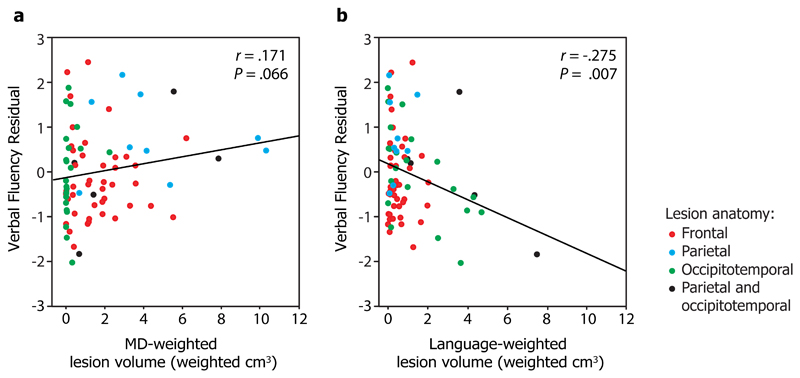
Finally, to test whether performance on a task that relies on the language system can be predicted from language-weighted lesions, we examined our patients’ performance on a test of verbal fluency [52], after regressing out variation attributable to IQ [see 53]. Indeed, we found that language-weighted lesion volume predicted verbal fluency (r = -.275, p = 0.007) whereas MD-weighted lesion volume did not (r = .171, p = .066) (Fig. 2). Moreover, language-weighted lesion volume predicted verbal fluency residual after MD-weighted lesion volume was partialled out (r = -.269, p = 0.009). In our sample, large language-system lesions were usually posterior (occipitotemporal and parietal/occipitotemporal), and more data would be needed to examine the specific role of frontal language regions in fluency. Nonetheless, in the group as a whole we observed a double dissociation between the MD and language systems and performance on fluid intelligence and language tasks.
Figure 2. Correlation of a) MD-weighted lesion volume, and b) language-weighted lesion volume with verbal fluency scores.
Verbal fluency residuals are standardized residuals in the regression of Cattell Culture Fair scores against verbal fluency scores (a more negative score indicates poorer performance). Point colour indicates lesion anatomy: frontal (red), parietal (blue), occipitotemporal (green), or parietal and occipitotemporal (black). r is Pearson’s correlation coefficient, P is the corresponding one-tailed P-value of the correlation. r, P, and fit lines are shown for the whole group (N=79). After partialling out variance attributable to IQ, verbal fluency is predicted by the extent to which lesions affect the language, but not the MD, system.
Whereas our analyses point to the MD, and not language-selective, regions as central to fluid intelligence, they do not rule out the contribution of brain regions outside the boundaries of these two networks. A simple explanation based on total lesion volume is ruled out by the double dissociation and our previous observation that, for example, lesion volume in occipitotemporal patients does not predict fluid intelligence deficit [5]. However, contributions from other parts of the brain remain to be evaluated. For example, damage to white matter tracts plausibly plays an important role in fluid intelligence function [54].
Our results disentangle the relative causal contributions of domain-general MD regions and language-selective regions to fluid intelligence. We show that damage to the MD regions, but not to the language regions, causes fluid intelligence impairments. This work fits well with findings that individuals with severe aphasia retain the ability to engage in many forms of complex thought [25, 26], with findings that show age-related decay in executive function in the presence of preservation, or even improvement, in verbal abilities [55], with the finding that executive function is unrelated to language ability in deaf pre-schoolers [56], and with fMRI findings that language-responsive brain regions are not active when individuals engage in diverse executive function and problem-solving tasks [25, 57]. Thus, although linguistic abilities may be important in the development of certain cognitive abilities [e.g. 19, 25, 58, 59–62], our data suggest that in mature human brains the language system is not causally important for fluid intelligence.
Methods
Participants
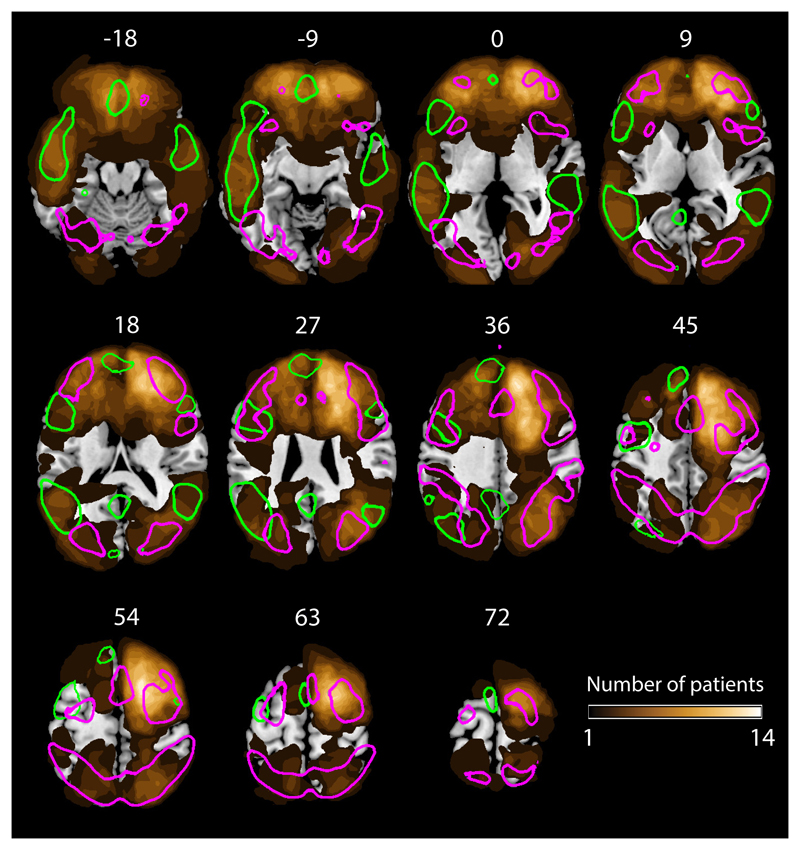
Eighty (34 female and 46 male; mean age 51.3 (SD = 12.9) years) patients with chronic, focal, adult-onset lesions (onset min 2 years prior to behavioural testing) of varied aetiology [see [5] for details; where the same group of participants were used] were recruited from the Cambridge Cognitive Neuroscience Research Panel (N=70) and the Institute of Cognitive Neurology Research Database (Buenos Aires, Argentina) (N=10). A sample size of 80 is sufficient to detect a correlation of .3 with a one-tailed alpha of .05 and a type II error rate of .15 [63]. Participants were not included if they had a visual field cut, overt aphasia, pre-insult history of epilepsy, or were unsuitable for MRI, or if their lesion comprised both frontal and posterior (parietal, occipital, temporal) cortices. Lesions were traced by F.M. who was blind to the behavioural scores of the participants and experimental aims. Group lesion anatomy provided good coverage of the MD and language regions (Fig. 3). Mean premorbid IQ, assessed using either the revised National Adult Reading Test [50] or the equivalent Word Accentuation Test [51], as appropriate for the participant’s first language, was 109.1 (SD = 13.1).
Figure 3. Anatomical distribution of lesions.
Gold colours indicate number of participants with a lesion at each voxel. Coloured outlines indicate regions of probability > 5% in the probabilistic MD (magenta, N = 63) and language (green, N = 220) maps which we used to derive MD and language weighted lesion volume. Our patient sample (N=80) provided good coverage of both the MD and language systems, with the exception of superior lateral regions of the left frontal cortex.
33 healthy control participants (21 female, 12 male), were used to create the multiple regression predicting fluid intelligence from age and premorbid IQ. These controls were recruited from the Medical Research Council Cognition and Brain Sciences Unit Volunteer Panel. They were selected to match the patient group on age (mean = 48.4 years; SD = 12.9 years) and premorbid IQ (mean = 109.5; SD = 12.3). All participants gave written informed consent and were paid under the approval of the Cambridge Local Research Ethics Committee, Cambridge, UK.
Probabilistic activation overlap maps
We created probabilistic maps for the MD and language system based on extant fMRI activation data. For the MD system map, we used data from 63 healthy participants (47 female and 16 male, mean age 27.6, SD = 4.31, partially overlapping with datasets from [12, 46]). Participants performed a spatial working memory task in which they had to remember a set of four vs. eight locations in a 3x4 grid in the easy and hard conditions, respectively. The hard > easy contrast robustly and reliably activates the fronto-parietal MD network, and the activations for this contrast overlap with hard > easy contrasts from numerous other tasks [12]. For the language map, we used data from 220 healthy participants (146 female and 74 male, mean age 29.1, SD = 5.09). Participants read sentences vs. lists of pseudowords (participants either read these materials passively, or performed a memory probe task at the end of each sentence/sequence; see [47, 64] for evidence that similar activations obtain regardless of the task). The sentence > pseudoword-list contrast robustly and reliably activates the fronto-temporo-parietal language system [45, 47]. For each contrast, individual participants’ maps were thresholded voxelwise at p<0.001 uncorrected, normalized, and overlaid in template space to create probabilistic activation overlap maps. In these maps, the value at each voxel indicates the proportion of participants that show an effect at the specified threshold, indicating the probability that the voxel falls within the MD system vs. within the language system. The maps are available for download from the Fedorenko laboratory website https://evlab.mit.edu/.
Lesion weighting
All patients had normalized lesion tracings drawn from T1-weighted Spoiled Gradient Echo (SPGR) MRI scans (1x1x1mm resolution) as part of previous participation in the Panel. Each lesion was weighted twice, once for each of the probabilistic activation maps. At each voxel, the lesion (0 or 1) was multiplied by the value in the relevant probabilistic overlay map, and these values were summed to give MD-weighted and language-weighted lesion volume. This calculation was carried out in MATLAB using routines from SPM (Wellcome Department of Imaging Neuroscience, London, UK; www.fil.ion.ucl.ac.uk/spm; script available at osf.io/wm8a3). Values were converted to cm3 by multiplying by the volume of one voxel.
Assessment of fluid intelligence
We assessed current fluid intelligence functioning using two problem-solving tests which are known to load strongly on fluid intelligence: Cattell Culture Fair (Scale 2, Form A) [48], and Letter Sets from the Educational Testing Service Kit of Factor-Referenced Tests [49]. The tests consist of timed puzzles involving geometrical figures (Cattell) or sets of letters (Letter Sets). In Cattell, participants must determine the next in a series, odd-one-out, completion of a matrix or topological relations; in Letter Sets, they determine the odd-one-out. Patient and control participants had scores on file as part of previous participation in our Panel.
Postmorbid change in fluid intelligence
We estimated postmorbid change in fluid intelligence from the discrepancy between predicted premorbid scores, and observed postmorbid scores, on the Cattell and Letter Sets tests, as in our previous work [5]. First, we used control data to derive multiple regressions predicting Culture Fair and Letter Sets scores from age and premorbid IQ (R = .682 in the regression for Culture Fair, R = .712 in the regression for Letter Sets). Then, we used these equations to predict premorbid Cattell and Letter Sets scores for each patient. Next, we subtracted the estimated premorbid score from the actual observed score and transformed the resulting score to a z-score by dividing it by the standard deviation of residuals in the relevant control group regression. Finally we averaged the discrepancies from the two tests together to give a single measure of postmorbid fluid intelligence change, in which a negative score indicates behavioural deficit.
Assessment of verbal fluency
We assessed verbal fluency using the standard phonemic version of the Verbal Fluency task [52], in which participants generate as many words as they can beginning with the letters F, A, and S, in blocks of one minute per letter. Data were available for 79/80 patients.
Factoring out the contribution of fluid intelligence from verbal fluency scores
As is the case with scores on many tasks across domains, verbal fluency scores are known to be predicted by fluid intelligence [see 53]. Indeed, this relationship obtained in our sample: regression of Cattell Culture Fair against Verbal Fluency was reliable (r = .412, F(1,77) = 15.776, p = .0002, two-tailed). In order to test for the impact of brain lesions on the component of verbal fluency that is not attributable to fluid intelligence, we used the residuals of this regression in our correlations with language and MD-weighted lesion volumes.
Correlation of weighted lesion volume with behavioural scores
We assessed the correlation between weighted lesion volumes and derived behavioural scores by calculating Pearson’s correlation coefficient (as appropriate for a linear relationship between continuous variables) and testing its significance. Reported P-values are one-tailed as the direction of the effect was pre-specified (larger lesions leading to poorer performance). The data met the assumptions of the test, and additional analyses excluding points with high leverage and/or Cook’s scores did not change the results.
Supplementary Material
Acknowledgements
A.W. was supported by ARC Fellowships (Discovery Early Career Researcher Award, DECRA, Grant Number DE120100898, and Future Fellowship, Grant Number FT170100105) and an ARC Discovery Project research grant (DP12102835). J.D. was supported by the Medical Research Council (United Kingdom) intramural program (grant no. SUAG/002/RG91365 ). E.F. was supported by a R00 award HD 057522 from NICHD, and by grants ANR-11-LABX-0036 (BLRI) and ANR-11-IDEX-0001-02 (A*MIDEX). We thank Nadene Dermody for help with analysis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing Interests
The authors declare no competing interests.
Author Contributions
Conceptualization, E.F.; Methodology, E.F. and A.W.; Formal Analysis, A.W.; Writing – Original Draft, E.F. and A.W.; Writing – Reviewing and Editing, J.D., E.F., and A.W; Visualization, A.W.; Supervision, E.F. and J.D. F.M. traced the patient lesions.
Data availability
The probabilistic maps used in the current study are available for download from the Fedorenko laboratory website https://evlab.mit.edu/. The datasets generated and/or analysed during the current study are available from the corresponding author on request.
Code availability
Code used to calculate the weighted lesion volumes in this study is available on the Open Science Framework at osf.io/wm8a3.
References
- 1.Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Duncan J. The structure of cognition: attentional episodes in mind and brain. Neuron. 2013;80:35–50. doi: 10.1016/j.neuron.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- 4.Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H. A neural basis for general intelligence. Science (New York, N.Y) 2000;289:457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- 5.Woolgar A, Parr A, Cusack R, Thompson R, Nimmo-Smith I, Torralva T, Roca M, Antoun N, Manes F, Duncan J. Fluid intelligence loss linked to restricted regions of damage within frontal and parietal cortex. Proc Natl Acad Sci. 2010;107:14899–14902. doi: 10.1073/pnas.1007928107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan J, Burgess P, Emslie H. Fluid intelligence after frontal lobe lesions. Neuropsychologia. 1995;33:261–268. doi: 10.1016/0028-3932(94)00124-8. [DOI] [PubMed] [Google Scholar]
- 7.Glascher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, Adolphs R. Distributed neural system for general intelligence revealed by lesion mapping. Proc Natl Acad Sci. 2010;107:4705–4709. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amunts K, Lenzen M, Friederici AD, Schleicher A, Morosan P, Palomero-Gallagher N, Zilles K. Broca's region: novel organizational principles and multiple receptor mapping. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fedorenko E, Duncan J, Kanwisher N. Language-selective and domain-general regions lie side by side within Broca's area. Curr Biol. 2012;22:2059–2062. doi: 10.1016/j.cub.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wise RJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within 'Wernicke's area'. Brain : a journal of neurology. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- 11.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 12.Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci. 2013;110:16616–16621. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedorenko E, Behr MK, Kanwisher N. Functional specificity for high-level linguistic processing in the human brain. Proc Natl Acad Sci. 2011;108:16428–16433. doi: 10.1073/pnas.1112937108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carruthers P. The cognitive functions of language. Behav Brain Sci. 2002;25:657–674. doi: 10.1017/s0140525x02000122. discussion 674-725. [DOI] [PubMed] [Google Scholar]
- 15.Hinzen W. The philosophical significance of Universal Grammar. Lang Sci. 2012;34:635–649. [Google Scholar]
- 16.Hinzen W. Narrow syntax and the language of thought. Philos Psychol. 2013;26:1–23. [Google Scholar]
- 17.Dennett DC. The role of language in intelligence. Sprache und Denken/Language and Thought. 1997:42. [Google Scholar]
- 18.Bickerton D. Language and human behavior. University of Washington Press; 1995. [Google Scholar]
- 19.Gentner D. Why we're so smart. In: Gentner D, Goldin-Meadow S, editors. Language in mind: Advances in the study of language and thought. Cambridge: MIT Press; 2003. [Google Scholar]
- 20.Kuczaj SA, Hendry JL. Does language help animals think? In: Gentner D, Goldin-Meadow S, editors. Language in mind: Advances in the study of language and thought. Cambridge: MA: MIT Press; 2003. [Google Scholar]
- 21.Bermúdez JL. Thinking without words. Oxford University Press; 2003. [Google Scholar]
- 22.Baldo JV, Bunge SA, Wilson SM, Dronkers NF. Is relational reasoning dependent on language? A voxel-based lesion symptom mapping study. Brain Lang. 2010;113:59–64. doi: 10.1016/j.bandl.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldo JV, Dronkers NF, Wilkins D, Ludy C, Raskin P, Kim J. Is problem solving dependent on language? Brain Lang. 2005;92:240–250. doi: 10.1016/j.bandl.2004.06.103. [DOI] [PubMed] [Google Scholar]
- 24.Baldo JV, Paulraj SR, Curran BC, Dronkers NF. Impaired reasoning and problem-solving in individuals with language impairment due to aphasia or language delay. Front Psychol. 2015;6:1523. doi: 10.3389/fpsyg.2015.01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedorenko E, Varley R. Language and thought are not the same thing: evidence from neuroimaging and neurological patients. Ann N Y Acad Sci. 2016;1369:132–153. doi: 10.1111/nyas.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varley R. Reason without much language. Lang Sci. 2014;46:232–244. [Google Scholar]
- 27.Tomasello M, Herrmann E. Ape and Human Cognition What's the Difference? Current Directions in Psychological Science. 2010;19:3–8. [Google Scholar]
- 28.Sherwood CC, Subiaul F, Zawidzki TW. A natural history of the human mind: tracing evolutionary changes in brain and cognition. J Anat. 2008;212:426–454. doi: 10.1111/j.1469-7580.2008.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Premack D. Human and animal cognition: continuity and discontinuity. Proc Natl Acad Sci. 2007;104:13861–13867. doi: 10.1073/pnas.0706147104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penn DC, Holyoak KJ, Povinelli DJ. Darwin's mistake: explaining the discontinuity between human and nonhuman minds. Behav Brain Sci. 2008;31:109–130. doi: 10.1017/S0140525X08003543. discussion 130-178. [DOI] [PubMed] [Google Scholar]
- 31.Broca P. Remarks on the seat of the faculty of articulated language, following an observation of aphemia (loss of speech) Bulletin de la Société Anatomique. 1861;6:330–357. [Google Scholar]
- 32.Monti MM, Parsons LM, Osherson DN. Thought beyond language: neural dissociation of algebra and natural language. Psychol Sci. 2012;23:914–922. doi: 10.1177/0956797612437427. [DOI] [PubMed] [Google Scholar]
- 33.Woolgar A, Jackson J, Duncan J. Coding of Visual, Auditory, Rule, and Response Information in the Brain: 10 Years of Multivoxel Pattern Analysis. J Cogn Neurosci. 2016:1–22. doi: 10.1162/jocn_a_00981. [DOI] [PubMed] [Google Scholar]
- 34.Yeo BT, Krienen FM, Eickhoff SB, Yaakub SN, Fox PT, Buckner RL, Asplund CL, Chee MW. Functional Specialization and Flexibility in Human Association Cortex. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Consciousness and cognition. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 40.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Power JD, Petersen SE. Control-related systems in the human brain. Current opinion in neurobiology. 2013;23:223–228. doi: 10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 43.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 44.Gray JR, Thompson PM. Neurobiology of intelligence: science and ethics. Nature reviews. 2004;5:471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- 45.Fedorenko E, Hsieh PJ, Nieto-Castanon A, Whitfield-Gabrieli S, Kanwisher N. New method for fMRI investigations of language: defining ROIs functionally in individual subjects. J Neurophysiol. 2010;104:1177–1194. doi: 10.1152/jn.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blank I, Kanwisher N, Fedorenko E. A functional dissociation between language and multiple-demand systems revealed in patterns of BOLD signal fluctuations. J Neurophysiol. 2014;112:1105–1118. doi: 10.1152/jn.00884.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahowald K, Fedorenko E. Reliable individual-level neural markers of high-level language processing: A necessary precursor for relating neural variability to behavioral and genetic variability. Neuroimage. 2016;139:74–93. doi: 10.1016/j.neuroimage.2016.05.073. [DOI] [PubMed] [Google Scholar]
- 48.Institute for Personality and Ability Testing. Measuring Intelligence with the Culture Fair Tests. Champaign, IL: Institute for Personality and Ability Testing; 1973. [Google Scholar]
- 49.Ekstron RB, French JW, Harmon HH, D D. ETS Kit of Factor-Referenced Cognitive Tests. Educational Testing Service; Princeton, NJ: 1976. [Google Scholar]
- 50.Nelson HE, Willison JR. The Revised National Adult Reading Test-Test manual. Windsor, UK: NFER-Nelson; 1991. [Google Scholar]
- 51.Burin DI, Jorge RE, Arizaga RA, Paulsen JS. Estimation of premorbid intelligence: the word accentuation test--Buenos Aires version. J Clin Exp Neuropsychol. 2000;22:677–685. doi: 10.1076/1380-3395(200010)22:5;1-9;FT677. [DOI] [PubMed] [Google Scholar]
- 52.Benton AL, Hamsher K. Multilingual aphasia examination. Iowa City: University of Iowa Press; 1976. [Google Scholar]
- 53.Roca M, Parr A, Thompson R, Woolgar A, Torralva T, Antoun N, Manes F, Duncan J. Executive function and fluid intelligence after frontal lobe lesions. Brain : a journal of neurology. 2010;133:234–247. doi: 10.1093/brain/awp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glascher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, Adolphs R. Distributed neural system for general intelligence revealed by lesion mapping. Proc Natl Acad Sci. 2010;107:4705–4709. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartshorne JK, Germine LT. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol Sci. 2015;26:433–443. doi: 10.1177/0956797614567339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vernon M. Relationship of language to the thinking process. Arch Gen Psychiatry. 1967;16:325–333. doi: 10.1001/archpsyc.1967.01730210065011. [DOI] [PubMed] [Google Scholar]
- 57.Monti MM, Parsons LM, Osherson DN. The boundaries of language and thought in deductive inference. Proc Natl Acad Sci. 2009;106:12554–12559. doi: 10.1073/pnas.0902422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Villiers J. Language and theory of mind: What are the developmental relationships. Understanding other minds: Perspectives from developmental cognitive neuroscience. 2000;2:83–123. [Google Scholar]
- 59.Piaget J. The language and thought of the child. Oxford, England: Harcourt, Brace; 1926. [Google Scholar]
- 60.Vygotskiĭ LS. Thought and language. MIT press; 2012. [Google Scholar]
- 61.Gentner D, Loewenstein J. Relational language and relational thought. Language, literacy, and cognitive development: The development and consequences of symbolic communication. 2002:87–120. [Google Scholar]
- 62.Winsler A, Naglieri J. Overt and covert verbal problem-solving strategies: Developmental trends in use, awareness, and relations with task performance in children aged 5 to 17. Child Dev. 2003;74:659–678. doi: 10.1111/1467-8624.00561. [DOI] [PubMed] [Google Scholar]
- 63.Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing clinical research. Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 64.Fedorenko E. The role of domain-general cognitive control in language comprehension. Front Psychol. 2014;5:335. doi: 10.3389/fpsyg.2014.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The probabilistic maps used in the current study are available for download from the Fedorenko laboratory website https://evlab.mit.edu/. The datasets generated and/or analysed during the current study are available from the corresponding author on request.
Code availability
Code used to calculate the weighted lesion volumes in this study is available on the Open Science Framework at osf.io/wm8a3.