Abstract
Stroke is the second leading cause of death worldwide accounting for >6M deaths annually (including 2M stroke deaths in China)1,2. Both ischaemic stroke (IS) and haemorrhagic stroke (chiefly intracerebral haemorrhage [ICH]), account for an equal number of stroke deaths in China, despite the incidence of IS being about 4-fold greater than ICH1,2. China also has a higher incidence of stroke and a higher proportion of ICH, compared with Western populations3–5, despite having a lower mean low-density lipoprotein cholesterol (LDL-C) concentration. Observational studies reported weaker positive associations of LDL-C with IS than with coronary heart disease (CHD)6,7, but LDL-C-lowering trials demonstrated similar risk reductions for IS and CHD8–10. Mendelian randomisation (MR) studies of LDL-C and IS have reported conflicting results11–13, prompting questions about the importance of LDL-C for IS. Concerns about the excess risks of ICH associated with lowering LDL-C14,15, may have prevented the more widespread use of statins for prevention of cardiovascular disease (CVD) in China. We examined the associations of biochemically-measured LDL-C, and of other major lipids, with IS and ICH in a nested case-control study in the China Kadoorie Biobank (CKB), and compared the risks for both stroke types associated with equivalent differences in LDL-C in MR analyses, and with worldwide LDL-C-lowering trials. The results demonstrated strong positive associations of LDL-C with IS and equally strong inverse associations with ICH, that were confirmed by genetic analyses and by LDL-C-lowering trials, but lowering LDL-C is still likely to have net benefit for prevention of overall stroke and CVD in China.
A total of 512,891 adults from 10 diverse areas in China were recruited into the CKB prospective study. Among the subset of 489,762 individuals with no prior history of stroke, transient ischaemic attack, or CHD at baseline, the mean (standard deviation [SD]) age was 51 (11) years and 59% were women. Overall, after a median follow-up duration of 9 years, a total of 32,869 incident IS cases and 8,270 incident ICH cases were recorded, yielding age- and sex-adjusted incidence rates of 761 and 187 cases per 100,000 person-years, respectively.
Among individuals with no prior history of CVD, cancer, lipid-lowering, anticoagulant, or antiplatelet treatment at baseline, 5475 IS cases, 4776 ICH cases and 6290 healthy controls were selected for a nested case-control study of incident stroke. At baseline, IS cases, compared with controls, were more likely to be urban residents and to smoke, but had similar dietary patterns. Regular consumption of certain animal-based foods, e.g., meat and eggs, was less common in ICH cases than in controls, but the distribution of other socio-economic and lifestyle factors were similar (Table 1). The overall mean (SD) plasma concentrations of total cholesterol, LDL-C, and HDL-C were 4.6 (0.9) mmol/L, 2.4 (0.6) mmol/L, and 1.2 (0.3) mmol/L, respectively. The median (inter-quartile range) concentration of triglycerides was 1.6 (1.3) mmol/L. Stroke cases had higher mean levels of systolic blood pressure (SBP) than controls, but LDL-C and SBP were only weakly correlated (r=0.06).
Table 1. Baseline characteristics of participants in the nested case-control study of strokea.
| Ischaemic stroke cases | Intracerebral haemorrhage cases | Controls | |
|---|---|---|---|
| Number of participants | 5475 | 4776 | 6290 |
| Demographic factors | |||
| Age at baseline, Mean (SD), years | 54.3 (10.7) | 58.8 (10.7) | 56.7 (11.6) |
| Female, % | 53.1 | 47.8 | 47.9 |
| Urban, % | 44.9 | 22.5 | 21.2 |
| ≥6 years of education, % | 38.6 | 36.1 | 38.6 |
| Household income (>20,000 yuan/year), % | 33.1 | 29.2 | 29.5 |
| Lifestyle factors | |||
| Male ever smokers, % | 64.2 | 60.6 | 60.3 |
| Female ever smokers, % | 3.7 | 3.3 | 3.2 |
| Male ever drinkers, % | 29.1 | 30.6 | 29.7 |
| Female ever drinkers, % | 2.5 | 2.6 | 1.9 |
| Physical activity, Mean (SD), MET-h/day | 18.1 (14.2) | 18.1 (13.1) | 18.9 (12.0) |
| Regular consumption of certain foodsb, % | |||
| Meat or poultry | 38.5 | 35.6 | 37.8 |
| Fish or other seafood | 5.1 | 3.8 | 5.1 |
| Eggs | 25.1 | 21.8 | 26.8 |
| Fresh fruit | 19.9 | 17.9 | 21.6 |
| Dairy products | 10.0 | 7.5 | 10.3 |
| Physical and blood measurements, Mean (SD) | |||
| SBP, mmHg | 144.1 (34.2) | 152.0 (29.5) | 134.0 (21.1) |
| DBP, mmHg | 83.0 (18.8) | 87.0 (15.8) | 77.3 (11.7) |
| BMI, kg/m2 | 23.9 (4.4) | 23.5 (3.8) | 23.2 (3.4) |
| Random blood glucose, mmol/L | 6.6 (3.9) | 6.5 (3.4) | 6.0 (2.8) |
| Lipid measurements | |||
| Total cholesterol, mmol/L | 4.8 (1.0) | 4.5 (1.0) | 4.5 (0.9) |
| LDL-cholesterol, mmol/L | 2.5 (0.7) | 2.3 (0.7) | 2.3 (0.7) |
| HDL-cholesterol, mmol/L | 1.2 (0.3) | 1.3 (0.3) | 1.3 (0.3) |
| Triglycerides, mmol/L c | 1.7 (1.4) | 1.5 (1.2) | 1.5 (1.2) |
| Apolipoprotein B, mg/dL | 85.8 (27.1) | 81.7 (21.4) | 82.8 (20.4) |
| Apolipoprotein A1, mg/dL | 133.9 (29.1) | 134.4 (22.7) | 134.7 (21.9) |
| Lipoprotein (a), nmol/L c | 18.3 (36.5) | 18.7 (33.3) | 18.4 (35.5) |
| Medical history and health status, % | |||
| Diabetes | 10.5 | 8.6 | 5.1 |
| Hypertension d | 56.8 | 69.3 | 37.6 |
| Self-rated poor health status e | 14.2 | 15.1 | 9.4 |
SD=Standard deviation; MET=Metabolic equivalent; SBP=Systolic blood pressure; DBP=Diastolic blood pressure; BMI=Body mass index. Mean (SD) values were directly standardised to the age (at baseline, 10-year range), sex, and study area structure of the entire study population included, unless otherwise stated.
Regular consumption was defined as consumption of the food groups on at least four days per week.
Estimates were medians (inter-quartile range) for triglycerides, and lipoprotein (a).
Participants were considered to be hypertensive if they had a measured SBP of at least 140 mmHg, or a measured DBP of at least 90 mmHg, or were receiving treatment for hypertension. The latter was defined as those who reported a diagnosis of hypertension by a physician and use of anti-hypertensives at baseline.
Individuals were asked to classify their current general health status compared with others of the same age by responding to the question “How is your current health status?” If they replied that it was “Poor”, they were classified as having “Self-rated poor health status”.
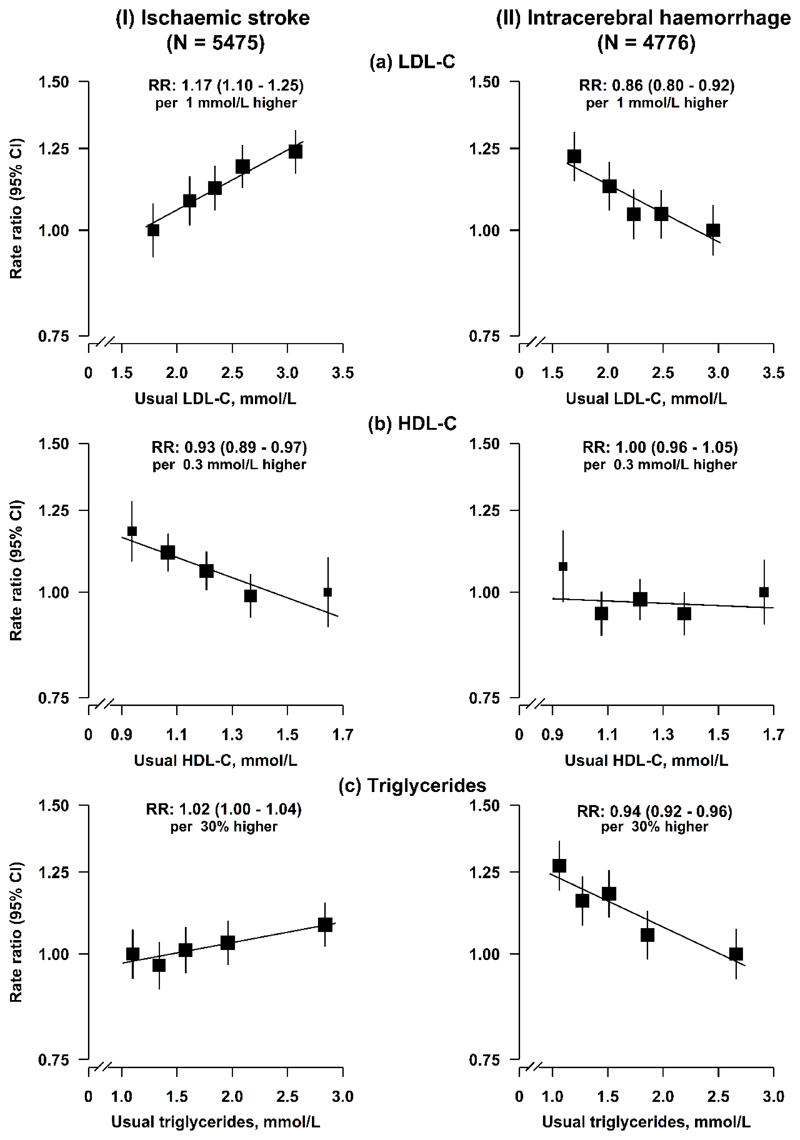
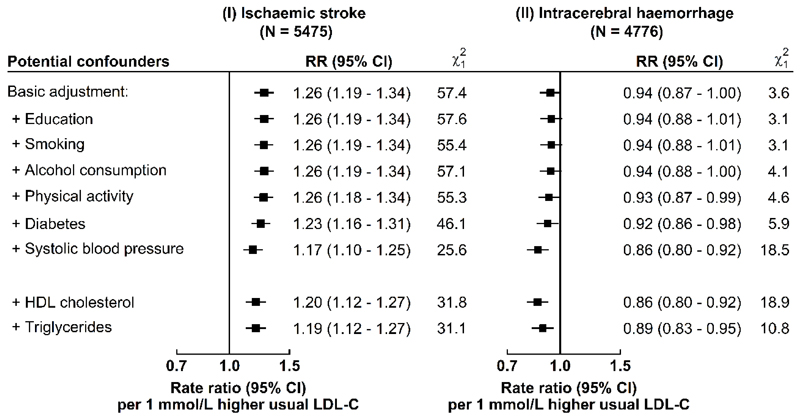
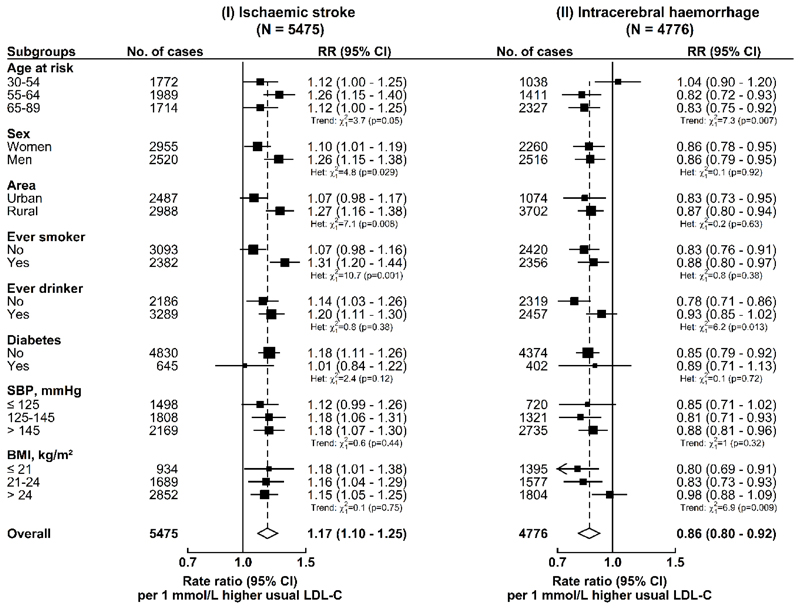
Plasma concentrations of LDL-C were positively associated with risk of IS and inversely associated with risk of ICH, after stratification for age-at-risk (5-year intervals), study area, and sex, and adjustment for education, smoking, alcohol consumption, physical activity, diabetes, and baseline SBP. Throughout the range examined, i.e., 1.7-3.2 mmol/L, each 1 mmol/L higher usual LDL-C was associated with a 17% (rate ratio [RR]=1.17, 95% confidence intervals [CI]: 1.10-1.25) higher risk of IS, and a 14% (0.86, 0.80-0.92) lower risk of ICH (Fig. 1), which translated into an RR of 0.85 (0.80-0.91) for IS and 1.16 (1.08-1.25) for ICH, for each 1 mmol/L lower LDL-C. These results were unaltered by further adjustment for other lipid fractions (Extended Data Fig. 1) and were generally similar in different subgroups (except for sex, area, and smoking for IS; age and body mass index [BMI] for ICH) (Extended Data Fig. 2).
Fig. 1. Adjusted rate ratios (RR) for risk of ischaemic stroke and intracerebral haemorrhage by fifths of usual concentrations of LDL-C, HDL-C, and triglycerides in observational analyses in CKB.
Cox regression was used to estimate the rate ratios (RR) and 95% confidence intervals (CI) for ischaemic stroke (N = 5475) and intracerebral haemorrhage (N = 4776) by fifths of (a) usual LDL-C, (b) usual HDL-C, and (c) usual triglycerides. Each square has an area inversely proportional to the variance of the log risk in the specific group. The line represents the slope from a weighted linear regression with the weights based on the inverse variance of the log RR.
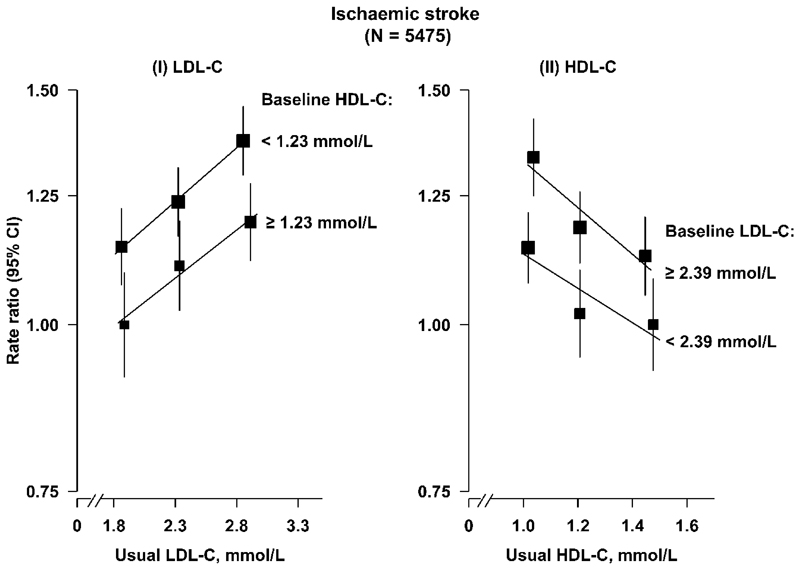
Plasma concentrations of HDL-C were inversely associated with risk of IS (0.93, 0.89-0.97 per 0.3 mmol/L higher HDL-C), but not with ICH (1.00, 0.96-1.05) (Fig. 1). The associations of LDL-C and HDL-C with IS were independent of each other (Extended Data Fig. 3).
Plasma concentrations of triglycerides were weakly positively associated with risk of IS (1.02, 1.00-1.04 per 30% higher triglycerides), but were inversely associated with ICH (0.94, 0.92-0.96) (Fig. 1). The risk estimates for IS and ICH for all major blood lipids were largely unaltered after additional adjustment for BMI (Supplementary Table 1). Overall, the associations of LDL-C, HDL-C, and triglycerides with IS differed qualitatively from those for ICH (Pheterogeneity between IS and ICH: 4.2 × 10-11, P=0.01, 1.3 × 10-8, respectively) (Supplementary Table 1).
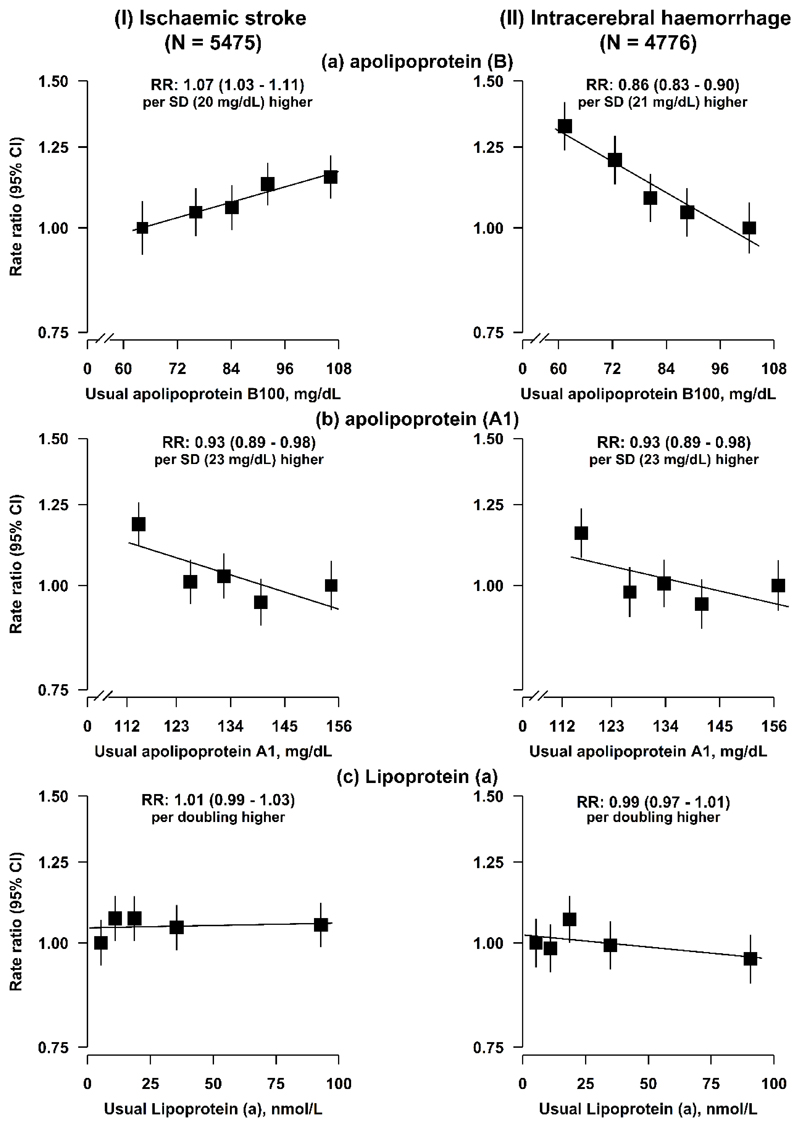
Plasma concentrations of LDL-C were strongly correlated with apolipoprotein B (r=0.92), and weakly correlated with lipoprotein (a) (r=0.22). The associations of apolipoprotein B with stroke types were consistent with those for LDL-C. However, lipoprotein (a) was not significantly associated with either IS or ICH (Extended Data Fig. 4), and the risk estimates of LDL-C for both stroke types were unaltered after further adjustment for lipoprotein (a).
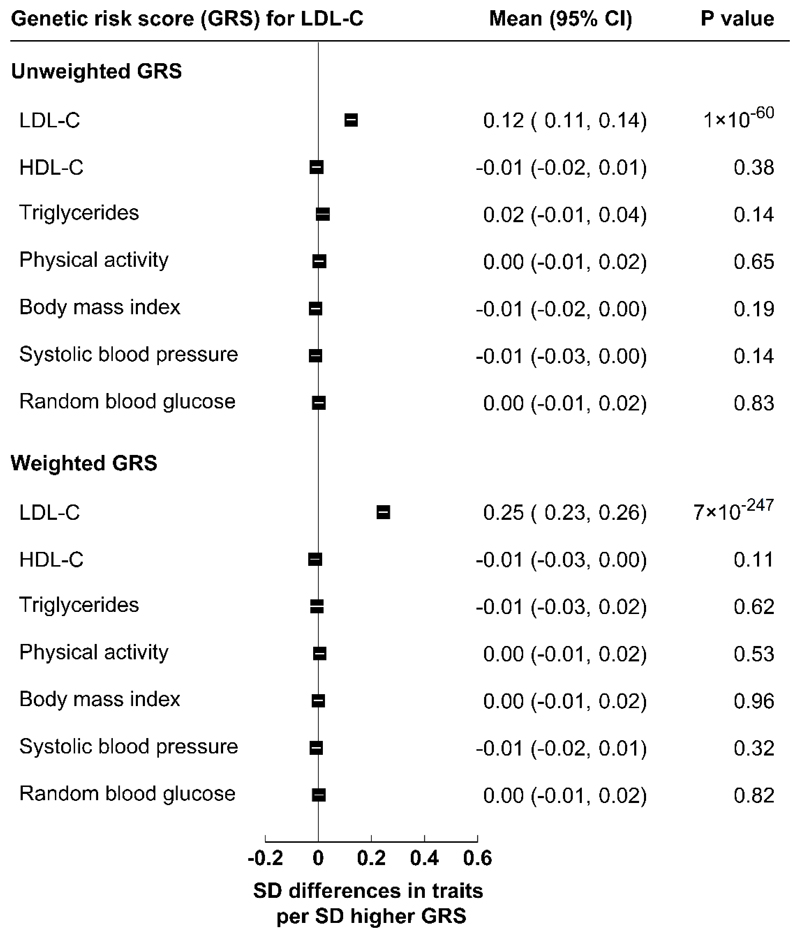
A genetic risk score (GRS) comprising 46 single-nucleotide polymorphisms (SNPs) most strongly associated with plasma LDL-C concentrations in the Global Lipids Genetics Consortium (GLGC)16,17 was constructed as an instrumental variable for LDL-C using previously published methods18 (see Methods). Supplementary Table 2 also compares the effect sizes of the 46 SNPs on plasma LDL-C concentrations in CKB with those in the GLGC17, and shows good concordance for genetically-instrumented differences in LDL-C in both Chinese and Western populations. In CKB, the GRS for LDL-C strongly predicted plasma concentrations of LDL-C (P=7×10-247), but not HDL-C, triglycerides, physical activity, BMI, SBP, or random blood glucose (Extended Data Fig. 5).
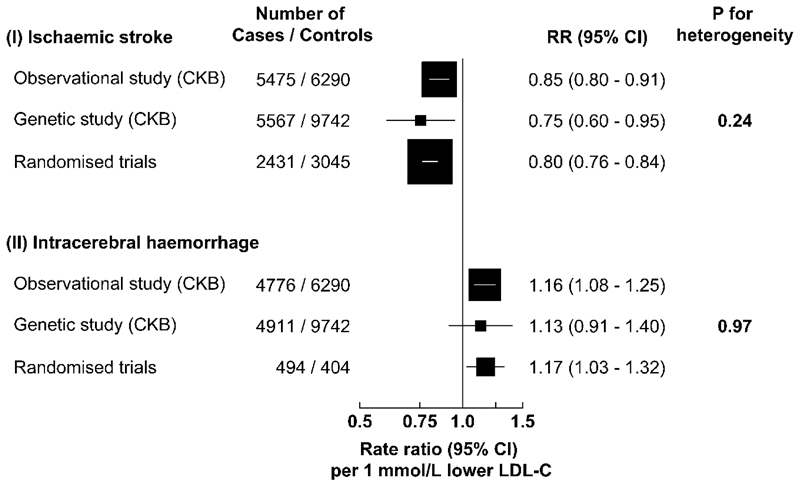
Each 1 mmol/L lower genetically-instrumented LDL-C was associated with RRs of 0.75 (0.60-0.95) for IS and 1.13 (0.91-1.40) for ICH (Fig. 2). Sensitivity analyses including median-weighted or inverse-variance weighted MR and MR-Egger approaches indicated similar results to those obtained by the main GRS for stroke types (Supplementary Table 3).
Fig. 2. Adjusted rate ratios (RR) for risk of ischaemic stroke and intracerebral haemorrhage associated with 1 mmol/L lower LDL-C in observational and genetic analyses in CKB, and in randomised trials of LDL-C-lowering drug treatment in Western populations.
The values shown are the RR (95% CI) per 1 mmol/L lower LDL-C concentrations. The number of cases of ischaemic stroke and of intracerebral haemorrhage, and controls in the observational analyses were 5475, 4776, and 6290, respectively; and in the genetic analyses were 5567, 4911, and 9742, respectively. In the randomised trials, the number of ischaemic stroke cases were 2431 in the treated and 3045 in the placebo groups, and the corresponding numbers of intracerebral haemorrhage cases were 494 in the treated and 404 in the placebo groups, respectively. Chi-square tests were used to test for heterogeneity. P-values (two-sided) were uncorrected for multiple testing.
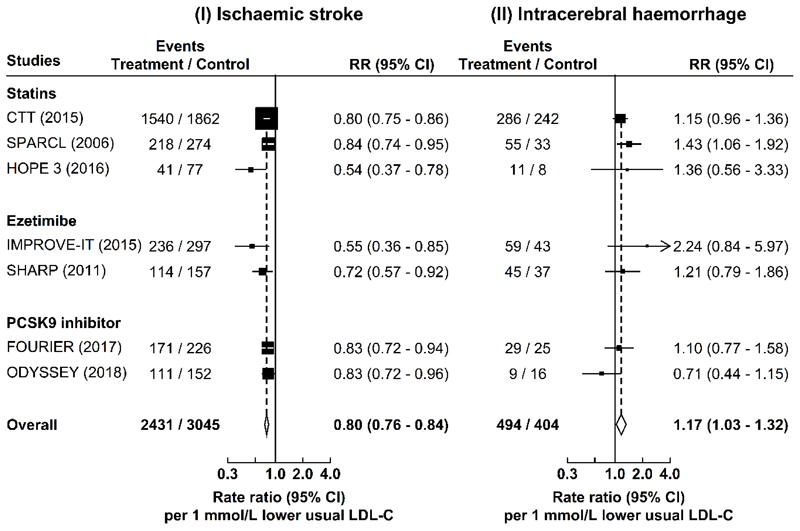
In a meta-analysis of the worldwide randomised trials of LDL-C-lowering drug treatment, each 1 mmol/L lower LDL-C was associated with RRs of 0.80 (0.76-0.84) for IS and 1.17 (1.03-1.32) for ICH (Fig. 2 and Extended Data Fig. 6). The risk estimates obtained from trials were highly consistent with those in the observational and genetic studies in CKB (Pheterogeneity: 0.24 and 0.97, respectively) (Fig. 2).
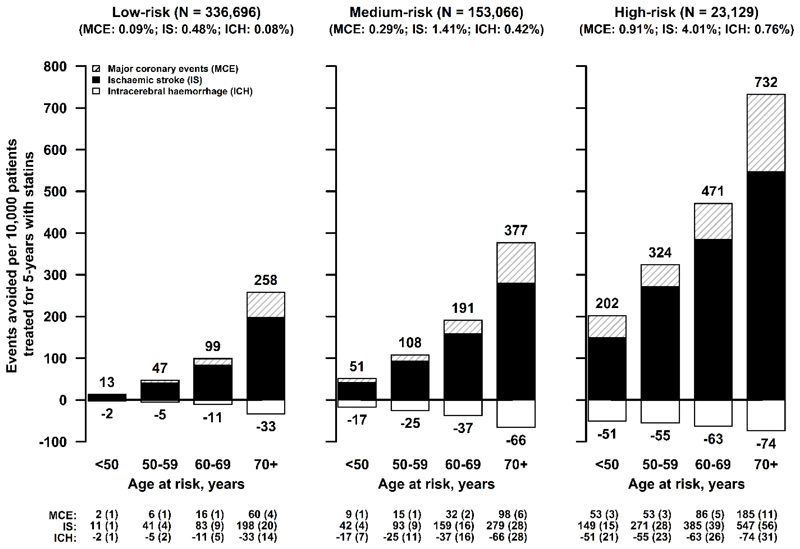
To assess the net effects (benefits vs hazards) of LDL-C-lowering drug treatment in the Chinese population, we applied the relative risk estimates from the LDL-C-lowering trials to the age-specific absolute risks of stroke types and major coronary events (including myocardial infarction and fatal ischaemic heart disease) in all CKB participants. Fig. 3 demonstrates that the predicted number of incident events of IS and major coronary events avoided greatly exceeds the excess ICH events by lowering LDL-C by 1 mmol/L per 10,000 patients treated for 5 years in Chinese adults. The results suggest a net benefit for prevention of overall stroke and major coronary events in both primary (low-risk individuals) and in secondary (high-risk of recurrent vascular events) prevention settings. Moreover, the net benefits are likely to be greater if all atherosclerotic vascular diseases were also to be included.
Fig. 3. Predicted number of events avoided for ischaemic stroke, major coronary events, and intracerebral haemorrhage per 10,000 patients treated by lowering LDL-C by 1 mmol/L with statins for 5 years in Chinese adults with different levels of vascular risk.
The estimated number of events (and their standard deviations) avoided by lowering LDL-C by 1 mmol/L, obtained by applying the rate ratios from the LDL-C-lowering trials to low, medium and high-risk population subgroups in CKB, are shown below the figure.
Discussion
The present study, including a large number of brain image-confirmed IS and ICH cases in populations without prior history of chronic disease or statin use, demonstrated strong positive associations of LDL-C with IS and equally strong inverse associations with ICH. The causal relevance of LDL-C for both IS and ICH was confirmed by MR analyses in the same study population, which was less susceptible to reverse causality and confounding factors. For LDL-C, the risk estimates for IS were consistent with those observed in Western populations7, but extended the lower range of LDL-C in the general population down to 1.7 mmol/L, i.e., well below the concentrations typically seen in Western populations. These results suggest that even among those with what is by Western standards, a normal or low LDL-C concentration, lower LDL-C is associated with a lower risk of IS, as it is for CHD19. Conversely, lower LDL-C was associated with a higher risk of ICH, irrespective of baseline levels of blood pressure, BMI, or other vascular risk factors. The risk estimates for different stroke types in both observational and genetic analyses in CKB were similar for equivalent differences in LDL-C in the LDL-C-lowering trials conducted predominantly in Western populations.
Large-scale trials have demonstrated that lowering LDL-C by 1 mmol/L with statins reduces the risk of IS by about one-fifth8,15, with similar effect estimates observed for other LDL-C-lowering drug treatments, e.g., ezetimibe or evolocumab9,20,21. The risk reductions associated with LDL-C-lowering drug treatment observed in the trials were not reliably predicted by previous observational studies6,22, which included studies predating the widespread use of brain imaging for stroke diagnosis. In contrast, recent reports of MR analyses of LDL-C and IS23,24, demonstrated significant associations of genetically-instrumented LDL-C with IS, consistent with the results of the present study. The highly consistent results from the observational and genetic analyses in China and randomised trials conducted chiefly in Western populations now provide reliable evidence that lower LDL-C is causally associated with a higher risk of ICH. Previous studies have suggested that the proportional excess risk of ICH associated with lower LDL-C was confined to individuals with elevated blood pressure25,26, but this is not supported by the present study, suggesting that the previous reported interaction between cholesterol and SBP for ICH25,26 could be a chance finding27. Randomised trials have reported similar proportional reductions in risk of total stroke with LDL-C-lowering treatment in individuals with hypertension versus those without28, and with different levels of total cardiovascular risk8,15. The mechanisms by which low LDL-C causes ICH are not fully understood. Histopathologic studies have suggested that lower cholesterol concentrations may increase permeability of the vessel walls29,30, cause arterionecrosis, microaneurysms, and ICH25,30,31.
The present study estimated that each 1 mmol/L lower LDL-C was associated with approximately 10-20 excess ICH cases in Chinese adults per 10,000 individuals treated for 5 years with commonly available statins, compared with 5-10 ICH cases in North American or European populations8. Concerns about the excess risk of ICH associated with LDL-C-lowering treatment have been an obstacle to the more widespread use of statins in China. For example, only <5% of the individuals at high risk of CVD reported regular use of statins in CKB and other studies in China32, compared with 66% in most Western countries (e.g., Sweden and Canada)32. However, in Chinese adults, with higher rates of stroke4,33,34, and a higher proportion of ICH4,33, the present study demonstrated that lowering LDL-C still has a net benefit on prevention of overall stroke, irrespective of age, prior history of hypertension or CVD. Moreover, any net beneficial effects of LDL-C-lowering are likely to be greater if the trends of increasing IS incidence and decreasing ICH incidence observed over the last two decades continues5,35, or if the beneficial effects on other occlusive vascular diseases are also included8,15.
In conclusion, the associations of major blood lipids with stroke differed qualitatively by stroke type. Lower LDL-C concentrations were associated with lower risks of IS and higher risks of ICH, and the causal relevance of these associations was confirmed by genetic analyses in the same population and by LDL-C-lowering trials in Western populations. Thus, the highly consistent results of observational and genetic analyses in the Chinese population and those of the LDL-C-lowering trials in Western populations suggest that the excess risk of ICH observed in the trials is probably due to lower concentrations of LDL-C, rather than from some other factors. Importantly, the results also suggest that lower LDL-C concentrations are still likely to have net benefit for prevention of overall stroke and CVD in the Chinese population with high stroke rates. Hence, the results provide support for more widespread use of LDL-C-lowering drug treatment for prevention of overall stroke and other vascular diseases both in Chinese and other populations worldwide with low mean LDL-C concentrations.
Methods
CKB Study Population
The CKB study recruited 512,891 adults aged 30-79 years from 10 diverse areas in China during 2004-200836. At baseline and subsequent resurveys in a 5% random subset, detailed data were collected on medical history (including use of statins), lifestyle characteristics (including smoking, alcohol consumption, physical activity, and diet), and clinical measurements (including blood pressure and anthropometry)37. A blood sample was collected from all participants and plasma was separated for long-term storage at -196°C. All participants were followed-up by electronic linkage, via a unique personal identification number, to death and disease registries, and to nationwide health insurance agencies, for cause-specific mortality and morbidity. The accuracy of reported stroke types (IS and ICH), was verified by a review of the original medical records by a panel of certified neurologists and stroke physicians in China. Among the stroke cases selected, >90% were confirmed by brain imaging. The periodic resurvey data were used to correct for regression dilution bias38. Approval was obtained from relevant international, national, and local ethics committees, and all participants provided written informed consent.
Nested Case-Control Study of Stroke Types
The nested case-control study of incident stroke types included 5,475 IS cases, 4,776 ICH cases, and 6,290 healthy controls. Participants had no prior history of stroke, CHD, cancer, or use of lipid-lowering, antiplatelet, or anticoagulant drug treatment. Controls were selected among those who were free of diagnosis of stroke of any type, or unspecified type, myocardial infarction, or other CHD, by the censoring date. To avoid selecting any individual as a control who later became a case, the cases were ranked by the reverse of the dates on which they developed an ICH event (starting with the most recent, and working backwards to the earliest cases)39. The same controls were used for both IS and ICH cases. Plasma lipid concentrations were measured, with samples randomly ordered by disease status, using AU680 Chemistry Analyzers (Beckman-Coulter), which provided direct homogenous assays for LDL-C and HDL-C, and enzymatic colour assays for total cholesterol and triglycerides. Plasma concentrations of apolipoprotein B, apolipoprotein A1, and lipoprotein (a) were measured by immune turbidimetric assays. Genotyping was carried out using an Affymetrix Axiom® array, involving 800,000 SNPs, customised for the Chinese population.
Genetic Risk Score for LDL-C
In the MR analyses, a GRS for LDL-C was constructed using all available SNPs from the largest published genome-wide meta-analysis (GLGC)17 on lipids, which discovered 157 loci associated with lipids. Within 1-Mb intervals of these 157 loci, 185 independent (r2<0.05) SNPs were associated (P<5x10-8) with LDL-C, HDL-C, or triglycerides16, including 76 SNPs associated with LDL-C (P<5x10-8), of which 68 had the most extreme p-values for LDL-C. Only 46 of these 68 SNPs were directly genotyped on the Affymetrix array in CKB. Hence, the GRS for LDL-C was restricted to those 46 SNPs most strongly associated with LDL-C and having the largest differences between LDL-C and the other lipid fractions (Supplementary Table 2). For each variant, the effect allele was defined as the allele associated with higher LDL-C concentrations in GLGC. The GRS was calculated by summing the number of effect alleles carried by each participant, weighted by the reported effect size of each variant on LDL-C concentrations in GLGC.
LDL-C Lowering Trials
LDL-C-lowering trials were identified by searching PubMed, Cochrane Central Register of Controlled Trials, and the ClinicalTrial.gov database, from 1994-2008 using terms “statin”, “ezetimibe”, “PCSK9”, and “cardiovascular disease”. Consistent with the criteria used in the Cholesterol Treatment Trialists’ Collaboration (CTT meta-analysis of 27 trials of 174,000 participants)15, additional trials (published before 16 November 2018) were identified if they: (i) assessed an unconfounded intervention to lower LDL-C concentrations; (ii) had scheduled duration ≥2 years; and (iii) included ≥1,000 participants. Overall, nine studies (CTT meta-analysis15 plus eight additional9,14,20,21,40–43 were identified, but two trials were excluded due to a lack of information on different stroke types40,41.
Statistical Methods
For observational analyses, a Cox regression analysis was used to calculate the RR and 95% CI of incident stroke types associated with usual plasma lipid concentrations after correction for regression dilution bias. Participants were categorised into fifths of usual lipid concentrations to assess the shape of associations with different stroke types. General linear regression was used to estimate the strength of such associations, weighted by the inverse variance of log RR. All analyses were stratified by age-at-risk (5-year), sex, and study area, with adjustment for education, smoking, alcohol consumption, physical activity, diabetes, and baseline SBP. For categorical variables with more than two levels, risk estimates were accompanied by a group-specific 95% CI, representing the statistical information derived only for such groups44. The RRs were reported for clinically-achievable differences of 1 mmol/L for LDL-C, 0.3 mmol/L for HDL-C, and 30% for triglycerides, and also for a 1 SD higher plasma concentration for each lipid fraction. Additional sensitivity analyses included adjustment for adiposity (to avoid over-adjustment for blood lipids in the primary analyses)45.
For genetic analysis, linear or Cox regression analyses were used to assess associations of GRS with continuous or binary traits, after adjustment for sex, age, and age-squared. All analyses were conducted separately by study area, with overall effects estimated using an inverse-variance-weighted meta-analysis of the area-specific results. The effects of each 1 mmol/L lower genetically-instrumented LDL-C on different stroke types were estimated using the ratio method46. Sensitivity analyses included median-weighted inverse-variance weighted MR and MR-Egger approaches that provide consistent causal estimates from summary data for multiple genetic variants under different statistical assumptions.
For a meta-analysis of randomised trials, the study-specific RR were scaled to each 1 mmol/L lower LDL-C for risk of IS and ICH, using mean LDL-C differences between allocated treatment groups at about 1 year of follow-up. Summary RRs were estimated using an inverse-variance-weighted-average of the study-specific results47.
In order to predict the number of events avoided by lowering LDL-C by 1 mmol/L, the age-specific rates of IS, ICH, and major coronary events (including myocardial infarction and fatal ischaemic heart disease) in CKB were estimated for different levels of background vascular risk (Supplementary Table 4). Hypertension was defined as measured systolic blood pressure of at least 140 mmHg, or a measured diastolic blood pressure of at least 90 mmHg, or receiving drug treatment for hypertension48. Low-risk populations were defined as those with no measured hypertension, or prior history of CVD. Medium-risk populations were defined as those with measured hypertension, but with no prior history of CVD. High-risk populations were defined as those with prior history of CVD. The absolute numbers were calculated assuming that lowering LDL-C by 1 mmol/L reduces the risk of IS and major coronary events by 20% (95% CI: 16-24%) and 24% (95% CI: 21-27%), respectively15, and increases the risk of ICH by 17% (95% CI: 3-32%) as results from LDL-C-lowering trials in this report, with the incidence rates of events reported in all individuals in CKB. All P values were two-sided. All analyses were conducted using SAS® v9.3 and all Figures were produced using R v3.3.
Extended Data
Extended Data Fig. 1. Effect of progressive adjustment for potential confounders on risk of ischaemic stroke and intracerebral haemorrhage with usual LDL-C.
Cox regression was used to estimate adjusted rate ratios (RR) (95% confidence intervals [CI]) for risk of different stroke types per 1 mmol/L higher concentrations of usual LDL-C. Each square has an area inversely proportional to the variance of the log risk. The horizontal lines represent the 95% CI.
Extended Data Fig. 2. Associations of usual LDL-C with risk of ischaemic stroke and intracerebral haemorrhage in population subgroups at baseline.
Cox regression was used to estimate the adjusted rate ratios (RR) (95% CI) for risk of different stroke types per 1 mmol/L higher concentrations of usual LDL-C. Chi-square tests were used to assess heterogeneity and trend, and the degrees of freedom are provided as subscripts. All P-values (two-sided) were uncorrected for multiple testing. Symbols and conventions as in Extended Data Fig. 1.
Extended Data Fig. 3. Adjusted rate ratios (RR) for risk of ischaemic stroke by usual concentrations of LDL-C and HDL-C in observational analyses in CKB.
Symbols and conventions as in Extended Data Fig. 1. The number of ischaemic stroke cases and controls were 5475 and 6290, respectively.
Extended Data Fig. 4. Adjusted rate ratios (RR) for risk of ischaemic stroke and intracerebral haemorrhage by usual concentrations of apolipoprotein B, apolipoprotein A1, and lipoprotein (a) in observational analyses in CKB.
Cox regression was used to estimate the rate ratios (RR) (95% CI) for ischaemic stroke (N = 5475) and intracerebral haemorrhage (N = 4776) by fifths of (a) usual apolipoprotein B, (b) usual apolipoprotein A1, and (c) usual lipoprotein (a), respectively. The line represents the slope from a weighted linear regression with weights based on the inverse variance of the log RR. Symbols and conventions as in Extended Data Fig. 1.
Extended Data Fig. 5. Associations of the genetic risk score (GRS) for LDL-C with major vascular risk factors.
SD=Standard deviation. The analyses were conducted in 17,567 CKB participants with available data, adjusted for sex, age, age-squared, and case status. General linear regression was used to estimate SD differences in all traits (after rank-inverse-normal transformation) per 1 SD higher GRS. All P-values (two-sided) were uncorrected for multiple testing.
Extended Data Fig. 6. Meta-analysis of randomised trials of LDL-C-lowering treatment with statins, ezetimibe, or PCSK9 inhibitor and risk of ischaemic stroke and intracerebral haemorrhage.
The study-specific rate ratios (RR) (95% CI) were obtained from the published results of the LDL-C-lowering trials. The overall RR (95% CI) were obtained by inverse-variance-weighted meta-analysis of the study-specific RR per 1 mmol/L lower LDL-C concentration.
Supplementary Material
Acknowledgments
The chief acknowledgment is to the participants, the project staff, staff of the China CDC and its regional offices for access to death and disease registries. The Chinese National Health Insurance scheme provided electronic linkage to all hospitalization data. The China Kadoorie Biobank study is jointly coordinated by the University of Oxford and the Chinese Academy of Medical Sciences. The funding body for the baseline survey was the Kadoorie Charitable Foundation, Hong Kong, China and the funding sources for the long-term continuation of the study include UK Wellcome Trust (202922/Z/16/Z, 104085/Z/14/Z, 088158/Z/09/Z), Chinese National Natural Science Foundation (81390540, 81390541, 81390544), and the National Key Research and Development Program of China (2016YFC0900500, 2016YFC0900501, 2016YFC0900504, 2016YFC1303904). Core funding was provided to the CTSU, University of Oxford, by the British Heart Foundation, the UK Medical Research Council, and Cancer Research UK. LS received a Clarendon Scholarship at University of Oxford. The funders played no role in the design or conduct of the study, including data collection, management, analysis, or interpretation of the results; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Author contributions: LS, RCl, DB, RP and ZC designed and planned the paper. LS performed data analyses and wrote the first draft of the manuscript. RCl, DB, RP, and ZC helped with the scientific interpretation of the results and revisions of the manuscript. RCl, ZC, LL, RP, RC, RW, JL, and JC, as the members of CKB steering committee, designed and supervised the overall conduct of the study and obtained funding. YG, YC, ZB, and ZC coordinated the data acquisition (for baseline, resurveys, and long-term follow-up). RW, YG, IM, ZB, and MH coordinated the genotyping work in China and laboratory assays in Oxford. All authors provided critical comments on the manuscript.
Access to data and data analysis: RCl, DB, LL and ZC had full access to all the data in the study and take responsibility for the integrity of all data and accuracy of the data analysis. Data from the baseline survey, first resurvey, and cause-specific mortality are available to all bona fide researchers (www.ckbiobank.org). Additional data are also made available on a collaborative basis by contacting the study investigators. All data requests are reviewed monthly by the CKB Data Access Committee, which is composed of senior scientists from Beijing and Oxford.
Competing interests: We declare that we have no conflicts of interest.
References
- 1.Roth GA, et al. Demographic and Epidemiologic Drivers of Global Cardiovascular Mortality. N Engl J Med. 2015;372:1333–1341. doi: 10.1056/NEJMoa1406656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study 2016. Global Burden of Disease Study 2016 (GBD 2016) Results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME); 2016. [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PLOS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81:264–272. doi: 10.1212/WNL.0b013e31829bfde3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou M, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2015;387:251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 6.Lewington S, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 7.Di Angelantonio E, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins R, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 9.Sabatine MS, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM. LDL cholesterol: controversies and future therapeutic directions. Lancet. 2014;384:607–617. doi: 10.1016/S0140-6736(14)61009-6. [DOI] [PubMed] [Google Scholar]
- 11.Hopewell J, Stari T, Parish S, Collins R, Clarke R. Abstract 11959: The Impact of Genetic Variants Related to LDL-cholesterol on Risk of Ischemic Stroke and Coronary Heart Disease. Circulation. 2012;126 [Google Scholar]
- 12.Ference BA, et al. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N Engl J Med. 2016;375:2144–2153. doi: 10.1056/NEJMoa1604304. [DOI] [PubMed] [Google Scholar]
- 13.Hopewell JC, et al. Differential effects of PCSK9 variants on risk of coronary disease and ischaemic stroke. Eur Heart J. 2017;39:354–359. doi: 10.1093/eurheartj/ehx373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-Dose Atorvastatin after Stroke or Transient Ischemic Attack. N Engl J Med. 2006;355:549–559. [Google Scholar]
- 15.Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 16.Do R, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–1352. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer TM, et al. Instrumental Variable Estimation of Causal Risk Ratios and Causal Odds Ratios in Mendelian Randomization Analyses. Am J Epidemiol. 2011;173:1392–1403. doi: 10.1093/aje/kwr026. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, et al. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ. 1991;303:276–282. doi: 10.1136/bmj.303.6797.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baigent C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannon CP, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, et al. Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int J Epidemiol. 2003;32:563–572. doi: 10.1093/ije/dyg106. [DOI] [PubMed] [Google Scholar]
- 23.Hindy G, et al. Role of Blood Lipids in the Development of Ischemic Stroke and its Subtypes: A Mendelian Randomization Study. Stroke. 2018;49:820–827. doi: 10.1161/STROKEAHA.117.019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao AS, et al. Large-Scale Phenome-Wide Association Study of PCSK9 Variants Demonstrates Protection Against Ischemic Stroke. Circ Genom Precis Med. 2018;11:e002162. doi: 10.1161/CIRCGEN.118.002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iso H, Jacobs DR, Jr, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med. 1989;320:904–910. doi: 10.1056/NEJM198904063201405. [DOI] [PubMed] [Google Scholar]
- 26.Ebrahim S, et al. Serum cholesterol, haemorrhagic stroke, ischaemic stroke, and myocardial infarction: Korean national health system prospective cohort study. BMJ. 2006;333:22. doi: 10.1136/bmj.38855.610324.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peto R. Current misconception 3: that subgroup-specific trial mortality results often provide a good basis for individualising patient care. Br J Cancer. 2011;104:1057–1058. doi: 10.1038/bjc.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sever PS, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 29.Ooneda G, et al. Smooth muscle cells in the development of plasmatic arterionecrosis, arteriosclerosis, and arterial contraction. Blood Vessels. 1978;15:148–156. doi: 10.1159/000158160. [DOI] [PubMed] [Google Scholar]
- 30.Konishi M, et al. Associations of serum total cholesterol, different types of stroke, and stenosis distribution of cerebral arteries. The Akita Pathology Study. Stroke. 1993;24:954–964. doi: 10.1161/01.str.24.7.954. [DOI] [PubMed] [Google Scholar]
- 31.MacKenzie JM. Intracerebral haemorrhage. J Clin Pathol. 1996;49:360–364. doi: 10.1136/jcp.49.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yusuf S, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378:1231–1243. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 33.Liu M, et al. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurology. 2007;6:456–464. doi: 10.1016/S1474-4422(07)70004-2. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, et al. Prevalence, Incidence and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480,687 Adults. Circulation. 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 35.Zhao D, et al. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing Project. Stroke. 2008;39:1668–1674. doi: 10.1161/STROKEAHA.107.502807. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, et al. Cohort Profile: The Kadoorie Study of Chronic Disease in China (KSCDC) Int J Epidemiol. 2005;34:1243–1249. doi: 10.1093/ije/dyi174. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke R, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 39.Peto R. The marked differences between carotenoids and retinoids - methodological implications for biochemical epidemiology. Cancer Surveys. 1983;2:327–340. [Google Scholar]
- 40.Athyros VG, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus' usual'care in secondary coronary heart disease prevention. Current Medical Research and Opinion. 2002;18:220–228. doi: 10.1185/030079902125000787. [DOI] [PubMed] [Google Scholar]
- 41.Rossebø AB, et al. Intensive Lipid Lowering with Simvastatin and Ezetimibe in Aortic Stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 42.Yusuf S, et al. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med. 2016;374:2021–2031. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz GG, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 44.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10:1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 45.The Global BMI Mortality Collaboration. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wald A. The Fitting of Straight Lines if Both Variables are Subject to Error. Ann Math Statist. 1940:284–300. [Google Scholar]
- 47.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–371. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 48.Lewington S, et al. The Burden of Hypertension and Associated Risk for Cardiovascular Mortality in China. JAMA Intern Med. 2016;176:524–532. doi: 10.1001/jamainternmed.2016.0190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.