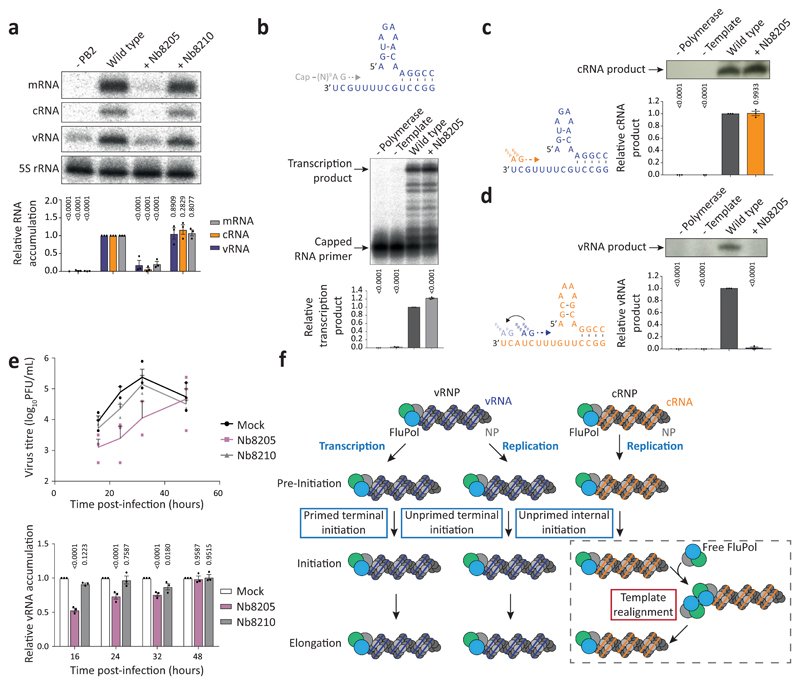
Fig. 4. Nanobody Nb8205 that binds FluPolA at the dimer interface inhibits cRNA to vRNA replication and virus growth.
a, Effect of nanobodies on FluPolA activity in a vRNP reconstitution assay. Data are mean ± s.e.m., n=3 independent transfections. Two-way ANOVA. P < 0.05 is considered significant. b, Effect of nanobody on in vitro transcription by FluPolA primed with a capped RNA primer. Data are mean ± s.e.m., n=3 independent reactions. One-way ANOVA. P < 0.05 is considered significant. c, d, Effect of nanobody on in vitro primer-independent replication by FluPolA on a vRNA (c) and cRNA (d) template. Data are mean ± s.e.m., n=3 independent reactions. One-way ANOVA. P < 0.05 is considered significant. e, Effect of nanobodies on the growth of influenza A/WSN/33 virus and vRNA levels in infected HEK-293T cells. Data are mean ± s.e.m., n=3 independent transfections and infections. Two-way ANOVA (Nb8210: P = 0.8126; 0.4390; 0.8496; 0.8489, Nb8205: P = 0.1075; 0.0096; 0.0217; 0.9828, for 16, 24, 32, 48 hours post-infection). P < 0.05 is considered significant. For gel source data, see Supplementary Fig. 2. f, Model for the role of polymerase dimerisation in influenza virus genome replication.