Abstract
The mimicking enzyme activities of eighteen classic POMs with different structures, Keggin (H3PW12O40, H4SiW12O40, H4GeW12O40, K4GeW12O40, H3PMo12O40, H4SiMo12O40 and Eu3PMo12O40), Wells-Dawson (H6P2Mo18O62, α-(NH4)6P2W18O62 and α-K6P2W18O62·14H2O), lacunary-Keggin (Na8H[α-PW9O34], Na10[α-SiW9O34], Na10[α-GeW9O34] and K8[γ-SiW10O36]), the transition-metal substituted-type (α-1,2,3-K6H[SiW9V3O34] and H5PMo10V2O40), sandwich-type (K10P2W18Fe4(H2O)2O68) and an isopolyoxotungstate (Na10H2W12O42) were screened and compared. The mechanisms and reaction conditions of POMs with mimicking enzyme-like activities were also analyzed. The results shown that the structures, the hybrid atoms, the coordination atoms, the substituted metal atoms, pH and substrate are the effect factors for the enzyme mimic activities of POM. Among the eighteen POMs, H3PW12O40, H4SiW12O40, H4GeW12O40, α-(NH4)6P2W18O62, α-K6P2W18O62·14H2O, Na8H[α-PW9O34], Na10[α-SiW9O34], Na10[α-GeW9O34], K8[γ-SiW10O36], K10P2W18Fe4(H2O)2O68 and Na10H2W12O42 had the peroxidase activities. Eu3PMo12O40, H3PMo12O40, H4SiMo12O40, α-1,2,3-K6H [SiW9V3O34], H6P2Mo18O62 and H5PMo10V2O40 showed the oxidase-like activities. K4GeW12O40 did not show the peroxidase and oxidase activities. The Na8H[α-PW9O34], Na10[α-SiW9O34] and Na10[α-GeW9O34] showed intrinsic enzyme activities at alkaline conditions, which were different from other type of POMs. The sandwich-type K10P2W18Fe4(H2O)2O68 displayed the strongest peroxidase activity, which is similar to natural horseradish peroxidase.
Subject terms: Analytical chemistry, Inorganic chemistry
Introduction
Natural enzymes with high substrate specificity, activities and yields have attracted continuous scientific research interest. However, their intrinsic drawbacks, such as poor substrate versatility and assortment, low operational stabilities and low tolerance to environment conditions, limited their applications1,2. Therefore, artificial enzymes, as highly stable and low-cost alternatives to nature enzymes, attract continuing attention3,4. Constructing and screening highly efficient enzyme mimics is a tremendous motivator for researchers. To date, impressive development has been made in the field of artificial enzymes, and numerous diversity materials, such as supramolecular, porphyrins, nanoenzyme, and metal complexes have been extensively explored to mimic natural enzymes5–9. Generally, the efforts toward designing artificial enzymes with high activity can be divided in to two groups: the first is ‘structural mimicking’, which is to mimic the structure of enzymes, and the second is ‘functional mimicking’, which is to mimic enzymes that have similar activities10. Functional mimicking offers a straight forward method to discover new properties of functional materials, such as the discovery of nanoparticles with peroxidase-like activity, later referred to as nanozymes9,11.
Polyoxometalates (POMs), a metal oxide cluster compounds, are combinations between oxygen and early transition metals at their high oxidation states12,13. The majority of the applications of POMs are found in the area of catalysis14–16. It is reported that POMs can catalyze H2O2-based epoxidation and oxidation of organic substrates by O2 and H2O2 by multistep electron-transfer processes17–19. Therefore, it is not astonishing that POMs can used as enzyme mimics to catalyze H2O2-based oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) and Ortho-Phenylenediamine (OPD) to a colored complex which can be applied in bio- and chem-sensing, i.e., colorimetric detection of tumor cells and glucose. Wang et al. firstly found that folate-functionalized polyoxometalate nanoparticles have unique oxidase-like activity in colorimetric multiplexed immunoassay20. Moreover, they investigated the peroxidase mimetics of POMs (H3PW12O40, H4SiW12O40 and H3PMo12O40) and H3PW12O40/graphene in detection of glucose and H2O221. Meanwhile, Sun et al. reported a simple, fast and sensitive colorimetric method to detect H2O2 based on H4SiW12O4019. Furthermore, Wang et al. synthesized folate-functionalized POM hybrid nanoparticles (FA-g-[(FeOH2)2SiW10O36] and FAnPMo12−nVnO40, n = 1–3) and studied the peroxidase-like activity in colorimetric assay of H2O2 and cancer cells22. These above early findings proved that the typical polyoxometalates and their hybrid nanoparticles have the enzyme mimics activities.
POMs with different structural morphologies can incorporated with different metal atoms. The new inorganic compositions have presented attractive enzyme mimic features. For example, Li et al. synthesized three new tetra-nuclear ZrIV- substituted POMs, which exhibit peroxidase-like activities23. Wang et al. established a colorimetric detection method based on the metal-substituted polyoxometalates of SiW9M3 (M = Co2+, Fe3+, Cu2+ and Mn2+)24. Xu et al. developed a Fe-containing heteropolyacid by cation-exchange and employed KFePW12O40 nanostructures for Fenton, photo-Fenton and enzyme-mimetic reactions25.
POMs can assemble with functional materials to improve their properties and potential practical applications26. For instance, the dipeptide-POMs-graphene oxide ternary hybrid prepared by a precipitation method show a higher peroxidase activity compared to POMs alone27. Inorganic-organic hybrids based on POMs and transition-metal complexes are another similar strategy to construct new enzyme mimics. Gao et al. synthesized and structurally characterized two new hybrids based on copper(II)-imidazole complex modified sandwich-type tungstobismuthate or tungstoantimonite, Na4H2[Cu4(H4im)12(H3im)2][Cu3(H2O)3(XW9O33)2] · nH2O (H4im = imidazole, H3im = deprotonated imidazole, X = Bi, Sb), which demonstrate higher peroxidase-like activity than Keggin-type POMs around physiological pH values in a heterogeneous phase28. Sha et al. isolated two new POM involved hybrids containing helix/nanocages ([CuI2CuII2(fkz)2(H2O)7(SiW12O40)] and [(Hfkz)3(H4SiW12O40)]) and systematically studied their peroxidase-like activities29. Rao et al. investigated the enzyme mimetic activity of a new inorganic-organic covalent hybrid of POM-calixarene30. Wei et al. reported the improved peroxidase-mimic property of the vesicles of hexavanadate-organic hybrid surfactants31. Metal-organic framework (MOF) based bio-sensing is a new interesting field. Pillar-layered MOFs have been proven to be an effective route to construct enzyme mimics with high stability and multifunction. The advancement of MOF structure is also help in design of new enzymes with POMs moiety. For example, Qin et al. report a novel efficient peroxidase mimic POM-pillared MOF, Cu6(Trz)10(H2O)4[H2SiW12O40] ·8H2O32. Recently, Sha et al. reported a stable peroxidase mimic POM-pillared metal–MOF with 6-nuclear Cu-pz and 10-nuclear Cu-pz-Cl cycles, [Cu5(pz)6Cl] [SiW12O40]33.
Most of the POMs are stable and show higher enzyme activities at acid condition (about pH value 3 or 4). However, in the physiological solutions (pH 7.0–7.5), for most bioanalytical applications, the POM nanozymes become catalytically inactive. Fortunately, POMs show a great diversity in its structure derived from its multiple oxidation states and coordination geometries30,34. These features make it much easier to control the size, shape, and charge distribution at the molecular level. Flexibility in the structure makes it possible to fine-tune the redox potentials, acidities, and enzyme activities of POMs. For example, the trivacant Keggin Na10[α-SiW9O34] exhibits unusual peroxidase-like activity at basic condition35. Therefore, it is necessary to systematic investigate of POMs mimic enzymes activity with different structure category. Herein, the mimicking enzyme activities of classic polyoxometalates with different classic structures and different element atoms were screened and compared.
Results and Discussion
Characterization of POMs
The POMs were prepared according to the literature and identified by FI-IR spectra, UV-Vis spectra, as shown in Supplementary Figs S1 and S2.
Enzyme mimetic activities of POMs
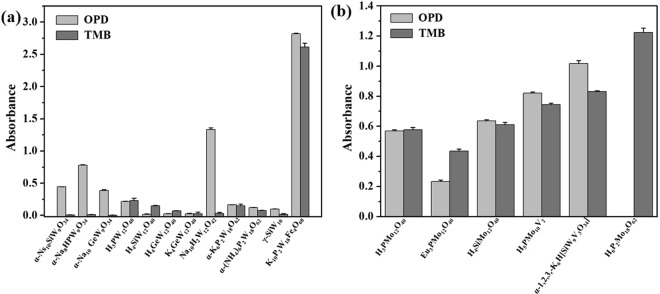
As shown in Fig. 1, the enzyme mimetic activities of 18 POMs with Keggin structures (H3PW12O40, H4SiW12O40, H4GeW12O40, K4GeW12O40, H3PMo12O40, H4SiMo12O40 and Eu3PMo12O40), Dawson structures (H6P2Mo18O62, α-(NH4)6P2W18O62 and α-K6P2W18O62·14H2O), lacunary-Keggin structures (Na8H[α-PW9O34], Na10[α-SiW9O34], Na10[α-GeW9O34] and K8[γ-SiW10O36]), the transition-metal substituted-type structures (α-1,2,3-K6H[SiW9V3O34], H5PMo10V2O40) and sandwich-type K10P2W18Fe4(H2O)2O68) were studied and compared at the same concentration with OPD and TMB as substrates. It was found that the coordination atoms, Mo and W, have effect on the enzyme mimic activity. All the polyoxotungstates H3PW12O40, H4SiW12O40, H4GeW12O40, K4GeW12O40, Na10[α-GeW9O34], Na8H[α-PW9O34], Na10[α-SiW9O34], K8[γ-SiW10O36], α-(NH4)6P2W18O62, α-K6P2W18O62·14H2O, Na10H2W12O42 and K10P2W18Fe4(H2O)2O68 are capable of catalyzing typical peroxidase reactions using both chromogenic hemeperoxidase substrates TMB and OPD in the presence of H2O2 to produce a blue color (maximum absorbance 650 nm) and brown color (maximum absorbance 450 nm) reaction, respectively, as shown in Fig. 1a. Initially, these reactions were carried out by adding 200 μM of POMs with the substrates OPD (576 μM) and H2O2 (200 mM) at room temperature in a buffered solution. It indicates that these POMs have intrinsic peroxidase-like activities towards these substrates (Fig. 1). However, the peroxidase-like activities of these POMs are different in the present of different substrates at the same concentration. For TMB as organic substrate, the absorption values of TMB+ indicated that the order of peroxidase-like activities from high to low was K10P2W18Fe4(H2O)2O68 > H3PW12O40 > H4SiW12O40 > α-K6P2W18O62·14H2O > α-(NH4)6P2W18O62 > H4GeW12O40 > Na10H2W12O42 > K4GeW12O40 > K8[γ-SiW10O36] > Na8H[α-PW9O34] ≈ Na10[α-SiW9O34] ≈ Na10[α-GeW9O34]. For OPD as organic substrate, the order of peroxidase-like activities from high to low was K10P2W18Fe4(H2O)2O68 > Na10H2W12O42 > Na8H[α-PW9O34] > Na10[α-SiW9O34] > Na10[α-GeW9O34] > H3PW12O40 > α-K6P2W18O62·14H2O > α-(NH4)6P2W18O62 > K8[γ-SiW10O36] > H4GeW12O40 ≈ K4GeW12O40 ≈ H4SiW12O40. From the results, the lacunary-Keggin POMs, Na10[α-GeW9O34], Na8H[α-PW9O34], Na10[α-SiW9O34] and Na10H2W12O42 are higher affinity to the substrate of OPD than TMB. There are tiny absorbance peaks (OD values of 0.0100, 0.0070, 0.0017 and 0.0317) can be found in the above four POMs with TMB as substrates. The result indicated that peroxidase-like activities of POMs may substrate-dependence. However, no matter which the substrate was, the sandwich-type K10P2W18Fe4(H2O)2O68 showed the highest peroxidase activity. Under the same substrate, it was found that the hybrid atoms had effect on the peroxidase activities of the POMs and the order was P > Si > Ge. In the same hybrid atom and TMB substrate, the peroxidase activity order is Keggin structure > Wells-Dawson > lacunary-Keggin. The reactions were also carried out in the absence of the POMs at their various suitable pH values, respectively. No significant unspecific oxidation reactions were observed [Supplementary Figs S3, S4] even after half an hour. Additional control experiments using POMs in absence of H2O2 showed that no oxidative reaction occurs. Hence, these POMs had only the peroxidase-like activities defeating many mimic enzyme peroxidases which also display oxidase-like activities. The enzymatic properties of these POMs are specificity and rarely reported in the literatures11.
Figure 1.
Comparison of enzyme mimic activities of polyoxometalates. (a) peroxidase-like activities with TMB or OPD as substrate. (b) oxidase-like activities with TMB or OPD as substrate. Conditions: 200 μM POMs, 200 mM H2O2 at room temperature for 10 minutes in the optimum pH.
The polyoxomolybdates (H3PMo12O40, H4SiMo12O40, Eu3PMo12O40, α-1,2,3,-K6H[SiW9V3O34], H5PMo10V2O40 and H6P2Mo18O62) are capable of catalyzing oxidase reactions with both substrates TMB and OPD in the absence of H2O2 to produce a blue color (maximum absorbance 650 nm) and orange color (maximum absorbance 450 nm) reaction, respectively, as shown in Fig. 1b. Furthermore, the oxidase-like activities of these POMs are similar in the present of different substrates. For TMB as organic substrate, the absorption values indicated that the order of oxidase-like activities from high to low was H6P2Mo18O62 > α-1,2,3-K6H[SiW9V3O34] > H5PMo10V2O40 > H4SiMo12O40 > H3PMo12O40 > Eu3PMo12O40. For OPD as substrate, the order of oxidase-like activities from high to low was α-1,2,3-K6H[SiW9V3O34] > H5PMo10V2O40 > H4SiMo12O40 > H3PMo12O40 > Eu3PMo12O40. In the OPD substrate, the color of H6P2Mo18O62 catalytic reaction solution became dark blue with a maximum absorption peak at 710 nm. The maximum absorption peak matched with the hybrid blue, the reduction product of H6P2Mo18O62, which covered the absorption peak of the oxide product of OPD. Therefore, only TMB was chosen as the substrate for H6P2Mo18O62. Finally, K4GeW12O40 did not show the peroxidase and oxidase activities.
Effect of pH
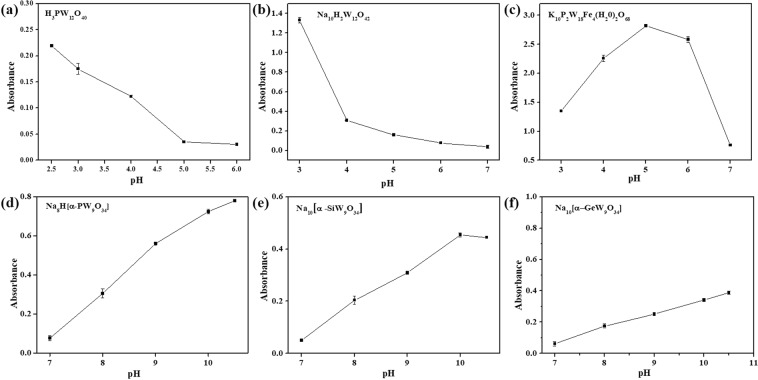
The effect of pH on the catalytic activities of different types of POMs was measured by varying the pH and keeping the OPD and H2O2 concentration constant. The absorbance value of DAB with H3PW12O40 and Na10H2W12O42 reached a maximum at the pH 2.5, as shown in Fig. 2a,b. After exceeding this point, the absorbance decreased gradually as increasing pH values. Therefore, pH 2.5 was selected as the optimal pH value for H3PW12O40 and Na10H2W12O42. Similarly, pH 5 was selected as the optimal pH value for K10P2W18Fe4(H2O)2O68, as shown in Fig. 2c. Interestingly, the catalytic activity of the Na10[α-GeW9O34], Na8H[α-PW9O34] and Na10[α-SiW9O34] shows a pH optimum at alkaline conditions (~10). At this pH and in the absence of Na10[α-GeW9O34], Na8H[α-PW9O34] and Na10[α-SiW9O34] the unspecific reaction between OPD (576 μM) and H2O2 (200 mM) was not observed. As mentioned, most of the known peroxidase-like POMs based artificial enzymes show their high activities at acid condition. Some POMs hybrids, such as inorganic-organic hybrids and FA functional particles exhibit oxidation catalyst at pH about 7.022,25. However, Na10[α-GeW9O34], Na8H[α-PW9O34] and Na10[α-SiW9O34] are highly active even at pH above 10, as shown in Fig. 2d–f. Based on the catalytic properties of these trivacant Keggin heterotungstates, we build a CdTe quantum dots (QDs)-based fluorometric method for sensitive detection of hydrogen peroxide35. The effect of pH on the catalytic activities of H3PW12O40 and K10P2W18Fe4(H2O)2O68 with TMB as substrate were also investigated, as shown in Supplementary Fig. S5. The optimal pH for H3PW12O40 and K10P2W18Fe4(H2O)2O68 were 2.5 and 4, respectively.
Figure 2.
Effects of pH on peroxidase-like enzymes with OPD as a substrate, respectively. (a) H3PW12O40; (b)Na10H2W12O42; (c) K10P2W18Fe4(H2O)2O68; (d) Na8H[α-PW9O34]; (e) Na10[α-SiW9O34]; (f) Na10[α-GeW9O34]; Conditions: 200 μM POMs, 200 mM H2O2, 0.2 mM Na2HPO4-citrate buffer for H3PW12O40, Na10H2W12O42 and K10P2W18Fe4(H2O)2O68; 0.1 mM Tris-HCl for Na8H[α-PW9O34], Na10[α-SiW9O34] and Na10[α-GeW9O34] at room temperature for 10 minutes. The error bars represent the standard deviation of three measurements.
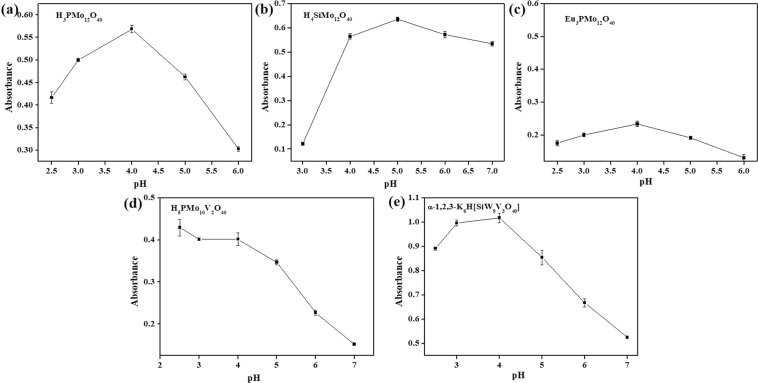
Analogously, the reaction pH-dependent response curves on oxidase mimics H3PMo12O40, H4SiMo12O40, Eu3PMo12O40, H5PMo10V2O40 and α-1,2,3-K6H-[SiW9V3O34] with OPD as substrates and H6P2Mo18O62 with TMB as substrate were shown in Fig. 3. When the pH value increased from 2.0 to 7, the absorbance reached the maximums at pH 4, 5, 3, 2.5, 4 and 5 for H3PMo12O40, H4SiMo12O40, Eu3PMo12O40, H5PMo10V2O40, α-1,2,3-K6H[SiW9V3O34] and H6P2Mo18O62, respectively. The effect of pH on the catalytic activities of H3PMo12O40, H4SiMo12O40, Eu3PMo12O40, H5PMo10V2O40 and α-1,2,3-K6H[SiW9V3O34] with TMB as substrate were also investigated, as shown in Supplementary Fig. S6. When TMB as substrates, H3PMo12O40, H4SiMo12O40, Eu3PMo12O40, H5PMo10V2O40 and α-1,2,3-K6H[SiW9V3O34] exhibited their strongest activities when the pH was 4, 5, 4, 2.5 and 4, respectively.
Figure 3.
Effects of pH on oxidase-like enzymes with OPD as a substrate. (a) H3PMo12O40; (b) H4SiMo12O40; (c) Eu3PMo12O40; (d) H5PMo10V2O40; (e) α-1,2,3-K6H[SiW9V3O34]; (f) H6P2Mo18O62 with TMB as substrates. Conditions: 200 μM POMs, 200 mM H2O2, 0.2 mM Na2HPO4-citrate buffer at room temperature for 10 minutes. The error bars represent the standard deviation of three measurements.
Effect of concentration of POMs
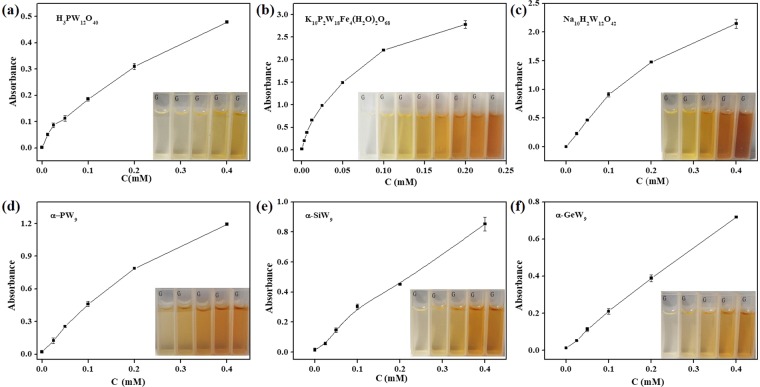
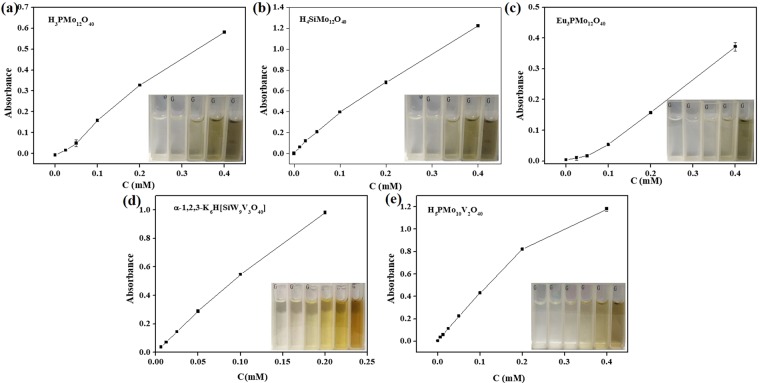
The OPD oxidation rate catalyzed by the peroxidase-like POMs was dependent on the concentration of these POMs with the other parameters were kept constant, as shown in Fig. 4. In a typical experiment, the POMs oxidation activity was determined by monitoring of the absorbance at 450 nm (which increases because of DAB, the oxidation product of OPD, formation) for 10 min at 25 °C in different buffer for varying concentrations of POMs with respect to OPD (576 μM) and H2O2 (200 mM). It can be found that the greater the amount of catalyst, the higher the yield of DAB. The increasing of the DAB yield becomes gentle when the amount of H3PW12O40, Na10H2W12O42, Na10[α-GeW9O34], Na8H[α-PW9O34], Na10[α-SiW9O34] and K10P2W18Fe4(H2O)2O68 exceeds 0.4, 0.4, 0.4, 0.4, 0.4 and 0.1 mM, respectively. Furthermore, Na10[α-GeW9O34], Na8H[α-PW9O34] and Na10[α-SiW9O34] were insoluble when the concentrations were beyond 0.4 mM. Therefore, 0.4 mM was chosen as the optimum concentrations of H3PW12O40, Na10H2W12O42, Na10[α-GeW9O34], Na8H[α-PW9O34] and Na10[α-SiW9O34] for the kinetics experiments. While, 0.1 mM was chosen for K10P2W18Fe4(H2O)2O68. The effect of the amounts of H3PW12O40 and K10P2W18Fe4(H2O)2O68 on peroxidase-like activities with TMB as substrates were also investigated, as shown in Supplementary Fig. S7. 0.4 mM and 0.1 mM were chosen for H3PW12O40 and K10P2W18Fe4(H2O)2O68 as the optimum concentrations, respectively.
Figure 4.
Effects of concentrations of peroxidase-like enzymes with OPD as a substrate. (a) H3PW12O40; (b) Na10H2W12O42; (c) K10P2W18Fe4(H2O)2O68; (d) Na8H[α-PW9O34]; (e) Na10[α-SiW9O34]; (f)Na10[α-GeW9O34]; Conditions: 200 mM H2O2, 0.2 mM Na2HPO4-citrate buffer for H3PW12O40, Na10H2W12O42 and K10P2W18Fe4(H2O)2O68; 0.1 mM Tris-HCl for Na8H[α-PW9O34], Na10[α-SiW9O34] and Na10[α-GeW9O34] at room temperature for 10 minutes. The error bars represent the standard deviation of three measurements.
As shown in Fig. 5, the effect of the amounts of POMs with oxidase-like activities on the yield of DAB was also investigated. The procedures were similar with above except H2O2. DAB is obtained without H2O2, while the absorbance of DAB increases along with the increase in the amount of H3PMo12O40, H4SiMo12O40 and Eu3PMo12O40 and reached the maximum at 0.4 mM POMs. The maximum concentrations of H5PMo10V2 and α-1,2,3-K6H[SiW9V3O34] were 0.2 mM. The maximum concentration of H6P2Mo18O62 with TMB as substrates was 50 μM. As shown in Fig. S8, when TMB were substrates, we found that the optimum concentration was 0.1 mM for H3PMo12O40, H5PMo10V2 and α-1,2,3- K6H[SiW9V3O34]. H4SiMo12O40 and Eu3PMo12O40 reached their maximum absorption values when the concentrations were 0.2 mM and 0.4 mM, respectively.
Figure 5.
Effects of concentrations of oxidase-like enzymes with OPD as a substrate. (a) H3PMo12O40; (b)H4SiMo12O40; (c)Eu3PMo12O40; (d)H5PMo10V2O40; (e)α-1,2,3-K6H[SiW9V3O34]; (f)H6P2Mo18O62 with TMB as substrates. Conditions: 0.2 mM Na2HPO4-citrate buffer at room temperature for 10 minutes.The error bars represent the standard deviation of three measurements.
Effect of reaction time
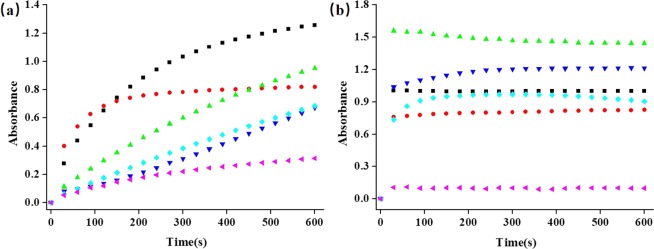
The variation of the DAB yield with increasing reaction time is shown in Fig. 6. It can be observed that absorption value of DAB reaches a maximum during a reaction time of less than 10 minutes and 1 minute for peroxidase and oxidase when 200 mM H2O2 and 576 μM OPD were used, respectively. After that, along with the increasing of reaction time, the yield of DAB remains nearly constant. This indicates that the optimized reaction time were 10 minutes for H3PW12O40, Na10[α-GeW9O34], Na8H[α-PW9O34] and Na10[α-SiW9O34], 5 minutes for K10P2W18Fe4(H2O)2O68 and 2 minutes for Na10H2W12O42, as shown in Fig. 6a. The reaction time for oxidase were less than 60 s for H3PMo12O40, H4SiMo12O40, Eu3PMo12O40, H5PMo10V2O40, α-1,2,3-K6H[SiW9V3O34] and H6P2Mo18O62.
Figure 6.
The reaction time of different-type POMs. (a) peroxidase (black: K10P2W18Fe4(H2O)2O68; red: Na10H2W12O42; green: Na8H[α-PW9O34]; light blue: [α-SiW9O34]; dark blue: Na10[α-GeW9O34]; pink: H3PW12O40); (b) oxidase (green: H6P2Mo18O62; dark blue: α-1,2,3-K6H[SiW9V3O34]; black: H5PMo10V2O40; light blue: H4SiMo12O40; red: H3PMo12O40; pink: Eu3PMo12O40).
Kinetic parameters
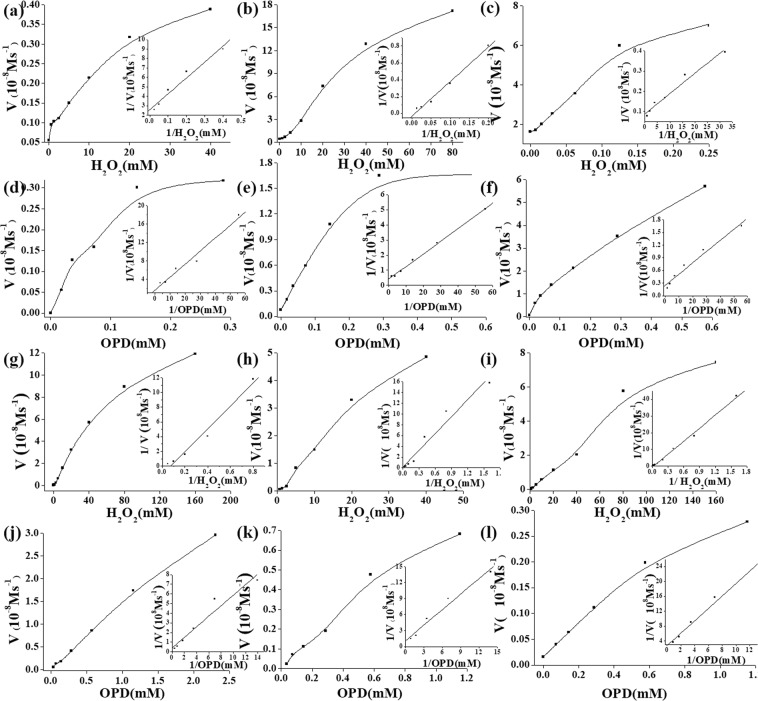
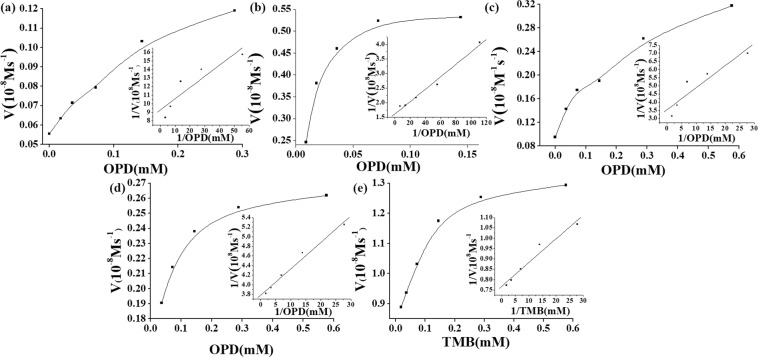
The mechanism of peroxidase-like catalytic activities of K10P2W18Fe4(H2O)2O68, H3PW12O40, H4SiW12O40, Na10[α-GeW9O34], Na8H[α-PW9O34], Na10[α-SiW9O34] and Na10H2W12O42 was further investigated using steady-state kinetics. It has been observed that OPD oxidation catalysis mediated by these POMs is dependent on the substrate concentration. In order to study activities of POMs, several experiments were performed whereby the concentration of either OPD or H2O2 was varied while keeping the other concentration constant. The concentration of the POMs was kept constant in all these experiments. The oxidation reaction catalyzed by K10P2W18Fe4(H2O)2O68, H3PW12O40, H4SiW12O40, Na10[α-GeW9O34], Na8H[α-PW9O34], Na10[α-SiW9O34] and Na10H2W12O42 follows a Michaelis-Menten behavior. The Km and Vmax of K10P2W18Fe4(H2O)2O68 and H3PW12O40 were obtained using a Line weaver-Burk plot with OPD as substrates, as shown in Fig. 7. The Km and Vmax of K10P2W18Fe4(H2O)2O68 with H2O2 as substrates were 0.113 mM and 1.13 × 10−9 M·S−1 in OPD system, as shown in Fig. 7a. The Km and Vmax of K10P2W18Fe4(H2O)2O68 with OPD as substrates were 0.109 mM and 4.11 × 10−8 M·S−1, as shown in Fig. 7b. The Kcat of K10P2W18Fe4(H2O)2O68 with H2O2 and OPD are 3.22 × 10−3 S−1 and 3.80 × 10−3 S−1. The Km and Vmax of H3PW12O40 with H2O2 as substrates were 6.624 mM and 3.89 × 10−9 M·S−1 in OPD system, as shown in Fig. 7c. The Km and Vmax of H3PW12O40 with OPD as substrates were 0.174 mM and 6.14 × 10−9 M·S−1, as shown in Fig. 7d. The Kcat of H3PW12O40 with H2O2 and OPD are 8.22 × 10−4 S−1 and 2.83 × 10−6 S−1. As shown in Fig. 8, the values for Km OPD of Na8H[α-PW9O34], Na10[α-SiW9O34], Na10[α-GeW9O34] and Na10H2W12O42 were 1.39, 0.854, 0.611 and 0.205 mM, respectively. The values for Vmax OPD of Na8H[α-PW9O34], Na10[α-SiW9O34], Na10[α-GeW9O34] and Na10H2W12O42 were 2.53 × 10−8, 8.79 × 10−9, 3.65 × 10−9 and 2.43 × 10−8 M·S−1, respectively. The Kcat OPD of Na8H[α-PW9O34], Na10[α-SiW9O34], Na10[α-GeW9O34] and Na10H2W12O42 were 4.912 × 10−4 S−1, 2.02 × 10−4 S−1, 7.03 × 10−4 S−1 and 1.46 × 10−4 S−1. The values for Km H2O2 of Na8H[α-PW9O34], Na10[α-SiW9O34], Na10[α-GeW9O34] and Na10H2W12O42 were 13.81, 63.55, 44.05, and 132.03 mM, respectively. The Vmax H2O2 of Na8H[α-PW9O34], Na10[α-SiW9O34], Na10[α-GeW9O34] and Na10H2W12O42 were 8.19 × 10−9, 6.07 × 10−8, 1.69 × 10−8 and 3.22 × 10−7 M·S−1, respectively. The values for Kcat H2O2 of Na8H[α-PW9O34], Na10[α-SiW9O34], Na10[α-GeW9O34] and Na10H2W12O42 were 9.73 × 10−6 S−1, 2.48 × 10−5 S−1, 1.58 × 10−4 S−1 and 4.26 × 10−5 S−1, respectively. The comparison of Km and Vmax of peroxidase-like enzymes were listed in Table 1. It was found that K10P2W18Fe4(H2O)2O68 had lower Km than other POMs-based peroxidases with OPD or H2O2 as substrates. It suggests that K10P2W18Fe4(H2O)2O68 might have a similar affinity for the surfaces of OPD with HRP. The mechanism of steady-state kinetics of K10P2W18Fe4(H2O)2O68 were also investigated with TMB as substrates, as shown in Supplementary Fig. S8. The values for Km and Vmax TMB was 0.25 mM and 2.42 × 10−7 M·S−1, respectively. The values for Km and Vmax H2O2 was 1.09 mM and 1.02 × 10−7 M·S−1. It suggests that K10P2W18Fe4(H2O)2O68 might have a similar affinity for the surfaces of TMB with HRP. The Kcat of K10P2W18Fe4(H2O)2O68 with H2O2 and TMB are 4.54 × 10−2 S−1 and 8.82 × 10−3 S−1. Comparing with the other organic-inorganic POM-based hybrids as shown in Table S1, the K10P2W18Fe4(H2O)2O68 also show strong peroxidase activities with TMB as a substrate. It can be selected to construct new materials with organic groups or different nanomaterials.
Figure 7.
The steady-state kinetic assay and catalytic mechanism of peroxidase-like enzymes. (a,b) K10P2W18Fe4(H2O)2O68, (c,d) H3PW12O40, (a,e) Na8H[α-PW9O34], (b,f) Na10[α-SiW9O34], (c,g) Na10[α-GeW9O34] and (d,h) Na10H2W12O42.
Figure 8.
The steady-state kinetic assay and catalytic mechanism of oxidase-like enzymes. (a)H3PMo12O40, (b) H4SiMo12O40, (c) Eu3PMo12O40, (d) H5PMo10V2O40, (e) α-1,2,3-K6H[SiW9V3O34], (f) H6P2Mo18O62.
Table 1.
Comparison of the Km and Vmax of peroxidase-like enzymes.
| Nanozymes | Substrate | Km (mM) | Vmax (M·S−1) |
|---|---|---|---|
| Na8H[α-PW9O34] | OPD | 1.39 | 6.14 × 10−9 |
| Na8H[α-PW9O34] | H2O2 | 13.81 | 3.89 × 10−9 |
| Na10[α-SiW9O34] | OPD | 0.85 | 2.53 × 10−8 |
| Na10[α-SiW9O34] | H2O2 | 63.55 | 8.19 × 10−9 |
| Na10[α-GeW9O34] | OPD | 0.61 | 8.79 × 10−9 |
| Na10[α-GeW9O34] | H2O2 | 44.05 | 6.07 × 10−8 |
| Na10H2W12O42 | OPD | 0.2 | 3.65 × 10−9 |
| Na10H2W12O42 | H2O2 | 132.03 | 1.69 × 10−8 |
| K10P2W18Fe4(H2O)2O68 | OPD | 0.10 | 2.43 × 10−8 |
| K10P2W18Fe4(H2O)2O68 | H2O2 | 0.11 | 3.22 × 10−7 |
| H3PW12O40 | OPD | 0.17 | 4.11 × 10−8 |
| H3PW12O40 | H2O2 | 6.62 | 1.13 × 10−9 |
| HRP | OPD | 0.59 | 4.65 × 10−8 46 |
| HRP | H2O2 | 0.34 | 9.48 × 10−8 46 |
The Km and Vmax were obtained for oxidase-like enzymes, as shown in Fig. 8. With OPD as substrates, the values of Km OPD were 0.014, 0.013, 0.038, 0.015 and 0.015 mM for H3PMo12O40, H4SiMo12O40, Eu3PMo12O40, H5PMo10V2O40 and α-1,2,3, −K6H[SiW9V3O34]. The Vmax OPD of H3PMo12O40, H4SiMo12O40, Eu3PMo12O40, H5PMo10V2O40 and α-1,2,3, -K6H[SiW9V3O34] were 1.08 × 10−9, 6.19 × 10−9, 2.83 × 10−9, 2.64 × 10−9 and 1.30 × 10−8 M·S−1, respectively. The Kcat OPD of H3PMo12O40, H4SiMo12O40, Eu3PMo12O40, H5PMo10V2O40 and α-1,2,3-K6H[SiW9V3O34] were 4.32 × 10−5, 3.09 × 10−4, 5.66 × 10−5, 1.32 × 10−4 and 1.73 × 10−3S−1, respectively. The comparison of Km and Vmax with different substrates were shown in Table 2. The value results of Km OPD suggested that OPD have similar affinity for the surface of the oxidase-like POMs except the Eu3PMo12O40. α-1,2,3-K6H[SiW9V3O34] has the fastest catalytic rate than the other POMs. Among the saturated Keggin-type POMs, the counter ion Eu3+ maybe inhibit the poly anions interactions with the organic component. The Km and Vmax were also obtained with TMB as substrate, as shown in Supplementary Fig. S9. The Km of oxidase-like enzymes was dramatically lower than natural enzyme HRP With TMB as substrates, the Km were 0.616, 0.49, 0.086, 0.55, 0.02, 0.75 mM for H3PMo12O40, H4SiMo12O40, Eu3PMo12O40, H5PMo10V2O40, α-1,2,3-K6H[SiW9V3O34] and H6P2Mo18O62. The Vmax of H3PMo12O40, H4SiMo12O40, Eu3PMo12O40, H5PMo10V2O40, α-1,2,3-K6H[SiW9V3O34] and H6P2Mo18O62 were 2.25 × 10−7, 2.25 × 10−7, 2.60 × 10−8, 2.30 × 10−7, 1.49 × 10−8, 5.30 × 10−7 M·S−1, respectively. The Kcat of H3PMo12O40, H4SiMo12O40, Eu3PMo12O40, H5PMo10V2O40, α-1,2,3-K6H[SiW9V3O34] and H6P2Mo18O62 were 9.00 × 10−3, 1.13 × 10−2, 5.20 × 10−4, 1.15 × 10−2, 1.98 × 10−3 and 2.12 × 10−7S−1, respectively.
Table 2.
Comparison of the Km and Vmax of oxidase-like enzymes.
| Nanozymes | Substrate | Km (mM) | Vmax (M·S−1) | Reference |
|---|---|---|---|---|
| H3PMo12O40 | OPD | 0.014 | 1.08 × 10−9 | This work |
| H3PMo12O40 | TMB | 0.625 | 2.25 × 10−7 | This work |
| H4SiMo12O40 | OPD | 0.013 | 6.19 × 10−9 | This work |
| H4SiMo12O40 | TMB | 0.522 | 2.25 × 10−7 | This work |
| Eu3PMo12O40 | OPD | 0.038 | 2.83 × 10−9 | This work |
| Eu3PMo12O40 | TMB | 0.086 | 2.60 × 10−8 | This work |
| H5PMo10V2O40 | OPD | 0.015 | 2.64 × 10−9 | This work |
| H5PMo10V2O40 | TMB | 0.553 | 2.30 × 10−7 | This work |
| H6P2Mo18O62 | TMB | 0.750 | 5.30 × 10−7 | This work |
| α-1,2,3-K6H[SiW9V3O34] | OPD | 0.015 | 1.30 × 10−8 | This work |
| α-1,2,3-K6H[SiW9V3O34] | TMB | 0.020 | 1.49 × 10−8 | This work |
| PMV-FA | TMB | 0.00041 | 4.70 × 10−6 | 20 |
| FAPMoV1 | TMB | 2.6 × 10−3 | 1.33 × 10−6 | 47 |
| FAPMoV3 | TMB | 0.32 × 10−3 | 1.46 × 10−5 | 47 |
Detection of the reactive hydroxyl radicals ·OH production
The mechanism of peroxidase-like activity of POMs originates from their catalytic ability to the decomposition of H2O2 to produce hydroxyl radical (·OH). Terephthalic acid (TA) can capture ·OH to generate 2-hydroxy terephthalic acid (HTA) which can be detected by fluorescence method. So we chose TA as fluorescence probe to detect the generation of ·OH. As show in Supplementary Fig. S10, the control groups (TA, TA with H2O2 and TA with POMs) did not show the significant intensity for HTA. Only in the presence of POMs and H2O2, the fluorescence can be remarkable found. This supported that the POMs can catalyze the H2O2 to generate ·OH, then justify the peroxidase-like activities of POMs.
Calibration curve for H2O2 detection
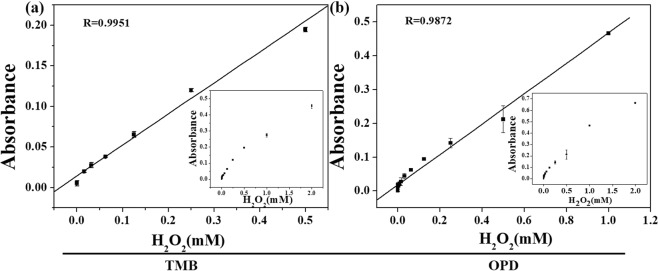
Because the K10P2W18Fe4(H2O)2O68 has the strongest the peroxidase mimetic activity, it can be used in the sensing for H2O2. Under optimum conditions, a colorimetric assay for the detection of hydrogen peroxide based on the peroxidase-like activity of K10P2W18Fe4(H2O)2O68 has been established with TMB and OPD as substrates, as shown in Fig. 9. When the substrate was TMB, the linear detection range was estimated to be from 15 to 1000 μM with correlation coefficients 0.9951. The lower limit of detection (LOD) of K10P2W18Fe4(H2O)2O68 was 10.43 μM. When the substrate was OPD, the correlation between the absorption value and H2O2 concentration were linear over the range of 15–500 μM with correlation coefficients 0.9872. The lower limit of detection (LOD) of K10P2W18Fe4(H2O)2O68 was 8.86 μM. In addition, the analytical performance of K10P2W18Fe4(H2O)2O68 as peroxidase mimics was compared with others reported POM, HRP and nanozymes, as summarized in Table 3. By comparing with other enzyme mimics, it revealed that the K10P2W18Fe4(H2O)2O68 sensor has wider linear range.
Figure 9.
A does-response curve for K10P2W18Fe4(H2O)2O68 depending of the absorbance at 450 nm with OPD as substrates and at 650 nm with TMB as substrates in the presence of diverse concentrations of H2O2. (a)TMB as substrates; (b) OPD as substrates.
Table 3.
Comparison of different nanozymes for the detection of H2O2.
| Nanozymes | Linear Range | Limit of Detection | Time | Reference |
|---|---|---|---|---|
| K10P2W18Fe4(H2O)2O68 | 15–1000 μM | 10.43 μM | 5 min | This worka |
| K10P2W18Fe4(H2O)2O68 | 15–500 μM | 8.86 μM | 5 min | This workb |
| Cu6(Trz)10(H2O)4[H2SiW12O40] | 10–60 μM | 1.37 μM | 1 min | 32 |
| Na4H2[Cu4(im)14][Cu3(H2O)3(BiW9O33)2] | 1–50 μM | 0.12 μM | 2 min | 28 |
| Na4H2[Cu4(im)14][Cu3(H2O)3(SbW9O33)2] | 1–50 μM | 0.12 μM | 2 min | 28 |
| FA-Fe2SiW10 | 0.13–67 μM | 0.13 μM | 1 min | 22 |
| FF@PW12 | 1–75 μM | 0.11 μM | 10 min | 27 |
| Na4(NH4)14[Zr4O6(OAc)2(P2W16O59)2]·51H2O | 100–1000 μM | 100 μM | 90 min | 23 |
| H4SiW12O40 | 1–20 μM | 0.4 μM | 5 min | 19 |
| H3PW12O40 | 0.1–67 μM | 0.13 μM | 10 min | 21 |
| HRP | 1–60 μM | 1 μM | — | 48 |
| Fe3O4 MNPs | 1–100 μM | 0.5 μM | — | 49 |
aTMB as the substrate; bOPD as the substrate.
Conclusion
In conclusion, the structures, the hybrid atoms, the coordination atoms, the substituted metal atoms are the effect factors for their enzyme mimic activities. The polyoxomolybdates (H3PMo12O40, H4SiMo12O40 and Eu3PMo12O40) have shown oxidase-like enzymes activities, while the polyoxotungstates shows peroxidase mimic activities. The substituted metals improved the enzyme mimic activities of POMs, which proved in H5PMo10V2O40, α-1,2,3-K6H[SiW9V3O34] and K10P2W18Fe4(H2O)2O68. The affinity of POM with substrate is another important factor on their enzyme mimic activities. For example, the Na10H2W12O42, Na8H[α-PW9O34], Na10[α-SiW9O34] and Na10[α-GeW9O34] possessed peroxidase-like activities only with OPD as the substrates. pH condition is the key impact factor not only in the stability of POM but also the enzymes activities of them. The lacunary-Keggin type POMs, Na10[α-GeW9O34], Na8H[α-PW9O34] and Na10[α-SiW9O34] were firstly found to own strong catalytic activities under alkaline conditions. Interesting, K10P2W18Fe4(H2O)2O68 showed the highest catalytic activities among the POMs. In the future, the further analysis the inorganic systems and functionalization of metal-substituted polyoxotungstates will be expected to have a potential application in biotechnology, clinical diagnosis and other industry to substitute natural enzymes.
Experimental
Chemicals and materials
All the chemicals used were of analysis and graded without further purification. OPD was purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). TMB was obtained from (Tokyo, Japan). H3PW12O40, H4SiW12O40 and H3PMo12O40 and Hydrogen peroxide (H2O2, 30%) were purchased from Beijing Chemical Works (Beijing, China). The water used in the experiments was purified. The POMs mimetic enzymes were characterized by IR and UV-vis spectrum. The UV-vis spectrum was recorded in the range of 200–600 nm on UV-Vis spectrophotometer (Puxi Inc., Beijing, China). Fourier-transform infrared spectrum (FT-IR) was collected in the range of 4000–400 cm−1 on an Alpha Centauri FT/IR spectrophotometer (Shizumi, Tokyo) using KBr pellets.
Synthesis of polyoxometalates
The 18 polyoxometalates including Keggin (H3PW12O40, H4SiW12O40, H4GeW12O40, K4GeW12O40, H4SiMo12O40, H3PMo12O40, Eu3PMo12O40), Dawson (H6P2Mo18O62, α-(NH4)6P2W18O62, α-K6P2W18O62·14H2O), lacunary-Keggin (Na10[α-GeW9O34], Na8H[α-PW9O34], Na10[α-SiW9O34], K8[γ-SiW10O36]) and the transition-metal substituted-type (α-1,2,3-K6H[SiW9V3O34], H5PMo10V2O40, Wells-Dawson (K10P2W18Fe4(H2O)2O68) investigated in this study were synthesized according to the literature36–43 or provide by prof. Yangguang Li.
Enzyme mimetic activities of POMs
The enzyme mimetic activities of POMs were determined spectrophotometrically by measuring the formation of DAB from OPD at 450 nm (ε = 2.1 × 104 mM−1 cm−1)44 or TMB+ from TMB at 650 nm (ε = 3.9 × 104 mM−1 cm−1)45 using UV-vis spectrophotometer in a 1 cm cuvette. Typically, the 480 μL of TMB (1.5 mM in ethanol) or OPD solution (3.6 mM in water) was added into 2400 μL different buffer solutions (NaH2PO4-citrate or Tris-HCl), followed by the addition of 60 μL of POMs (10 mM) and 60 μL of hydrogen peroxide (H2O2, 10 M). The mixed solution was incubated at room temperature. The oxidases activities of POMs (10 mM) were used under the same identical reaction conditions with the absence of H2O2.
pH measurements
The activities of the POMs at different pH values were performed using the same condition as above, except two different buffer compositions for the different pH ranges were employed. The reaction was carried out 200 μM POMs to which TMB (240 μM) or OPD (576 μM) and H2O2 (200 mM) were added. Between pH 2.5 to 7, 0.2 mM of Na2HPO4-citrate buffer was used; for pH 7–10, 0.1 mM of Tris-HCl buffer was used. The pH of the different buffers was adjusted using a pH meter (PHS-25 pH meter, Shanghai INESA Scientific Instrument Co., China).
Determination of kinetic parameters
The steady-state kinetics of POM-peroxidase were conducted by varying the concentrations of H2O2 (0–200 mM), or OPD/TMB (0–576 μM/0–240 μM) one at a time. The reaction was carried out in 0.2 mM Na2HPO4-citrate buffer and 0.1 mM Tris-HCl (at the optimum pH) and monitored spectrophotometrically by 300 s using a 1 cm cuvette. The kinetic curves were adjusted to the Michaelis-Menten model and linear weaver-Burk linearizations were performed using origin 7.0 software. The apparent kinetic parameters were calculated based on the equation v = Vmax × [S]/(Km + [S]), where v is the initial velocity, Vmax is the maximal reaction velocity, [S] is the concentration of substrate, and Km is the Michaelis constant.
Detection of the reactive hydroxyl radicals ·OH production
The hydroxyl radicals (·OH) production was measured by fluorescence method. The terephthalic acid was used as a fluorescence probe for detection the ·OH from the H2O2 for Na10[α-GeW9O34], Na8H[α-PW9O34], Na10[α-SiW9O34] and K10P2W18Fe4(H2O)2O68 as catalysts. 75 μL of 25 mM TA in NaOH (pH = 13) solution was added into the 3 mL of PBS (pH = 7.4) containing 100 mM H2O2 and/or 1 mM POMs. After 24 h incubation in the dark, the resulting solution was detected. The fluorescence spectra were obtained with excitation wavelength of 315 nm and the emission spectra were recorded in the wavelength of 425 nm.
Detection of H2O2
The detection of H2O2 was performed according to the following steps: 480 μL of OPD solution (576 μM) or TMB solution (240 μM), 60 μL of POMs (100 μM) and 60 μL H2O2 (200 mM) with various concentrations were added into 2400 μL of buffer solution, and the total volume of the mixed solution was 3 mL. After reacting 5 min under the optimum conditions, then the UV-Vis spectrophotometer was used to record the absorbance at 450 nm for OPD and 650 nm for TMB.
Supplementary information
Acknowledgements
This work was financially supported by NSFC (81402719) and Norman Bethune Program of Jilin University (2015228). We highly appreciate Prof. Yangguang Li from the Institute of Polyoxometalate Chemistry, Department of Chemistry, Northeast Normal University for the POMs. The open project of the CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety (NKSF201801).
Author contributions
Y.Q. conceived and designed the experiment, B.Z. performed the experiments and analyzed the data. Y.Q. contributed reagents/materials/analysis tools, B.Z., M.Z., R.T., B.C., H.Z., C.Z. and C.W. wrote the paper. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-50539-9.
References
- 1.Mozhaev VV, Sergeeva MV, Belova AB, Khmelnitsky YL. Multipoint attachment to a support protects enzyme from inactivation by organic solvents: α-chymotrypsin in aqueous solutions of alcohols and diols. Biotechnol. Bioeng. 1990;35:653–659. doi: 10.1002/bit.260350702. [DOI] [PubMed] [Google Scholar]
- 2.Burton SG, Cowan DA, Woodley JM. The search for the ideal biocatalyst. Nat. Biotechnol. 2002;20:37–45. doi: 10.1038/nbt0102-37. [DOI] [PubMed] [Google Scholar]
- 3.Rreslow R, Overman LE. “Artificial enzyme” combining a metal catalytic group and a hydrophobic binding cavity. J. Am. Chem. Soc. 1970;92:1075–1077. doi: 10.1021/ja00707a062. [DOI] [PubMed] [Google Scholar]
- 4.R. Breslow, Artificial enzymes, Wiley-VCH, Weinheim. (2005).
- 5.Raynal M, Ballester P, Vidal-Ferran A, Van Leeuwen PWNM. Supramolecular catalysis. Part 1: non-covalent interactions as a tool for building and modifying homogeneous catalysts. Chem. Soc. Rev. 2014;43:1734–1787. doi: 10.1039/C3CS60037H. [DOI] [PubMed] [Google Scholar]
- 6.Aiba Y, Sumaoka J, Komiyama M. Artificial DNA cutters for DNA manipulation and genome engineering. Chem. Soc. Rev. 2011;40:5657–5668. doi: 10.1039/c1cs15039a. [DOI] [PubMed] [Google Scholar]
- 7.Gao LZ, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007;2:577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 8.Wei H, Wang EK. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem. Soc. Rev. 2013;42:6060–6093. doi: 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- 9.Wei H, Wang EK. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008;80:2250–2254. doi: 10.1021/ac702203f. [DOI] [PubMed] [Google Scholar]
- 10.Kuah E, Toh S, Yee J, Ma Q, Gao ZQ. Enzyme mimics: advances and applications. Chem. Eur. J. 2016;22:8404–8430. doi: 10.1002/chem.201504394. [DOI] [PubMed] [Google Scholar]
- 11.Komkova MA, Karyakina EE, Karyakin AA. Catalytically synthesized prussian blue nanoparticles defeating natural enzyme peroxidase. J. Am. Chem. Soc. 2018;140:11302–11307. doi: 10.1021/jacs.8b05223. [DOI] [PubMed] [Google Scholar]
- 12.Long DL, Tsunashima R, Cronin L. Polyoxometalates: building blocks for functional nanoscale systems. Angew. Chem. Int. Edit. 2010;49:1736–1758. doi: 10.1002/anie.200902483. [DOI] [PubMed] [Google Scholar]
- 13.Pope MT, Müller A. Polyoxometalate chemistry: an old field with new dimensions in several disciplines. Angew. Chem. Int. Edit. 1991;30:34–48. doi: 10.1002/anie.199100341. [DOI] [Google Scholar]
- 14.Ma YY, et al. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energ. Environ. Sci. 2017;10:788–798. doi: 10.1039/C6EE03768B. [DOI] [Google Scholar]
- 15.Li JS, et al. Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution. Nat. Commun. 2016;7:11204. doi: 10.1038/ncomms11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang SS, Guo YY. Recent advance in polyoxometalate-catalyzed reactions. Chem. Rev. 2015;115:4893–4962. doi: 10.1021/cr500390v. [DOI] [PubMed] [Google Scholar]
- 17.Long DL, Tsunashima R, Cronin L. Polyoxometalates: building blocks for functional nanoscale systems. Angew. Chem. 2010;122:1780–1803. doi: 10.1002/ange.200902483. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno N, Yamaguchi K, Kamata K. Epoxidation of olefins with hydrogen peroxide catalyzed by polyoxometalates. Coord. Chem. Rev. 2005;249:1944–1956. doi: 10.1016/j.ccr.2004.11.019. [DOI] [Google Scholar]
- 19.Liu S, et al. Fast and sensitive colorimetric detection of H2O2 and glucose: a strategy based on polyoxometalate clusters. Chempluschem. 2012;77:541–544. doi: 10.1002/cplu.201200051. [DOI] [Google Scholar]
- 20.Wang JJ, Mi XG, Guan HY, Wang XH, Wu Y. Assembly of folate-polyoxometalate hybrid spheres for colormetric immunoassay like oxidase. Chem. Commun. 2011;47:2940–2942. doi: 10.1039/c0cc04850j. [DOI] [PubMed] [Google Scholar]
- 21.Wang JJ, Han DX, Wang XH, Qi B, Zhao MS. Polyoxometalates as peroxidase mimetics and their applications in H2O2 and glucose detection. Biosens. Bioelectron. 2012;36:18–21. doi: 10.1016/j.bios.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 22.Sun Z, et al. Fabrication of inorganic-organic hybrid based on polyoxometalates SiW10Fe2 and folate as peroxidases for colormetric immunoassay of cancer cells. Chinese Chem. Lett. 2013;24:76–78. doi: 10.1016/j.cclet.2012.12.002. [DOI] [Google Scholar]
- 23.Li D, et al. Modification of tetranuclear zirconium-substituted polyoxometalates- syntheses, structures, and peroxidase-like catalytic activities. Chem. Eur. J. 2013;24:1926–1934. [Google Scholar]
- 24.Duan XX, et al. Fabrication of metal-substituted polyoxometalates for colorimetric detection of dopamine and ractopamine. Materials. 2018;11:674–684. doi: 10.3390/ma11050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeb A, et al. Intrinsic peroxidase-like activity and enhanced photo-Fenton reactivity of iron-substituted polyoxometalate nanostructures. Dalton Trans. 2018;47:7344–7352. doi: 10.1039/C8DT01146J. [DOI] [PubMed] [Google Scholar]
- 26.She S, et al. Aliphatic organoimido derivatives of polyoxometalates containing a bioactive ligand. Chem. Eur. J. 2014;51:16987–16994. doi: 10.1002/chem.201404317. [DOI] [PubMed] [Google Scholar]
- 27.Ma Z, et al. Effective synergistic effect of dipeptide-polyoxometalate-graphene oxide ternary hybrid materials on peroxidase-like mimics with enhanced performance. ACS Appl. Mater. Interfaces. 2015;39:22036–22045. doi: 10.1021/acsami.5b07046. [DOI] [PubMed] [Google Scholar]
- 28.Chai DF, et al. Synergistic effect of sandwich polyoxometalates and copper-imidazole complexes for enhancing the peroxidase-like activity. RSC Adv. 2015;5:78771–78779. doi: 10.1039/C5RA13265G. [DOI] [Google Scholar]
- 29.Li X, et al. Keggin polyoxometalates based hybrid compounds containing helix/nanocages for colormetric biosensing. J. Solid State Chem. 2018;265:372–380. doi: 10.1016/j.jssc.2018.06.020. [DOI] [Google Scholar]
- 30.Narkhede N, Uttam B, Rao CP. Inorganic-organic covalent hybrid of polyoxometalate-calixarene: Synthesis, characterization and enzyme activity. Inorg. Chim. Acta. 2018;483:337–342. doi: 10.1016/j.ica.2018.08.034. [DOI] [Google Scholar]
- 31.Chen K, et al. Improved peroxidase-mimic property: Sustainable, high efficiency interfacial catalysis with H2O2 on the surface of vesicles of hexavanadate-organic hybrid surfactants. Nano Res. 2018;3:1313–1321. doi: 10.1007/s12274-017-1746-5. [DOI] [Google Scholar]
- 32.Zhou EL, et al. A stable polyoxometalate-pillared metal-organic framework for proton-conducting and colormetric biosensing. Chem. Eur. J. 2015;21:11894–11898. doi: 10.1002/chem.201501515. [DOI] [PubMed] [Google Scholar]
- 33.Li X, et al. Synthesis, structure and effective peroxidase-like activity of a stable polyoxometalate-pillared metal-organic framework with multinuclear cycles. Polyhedron. 2018;151:206–212. doi: 10.1016/j.poly.2018.05.046. [DOI] [Google Scholar]
- 34.Zhao JW, et al. Lanthanide-Connecting and Lone-Electron-Pair Active Trigonal-Pyramidal-AsO3 Inducing Nanosized Poly(polyoxotungstate) Aggregates and Their Anticancer Activities. Sci. Rep. 2016;6:26406–26418. doi: 10.1038/srep26406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian R, Zhang BY, Zhao MM, Ma Q, Qi YF. Polyoxometalates as promising enzyme mimics for the sensitive detection of hydrogen peroxide by fluorometric method. Talanta. 2018;188:332–338. doi: 10.1016/j.talanta.2018.05.085. [DOI] [PubMed] [Google Scholar]
- 36.Rocchiccioli-Deltcheff C, Fournier M, Franck R, Thouvenot R. Chemlnform abstract: vibrational investigations of polyoxometalates.2. evidence for anion-anion interactions in molybdenun(VI) compounds related to the keggin structure. Inorg. Chem. 1993;22:207. doi: 10.1021/ic00144a006. [DOI] [Google Scholar]
- 37.Romanelli GP, et al. Silicagel-supported H6P2W18O62.24H2O: a reusable catalyst to prepare diphenylmethyl (DPM) ethers. ARKIVOC. 2017;1:1. [Google Scholar]
- 38.Tézé A, Hervé G, Finke RG, Lyon DK. α-, β-, and γ-Dodecatungstosilicic acids: isomers and related lacunary compounds. Inorganic Syntheses. 2007;27:85–96. doi: 10.1002/9780470132586.ch16. [DOI] [Google Scholar]
- 39.Allmann R. Die Struktur des Ammoniumparawolframates (NH4)10[H2W12O42].10H2O. Acta. Cryst. 1971;B27:1393–1404. doi: 10.1107/S0567740871004047. [DOI] [Google Scholar]
- 40.Domaille PJ. 1- and 2-dimensinal tungsten-183 and vanadium-51 NMR characterization of isopolymetalates and heteropolymetalates. J. Am. Chem. Soc. 1984;106:7677–7687. doi: 10.1021/ja00337a004. [DOI] [Google Scholar]
- 41.Tang Y, Zhang J. Direct oxidation of benzene to phenol catalyzed by vanadium substituted heteropolymolybdic acid. Transit. Metal Chem. 2006;31:299–305. doi: 10.1007/s11243-005-6412-1. [DOI] [Google Scholar]
- 42.Finke RG, Droege MW, Domaille PJ. Trivacant heteropolytungstate derivative. 3 rational syntheses, characterization, two-dimensional 183W NMR, and properties of P2W18M4(H2O)2O6810− and P4W30M4(H2O)2O11216− (M=Co, Cu, Zn) Inorg. Chem. 1987;6:3886–3896. doi: 10.1021/ic00270a014. [DOI] [Google Scholar]
- 43.Barats D, Leitus G, Biro RP, Shimon LJW, Neurmann R. A stable ‘end-on’ iron(III)-hydroperoxo complex in water derived from a multi-iron(II)-substituted polyoxometalate and molecular oxygen. Angew. Chem. Int. Ed. 2008;47:9908–9912. doi: 10.1002/anie.200803966. [DOI] [PubMed] [Google Scholar]
- 44.Tyagi N, Mathur P. Iron (III) complexes of bis (benzimidazole-2-yl) methyl thiophene-2,5-dicarboxamide: synthesis, spectral and oxidation of o-phenylenediamine. Spectrochim. Acta. A. 2012;96:759–767. doi: 10.1016/j.saa.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Gao ZQ, Xu MD, Hou L, Chen GN, Tang DP. Irregular-shaped platinum nanoparticles as peroxidase mimics for highly efficient colorimetric immunoassay. Anal. Chim. Acta. 2013;776:79–86. doi: 10.1016/j.aca.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, et al. BSA-templated MnO2 nanoparticles as both peroxidase and oxidase mimics. Analyst. 2012;137:4552–4558. doi: 10.1039/c2an35700c. [DOI] [PubMed] [Google Scholar]
- 47.Ji Y, et al. Inorganic-bimolecular hybrids based on polyoxometalates: intrinsic oxidase catalytic activity and their application to cancer immunoassay. Sens. Actuators B. 2015;208:497–504. doi: 10.1016/j.snb.2014.11.058. [DOI] [Google Scholar]
- 48.Edgar P, Yona K. A Simple Colorimetric Method for the measurement of hydrgen peroxide produced by cells in culture. J. Immunol. Methods. 1980;38:161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- 49.Kim MI, Shim J, Li T, Lee J, Park HG. Fabrication of nanoporous nanocomposites entrapping Fe3O4 magnetic nanoparticles and oxidases for colorimetric biosensing. Chem. Eur. J. 2011;17:10700–10707. doi: 10.1002/chem.201101191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.