Abstract
AIM
To explore the effect of parthenolide (PTL) on human uveal melanoma (UM) cells (C918 and SP6.5 cells) and its molecular mechanism.
METHODS
Carboxyfluorescein succinimidyl amino ester (CFSE) assays and cell counting kit-8 (CCK-8) were performed to detect the cell viability. Flow cytometry was used to analyze cell cycle and apoptosis. Quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot assays were performed to measure proliferation-related and apoptosis-related factors.
RESULTS
Firstly, PTL decreased the viability of C918 and SP6.5 cells in a dose-dependent manner, and the effect of PTL on C918 cells was stronger than on SP6.5; however, it did not affect normal cells. Secondly, PTL increased the proportion of cell number at cell cycle G1 phase in C918 cells, and decreased the proportion of cell number at S phase, but the proportion did not change at G2 phase. In addition, PTL induced the apoptosis of C918 cells, and decreased the expressions of Cyclin D1, B-cell lymphoma-2 (Bcl-2) and B-cell lymphoma-extra large (Bcl-XL). Also, PTL increased Cyclin inhibition protein 1 (P21), Bcl-2-associated X protein (Bax), Cysteinyl aspartate specific proteinas-3 (Caspase-3) and Caspase-9 expression. However, the expression of Caspase-8 was not changed.
CONCLUSION
PTL inhibites proliferation and induces apoptosis in UM cells by arresting G1 phase and regulating mitochondrial pathway, however, it does not affect normal cells.
Keywords: parthenolide, uveal melanoma, proliferation, apoptosis, mitochondrial pathway
INTRODUCTION
Though uveal melanoma (UM) is rare tumor, however, it is the most common primary malignant tumor in adults' eyes[1]–[2]. Surveillance and epidemiology of the National Cancer Institute (NCI) reported that the incidence of UM in Caucasians is higher than in other colored population, thus, the occurrence of the disease is racially different[1],[3]. Meanwhile, the incidence of UM is gender different, and is higher in men than in women. The incidence of the disease is also positively correlated with age[4]–[5]. It has been found that the survival rate of UM was low, as the survival rates of 5, 10, 15, and 20y are 72%, 59%, 53% and 16%, respectively[1]. Moreover, UM is highly metastatic, and metastasis occurs in more than 50% of the cases. Liver metastasis is the most common, causing death within 2-4mo[6]–[7]. Although UM patients are treated with advanced drugs and technologies, the prognosis remains poor and half of UM patients die within 25y[8]–[9]. UM is characterized by a high malignancy, invasion, metastasis and poor prognosis, which can seriously affect the quality of life and even life of people[1],[7],[9]–[10]. Therefore, it is necessary to find effective drugs and treatment methods to prevent and treat UM.
In recent decades, many Chinese medicine researchers have carried out studies on anti-tumor screening in vivo and in vitro. Results show that many Chinese herbal medicines had anti-cancer effects at different levels[11]–[12]. Parthenolide (PTL) is the main extract of Chinese herbal medicine parthenium hysterophorus, which contains the component of sesquiterpene lactone[13] that is α-methylene-γ-lactone ring and has epoxide structure[14]. This structure can react with enzymes, which contain mercapto groups and other functional proteins, to interfere with the many key biological processes of cells, such as cell signaling pathways, mitochondrial respiration, proliferation and apoptosis[15]. In the past, PTL was primarily used to treat migraine, fever and rheumatoid arthritis[16]. In recent years, the studies find that PTL exerted anti-cancer effect in a variety of tumors, such as breast cancer, cholangiocarcinoma, pancreatic cancer, bladder cancer, prostate cancer, leukemia[17]–[24]. However, as far as we know, the potential effect of PTL on UM has not been investigated, and the molecular mechanism of PTL on UM remains to be studied.
PTL may control cell growth and apoptosis in tumor cells[24]–[27]. Cell cycle is the most important process of cellular activities. The regulation of cell cycle is achieved by the specific cell cycle protein in each phase of cell cycle. As we all known, cyclin D1 and Cyclin inhibition protein 1 (P21) played key roles in G1 phase[28]. So far, it has been reported that the Cyclin D1 and P21 genes were amplified or overexpressed in breast cancers, mammary hyperplasia and carcinoma[28]–[29]. According to the report, the family of Bcl-2 and Caspase proteins plays a vital role in the process of tumor apoptosis[30]. The members of Bcl-2 proteins family include, for example, Bax, Bcl-2, Bcl-XL. Bax is a protein that promotes apoptosis, while Bcl-2 and Bcl-XL are proteins that suppress apoptosis[31]. The members of Caspase proteins family include, for example, Caspase-3, Caspase-8 and Caspase-9, which are divided into initiators (Caspase-8, Caspase-9) and executors (Caspase-3), and the initiator can activate the executor[32]. Herein, we studied the effect of PTL on the proliferation of human UM (C918 and SP6.5 cells) and normal cells [human normal uveal melanocytes, retinal pigment epithelial (RPE)], and fibroblasts). Furthermore, whether PTL affected the apoptosis of C918 cells was also determined. We further explored the effect of PTL on the proliferation and apoptosis of C918 cells by arresting the corresponding stage of cell cycle and regulating corresponding pathway.
MATERIALS AND METHODS
Cell Lines and Cell Culture
Human UM (C918 and SP6.5), human normal uveal melanocyte, RPE and fibroblast cell lines were all purchased from American Type Culture Collection (ATCC, USA). C918 and SP6.5 cells were originated from a UM patient with liver metastasis[33] and a primary UM patient[34], respectively. C918 cells were epithelioid in morphology, which have highly an invasive and metastatic ability[33]. C918 and SP6.5 cells were cultured in Ham's F12 nutrient mixture (F12; Gibco, USA) containing 10% fetal bovine serum (FBS; Invitrogen, USA) and 50 µg/mL gentamicin (Solarbio, Beijin, China). Human normal uveal melanocytes, RPE, and fibroblasts were cultured in Dulbecco's modified Eagle medium (DMEM; Gibco, USA) containing 10% FBS and 50 µg/mL gentamicin. The cells were cultured in a 5% CO2 humidified incubator with at 37°C.
Drug Treatment
PTL was obtained from Desite Biotechnology Co., LTD. (Chengdu, China) and dissolved in absolute alcohol to form a concentration of 50, 100, and 200 µmol/L, respectively. In subsequent experiments, these different concentrations of PTL were used to treat cells respectively to explore the effect of PTL on C918 and SP6.5 cells.
CCK-8 Assay
C918, SP6.5, RPE, fibroblast and human normal uveal melanocyte cells were seeded in plates (96-well) at a density of 3×103 cell/well and incubated in 5% CO2 humidified incubator at 37°C for 24h. Different concentrations of PTL were added into the RPE, fibroblast, human normal uveal melanocyte cells, C918 and SP6.5 cells for 48h, respectively. Then, CCK-8 reagent (Solarbio, Beijing, China) was dropped into each well. The plates were again incubated in 5% CO2 humidified incubator at 37°C for 2h. Finally, absorbance of each group was measured at 450 nm using a microplate reader (Thermo, BD, USA, VL0LA0D2).
CFSE Assay
C918 and SP6.5 cells (2×104 cell/well) were seeded in plates (24-well) and cultured in 5% CO2 humidified incubator at 37°C for 24h. To explore the effect of PTL on the cell viability, the cells were divided into control group (0.1% PBS) and PTL treatment group (cells were treated with PTL 50, 100 and 200 µmol/L, respectively). Cells were treated with drugs for 24h. 0.25% EDTA-trypsin (Solarbio, Beijing, China) was used to digest the cells of each group, and then the cells were centrifuged (1000 rpm/min) for 5min and collected, and then resuspended by F12 medium. Next, CFSE solution (Thermo, Shanghai, China) was added into the cells, which were then incubated at 37°C for 15min. After incubation has been completed, FBS was added for terminating the reaction. The cells were centrifuged and resuspended by F12 medium again. Finally, cell viability was detected by a flow cytometer (Beckman Coulter, Gallios, USA).
Cell Cycle Assay
C918 cells (1×106 cell/well) were seeded in plates (6-well), and incubated in 5% CO2 humidified incubator at 37°C for 24h. The experiment was grouped in the same way as before. The cells were treated with drugs for 24h. Then, 40×RnaseA (Solarbio, Beijing, China) was dropped into each well at 37°C for 20min. The cells were stained in propidium iodide (PI) cell cycle solution (Solarbio, Beijing, China). Subsequently, the cells were incubated at 4°C for 15min. Finally, cell cycle was detected by a flow cytometer.
Cell Apoptosis Assay
C918 cells were seeded in plates (6-well, 1×106 cell/well) and incubated in 5% CO2 humidified incubator at 37°C for 24h. The experiment was grouped in the same way as before. The cells were treated with drugs for 24h. Then, the cells of each group were digested with 0.25% EDTA-trypsin, resuspended and centrifuged (1000 rpm/min) for 5min. Annexin V-FITC (Solarbio, Beijing, China) and PI were dropped into each well. Subsequently, the cells were incubated for 30min in the dark. Finally, a flow cytometer was used to measure the cell apoptosis of each group.
Quantitative Real-time Polymerase Chain Reaction Assay
C918 cells (1×106 cell/well) were seeded in plates (6-well), and cultured in 5% CO2 humidified incubator at 37°C for 24h. The experiment was grouped in the same way as before. The cells were treated with drugs for 24h. Then, total RNAs of the samples were collected by using RNAiso Plus (Takara, Beijing, China). RT Master Mix kit (Takara, Beijing, China) was applied to synthesize RNA into cDNA, and the reaction conditions were as follows: at 37°C for 60min, at 85°C for 5min and at 4°C for 5min. SYBR Premix Taq™ II kit (Takara, Beijing, China) was performed for amplifying cDNA. PCR reaction conditions were set at 95°C for 10min, (at 95°C for 15s, at 60°C for 45s) for 40 cycles, at 95°C for 15s, at 60°C for 1min, at 95°C for 15s and at 60°C for 15s. The primer sequence used in quantitative real-time polymerase chain reaction (qRT-PCR) experiment was presented in Table 1. The formula 2−ΔΔCT was used to calculate the gene expression. GAPDH was considered as an internal reference.
Table 1. Sequences of the primers used for qRT-PCR.
| Primer name | Forward sequence (5′-3′) | Reverse sequence (5′-3′) |
| Cyclin D1 | CCCTCGGTGTCCTACTTCAA | CTTAGAGGCCACGAACATGC |
| P21 | ACAAGAGGCCCAGTACTTCC | AGAAATCTGTCAGGCTGGTCT |
| Caspase-3 | TGCCCAAGTGACTGACATCA | CATCCCCATTGACTGTGCAG |
| Caspase-8 | TTTGGCTGGCATCATCTGTG | CATCCACATGTGTCCCGTTC |
| Caspase-9 | ATGCTCCGTGTCCATTGAGA | AGTCACTGTCCAAGGTCCTG |
| Bcl-XL | ATGCTCCGTGTCCATTGAGA | AGTCACTGTCCAAGGTCCTG |
| Bax | GACCCGGTGCCTCAGGATGC | AGGTCAGCTCATCATGCTTG |
| Bcl-2 | GTGGAGGAGCTCTTCAGGGA | GTCTGTGTCCACGGCGGCAA |
| GAPDH | CACCCACTCCTCCACCTTTG | CCACCACCCTGTTGCTGTAG |
qRT-PCR: Quantitative real-time polymerase chain reaction.
Western Blot Assay
C918 cells (1×106 cell/well) were seeded in plates (6-well), and cultured in 5% CO2 humidified incubator at 37°C for 24h. The experiment was grouped in the same way as before. The cells were first treated with drugs for 24h and then collected. Protein from the cells was extracted by using RIPA buffer (high), and then a BCA protein assay kit (Beyotime, Shanghai, China) was used to measure the contents of protein extracts. Next, 30 micrograms of each protein was separated by 10% SDS-PAGE, and transferred onto the PVDF membrane (Reno, Hangzhou, China). Subsequently, the membrane was hybridized to primary antibodies at 4°C overnight after blocking in 5% non-fat milk for 2h. The next day, the membrane were incubated in corresponding secondary antibodies (HRP goat anti-rabbit IgG, Invitrogen, A-11034, 1:500; HRP rabbit anti-mouse IgG, Invitrogen, A-11059, 1:500) at room temperature for 1.5h. Finally, the protein was exposed by ECL detection reagent (Weiao, Shanghai, China). The primary antibodies were as follows: anti-P21 (Invitrogen, 33-7000; dilution: 1:800), anti-Cyclin D1 (Invitrogen, 710428; dilution: 1:2000), anti-Bax (Invitrogen, MA5-14006; dilution: 1:1000), anti-Bcl-XL (Invitrogen, MA5-11950; dilution: 1:1500), anti-Bcl-2 (Invitrogen, MA5-11757; dilution: 1:1000), anti-Caspase-3 (Invitrogen, 700182; dilution: 1:700), anti-Caspase-8 (Invitrogen, 710535; dilution: 1:2000), anti-Caspase-9 (Invitrogen, PA5-16358; dilution: 1:1000) and anti-GAPDH (Invitrogen, 39-8600; dilution: 1:1000). GAPDH was used as an internal reference.
Statistical Analysis
All data were presented as mean±SD, all analysis was conducted using GraphPad Prism 6.0. The Student's t-test was used to assess difference between the experimental groups. The statistical difference was considered significant if P<0.05. Each experiment was implemented in triplicate.
RESULTS
Effect of PTL on the Viability of Human Uveal Melanoma Cells
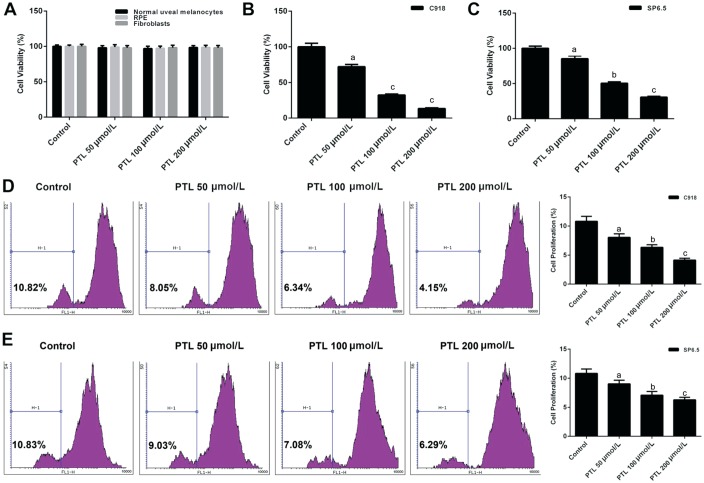
We explored how PTL affected the viability of human UM (C918 and SP6.5), human normal uveal melanocyte, RPE and fibroblast cells by CCK-8 and CFSE analysis, respectively. The viabilities of RPE, human normal uveal melanocytes and fibroblasts did not changed when cells were treated with PTL (Figure 1A). However, C918 and SP6.5 cells, which were treated with different concentration of PTL, reduced the cell viability in a dose-dependent manner (P<0.05; Figure 1B-1E). Meanwhile, we found that PTL inhibited the proliferation activity of C918 cells more strongly than that of SP6.5 cells, suggesting that PTL did not affect normal cells, however, it inhibited the proliferation of human UM (C918 and SP6.5) cells. Meanwhile, C918 cells were selected for later research.
Figure 1. PTL decreased the viability of human uveal melanoma cells (C918 and SP6.5).
A: The cell viabilities of human normal uveal melanocytes, fibroblasts and RPE treated with different concentration of PTL was detected by CCK-8 assay. B, C: CCK-8 assay was applied to test the viabilities of C918 and SP6.5 cells treat with different concentration of PTL. D, E: The viabilities of C918 and SP6.5 cells were further detected by CFSE assay. aP<0.05, bP<0.01, cP<0.001, compared with control.
Inhibitory Effect of PTL on the Viability of C918 Cells by Arresting G1 Phase
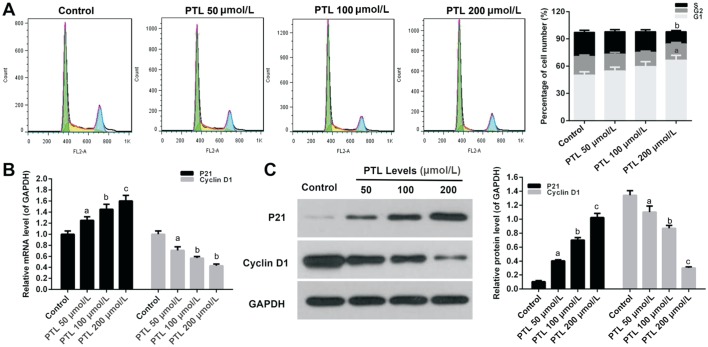
To analyze which phase of cell cycle was arrested after the viability of C918 cells was decreased by MTE, cell cycle was examined by quantitating the content cells' DNA using flow cytometry. The treatment of C918 cells with different concentration of PTLs decreased the percentage of cell number at S phase, and increased accumulation of the cell percentage at G1 phase, however, the percentage of cell number did not change at G2 phase (P<0.05; Figure 2A).
Figure 2. PTL decreased the viability of C918 cells by arresting G1 phase.
A: PI staining kit was used to measure the percentage of cell number at cell cycle S, G1, and G2 phase in C918 cells treat with different concentration of PTL. B, C: The relative mRNAs and protein expressions of P21 and Cyclin D1 were detected by qRT-PCR (B) and Western blot (C) assays, respectively. GAPDH served as an internal control. Quality one was applied to measure and count the gray value. aP<0.05, bP<0.01, cP<0.001, compared with control.
Cyclin D1 and P21 were measured by qRT-PCR and Western blot assay, respectively. PTL significantly stimulated the mRNA expression of Cyclin D1 in C918 cells in a dose-independent manner, and inhibited P21 mRNA expression (P<0.05; Figure 2B). The expression patterns of Cyclin D1 and P21 proteins were the same as mRNA expression in C918 cells treated with PTL (P<0.05; Figure 2C).
Promotive Effect of PTL on the Apoptosis of C918 Cells
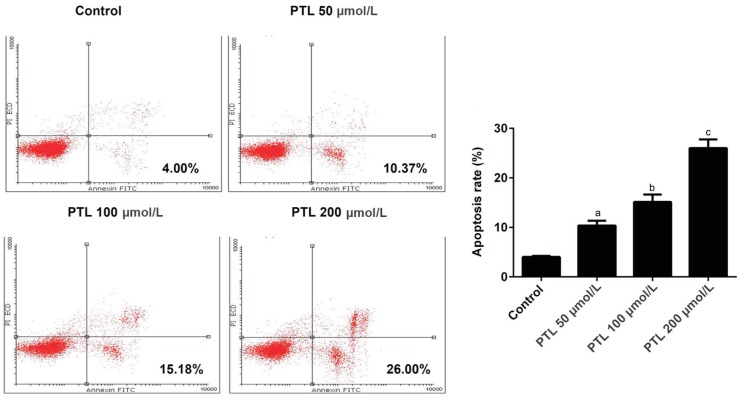
Annexin V-FITC apoptosis detection kit was applied to study the effect of PTL on the apoptosis of C918 cells. Our data showed that PTL increased apoptosis of C918 cells in a dose-dependent manner. When C918 cells were treated with different concentrations of PTL, the apoptosis rate increased by 2.60-folds, 3.80-folds, 6.50-folds, respectively (P<0.05; Figure 3).
Figure 3. PTL promoted the apoptosis of C918 cells.
Annexin V-FITC apoptosis detection kit assay was used to detect the apoptosis rate of C918 cells treated with different concentration of PTL. aP<0.05, bP<0.01, cP<0.001, compared with control.
Effect of PTL on the Expression of Bcl-2 Family Members in C918 Cells
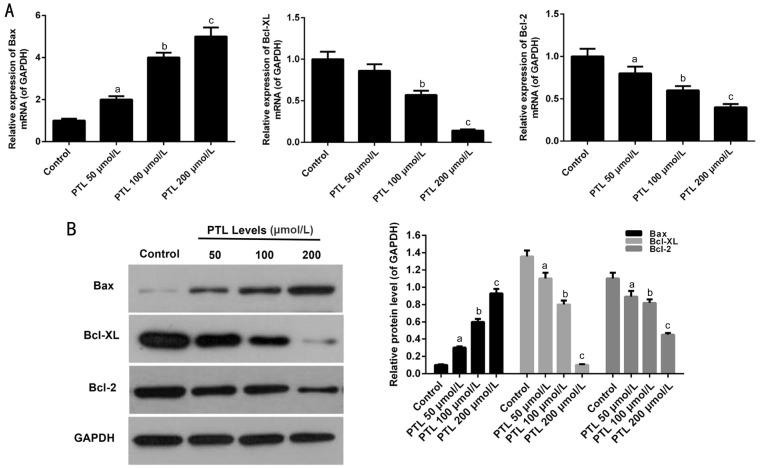
qRT-PCR and Western blot assays were performed to further explore whether PTL promoted apoptosis in C918 cells. PTL obviously enhanced the expressions of Bax in C918 cells in a dose-dependent manner (P<0.05; Figure 4). By contrast, the expression levels of Bcl-XL and Bcl-2 were decreased in C918 cells treat with PTL.
Figure 4. PTL regulated the members of Bcl-2 family expression in C918 cells.
A: qRT-PCR assay was applied to detect the mRNA expression levels of Bax, Bcl-2, and Bcl-XL in C918 cells. B: The protein expressions of Bax, Bcl-2, and Bcl-XL were detected by Western blot assays in C918 cells. Quality one was applied to detect count the gray value. aP<0.05, bP<0.01, cP<0.001, compared with control.
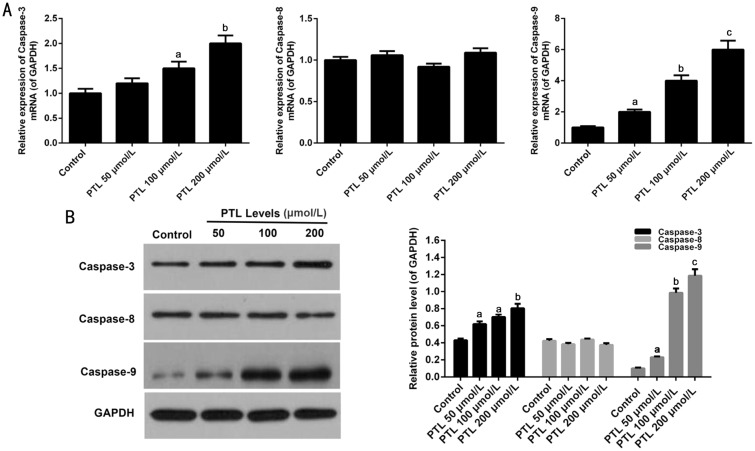
Effect of PTL on the Expression of Caspase Family Members in C918 Cells
qRT-PCR and Western blot analysis were used to explore the pathways of PTL-mediated C918 cells apoptosis. The results demonstrated that the mRNA expressions of Caspase-3 and Caspase-9 were obviously increased in C918 cells treat with different concentrations of PTL. PTL did not affect the mRNA expression level of Caspase-8 (P<0.05; Figure 5A). Similarly, the protein expressions of Caspase-3, Caspase-8 and Caspase-9 were the same as mRNA expressions (P<0.05; Figure 5B).
Figure 5. PTL regulated the members of Caspase family expression in C918 cells.
A: The mRNA expression levels of Caspase-3, Caspase-8, and Caspase-9 were surveyed by qRT-PCR assay in C918 cells. B: Western blot assay was used to survey the protein expressions of Caspase-3, Caspase-8, and Caspase-9 in C918 cells. GAPDH served as an internal control. Quality one was applied to detect count the gray value. aP<0.05, bP<0.01, cP<0.001, compared with control.
DISCUSSION
Recently, the extraction of new anti-tumor drugs from plants has drawn much research attention. Especially, studies have been increasingly carried out on the extraction of anticancer substances from Compositae Plants (Chrysanthemum Parthenium). PTL is one of the most important active ingredients in Chrysanthemum Parthenium, and it belongs to sesquiterpene lactone compounds[13]. In addition to immunomodulatory effects, PTL has been widely used to treat different kinds of tumors. Studies have shown that PTL has the effect of inhibiting proliferation and inducing apoptosis of tumor cells[24]–[27]. However, as far as we know, the effect of PTL on UM cells still remains unknown.
We explored the relationship between PTL and human UM cells (C918 and SP6.5) and normal cells (human normal uveal melanocytes, RPE, and fibroblasts). The results revealed that PTL decreased the viabilities of C918 and SP6.5 cells in a concentration-dependent manner. Therefore, the cytotoxic effect of PTL in C918 cells was stronger than in SP6.5 cells. However, the viability of human normal uveal melanocytes, RPE, and fibroblasts were not affected by PTL. So, it was suggested that PTL had a significant anti-tumor effect on human UM cells.
PTL inhibits anti-tumor activity through various molecular mechanisms[35]. It has been found that the cell cycle and apoptosis change partly made of anti-tumor mechanisms, and the cell cycle and apoptosis change may cause the corresponding protein change[18],[36]. Cell cycle is accomplished by the combination of Cyclin-dependent kinases (CDK) and Cyclins. Cyclin D1 is a member of Cyclins family, which affects G1 phase and has been recognized as a proto-oncogene. Overexpression of Cyclin D1 is closely related to the development of cancer, and it plays a key role in cell cycle regulation[37]–[39]. Besides, P21 is CDK inhibitor, and P21 and P53 are composed of the check point of cell cycle G1 phase[40]. Many researches demonstrated that anti-tumor drugs induced cell cycle by arresting G1 phase to up-regulate P21 expression in tumor cells[41]–[43]. Similar to previous studies, our data showed that PTL arrested cell cycle G1 phase to up-regulate P21 expression and down-regulate Cyclin D1 expression in C918 cells.
Apoptosis is a complex process in which multiple signaling proteins are transmitted via several pathways[31]. At present, it is clear that there are two characteristic pathways via which activated Caspase cascade regulate apoptosis, one is a death receptor pathway (external pathway), another is the mitochondrial pathway (internal pathway). Under certain circumstances, the two apoptotic pathways may cross each other in specific cases. External pathway activates death receptor to combine with corresponding ligands. Subsequently, it can further stimulate Caspase-8 to cause downstream events, including Caspase cleavage and apoptosis. The internal pathway is mediated by Bcl-2 family proteins (Bax, Bcl-2, etc.). The number of pro-apoptotic protein (Bax) is positively correlated with the mitochondrial membrane permeability. Bax can promote the mitochondrial membrane permeability by activating Caspase-3 and Caspase-9, eventually leading to apoptosis[31]–[32],[44]. It has been reported that Bcl-2 was up-regulated in 70% UMs, however, the anti-tumor drugs down-regulate Bcl-2 expression in tumor cells[45]. It has been proved that application of arsenic and other drugs can increase the expressions of Caspase-3 and Caspase-9 to promote tumor cells apoptosis[46]–[47]. Similar to previous studies, we found that PTL induced the apoptosis of C918 cells, therefore, the expressions of Bcl-2 and Bcl-XL were decreased and Bax, Caspase-3, and Caspase-9 expression were increased in C918 cells in a dose-dependent manner. Therefore, it was explained that PTL induced the apoptosis of C918 cells by regulating mitochondrial pathway.
In conclusion, PTL reduced the proliferation of human UM cells (C918 and SP6.5), and the reduction was more noticeable in C918 cells than in SP6.5 cells, however, PTL did not affect normal cells. PTL inhibited proliferation and induced apoptosis of C918 cells by arresting G1 phase and regulating mitochondrial pathway. Note that this conclusion still requires further investigation in vivo.
Acknowledgments
Foundation: Supported by the Health Special Project of Jilin Province Department of Finance (No.3D5177883429).
Conflicts of Interest: Che ST, None; Bie L, None; Li X, None; Qi H, None; Yu P, None; Zuo L, None.
REFERENCES
- 1.Kaliki S, Shields CL. Uveal melanoma: relatively rare but deadly cancer. Eye (Lond) 2017;31(2):241–257. doi: 10.1038/eye.2016.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang YC, Yang X, Wei WB, Xu XL. Role of microRNA-21 in uveal melanoma cell invasion and metastasis by regulating p53 and its downstream protein. Int J Ophthalmol. 2018;11(8):1258–1268. doi: 10.18240/ijo.2018.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu DN, Yu GP, McCormick SA, Schneider S, Finger PT. Population-based incidence of uveal melanoma in various races and ethnic groups. Am J Ophthalmol. 2005;140(4):612–617. doi: 10.1016/j.ajo.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Ophthalmic Oncology Task Force. Local recurrence significantly increases the risk of metastatic uveal melanoma. Ophthalmology. 2016;123(1):86–91. doi: 10.1016/j.ophtha.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Damato B. Detection of uveal melanoma by optometrists in the United Kingdom. Ophthalmic Physiol Opt. 2001;21(4):268–271. doi: 10.1046/j.1475-1313.2001.00595.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Shin SJ, Heo SJ, Choe EA, Kim CG, Jung M, Keum KC, Yoon JS, Lee SC, Shin SJ. Prognoses and clinical outcomes of primary and recurrent uveal melanoma. Cancer Res Treat. 2018;50(4):1238–1251. doi: 10.4143/crt.2017.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Shabat I, Belgrano V, Ny L, Nilsson J, Lindnér P, Olofsson Bagge R. Long-term follow-up evaluation of 68 patients with uveal melanoma liver metastases treated with isolated hepatic perfusion. Ann Surg Oncol. 2016;23(4):1327–1334. doi: 10.1245/s10434-015-4982-5. [DOI] [PubMed] [Google Scholar]
- 8.Lane AM, Kim IK, Gragoudas ES. Survival rates in patients after treatment for metastasis from uveal melanoma. JAMA Ophthalmol. 2018;136(9):981–986. doi: 10.1001/jamaophthalmol.2018.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(11):4651–4659. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- 10.Augsburger JJ, Corrêa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol. 2009;148(1):119–127. doi: 10.1016/j.ajo.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109(7):3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 12.Kingston DG. Modern natural products drug discovery and its relevance to biodiversity conservation. J Nat Prod. 2011;74(3):496–511. doi: 10.1021/np100550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li CJ, Guo SF, Shi TM. Culture supernatants of breast cancer cell line MDA-MB-231 treated with parthenolide inhibit the proliferation, migration, and lumen formation capacity of human umbilical vein endothelial cells. Chin Med J. 2012;125(12):2195–2199. [PubMed] [Google Scholar]
- 14.Shanmugam R, Kusumanchi P, Cheng L, Crooks P, Neelakantan S, Matthews W, Nakshatri H, Sweeney CJ. A water-soluble parthenolide analogue suppresses in vivo prostate cancer growth by targeting NFkappaB and generating reactive oxygen species. Prostate. 2010;70(10):1074–1086. doi: 10.1002/pros.21141. [DOI] [PubMed] [Google Scholar]
- 15.Wyrębska A, Gach K, Szemraj J, Szewczyk K, Hrabec E, Koszuk J, Janecki T, Janecka A. Comparison of anti-invasive activity of parthenolide and 3-isopropyl-2-methyl-4-methyleneisoxazolidin-5-one (MZ-6): a new compound with α-methylene-γ-lactone motif: on two breast cancer cell lines. Chem Biol Drug Des. 2012;79(1):112–120. doi: 10.1111/j.1747-0285.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- 16.Kishida Y, Yoshikawa H, Myoui A. Parthenolide, a natural inhibitor of nuclear factor-kappaB, inhibits lung colonization of murine osteosarcoma cells. Clin Cancer Res. 2007;13(1):59–67. doi: 10.1158/1078-0432.CCR-06-1559. [DOI] [PubMed] [Google Scholar]
- 17.Carlisi D, de Blasio A, Drago-Ferrante R, di Fiore R, Buttitta G, Morreale M, Scerri C, Vento R, Tesoriere G. Parthenolide prevents resistance of MDA-MB231 cells to doxorubicin and mitoxantrone: the role of Nrf2. Cell Death Discov. 2017;3:17078. doi: 10.1038/cddiscovery.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czyz M, Lesiak-Mieczkowska K, Koprowska K, Szulawska-Mroczek A, Wozniak M. Cell context-dependent activities of parthenolide in primary and metastatic melanoma cells. Br J Pharmacol. 2010;160(5):1144–1157. doi: 10.1111/j.1476-5381.2010.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holcomb BK, Yip-Schneider MT, Waters JA, Beane JD, Crooks PA, Schmidt CM. Dimethylamino parthenolide enhances the inhibitory effects of gemcitabine in human pancreatic cancer cells. J Gastrointest Surg. 2012;16(7):1333–1340. doi: 10.1007/s11605-012-1913-7. [DOI] [PubMed] [Google Scholar]
- 20.Cheng G, Xie L. Parthenolide induces apoptosis and cell cycle arrest of human 5637 bladder cancer cells in vitro. Molecules. 2011;16(8):6758–6768. doi: 10.3390/molecules16086758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YR, Eom JI, Kim SJ, Jeung HK, Cheong JW, Kim JS, Min YH. Myeloperoxidase expression as a potential determinant of parthenolide-induced apoptosis in leukemia bulk and leukemia stem cells. J Pharmacol Exp Ther. 2010;335(2):389–400. doi: 10.1124/jpet.110.169367. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz D, Bloom D, Castro R, Pagán OR, Jiménez-Rivera CA. Parthenolide blocks cocaine's effect on spontaneous firing activity of dopaminergic neurons in the ventral tegmental area. Curr Neuropharmacol. 2011;9(1):17–20. doi: 10.2174/157015911795017010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang WJ, Meng ZL, Mo YC, Liu JW, Sun CC, Hu SS, Zhang H. Unloading the infarcted heart affect MMPs-TIMPs axis in a rat cardiac heterotopic transplantation model. Mol Biol Rep. 2012;39(1):277–283. doi: 10.1007/s11033-011-0736-z. [DOI] [PubMed] [Google Scholar]
- 24.Yun BR, Lee MJ, Kim JH, Kim IH, Yu GR, Kim DG. Enhancement of parthenolide-induced apoptosis by a PKC-alpha inhibition through heme oxygenase-1 blockage in cholangiocarcinoma cells. Exp Mol Med. 2010;42(11):787–797. doi: 10.3858/emm.2010.42.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin X, Zhou J, Zhang Z, Lv H. The combined administration of parthenolide and ginsenoside CK in long circulation liposomes with targeted tLyp-1 ligand induce mitochondria-mediated lung cancer apoptosis. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S931–S942. doi: 10.1080/21691401.2018.1518913. [DOI] [PubMed] [Google Scholar]
- 26.Talib WH, Al Kury LT. Parthenolide inhibits tumor-promoting effects of nicotine in lung cancer by inducing P53-dependent apoptosis and inhibiting VEGF expression. Biomed Pharmacother. 2018;107:1488–1495. doi: 10.1016/j.biopha.2018.08.139. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Lu H, Lv M, Wang Q, Sun Y. Parthenolide facilitates apoptosis and reverses drug-resistance of human gastric carcinoma cells by inhibiting the STAT3 signaling pathway. Oncol Lett. 2018;15(3):3572–3579. doi: 10.3892/ol.2018.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto T, Tanikawa C, Yodsurang V, Zhang YZ, Imoto S, Yamaguchi R, Miyano S, Nakagawa H, Matsuda K. Identification of a p53-repressed gene module in breast cancer cells. Oncotarget. 2017;8(34):55821–55836. doi: 10.18632/oncotarget.19608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai M, Al-Odaini AA, Fils-Aimé N, Villatoro MA, Guo J, Arakelian A, Rabbani SA, Ali S, Lebrun JJ. Cyclin D1 cooperates with p21 to regulate TGFβ-mediated breast cancer cell migration and tumor local invasion. Breast Cancer Res. 2013;15(3):R49. doi: 10.1186/bcr3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao C, Cao X, Fu Z, Tian J, Dong W, Xu J, An K, Zhai L, Yu J. Boschniakia rossica polysaccharide triggers laryngeal carcinoma cell apoptosis by regulating expression of Bcl-2, Caspase-3, and P53. Med Sci Monit. 2017;23:2059–2064. doi: 10.12659/MSM.901381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Bhola PD, Letai A. Mitochondria-judges and executioners of cell death sentences. Mol Cell. 2016;61(5):695–704. doi: 10.1016/j.molcel.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabbagh L, Kaech SM, Bourbonnière M, Woo M, Cohen LY, Haddad EK, Labrecque N, Ahmed R, Sékaly RP. The selective increase in caspase-3 expression in effector but not memory T cells allows susceptibility to apoptosis. J Immunol. 2004;173(9):5425–5433. doi: 10.4049/jimmunol.173.9.5425. [DOI] [PubMed] [Google Scholar]
- 33.Daniels KJ, Boldt HC, Martin JA, Gardner LM, Meyer M, Folberg R. Expression of type VI collagen in uveal melanoma: its role in pattern formation and tumor progression. Lab Invest. 1996;75(1):55–66. [PubMed] [Google Scholar]
- 34.Soulieres D, Rousseau A, Deschenes J, Tremblay M, Tardif M, Pelletier G. Characterization of gangliosides in human uveal melanoma cells. Int J Cancer. 1991;49(4):498–503. doi: 10.1002/ijc.2910490404. [DOI] [PubMed] [Google Scholar]
- 35.Mathema VB, Koh YS, Thakuri BC, Sillanpää M. Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation. 2012;35(2):560–565. doi: 10.1007/s10753-011-9346-0. [DOI] [PubMed] [Google Scholar]
- 36.Sun J, Zhang C, Bao YL, Wu Y, Chen ZL, Yu CL, Huang YX, Sun Y, Zheng LH, Wang X, Li YX. Parthenolide-induced apoptosis, autophagy and suppression of proliferation in HepG2 cells. Asian Pac J Cancer Prev. 2014;15(12):4897–4902. doi: 10.7314/apjcp.2014.15.12.4897. [DOI] [PubMed] [Google Scholar]
- 37.Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med. 2016;94(12):1313–1326. doi: 10.1007/s00109-016-1475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortiz AB, Garcia D, Vicente Y, Palka M, Bellas C, Martin P. Prognostic significance of cyclin D1 protein expression and gene amplification in invasive breast carcinoma. PLoS One. 2017;12(11):e0188068. doi: 10.1371/journal.pone.0188068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiao X, Gan M, Wang C, Liu B, Shang Y, Li Y, Chen S. Tetracenomycin X exerts antitumour activity in lung cancer cells through the downregulation of cyclin D1. Mar Drugs. 2019;17(1):E63. doi: 10.3390/md17010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung YS, Qian Y, Chen X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal. 2010;22(7):1003–1012. doi: 10.1016/j.cellsig.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, Wang Z, Jin S, Hao H, Zheng L, Zhou B, Zhang W, Lv H, Yuan Y. Dux4 induces cell cycle arrest at G1 phase through upregulation of p21 expression. Biochem Biophys Res Commun. 2014;446(1):235–240. doi: 10.1016/j.bbrc.2014.02.105. [DOI] [PubMed] [Google Scholar]
- 42.Zhao L, Li J, Sun ZB, Sun C, Yu ZH, Guo X. Saikosaponin D inhibits proliferation of human osteosarcoma cells via the p53 signaling pathway. Exp Ther Med. 2019;17(1):488–494. doi: 10.3892/etm.2018.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishii K, Sasaki T, Iguchi K, Kato M, Kanda H, Hirokawa Y, Arima K, Watanabe M, Sugimura Y. Pirfenidone, an anti-fibrotic drug, suppresses the growth of human prostate cancer cells by inducing G1 cell cycle arrest. J Clin Med. 2019;8(1):pii: E44. doi: 10.3390/jcm8010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Lv B, Kong L, Xia J, Zhu M, Hu L, Zhen D, Wu Y, Jia X, Zhu S, Cui H. Nova1 mediates resistance of rat pheochromocytoma cells to hypoxia-induced apoptosis via the Bax/Bcl-2/caspase-3 pathway. Int J Mol Med. 2017;40(4):1125–1133. doi: 10.3892/ijmm.2017.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo LJ, Wu ZY, Zhang S, Zheng JL, Zheng HL. Effect of bcl-2 antisense oligonucleotides on multidrug resistance of cultured uveal melanoma cells. Zhonghua Yan Ke Za Zhi. 2003;39(2):73–76. [PubMed] [Google Scholar]
- 46.Chen MJ, Yang PY, Ye YZ, Hu DN, Chen MF. Arsenic trioxide induces apoptosis in uveal melanoma cells through the mitochondrial pathway. Am J Chin Med. 2010;38(6):1131–1142. doi: 10.1142/S0192415X10008524. [DOI] [PubMed] [Google Scholar]
- 47.Kemeny-Beke A, Berenyi E, Facsko A, Damjanovich J, Horvath A, Bodnar A, Berta A, Aradi J. Antiproliferative effect of 4-thiouridylate on OCM-1 uveal melanoma cells. Eur J Ophthalmol. 2006;16(5):680–685. [PubMed] [Google Scholar]