Abstract
AIM
To compare the effectiveness of postoperative adjunctive use of subconjunctival bevacizumab in altering the outcome of primary trabeculectomy in terms of sustained lowering of intraocular pressure (IOP) and reduction of postoperative bleb vascularization and fibrosis.
METHODS
A prospective, one center, randomized, placebo-control study. Fifty-nine patients (59 eyes) with uncontrolled IOP under maximal tolerated medical treatment (MTMT) were recruited. A primary trabeculectomy with mitomycin C (MMC) was done and the patients were randomized to either postoperative subconjunctival injection of bevacizumab (1.25 mg/0.05 mL) or balanced salt solution (BSS). Forty-seven patients (47 eyes) completed at least one year of follow up and were included in the study. The main outcome measure was the IOP, and secondary outcome measures include bleb morphology, vascularization, and fibrosis, as well as the need for glaucoma medications and 5-fluorouracil (5-FU) needling.
RESULTS
At 1-year follow up, there was no significant difference between groups for IOP (P=0.65), bleb morphology (P=0.65), and the need for glaucoma medications (P=0.65) or 5-FU needling requirements (P=0.11). However, the bevacizumab group had a higher rate of success results, lower use of glaucoma medications after surgery, and optimal bleb aspect in more patients, but more 5-FU needling procedures required.
CONCLUSION
A bigger sample size is needed in order to determine whether the differences found in the bevacizumab group are statistically significant.
Keywords: glaucoma, trabeculectomy, bevacizumab, bleb
INTRODUCTION
Glaucoma, remains a significant public health problem as a leading cause of irreversible visual loss[1]. In a recent systematic review and Meta-analysis it has been estimated that the overall global prevalence of glaucoma was 3.54%, with the highest prevalence in Africa, and that the number of people with glaucoma worldwide (aged 40-80y) will increase from 64.3 million in the year 2013 to 111.8 million in 2040, disproportionally affecting people residing in both Asia and Africa[2]. The single most effective therapeutic option currently availableaims at lowering the intraocular pressure (IOP) by medical, laser, or surgical techniques[3]. Glaucoma surgery is sought when further IOP lowering is required despite the use of maximal tolerable medical and indicated laser therapy. Trabeculectomy with intra-operative use of mitomycin C (MMC) is the current standard form of surgery performed. Trabeculectomy aims to create a functioning aqueous drainage channel into a filtering bleb from which aqueous subsequently flows transconjunctivally or is absorbed into the episcleral and subconjunctival capillaries. The success of bleb formation is depends on preventing wound scarring. The events of wound healing after trabeculectomy are mediated by both fibroblast activity and angiogenesis[4], which leads to increased vascular permeability, vascularization and fibrosis. The result of injury-mediated factors is conjunctival and episcleral fibrosis allowing the closure of the drainage space leading to premature surgery failure[5]. The ability to modulate and reduce these factors in the immediate postoperative period could lead to better long-term bleb sustainability. Currently, adjunctive medications which modulate wound healing such as anti-metabolites like 5-fluorouracil (5-FU) and anti-proliferative agents such as MMC target fibroblast activity and are effective in curbing the fibrosis process[6]–[9]. However, excessive inhibition of wound healing noted in antimetabolite-augmented trabeculectomy is frequently linked to complications such as postoperative infections, hypotony, corneal toxicity, and thin-walled avascular blebs, which are susceptible to leakage[10]–[11]. As a result, methods to tone down the wound-healing response with safer medications are under investigation.
Recent advances in ophthalmic wound healing research, including vascular endothelial growth factor (VEGF) inhibitors, antibodies, RNAi, gene therapy, nanoparticles, liposomes, dendrimers, proteoglycans and small molecule inhibitors are under development[12]. VEGF plays a central role in early phases of wound healing, including angiogenesis and vascular maintenance. Increased vascular permeability along with increased VEGF concentration has been shown to occur during the early phases of wound repair, allowing deposition of the fibrin matrix necessary for cellular migration. VEGF is produced or released locally in surgical wounds and its neutralization decreases angiogenesis and endothelial cell chemotaxis[13]–[14].
In light of this understanding of the wound healing process, the adjunctive use of VEGF inhibitors has been recently attempted in trabeculectomy. It has been postulated that the use of these selective wound modulators may enhance surgical efficacy and, at the same time, offer a more favorable outcome in regards of success and safety[15]–[17]. Bevacizumab (Avastin; Genetech, Inc,. South San Francisco, CA, USA) is a monoclonal full length recombinant antibody that binds all isoforms of VEGF. It is FDA approved for metastatic colorectal cancer treatment but has also been used for non-small cell lung cancer and breast cancer[18]. It is commonly used in ophthalmology off-label for the treatment of neovascular age related macular degeneration, but its use has also been extensively reported in the treatment of proliferative diabetic retinopathy, cystoid macular edema, and neovascular glaucoma[19]–[21].
Our hypothesis is that adjunctive subconjunctival single use of bevacizumab in the early postoperative period following trabeculectomy with MMC can modulate the wound healing and promote healthy filtering bleb formation. At the same time we postulate that adjunctive use of bevacizumab could potentially decrease long-term risk of bleb failure and hence result in sustained lower IOP.
The present study compares the efficacy of a single, postoperative subconjunctival bevacizumab injection versus placebo, in patients with different types of glaucoma that underwent a primary trabeculectomy with MMC.
SUBJECTS AND METHODS
Ethical Approval
This study was registered at the Clinical Trials Registry in July, 2010 (NCT01166594). This is a one center, prospective, randomized, placebo-controlled study, which was conducted in compliance with the tenets of Declaration of Helsinki and after approval of the Research Ethics Board of Sunnybrook Health Sciences Centre. Signed informed consent was obtained from all patients.
Between June 2010 and September 2013, 59 patients diagnosed with glaucoma and uncontrolled IOP, receiving maximal tolerated medical therapy (MTMT), and requiring a primary trabeculectomy, were recruited in the Glaucoma Clinic, Sunnybrook Health Sciences Centre, Toronto, Canada. Inclusion criteria: age more than 18 years old; inadequately controlled glaucoma on MTMT requiring a primary trabeculectomy; decision makers fluent in English and able to read and understand the consent form; no known contraindication for use of anti-VEGFs. Exclusion criteria: active intraocular inflammation/uveitis, neovascular glaucoma or aphakia; history of previous trabeculectomy surgery or prior retinal detachment with scleral buckle; pregnant and nursing women. Fifty-nine patients underwent a standard trabeculectomy with MMC 0.2 mg/mL and were randomized by use of a random number table to receive a single subconjunctival dose of bevacizumab 1.25 mg/0.05 mL (group A) or balanced salt solution (BSS) 0.05 mL (group B) between the first to fifth day after the surgery.
The age, sex, race, type of glaucoma, preoperative best corrected visual acuity (BCVA), IOP measured by calibrated Goldman applanation tonometer, central corneal thickness (CCT), number of glaucoma medications, number of previous laser trabeculoplasty treatments, previous cataract surgery, visual field mean deviation, pattern standard deviation and mean ocular coherence tomography (OCT) retinal nerve fiber layer (RNFL) thickness were recorded before surgery.
A standard fornix based trabeculectomy was performed by a single surgeon (Birt C) at Sunnybrook Health Sciences Centre between July 2010 and January 2013. The MMC exposure time varied between 60 and 150s depending on surgeon's judgment of the individual patient's risk factors (these primarily included age, race, preoperative and target IOP, use of topical medications and previous anterior segment surgery). A square 4×4 mm2 scleral flap and iridectomy was performed in all patients. The flap was secured with four 10-0 nylon sutures and the conjunctiva was closed using an 8-0 vicryl running suture. During the first to fifth postoperative day, patients were examined and in the absence of any contraindication including infection, bleb leak, hypotony, elevated IOP, uveitis or hyphema, the study nurse prepared a syringe containing the assigned treatment for the patient, either bevacizumab or BSS. In each case it was injected subconjunctivally in the normal fashion as for a bleb needling procedure using a 30 gauge needle and aseptic technique.
All patients received prednisolone acetate 1% drops, 10 times per day during the first week and approximately 6 times per day during the second week with slow taper for the next two months postoperatively. A fourth generation fluoroquinolone was also used 4 times per day for the first two weeks after surgery. Laser suture lysis was performed at the discretion of the treating physician.
On postoperative days 1, 7, 14, 30, 60, 90, 180 and 365, the IOP, BCVA, number of glaucoma medications and complications were recorded. The bleb appearance was formally graded according to the Indiana Bleb-Grading Scale (IBGS) at each visit[22]. This scale assesses bleb height, bleb extension, grade of vascularity and leak presence or absence. The use of 5-FU needling for additional wound modulation was also recorded. A window of ±7d was allowed for the 30, 60 and 90d visits and a range of ±14d was allowed for the 180 and 365d visits.
Success, Qualified Success, and Failure Criteria
Success was defined as postoperative IOP<18 mm Hg and a 20% IOP decrease from baseline without use of postoperative hypotensive drops, no need of 5-FU bleb needling later than 6wk after surgery and no laser trabeculoplasty needed post trabeculectomy. Qualified success was defined as a violation of any of above success criteria. The surgical failure category consisted of patients who received further incisional surgery, trans-scleral YAG laser treatment or who had chronic hypotony (IOP less than 5 mm Hg) with vision loss or hypotony maculopathy[23].
Statistical Analysis
Past studies measuring IOP post trabeculectomy result in average standard deviation of 4 mm Hg. Less than 3 mm Hg IOP difference is considered insignificant. We calculated a need for 30 patients in each group to show a difference in IOP of over 3 mm Hg (considering a P value <0.05 statistically significant) with a 95% confidence interval. Comparison between treatment groups was performed using the Student's t test for continuous variables. Categorical variables were compared using a continuity adjusted Chi-square test. Treatment comparisons using qualified success and failure definitions were assessed using the stratified Kaplan-Meier survival log-rank test. SPSS version 21.0 (Chicago, USA) was used.
RESULTS
Between June 2010 and September 2013, 59 patients were recruited. Randomization assigned 30 patients to the bevacizumab group and 29 patients to the placebo group. Five patients withdrew their consent after surgery, four patients were found to have had a violation of the inclusion criteria and three patients were lost to follow up. Forty-seven patients completed at least one year of follow up, 23 patients in group A (Avastin) and 24 patients in group B (BSS). The demographic characteristics of the study patients are summarized in Table 1.
Table 1. Demographics characteristics of the study patients.
| Parameters | Group A (n=23) | Group B (n=24) | P |
| Mean age (y) | 63.7±10.8 | 61.3±10.4 | 0.86 |
| Range | 36 to 85 | 29 to 76 | |
| Sex | |||
| Male | 9 (19.1) | 13 (27.7) | 0.36 |
| Female | 14 (29.8) | 11 (23.4) | |
| Race | |||
| White | 15 (31.9) | 17 (36.1) | 0.22 |
| Black | 6 (12.7) | 3 (6.3) | |
| Asian | 0 (0.0) | 3 (6.3) | |
| South Asian | 2 (4.2) | 1 (2.1) | |
| Type of glaucoma | |||
| POAG | 19 (40.4) | 11 (23.4) | 0.03 |
| PXFG | 2 (4.2) | 7 (14.9) | |
| Other | 2 (4.2) | 6 (12.8) |
n (%)
The groups were similar and comparable. No statistically significant differences were found at baseline other than a higher number of patients diagnosed with primary open angle glaucoma (POAG) present in the bevacizumab group.
Baseline clinical characteristics are shown in Table 2. No statistically significant differences were found between groups regarding age, visual acuity, IOP, CCT, number of glaucoma medications, 24-2 visual field parameters, OCT RNFL thickness, number of previous laser trabeculoplasty treatments, and the number of patients with previous cataract surgery.
Table 2. Baseline group comparison.
| Parameters | Group A (n=23) | Group B (n=24) | P |
| Mean age (y) | 63.7±10.8 | 61.3±10.4 | 0.86 |
| VA (logMAR) | 0.2±0.3 | 0.4±0.7 | 0.45 |
| IOP (mm Hg, range) | 23.2±7.2 (15-40) | 25.7±9.2 (14-44) | 0.26 |
| CCT | 540±38 | 552±39 | 0.30 |
| Meds (range) | 3.3±0.9 (1-5) | 3.5±1.0 (2-6) | 0.65 |
| MD (dB, range) | -10.3 (-0.9 to -24.5) | -14.9 (-2.1 to -30.5) | 0.34 |
| PSD (dB, range) | 7.53 (1.96-13.51) | 8.78 (1.18-13.44) | 0.25 |
| OCT RNFL thickness | 64.2±17.9 | 64.9±20.6 | 0.54 |
| Previous trabeculoplasty | |||
| No. of patients (%) | 6 (26) | 4 (16.6) | 0.67 |
| Previous cataract surgery | |||
| No. of patients (%) | 1 (4.3) | 2 (8.3) | 1.00 |
VA: Visual acuity; CCT: Central corneal thickness; Meds: No. of medications; MD: Mean deviation; PSD: Pattern standard deviation; OCT RNFL: Ocular coherence tomography retinal nerve fiber layer overall thickness.
mean±SD
The preoperative IOP was 23.2±7.2 mm Hg using 3.3±0.9 IOP-lowering medications in group A and 25.7±9.2 mm Hg using 3.5±1.0 IOP-lowering medications in group B. Postoperative IOP at 6-month follow-up was 12.9±4.2 mm Hg in group A and 13.2±6.8 mm Hg in group B. At 1-year follow-up the IOP was 13.8±4.5 mm Hg in group A using 0.4 IOP-lowering medications and 13.2±5.1 mmHg using 0.5 IOP-lowering medications in group B. No statistically significant differences were found between groups at 6mo (P=0.81) and 1-year follow-up (P=0.65). The bleb aspect was classified according to the IBGS in two groups, satisfactory versus non-satisfactory according to bleb height, extension, vascularity and leak presence. At 1-year follow-up 60.8% of the patients presented satisfactory blebs in group A and 50% of the patients in group B. This difference was not statistically significant (P=0.65).
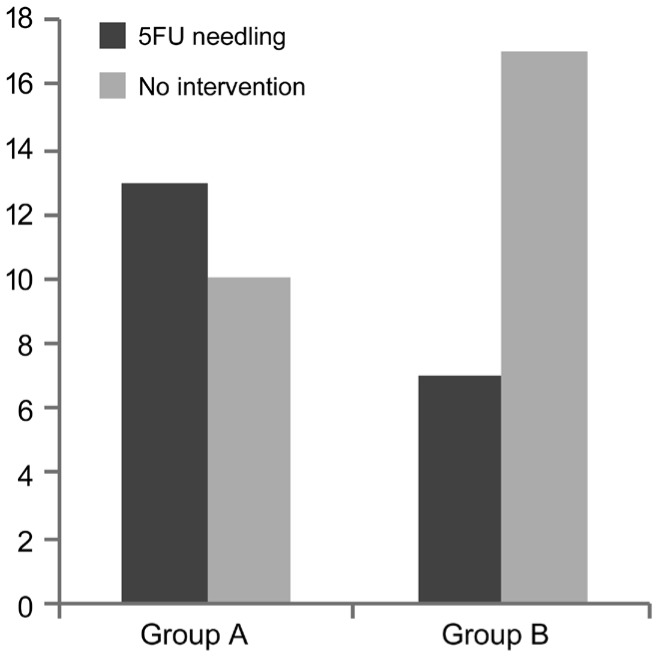
Both groups had a similar drop in number of glaucoma medications required (3.3 to 0.43 in group A, 3.54 to 0.58 in group B) and no statistically significant differences were found between groups. At 1-year follow-up 13 patients (56.5%) had required a 5-FU needling in group A and 7 patients (29.1%) in group B (P=0.10; Figure 1).
Figure 1. 5-FU Needling group comparison at 1-year follow-up.
Postoperative complications included bleb leaks, hypotony, choroidal detachments and others. During the first 2wk after surgery 8 patients (17%) presented with a bleb leak in group A and 7 patients (14.8%) in group B. It was resolved using one or more of the following techniques: 48h eye patch, bandage contact lens, additional suture to the wound, or full bleb revision. Only one patient (group B) presented a late bleb leak (1-year visit) that required a bleb revision. No statistically significant differences were found between groups comparing bleb leak rates (P=0.92). The 14 patients (29.8%) developed hypotony during the 1-year postoperative follow up in group A and 11 patients (23.4%) in group B (P=0.46). Six patients (12.8%) presented with choroidal detachments in group A and 4 patients (8.5%) in group B (P=0.67). Six patients (12.8%) developed other postoperative complications including 3 cases of anterior chamber shallowing that required reformation, 2 cases of hyphema and 1 case of iris blocking sclerostomy in group A. In group B, 7 patients (14.8%) presented with other complications including 2 cases of suprachoroidal hemorrhage, 1 case of branch retinal vein occlusion, 1 case of corneal abrasion, 1 case of anterior chamber shallowing that required reformation and 2 cases of hyphema (P=1.00; Table 3).
Table 3. Postoperative complications at 1-year follow up.
| Complications | Group A (n=23) | Group B (n=24) | P |
| Bleb leak | 8 (17) | 7 (14.8) | 0.92 |
| Hypotony | 14 (29.8) | 11 (23.4) | 0.46 |
| Choroidal detachment | 6 (12.8) | 4 (8.5) | 0.67 |
| Other | 6 (12.8) | 7 (14.8) | 1.00 |
n (%)
The preoperative BCVA was 0.2±0.3 in group A and 0.4±0.7 in group B, being at 1-year follow-up 0.66±1.8 logMAR for group A and 0.47±0.66 logMAR for group B (P=0.65).
The preoperative mean deviation in 24-2 Humphrey visual field was -10.3±7.21 in group A and -14.9±8.78 in group B. At 1-year follow-up it was -10.4 in group A and -13.5 in group B (P=0.32).
The mean OCT RNFL thickness was 64.2±17.9 in group A and 64.9±20.6 in group B. Non statistically significant differences were found at 1-year follow-up with a mean of 74.6±21.1 in group A and 70.9±16.1 in group B (P=0.60; Table 4).
Table 4. Group comparison at baseline and 1-year follow up.
| Baseline and 1-year group comparison | Group A (baseline) | Group B (baseline) | Group A (1-year) | Group B (1-year) | P (baseline to 1-year) |
| VA (logMAR) | 0.2±0.3 | 0.4±0.7 | 0.66±1.8 | 0.47±0.66 | 0.65 |
| IOP (mm Hg, range) | 23.2±7.2 (15-40) | 25.7±9.2(14-44) | 13.8±4.5 (8-24) | 13.2±5.1 (4-24) | 0.34 |
| Meds (range) | 3.3±0.9 (1-5) | 3.5±1.0 (2-6) | 0.4±1.1 (0-4) | 0.5±1.1 (0-5) | 0.65 |
| MD (dB, range) | -10.3 (-0.9 to -24.5) | -14.9 (-2.1 to -30.5) | -10.4 (-2.1 to -23.5) | -13.5 (-1.6 to -30.2) | 0.32 |
| PSD (dB, range) | 7.53 (1.96 to 13.51) | 8.78 (1.18 to 13.44) | 8.74 (1.27 to 19.01) | 7.89 (2.4 to 13.12) | 0.56 |
| OCT RNFL thickness | 64.2±17.9 | 64.9±20.6 | 74.6±21.1 | 70.9±16.1 | 0.60 |
mean±SD
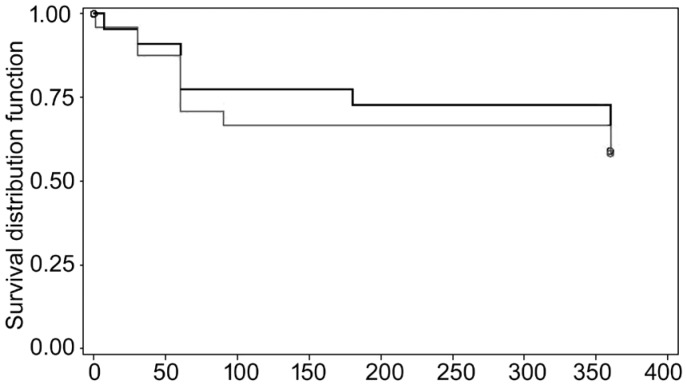
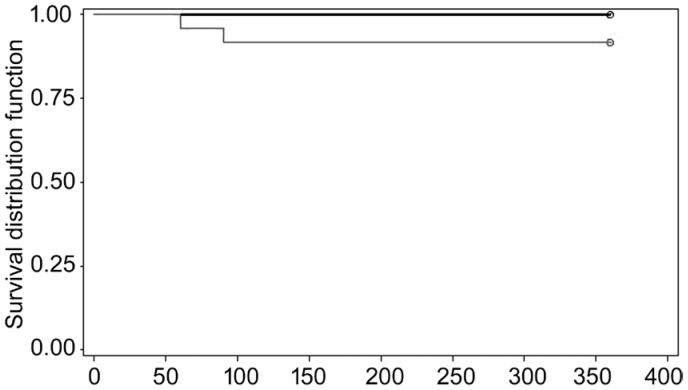
At 1-year follow-up in group A, 14 patients (29.8%) were considered a success and 9 patients (19.1%) a qualified success. No clinical failures were noticed in this group. In group B there was 12 cases of success (25.5%), 10 cases of qualified success (21.3%) and 2 cases of failure (4.2%). Both failure patients developed a suprachoroidal hemorrhage that required drainage and a pars plana vitrectomy. The first case developed late corneal decompensation and the second case developed chronic hypotony after vitrectomy. No significant differences were found between groups in terms of qualified success (P=0.87) or failure (P=0.16).
Kaplan-Meier survival analysis was used to compare the time to qualified success or to failure between the 2 treatment groups (Figures 2 and 3).
Figure 2. Kaplan Meyer survival curve for qualified success at 1-year follow-up.
Figure 3. Kaplan Meier survival curve for failure at 1-year follow-up.
DISCUSSION
Glaucoma filtration surgeries that function by draining aqueous humor to the sub-Tenon's space, including trabeculectomy, some varieties of nonpenetrating surgery, and glaucoma drainage devices, rely upon the external resistance created by wound healing to prevent hypotony. However, the sustainability of success of the surgical procedure depends on maintaining the patency of the fistula tract created during surgery. Failure of the procedure is usually the result of conjunctival scarring to the episclera leading to aqueous outflow restriction. Histologic studies have demonstrated that the utmost proliferation of subconjunctival fibroblasts happens between the third and fifth surgical day[19].
Pharmacologic enhancement of trabeculectomy using either 5-FU or MMC has improved rates of trabeculectomy success considerably. However, the nonspecific mechanism of action of those agents might lead to widespread cell death and thin-walled avascular blebs that are vulnerable to leak[20]–[21], infection[24]–[25], and dysaesthesia[26]. With lower levels of exposure, the frequency of bleb-related complications is less, however episcleral fibrosis and bleb failure is more likely to occur.
The search for selective wound modulators that have an acceptable risk profile and can replace the current antifibrotics that are notorious for their nonspecific inhibitory activity and continued risk of failure has been underway for the last few years. VEGF plays a pivotal role in both physiological and pathological angiogenesis throughout the body. Different VEGF isoforms may be responsible for different roles in the process of ocular wound healing. van Bergen et al[27] studied the presence of VEGF in trabeculectomy blebs and reported VEGF (165) and VEGF (121) to be mainly involved in angiogenesis, and VEGF (189) to be predominantly responsible for fibrous tissue proliferation. Lopilly Park et al[28] examined the levels of VEGF in both the aqueous humor and Tenon's tissue in eyes with POAG and compared their levels with the surgical outcome. They identified a significant correlation between the VEGF level in Tenon's tissue at the time of filtration surgery with the 1-year IOP and the final success of the surgery. Therefore by downgrading angiogenesis, resultant inflammation and collagen buildup, VEGF inhibition may have a favorable effect on the outcomes of filtration surgery[29]–[30].
In 2009, Li et al[31] reported a rise in aqueous humor VEGF protein concentration post trabeculectomy in rabbits, which was inhibited partially for up to 6d by a single subconjunctival and intracameral injection of bevacizumab at the time of surgery. No substantial advantage was observed in terms of IOP lowering up to 29d post trabeculectomy, however larger bleb area was documented using the Moorfields bleb classification in eyes receiving bevacizumab. One year later, Miyake et al[32] reported that intravitreal injection of 1.25 mg bevacizumab in 3 macaque monkey eyes resulted in deceased aqueous VEGF concentrations 1 to 28d after injection. They also noted that this decrease was maintained until 42d after injection. Memarzadeh et al[33] randomized 42 rabbits to trabeculectomy using a more intensive regimen of 7 subconjunctival injections of 1.25 mg bevacizumab, 5 mg 5-FU, or 0.1 mL BSS, given during the first 14 postoperative days. They documented no differences in mean IOP during the study period, but bevacizumab significantly improved bleb survival and resulted in less scarring in comparison with the 5-FU and BSS groups. Similarly, How et al[34] reported 100% bleb survival in the rabbit model at 28d when subconjunctival bevacizumab (2.5 mg) was combined with 5-FU (5 mg), 50% in the bevacizumab only group, 25% in the 5-FU-only group, and 0 in the control group (0.1 mL phosphate-buffered saline).
A limitation of animal studies of glaucoma filtration surgery in general was the difficulty in getting demonstrable IOP differences as primary outcomes, necessitating the use of bleb morphologic features instead.
Grewal et al[35] published a prospective, non-randomized small case series in 2008, analyzing the postoperative effect of a single subconjunctival dose of bevacizumab in patients who underwent a primary trabeculectomy without MMC or 5-FU. All eyes had an IOP reduction of 52% after 6mo. However, the authors noticed increases in bleb vascularity 3mo after surgery.
Kahook[36] published a prospective, randomized, case control study in 2010, comparing the use of a single intravitreal injection of bevacizumab versus placebo in 10 patients who had a primary trabeculectomy with MMC. At 6mo follow-up, there were no differences in terms of success or IOP, however, the authors mentioned more diffuse and less vascularized blebs in the bevacizumab group. In 2011, Sedghipour et al[37] published a prospective, randomized, placebo controlled trial comparing the use of single subconjunctival dose of bevacizumab (0.2 mg) vs placebo (BSS) in 37 patients with glaucoma that had a primary trabeculectomy without MMC, finding no differences between groups in terms of IOP after 3mo follow-up. During the same year, Ghanem[38] published a similar study including 55 patients comparing the single use of subconjunctival bevacizumab (1.25 mg/0.05 mL) versus placebo (BSS) in patients that had a primary trabeculectomy with MMC. Similar to the findings of Sedghipour et al[37], they didn't find differences in IOP or success rates at 1-year follow up, however the reduction in vascularity of the filtering bleb was statistically significant in the bevacizumab group. In 2014, Vandewalle et al[39] published a prospective, randomized, placebo controlled study comparing the use of a single dose of intracameral bevacizumab (1.25 mg/0.05 mL) versus placebo (BSS) in patients with open angle glaucoma scheduled for a primary trabeculectomy with or without MMC. A total of 138 patients were analyzed one year after surgery and no statistically significant differences in IOP were noticed between groups, however absolute success was reached in more patients in the bevacizumab group and also the need for needling was lower in this group. Kiddee et al[40] in October 2015, published a prospective, randomized, placebo-control study comparing the single use of subconjunctival bevacizumab (1.25 mg/0.05 mL) versus placebo (BSS) in patients with POAG that had a primary trabeculectomy with MMC. The authors analyzed a total of 39 patients, finding no statistically significant differences in IOP reduction or success rates between groups after 1-year follow up. The bleb vascularity score was significantly lower in bevacizumab during the first month but the difference was not sustained throughout the follow-up period.
In 2016, Fakhraie et al[41] published a prospective, randomized, placebo-controlled study using a different technique. They compared a single dose of intracameral bevacizumab (1.25 mg/0.05 mL) versus placebo (BSS) at the end of a primary trabeculectomy without MMC in patients with open angle glaucoma who had a primary trabeculectomy. The authors intentionally included only patients who were 65 years of age or more. Sixty-five patients completed a six months follow-up and were analyzed. They observed that bevacizumab significantly improved the short-term success rate in these patients and the rate of encapsulated bleb formation was clinically more significant in placebo group but it didn't reach a statistically significant difference. On the other hand, early filtering bleb leakage was significantly more common in the bevacizumab group. The authors concluded that bevacizumab could be as effective as MMC or 5-FU preventing bleb failure in primary trabeculectomy; however bleb morphology was not analyzed and long term results have not been published so far. On the other hand, previous studies that compared the use of 5-FU or MMC to subconjunctival bevacizumab in primary trabeculectomy found lower rates of success when bevacizumab was used as single agent[42]–[44].
Our study is, so far, the largest prospective, randomized, placebo-control study published comparing the adjunctive use of a standard dose of subconjunctival bevacizumab (1.25 mg/0.5 mL) in primary trabeculectomy surgery using MMC. Previous prospective and randomized studies analyzed smaller groups of patients[40], included refractory glaucomas [neovascular, post-penetrating keratoplasty (PKP), uveitis, post-vitrectomy] tested lower dose of bevacizumab[37], or used different routes of administration[39]–[41].
In our study no statistically significant differences were found at 1-year follow up in terms of IOP between bevacizumab and control group. The bleb aspect was optimal in more patients in the bevacizumab group, however this was not statistically significant and not related to a lower intraocular pressure at 1 year-follow-up. Although the bevacizumab group had a tendency to have more successful results and a lower use of glaucoma medications after surgery, this group of patients also required more 5-FU needling procedures. None of these differences, however, were statistically significant.
The fact that we didn't find any statistically significant difference between groups despite the tendency to get a better quality blebs and more success results in the bevacizumab group could be explained by our sample size. This study was originally powered for 60 subjects for a 95% of significance level. Unfortunately although 59 patients were randomized, for various reasons only 47 patients completed the follow up and were analyzed. The lower power obtained due to the loss of subjects is a weakness of our paper. A bigger sample size that fulfilled the requirements for a fully powered study is needed in order to determine whether the differences found between groups are in fact statistically significant.
The fact that several other researchers have reported similar results with little difference between treatment and placebo groups, however, suggests the possibility that the BSS injected subconjunctivally for placebo group may have an active effect on surgery outcomes. The saline bolus may help in bleb formation, either by breaking fibrin adhesions and/or diluting adverse growth factors within the bleb. We did not include a sham injection control group, but this might be a good idea for future studies.
Furthermore, the pharmacokinetics of subconjunctival bevacizumab have not been well studied[45]. A single dose of 1.25 mg intravitreal bevacizumab is likely to result in complete intravitreal VEGF blockade for 4 to 6wk[19],[46]. The fact that no statistically significant differences between the bevacizumab and placebo groups were found with a single dose of sub-conjunctival bevacizumab, may justify future trials using repeated injections of bevacizumab or a comparison of bevacizumab vs 5-FU in patients with severely vascularized blebs in the early postoperative period after trabeculectomy.
Of importance, the optimal route of administration and dosing frequency are still undetermined for bevacizumab[45]–[49]. Surprisingly, results from animal studies suggest that there is not a major advantage for intravitreal use over subconjunctival. Intravitreal administration reaches higher concentrations inside the eye, although there is some evidence that subconjunctival injection may result in high tissue levels for periods as long as those associated with intravitreal injection[45].
The utilization of bevacizumab in trabeculectomy is an off-label treatment, and several issues need to be addressed, such as the best administration route (intravitreal, anterior chamber or subconjunctival), duration of action, dosage and toxicity. In this study, the complication rate was comparable in both groups for bleb leak, hypotony and choroidal detachments, and no systemic side effects were reported. The goal of modulating wound healing to provide safe and effective IOP control in our surgical patients' remains highly desirable, and anti-VEGF antibody treatment, such as with bevacizumab continues to be a possible addition to our armamentarium in this regard. Further work exploring the options available for treatment is indicated.
Acknowledgments
This study was presented as a poster at the World Glaucoma Congress 2019.
This study was presented as an abstract at ARVO annual meeting in April, 2014.
Foundation: Supported by the Glaucoma Research Society of Canada.
Conflicts of Interest: Muhsen S, None; Compan J, None; Lai T, None; Kranemann C, None; Birt C, None.
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien C, Schwartz B, Takamoto T, Wu DC. Intraocular pressure and the rate of visual field loss in chronic open-angle glaucoma. Am J Ophthalmol. 1991;111(4):491–500. doi: 10.1016/s0002-9394(14)72386-4. [DOI] [PubMed] [Google Scholar]
- 4.Khaw PT, Occleston NL, Schultz G, Grierson I, Sherwood MB, Larkin G. Activation and suppression of fibroblast function. Eye (Lond) 1994;8(Pt 2):188–195. doi: 10.1038/eye.1994.44. [DOI] [PubMed] [Google Scholar]
- 5.Schlunck G, Meyer-ter-Vehn T, Klink T, Grehn F. Conjunctival fibrosis following filtering glaucoma surgery. Exp Eye Res. 2016;142:76–82. doi: 10.1016/j.exer.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Fontana H, Nouri-Mahdavi K, Lumba J, Ralli M, Caprioli J. Trabeculectomy with mitomycin C: outcomes and risk factors for failure in phakic open-angle glaucoma. Ophthalmology. 2006;113(6):930–936. doi: 10.1016/j.ophtha.2006.01.062. [DOI] [PubMed] [Google Scholar]
- 7.Singh RP, Goldberg I, Mohsin M. The efficacy and safety of intraoperative and/or postoperative 5-fluorouracil in trabeculectomy and phacotrabeculectomy. Clin Exp Ophthalmol. 2001;29(5):296–302. doi: 10.1046/j.1442-9071.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 8.Cabourne E, Clarke JC, Schlottmann PG, Evans JR. Mitomycin C versus 5-Fluorouracil for wound healing in glaucoma surgery. Cochrane Database Syst Rev. 2015;(11):CD006259. doi: 10.1002/14651858.CD006259.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green E, Wilkins M, Bunce C, Wormald R. 5-Fluorouracil for glaucoma surgery. Cochrane Database Syst Rev. 2014;(2):CD001132. doi: 10.1002/14651858.CD001132.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jampel HD, Solus JF, Tracey PA, Gilbert DL, Loyd TL, Jefferys JL, Quigley HA. Outcomes and bleb-related complications of trabeculectomy. Ophthalmology. 2012;119(4):712–722. doi: 10.1016/j.ophtha.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 11.Saeedi OJ, Jefferys JL, Solus JF, Jampel HD, Quigley HA. Risk factors for adverse consequences of low intraocular pressure after trabeculectomy. J Glaucoma. 2014;23(1):e60–e68. doi: 10.1097/IJG.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 12.Yu-Wai-Man C, Khaw PT. Developing novel anti-fibrotic therapeutics to modulate post-surgical wound healing in glaucoma: big potential for small molecules. Expert Rev Ophthalmol. 2015;10(1):65–76. doi: 10.1586/17469899.2015.983475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152(6):1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 14.Kim M, Lee C, Payne R, Yue BY, Chang JH, Ying HY. Angiogenesis in glaucoma filtration surgery and neovascular glaucoma: a review. Surv Ophthalmol. 2015;60(6):524–535. doi: 10.1016/j.survophthal.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrés-Guerrero V, Perucho-González L, García-Feijoo J, Morales-Fernández L, Saenz-Francés F, Herrero-Vanrell R, Júlvez LP, Llorens VP, Martínez-De-la-casa JM, Konstas AGP. Current perspectives on the use of anti-VEGF drugs as adjuvant therapy in glaucoma. Adv Ther. 2017;34(2):378–395. doi: 10.1007/s12325-016-0461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozgonul C, Mumcuoglu T, Gunal A. The effect of bevacizumab on wound healing modulation in an experimental trabeculectomy model. Curr Eye Res. 2014;39(5):451–459. doi: 10.3109/02713683.2013.851704. [DOI] [PubMed] [Google Scholar]
- 17.Pro MJ, Freidl KB, Neylan CJ, Sawchyn AK, Wizov SS, Moster MR. Ranibizumab versus mitomycin C in primary trabeculectomy-a pilot study. Curr Eye Res. 2015;40(5):510–515. doi: 10.3109/02713683.2014.935441. [DOI] [PubMed] [Google Scholar]
- 18.Quiram PA, Hassan TS, Williams GA. Treatment of naïve lesions in neovascular age-related macular degeneration with pegaptanib. Retina. 2007;27(7):851–856. doi: 10.1097/IAE.0b013e31806458f0. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi S, Inoue M, Yamane S, Sakamaki K, Arakawa A, Kadonosono K. Long-term outcomes after preoperative intravitreal injection of bevacizumab before trabeculectomy for neovascular glaucoma. J Glaucoma. 2016;25(3):281–284. doi: 10.1097/IJG.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 20.Sun JT, Liang HJ, An M, Wang DB. Efficacy and safety of intravitreal ranibizumab with panretinal photocoagulation followed by trabeculectomy compared with Ahmed glaucoma valve implantation in neovascular glaucoma. Int J Ophthalmol. 2017;10(3):400–405. doi: 10.18240/IJO.2017.03.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun YY, Liang Y, Zhou P, Wu HJ, Hou XR, Ren ZQ, Li XX, Zhao MW. Anti-VEGF treatment is the key strategy for neovascular glaucoma management in the short term. BMC Ophthalmol. 2016;16(1):150. doi: 10.1186/s12886-016-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand N, Arora S, Clowes M. Mitomycin C augmented glaucoma surgery: evolution of filtering bleb avascularity, transconjunctival oozing, and leaks. Br J Ophthalmol. 2006;90(2):175–180. doi: 10.1136/bjo.2005.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC, Tube Versus Trabeculectomy Study Group Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–814.e1. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenfield DS, Suñer IJ, Miller MP, Kangas TA, Palmberg PF, Flynn HW., Jr Endophthalmitis after filtering surgery with mitomycin. Arch Ophthalmol. 1996;114(8):943–949. doi: 10.1001/archopht.1996.01100140151007. [DOI] [PubMed] [Google Scholar]
- 25.Higginbotham EJ, Stevens RK, Musch DC, Karp KO, Lichter PR, Bergstrom TJ, Skuta GL. Bleb-related endophthalmitis after trabeculectomy with mitomycin C. Ophthalmology. 1996;103(4):650–656. doi: 10.1016/s0161-6420(96)30639-8. [DOI] [PubMed] [Google Scholar]
- 26.Budenz DL, Hoffman K, Zacchei A. Glaucoma filtering bleb dysesthesia. Am J Ophthalmol. 2001;131(5):626–630. doi: 10.1016/s0002-9394(00)00901-6. [DOI] [PubMed] [Google Scholar]
- 27.van Bergen T, Vandewalle E, van de Veire S, Dewerchin M, Stassen JM, Moons L, Stalmans I. The role of different VEGF isoforms in scar formation after glaucoma filtration surgery. Exp Eye Res. 2011;93(5):689–699. doi: 10.1016/j.exer.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Lopilly Park HY, Kim JH, Ahn MD, Park CK. Level of vascular endothelial growth factor in tenon tissue and results of glaucoma surgery. Arch Ophthalmol. 2012;130(6):685–689. doi: 10.1001/archophthalmol.2011.2799. [DOI] [PubMed] [Google Scholar]
- 29.Cheng JW, Cheng SW, Wei RL, Lu GC. Anti-vascular endothelial growth factor for control of wound healing in glaucoma surgery. Cochrane Database Syst Rev. 2016;(1):CD009782. doi: 10.1002/14651858.CD009782.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan Gaskin JC, Nguyen DQ, Soon Ang G, O'Connor J, Crowston JG. Wound healing modulation in glaucoma filtration surgery-conventional practices and new perspectives: antivascular endothelial growth factor and novel agents (part II) J Curr Glaucoma Pract. 2014;8(2):46–53. doi: 10.5005/jp-journals-10008-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li ZQ, van Bergen T, van de Veire S, van de Vel I, Moreau H, Dewerchin M, Maudgal PC, Zeyen T, Spileers W, Moons L, Stalmans I. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2009;50(11):5217–5225. doi: 10.1167/iovs.08-2662. [DOI] [PubMed] [Google Scholar]
- 32.Miyake T, Sawada O, Kakinoki M, Sawada T, Kawamura H, Ogasawara K, Ohji M. Pharmacokinetics of bevacizumab and its effect on vascular endothelial growth factor after intravitreal injection of bevacizumab in macaque eyes. Invest Ophthalmol Vis Sci. 2010;51(3):1606–1608. doi: 10.1167/iovs.09-4140. [DOI] [PubMed] [Google Scholar]
- 33.Memarzadeh F, Varma R, Lin LT, Parikh JG, Dustin L, Alcaraz A, Eliott D. Postoperative use of bevacizumab as an antifibrotic agent in glaucoma filtration surgery in the rabbit. Invest Ophthalmol Vis Sci. 2009;50(7):3233–3237. doi: 10.1167/iovs.08-2441. [DOI] [PubMed] [Google Scholar]
- 34.How A, Chua JL, Charlton A, Su R, Lim M, Kumar RS, Crowston JG, Wong TT. Combined treatment with bevacizumab and 5-fluorouracil attenuates the postoperative scarring response after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2010;51(2):928–932. doi: 10.1167/iovs.09-3949. [DOI] [PubMed] [Google Scholar]
- 35.Grewal DS, Jain R, Kumar H, Grewal SP. Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy a pilot study. Ophthalmology. 2008;115(12):2141–2145.e2. doi: 10.1016/j.ophtha.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Kahook MY. Bleb morphology and vascularity after trabeculectomy with intravitreal ranibizumab: a pilot study. Am J Ophthalmol. 2010;150(3):399–403.e1. doi: 10.1016/j.ajo.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Sedghipour MR, Mostafaei A, Taghavi Y. Low-dose subconjunctival bevacizumab to augment trabeculectomy for glaucoma. Clin Ophthalmol. 2011;5:797–800. doi: 10.2147/OPTH.S17896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghanem AA. Trabeculectomy with or without intraoperative sub-conjunctival injection of bevacizumab in treating refractory glaucoma. J Clin Exp Ophthalmol. 2011;2(2):1–4. [Google Scholar]
- 39.Vandewalle E, Abegão Pinto L, van Bergen T, Spielberg L, Fieuws S, Moons L, Spileers W, Zeyen T, Stalmans I. Intracameral bevacizumab as an adjunct to trabeculectomy: a 1-year prospective, randomised study. Br J Ophthalmol. 2014;98(1):73–78. doi: 10.1136/bjophthalmol-2013-303966. [DOI] [PubMed] [Google Scholar]
- 40.Kiddee W, Orapiriyakul L, Kittigoonpaisan K, Tantisarasart T, Wangsupadilok B. Efficacy of adjunctive subconjunctival bevacizumab on the outcomes of primary trabeculectomy with mitomycin C: a prospective randomized placebo-controlled trial. J Glaucoma. 2015;24(8):600–606. doi: 10.1097/IJG.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fakhraie G, Ghadimi H, Eslami Y, Zarei R, Mohammadi M, Vahedian Z, Mafi M, Moghimi S. Short-term results of trabeculectomy using adjunctive intracameral bevacizumab: a randomized controlled trial. J Glaucoma. 2016;25(3):e182–e188. doi: 10.1097/IJG.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 42.Akkan JU, Cilsim S. Role of subconjunctival bevacizumab as an adjuvant to primary trabeculectomy: a prospective randomized comparative 1-year follow-up study. J Glaucoma. 2015;24(1):1–8. doi: 10.1097/IJG.0b013e318287abf3. [DOI] [PubMed] [Google Scholar]
- 43.Jurkowska-Dudzińska J, Kosior-Jarecka E, Zarnowski T. Comparison of the use of 5-fluorouracil and bevacizumab in primary trabeculectomy: results at 1 year. Clin Exp Ophthalmol. 2012;40(4):e135–e142. doi: 10.1111/j.1442-9071.2011.02608.x. [DOI] [PubMed] [Google Scholar]
- 44.Nilforushan N, Yadgari M, Kish SK, Nassiri N. Subconjunctival bevacizumab versus mitomycin C adjunctive to trabeculectomy. Am J Ophthalmol. 2012;153(2):352–357.e1. doi: 10.1016/j.ajo.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Nomoto H, Shiraga F, Kuno N, Kimura E, Fujii S, Shinomiya K, Nugent AK, Hirooka K, Baba T. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci. 2009;50(10):4807–4813. doi: 10.1167/iovs.08-3148. [DOI] [PubMed] [Google Scholar]
- 46.Grisanti S, Biester S, Peters S, Tatar O, Ziemssen F, Bartz-Schmidt KU, Tuebingen Bevacizumab Study Group Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol. 2006;142(1):158–160. doi: 10.1016/j.ajo.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 47.Chalam KV, Gupta SK, Grover S, Brar VS, Agarwal S. Intracameral Avastin dramatically resolves iris neovascularization and reverses neovascular glaucoma. Eur J Ophthalmol. 2008;18(2):255–262. doi: 10.1177/112067210801800214. [DOI] [PubMed] [Google Scholar]
- 48.Paula JS, Secches DJ, Silva MJ, Rodrigues Mde L, Cunha Junior Ada S. Safety and feasibility of the use of a bevacizumab-methylcellulose mixture as an adjunct to glaucoma surgery: a pilot study. Arq Bras Oftalmol. 2015;78(3):194–196. doi: 10.5935/0004-2749.20150050. [DOI] [PubMed] [Google Scholar]
- 49.Slabaugh M, Salim S. Use of anti-VEGF agents in glaucoma surgery. J Ophthalmol. 2017;2017:1645269. doi: 10.1155/2017/1645269. [DOI] [PMC free article] [PubMed] [Google Scholar]