Abstract
Psychotic disorders are not uncommon in late life. These disorders often have varied etiologies, different clinical presentations, and are associated with significant morbidity and mortality among the older adult population. Psychotic disorders in late life develop due to the complex interaction between various biological, psychological, social, and environmental factors. Given the significant morbidity and mortality associated with psychotic disorders in late life, a comprehensive work-up should be conducted when they are encountered. The assessment should not only identify the potential etiologies for the psychotic disorders, but also recognize factors that predicts possible outcomes for these disorders. Treatment approaches for psychotic disorders in late life should include a combination of nonpharmacological management strategies with the judicious use of psychotropic medications. When antipsychotic medications are necessary, they should be used cautiously with the goal of optimizing outcomes with regular monitoring of their efficacy and adverse effects.
Keywords: delusional disorder, elderly, geriatric, late life, late-onset schizophrenia, psychotic disorders, schizoaffective disorder, schizophrenia, very late-onset schizophrenia-like psychosis
Introduction
Although a formal definition of ‘psychosis’ is not stated in either the DSM-5 or the ICD classification systems, psychotic features include the presence of delusions, hallucinations, disorganized thinking (speech), grossly disorganized motor behavior (including catatonia), or negative symptoms.1–3 When disorders are associated with psychotic features, they are termed psychotic disorders, for example, Schizophrenia Spectrum and Other Psychotic Disorders (DSM-5). The etiologies for psychosis in late life differs from psychosis in younger individuals, with a greater incidence of secondary causes for psychosis among older adults.4 In addition, psychosis in late life is associated with higher rates of morbidity and mortality when compared with psychosis among younger adults.5 Furthermore, the treatment of psychosis is complicated by the higher incidence of adverse effects when antipsychotic medications are prescribed to older adults when compared with younger adults.6
This paper intends to provide a comprehensive narrative review of the epidemiology, diagnostics, risks factors, and pathophysiology, as well as the treatment of psychotic disorders of late life. This review includes studies obtained through a literature search of the PubMed, MEDLINE, and Cochrane collaboration databases on 20 March 2019 using keywords related to each section about psychotic disorders in elderly patients (e.g. epidemiology of psychosis in late life, treatment of psychotic disorders in late life, psychosis in neurocognitive disorders, affective psychosis in late life, schizophrenia in late life, ICD-11 schizophrenia, etc.). The search was conducted by all authors, and if there were disagreements regarding the inclusion or exclusion of papers, a consensus was reached through discussion amongst all the authors. The authors included studies that they thought would be beneficial in educating practitioners about psychotic disorders in late life. The search was not restricted by the age of the participants. This review only included studies in human subjects published in English-language journals or those with official English translations. Studies that were included in this manuscript were not restricted by date of publication.
Epidemiology
Understanding the possible etiologies for psychotic disorders in late life is important as they can have significant implications in both the presentation and treatment for these conditions. The initial primary distinction to be made is between primary psychotic disorders and secondary psychotic disorders.7
Primary psychotic disorders comprise illnesses where the psychotic symptoms appear as part of the core symptoms of the disorder, and include conditions like schizophrenia spectrum illnesses (which can be further subdivided in to categories of late-onset (LOS) and very-late onset schizophrenia-like psychosis (VLOS), as well as psychosis as a symptom of affective disorders like major depressive disorder or bipolar disorder. Secondary psychotic disorders include psychosis as a symptom of another disorder including neurocognitive disorders, delirium, illicit substance use, prescribed medications, or other medical and neurological disorders.8,9 It is very important to identify the etiologies for psychosis among older adults as a majority of the cases (approximately 60%) of psychosis occur as a result of these disorders.8,10,11
Schizophrenia has a prevalence among older adults of approximately 0.1–0.5%,12,13 which contrasts with the overall lifetime prevalence of schizophrenia at about 1%. The relatively lower prevalence of schizophrenia among older adults can be accounted for by the increased premature mortality (due to various causes) among individuals with this illness.14,15 However, one should keep in mind that the cited prevalence of schizophrenia among older adults does not distinguish between younger adults with a diagnosis of schizophrenia who grew older versus incident cases of schizophrenia among older adults.
Schizophrenia can be further subdivided in to categories of early-onset (EOS, onset before age 40 years old), late-onset (LOS, onset at 40–60 years old), and very-late-onset schizophrenia-like psychosis (VLOS, onset after age 60).4 Approximately 75–80% of cases of schizophrenia are of early onset by this definition, with 20–25% of cases being incident in late or very-late life.16 The estimated incidence of schizophrenia after the age of 65 years is 7.5 per 100,000 person-years.17 In the largest cohort study to date investigating epidemiology of VLOS, Stafford and colleagues found a crude incidence rate of VLOS to be 37.66 per 100,000 person-years at-risk.18 They also found VLOS to have a preponderance of women (60%) with an accelerating incidence rate after age 80. There are notable differences in the symptomology and neuropsychiatric sequelae between EOS, LOS, and VLOS. It should be noted that risk of dementia has been shown to be increased in people with any history of schizophrenia.19,20 In fact, a recent meta-analysis found a significantly greater risk of developing dementia [relative risk (RR) 2.29; 95% confidence interval (CI) 1.35–3.88] in individuals with schizophrenia.21 Table 1 delineates the notable differences between each of these conditions.
Table 1.
| Features | EOS | LOS | VLOS |
|---|---|---|---|
| Age of onset | <40 years | 40–60 years | >60 years |
| Female preponderance | No | Yes | Definitely |
| Negative symptoms | Definitely | May be | Less likely |
| Learning | Ok | Ok | Impaired |
| Retention | Ok | Ok | Impaired |
| Progressive cognitive deterioration | Yes | Yes | Yes (very high) |
| Brain structural abnormalities | No | No | Yes |
| Family history | Present | Present | Weak association |
| Early childhood maladjustments | Present | Present | Absent |
| Antipsychotic dosing | Higher | Lower | Lower |
| Risk of Tardive Dyskinesia | Present | Present | Very high |
EOS, early onset schizophrenia; LOS, late onset schizophrenia; VLOS, very late onset schizophrenia like psychosis.
A study by Meesters and colleagues estimated the 12-month prevalence of schizoaffective disorder among adults ⩾60 years to be at 0.14%.24 Older adults with schizoaffective disorder appear to have greater severity of illness with worse outcomes, including greater treatment resistance and risk for suicide.25 Older adults with schizophrenia and schizoaffective disorders have similar mortality rates, negative symptom burden, and clinical global impression of impairment.26 In addition, older adults with schizophrenia or schizoaffective disorder are less likely to drive, are less likely to be married, and less likely to live independently compared with age-matched individuals with bipolar disorder. Also, individuals with the depressive-type of schizoaffective disorder more often had a history of suicide attempts and were more likely to be treated with an antidepressant medication compared with those with the bipolar-type illness.26
Data on delusional disorder among older adults is limited, but, typically, delusional disorder tends to occur in mid-to-late life (average age of onset being about 49 years old).27 The estimated prevalence of delusional disorder among older adults is 0.03%.28 From what is known about delusional disorder among older adults, it will typically cause social dysfunction, but otherwise these individuals appear to have normal cognitive, personal, and occupational functioning.15
Psychosis can also arise as a complication of affective disorders including both depression and bipolar disorder. The overall incidence of affective psychosis among older adults is estimated to be about 30.9 per 100,000 person-years.17 However, it is important to consider that psychotic depression actually becomes more common in later life, with an estimated average age of onset of 51.2 years of age and delusions estimated to occur in as high as 45% of elderly patients admitted to hospitals for reasons related to depression.29 It has also been observed that, among older adults with psychotic depression, there was a higher prevalence of comorbid anxiety and somatic symptoms.30
The 1-year prevalence rate of bipolar disorders among older adults is 0.1%, which is lower than the prevalence rates among adults aged 18–44 years (1.4%) and 45–64 years (0.4%).31 Much of the data reporting psychotic features in bipolar related illnesses among the elderly do not distinguish psychosis related to mania versus psychosis related to depression.32 The mean frequency of psychotic features in bipolar disorder among the elderly that was pooled from a review of five studies was found to be 64%.30 This frequency is actually similar to the frequency of psychotic symptoms seen among middle-aged adults with bipolar disorder, but older adults were noted to have more paranoia when compared with younger individuals.32
The overall pooled prevalence of psychotic symptoms among individuals with major neurocognitive disorders is estimated to be as high as 40%. In a literature review by Ropacki and Jeste, the investigators found a mean pooled prevalence of hallucinations or delusions among individuals with Alzheimer’s disease (AD) to be 41.1%.33 In the largest United States (US) population-based study to date, conducted by Leroi and colleagues, the investigators found the prevalence of hallucinations or delusions among individuals with vascular dementias to be about 15%.34 In addition, in the same study, they delineated types of delusions, and found persecutory delusions to be the most common type of delusion in both AD and vascular neurocognitive disorders. They also found that visual hallucinations appeared to be more common than auditory hallucinations in both AD and vascular neurocognitive disorders. Among individuals with neurocognitive disorders with Lewy bodies, Nagahama and colleagues found the prevalence of visual and auditory hallucinations and delusions to be 78% and 25%, respectively.35 Table 2 describes the differences between psychosis seen in individuals with dementia and schizophrenia.
Table 2.
| Features | Psychosis of dementia | Schizophrenia |
|---|---|---|
| Prevalence | 15–78% of patients | <1% of general population |
| Bizarre or complex delusions | Rare | Frequent |
| Misidentification | Frequent | Rare |
| Common form of hallucination | Visual | Auditory |
| Schneiderian First rank symptoms ABCD:Auditory hallucinations, Broadcasting of thought, Controlled thought (delusions of control), Delusional perception | Rare | Frequent |
| Past history of psychosis | Rare | Common |
| Eventual remission of psychosis | Frequent | Uncommon |
| Need for maintenance antipsychotic therapy | Uncommon | Common |
The prevalence of any psychotic symptoms in delirium is estimated to be 42.7%, with a specific prevalence of visual hallucinations being 27%, auditory hallucinations 12.4%, tactile hallucinations 2.7%, and delusions 25.6%.9 Older adults are at particular risk for developing psychosis secondary to drugs, toxins, and other underlying medical illnesses due to higher medical comorbidity, polypharmacy, and sensitivity to drug effect.38 Due to the diversity of secondary causes of psychosis in older adults, it is difficult to elucidate epidemiologic statistics for all secondary psychoses (excluding the neurocognitive disorders as discussed earlier). Table 3 describes the differences between the presentation of delirium, AD, Lewy body dementia (LBD), and depression.
Table 3.
| Characteristics | Delirium | AD | LBD | Depression |
|---|---|---|---|---|
| Presenting symptoms | Unfamiliarity with the environment with short term memory loss; “confusion” | Short term memory loss | Motor symptoms may appear before cognitive impairment; fluctuating cognition, visual hallucinations, and REM-sleep behavior disorder are part of core clinical features | Subjective complaints of poor memory and concentration |
| Onset | Sudden | Insidious | Insidious | Recent |
| Alertness | Fluctuating | Normal except in late phases | Fluctuating | Preserved |
| Duration | Hours to weeks | Months to years | Months to years | Variable |
| Orientation | Disorientation with onset | Disorientation occurs late in course | Fluctuating | Intact |
| Hallucinations | From onset | May occur late in course | From onset; visual hallucinations well-formed | Could occur in depression with psychotic features |
| Cognitive functioning | Fluctuating with alertness | Progressive deterioration | Progressive deterioration | Initially intact with efforts to perform cognitive tasks. May deteriorate without treatment progression |
| Mood | Fluctuate | Labile | Labile | Usually sad |
| Sundowning | Present | Present | Present | Absent, mood improve as day progress |
| Course | Usually reversible with treatment | Irreversible with progressive deterioration | Irreversible with progressive deterioration | Completely reversible |
AD, Alzheimer’s disease, LBD, Lewy Body dementia; REM, rapid eye movement.
Risk factors and pathophysiology
Risk factors that are associated with developing psychotic disorders among older adults include cognitive decline, poor health status, visual impairment, and negative life events.39 In addition, female gender appears to be a risk factor for developing late-onset schizophrenia and VLOS.40 Furthermore, being from an immigrant population, greater abnormalities in brain structures, family history of schizophrenia or avoidant personality, paranoid or schizoid personality disorder, hearing loss, and being from a lower socioeconomic status are risk factors for VLOS.4 Premorbid educational, occupational, and psychosocial functioning appears to be less impaired among late-onset than among early-onset individuals with schizophrenia.11
A recent study that compared the key risk factors among individuals with schizophrenia to identify trends according to the age of onset, comparing presentations prior to 26 years (youth onset), between 26 and 40 years (middle onset), and after 40 years of age (late onset) found that the older age of onset was associated with a weaker family history of schizophrenia, lower rates of substance use, better early psychosocial functioning, and higher educational achievement. In addition, female preponderance and comorbid physical health problems were notable among the late onset group compared with the other groups.23 Furthermore, individuals in the later life schizophrenia group showed a relatively greater association with psychosocial factors proximal to the onset of psychosis, including unemployment.
One study found that the genetic variant in Dopamine D2 receptor (DRD2), rs2734839, was significantly associated with schizophrenia as well as late onset age.41 Individuals who were carrying this genetic variation were more than twice as likely to have schizophrenia when compared with controls. Available evidence indicates that individuals with LOS appear to have larger thalamic volumes and abnormalities in white matter integrity when compared with individuals with EOS.42–44 In addition, reduced cerebral blood flow has been noted in both postcentral gyri among individuals with LOS when compared with individuals with EOS, who had reduced blood flow to the precentral gyrus and inferior frontal gyrus.45 One study found that older adults with delusional disorders had lateral ventricular volumes that were larger than those among older adults with schizophrenia and almost twice those of age-matched controls.46
Changes in dopaminergic, glutamatergic, and serotonergic systems associated with aging, including changes in the concentrations of these neurotransmitters, decline in serotonin receptors (5-HT2) and transporters (5-HTT), decreased dopamine and serotonin binding capacities, and reduced D1, D2, and D3 receptor densities are thought to be responsible extrapyramidal signs and symptoms including frequent falls, reduced cognitive flexibility, and sensitivity to psychotropic medications among older adults.47
Diagnosis
Diagnosing psychotic disorders among older adults can be difficult given the multitude of etiologies that can result in psychotic symptoms among these vulnerable individuals.48 Differentiating between LOS and VLOS could be helpful in these guiding diagnostic efforts. Similar efforts have been made to categorize diagnosis of psychotic disorder in other age groups, including early-onset (psychotic symptoms present before the age of 17) and very early-onset (referring to psychotic symptoms present before the age of 12).49 The initial diagnostic dilemma is to differentiate between the symptoms of psychosis due to a primary psychotic disorders versus psychotic symptoms that are secondary to medical/neurological disorders or due to the effect of medications or illicit drugs.50 The World Health Organization recently has made efforts in the ICD-11 to address this challenge by renaming ‘F2 Schizophrenia, schizotypal, and delusional disorders’ to ‘Schizophrenia spectrum and other primary psychotic disorders’, to drive differentiation of these etiologies.49
A thorough history can help differentiate between primary and secondary causes psychosis in late life.51 Acute or subacute onset of symptoms might suggest a secondary cause for the psychosis (e.g. delirium with an onset of days to weeks, or substance/medication-induced psychosis, with an onset of days to months). Neurocognitive disorders, on the other hand, may result in psychotic symptoms features that tend to have an insidious onset of symptoms that may take months to years for the progressive development of symptoms. Primary psychotic disorders such as schizophrenia spectrum disorders or affective disorders with psychotic symptoms, or delusional disorders, may have onset varying from weeks (especially in the case of mood disorder) to decades. Older adults who are have a history of abuse or neglect may have paranoia towards others that may be justifiable or not pathological given their history.52
Two important principles that may help guide the diagnosis of psychosis in later life include:
(1) Assume until proven otherwise that new-onset of psychotic symptoms among older adults is secondary in nature, as approximately 60% of psychosis in late-life has a secondary cause.
(2) There are no pathognomonic findings for any given psychiatric illness in late life; hence, a broad differential diagnosis should be entertained.
In order to differentiate primary and secondary psychotic disorders, scrutiny of certain characteristics can be helpful. Secondary psychotic disorders tend to have atypical age of onset of symptoms.39 If visual hallucinations are present independently of auditory hallucinations, suspect a secondary psychotic disorder. Psychotic symptoms in an individual with no previous psychiatric history or no family history may be suggestive of secondary cause for psychosis. Psychosis along with abnormalities in physical examinations also suggests a secondary psychotic disorder. Medical or neurological disorders with known psychiatric sequelae, or the presence of medication or illicit substances misuse, also suggests a secondary cause for psychotic symptoms among older adults.53
Clinicians should also gather collateral history through interviewing family members and discussing salient features of cases with other providers. In addition, conducting thorough physical examinations and cognitive assessments will help differentiate the various etiologies for the psychotic symptoms. Brain imaging such as magnetic resonance imaging (MRI) or computerized tomography (CT) scans can rule out structural brain abnormalities in the brain that could manifest psychotic symptoms. CT scans are faster to acquire than MRIs and would be more beneficial when examining anxious or claustrophobic individuals. In contrast, MRIs are more likely to be utilized in patients with focal neurological findings suggestive of an organic cause for psychotic symptoms, and when higher detail of soft tissue is required.54 Regular screening with imaging in first-episode psychosis patients is generally not recommended as a recent meta-analysis has indicated minimal diagnostic yield and clinical usefulness.55 Common laboratory studies including complete blood count (CBC), complete metabolic panel (CMP), thyroid stimulating hormone (TSH), vitamin B12, folate level, rapid plasma reagin (RPR), erythrocyte sedimentation rate (ESR), urine toxicology and autoimmune panels, and HIV panels can identify causes for the psychotic symptoms among older adults.56 Neuropsychological testing can help differentiate the etiologies and the extent of psychotic symptoms among older individuals.57 Figure 1 describes the pathway for identifying the etiologies for psychotic symptoms in late life.
Figure 1.
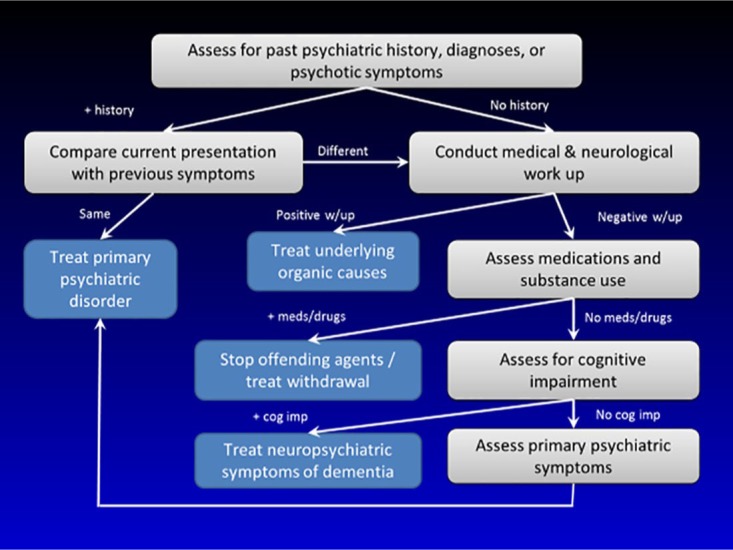
Pathway for identifying the etiologies for psychotic symptoms in late life.
Treatment
Initial treatment of psychosis in late life often requires the elimination of possible causes of secondary psychotic symptoms, including offending medications or organic causes for these symptoms. Thus, the treatment of neurological disorders, delirium, and substance intoxication/withdrawals contributing to, or exacerbating, psychosis would be the first step in improving secondary psychotic symptoms. If psychotic symptoms are due to a primary psychiatric disorder, then pharmacological treatment becomes essential when these symptoms do not respond to nonpharmacological treatment strategies.58 In addition, pharmacotherapy is necessary when the safety and well-being of the older individual or of others is being compromised. However, antipsychotic medications should be prescribed cautiously as their long-term use among older adults may result in significant negative health outcomes.59,60
Contemporary guidelines recommend the short-term use of antipsychotics to alleviate problematic symptoms to avoid long-term and nonreversible side effects.6 Notably, while typical antipsychotics may exhibit a smaller metabolic side effect burden compared with atypical drugs, they do exhibit an increased risk of tardive dyskinesia.61 In contrast, atypical antipsychotics often have the opposite side effect profile with metabolic adverse effects classically being more prominent while exhibiting a decreased risk of tardive dyskinesia as a result of more variable D2 receptor blockade. Thus, there should always be a risk vs. benefits analysis when employing psychotropics including a thorough evaluation of a patient’s physical state and medical comorbidities prior to initiation of treatment.6,62 In addition, lower dose initiation (1/2 the starting dose for adults) and gradual dose titration to the optimal dose may reduce the incidence of adverse effects.63 Furthermore, older adults may require liquid preparations of psychotropic medications if they are unable to swallow tablets or capsules.64
There is a dearth of high-quality pharmacotherapy studies among individuals with LOS and VLOS. During our literature review, we only found two prospective/population based studies,65,66 [Level 3 evidence according to the Centre for Evidence-Based Medicine (CEBM)],67 two randomized controlled trials (RCTs) (Level 2 evidence),68,69 and one expert consensus guideline (Level 5 evidence).70 A Cochrane review also found only one study that they could include in their review.71
More recently, Howard and colleagues published an RCT investigating the effect of low-dose amisulpride (a D2/D3 receptor blocker) at 100 mg daily in patients diagnosed with VLOS.68 The investigators found a significant improvement in the Brief Psychiatric Rating Scale (BPRS) in the amisulpride group compared with placebo, although it should be noted that the authors found no improvement in the secondary outcomes measuring patient-rated health-related quality of life, possibly reflecting the poor insight patients have regarding their psychotic symptoms.
There exists some data regarding the treatment of EOS in late-life using risperidone and olanzapine (Level 3 evidence),65 although there is little evidence to support the use of clozapine in treatment-resistance geriatric patients due to the drug’s significant side effect profile.72 Paliperidone was also shown to demonstrate some efficacy compared with placebo in the treatment of elderly patients with schizophrenia (Level 2 evidence).69 Recommended dosing is based on expert consensus. For example, risperidone dosage recommendations suggest administering 1.25 to 3.5 mg/day as the first-line agent. Alternatively, quetiapine may be dosed at 100–300 mg/day, olanzapine at 7.5–15 mg/day, and aripiprazole at 15–30 mg/day (Level 5 evidence).70 Lastly, a recent population-based study (Level 3 evidence) investigating the effect of optimized dose adjustments of amisulpride for psychosis symptoms (i.e. delusions) in elderly patients with AD suggests that the 50 mg/day of amisulpride is the minimally clinically effective dose in that target population.66 One caveat the authors reported though was that, in patients >75 years of age, 50 mg/day was also the maximally tolerated dose.73
Psychosis symptoms and agitation due to LBD are difficult to treat due to the possibility of worsening Parkinsonian symptoms with antipsychotic administration. In addition, there is a paucity of data concerning the safety and efficacy of antipsychotics in LBD.74 However, studies indicate some utility for Clozapine in treating LBD-related psychosis (Level 2 evidence).75–77 This is due to Clozapine’s greater serotonergic affinity and selective binding of D1 mesolimbic receptors while sparing striatal D2 receptors implicated in the deterioration of motor functions.78 However, clinicians should remain mindful of Clozapine’s significant side effect profile, which includes orthostatic hypotension, sedation, and agranulocytosis.78 In contrast, studies and reviews investigating the efficacy and tolerability of other antipsychotics have reported mixed results. One systematic review investigating the efficacy of quetiapine in patients with LBD found only one open-label trial that was able to report a significant reduction in psychotic symptoms when patients were prescribed quetiapine at tolerable doses (Level 3 evidence).79 Alternatively, one RCT investigating the efficacy of Olanzapine administration in LBD patients found that individuals treated with 5 mg daily dosages demonstrated improvements in hallucinations and delusions compared with the placebo group (Level 2 evidence).80 Higher doses were not found be more effective than placebo. Caution should be exercised when prescribing neuroleptics in patients with LBD due to their potential to cause rigidity, neuroleptic malignant syndrome, and death as a consequence of the underlying disruption of dopaminergic neurotransmission.81 If risks of neuroleptic administration outweigh any potential benefits they may produce, another option would be the use of a benzodiazepine such as clonazepam, which has the added benefit of treating the rapid eye movement (REM) sleep behavior disorder commonly found in LBD patients.82 The 5HT2A receptor inverse agonist, Pimavanserin, has demonstrated efficacy in improving psychosis symptoms in Parkinson’s disease,83 but no trials or studies have yet to be conducted in LBD populations.
Despite being the drugs of choice in treating psychotic symptoms and severe agitation associated with dementia and delirium states, there are controversies regarding the use of antipsychotics among older adults who present with these symptoms.84,85 This is due to the Food and Drug Administration (FDA) black box warning indicating an association between the administration of antipsychotics in geriatric patients and increased mortality risk.62 Studies have also shown an increased risk of cerebrovascular events, metabolic side effects, and pneumonia with the prescribing of antipsychotics in older adults compared with same-age cohorts not being prescribed these drugs.62,86 More recently, a systematic review and meta-analysis of the prevention and treatment of delirium with antipsychotics in adult surgical and medical patients suggests that antipsychotics may be more limited in their efficacy in treating delirium than once thought (Level 1 evidence).87 Thus, careful consideration should be conducted before initiating medications that may produce serious harmful effects relative to the moderately beneficial effects they could potentially generate.85
Although there is an impetus to discontinue antipsychotics as soon as safely possible due to reports of increased risk of cerebrovascular adverse events and intolerable side effects in geriatric patients,88,89 there is limited data regarding the discontinuation of antipsychotic medications in older adults diagnosed with chronic schizophrenia (Level 2 evidence).90 As a result, it is imperative to attempt discontinuation of antipsychotics only in older patients who do not respond to these classes of medications or if they have demonstrated long-standing clinical remission. If discontinuation is not possible due to persistent psychotic symptoms, decreasing the dose of medications to the lowest effective dosage should be done to minimize risk of adverse events.
There is a paucity of studies investigating the treatment of psychosis symptoms using electroconvulsive therapy (ECT) in the geriatric population. However, one selective review reports that prospective trials of ECT in psychotic elderly patients (Level 3 evidence) have indicated that bilateral ECT is a safe and effective treatment for older patients with schizophrenia91; it was also found to be synergistic with concurrent antipsychotic therapy. The authors also noted that the best evidence for ECT treatment is its utilization in patients presenting with aggression, catatonia, and in other cases that require rapid response such as acute suicidality or an acute onset of illness.
Alternatively, the combination of pharmacotherapy and psychosocial modalities are the most likely to alleviate psychotic symptoms with the least risk of severe side effects associated with pharmacological therapy alone. Although the number of studies investigating these psychosocial modalities are limited, there is existing high quality data (Level 2 evidence) indicating some benefit from cognitive behavioral social skills training (CBSST), Functional Adaptation Skills Training (FAST), supported employment, social skills training, and preventative healthcare programs.92–97 CBSST, for example, utilizes group therapy to promote cognitive and behavioral coping skills, problem solving skills, and improved social functioning in order to compensate for neurocognitive deficits.92 In contrast, FAST interventions focus more on improving daily living skills in middle-aged and older adults diagnosed with a psychotic disorder who live in the community. These interventions have been reported to help improve patient organization, arranging transportation, social skills, managing finances, and medication management.94 In addition, combined skills training along with preventative health care and health management could be employed to promote social functioning and independent living skills.95 Studies have also indicated that individuals with schizophrenia who received supported employment experienced better work outcomes and achieved superior quality of life measures compared with conventional vocational rehabilitation programs.98,99 Hence, psychosocial treatment modalities have the potential to improve many aspects of life and daily living among older adults with psychotic symptoms. Their implementation in any treatment plan is likely to produce benefits if utilized in appropriate situations based on the needs of individual.
Treatment of individuals with late life psychotic disorders should always be individualized, with careful attention to comorbidities. In addition, the use of evidence-based nonpharmacological treatments in combination with pharmacological strategies can optimize outcomes in these cases. Nonpharmacological treatments for psychotic disorders in late life have the highest quality of evidence when investigating their efficacy and include cognitive skills training, functional adaptation skills training, social rehabilitation, supported employment, and work rehabilitation. For more resistant and severe psychotic presentations, or in cases where there is need for emergency treatment, the use of psychotropics is indicated. When using antipsychotic medications in older adults, their use should be optimized, wherein the efficacy of these medications is maximized and adverse-effect profile is minimized, and they should be employed only for an appropriate period of time. However, it should be noted that data for the use of pharmacological agents in late-life psychotic disorders is limited, with this review finding only two RCTs investigating antipsychotic efficacy. The highest level of evidence supports the utilization of amisulpride and paliperidone for psychosis in elderly patients, although there is some evidence for the use of olanzapine and risperidone in this population. The use of clozapine should be restricted due to its significant side effect profile.
Conclusion
Psychotic disorders in late life represent a diverse group of illnesses with varied etiologies. In addition, they often have different clinical presentations and outcomes when compared with younger adults. In a majority of cases, psychosis in late life occurs due to underlying medical illnesses, or medications or illicit drug effects. It is important for secondary causes of psychosis to be identified and treated in order to reduce suffering among vulnerable older adults. Although differentiating between primary and secondary causes of psychosis can be challenging, it can be accomplished by obtaining a thorough history, by completing a focused physical examination, by using neuropsychological assessments, and by the appropriate use of laboratory data.
In producing this report, we were constrained by the relative scarcity of well-powered studies to conduct a systematic review, which is why we elected to produce a narrative review. This review is intended to provide an overall diagnostic and treatment guideline for clinicians to utilize in their everyday practice. As most studies investigating pharmacological treatments for psychotic disorders in late life were commonly low in power, future studies should endeavor to recruit larger cohorts to further examine the potential benefits and adverse events of prolonged psychotropic treatment. In addition, as Howard and colleagues have indicated in their report,68 further study of patient insight into psychotic disorders in late life would be beneficial in improving health outcomes by enhancing our understanding of patient perception of the disorder and developing methods to improve treatment compliance.
Acknowledgments
The authors would like to credit Ilse Wiechers for developing the pathway for identifying the etiologies for psychotic symptoms in late life (Figure 1) and would like to thank her for letting us use it for this article.
Footnotes
Funding: The authors received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical statement: Approval of an ethical committee was not required as no patients or human subjects participated in the production of this manuscript.
ORCID iD: Rajesh R Tampi  https://orcid.org/0000-0002-3770-4074
https://orcid.org/0000-0002-3770-4074
Contributor Information
Rajesh R. Tampi, Department of Psychiatry and Behavioral Sciences, Cleveland Clinic Akron General, Akron, OH 44307, USA; Section for Geriatric Psychiatry, Cleveland Clinic, Cleveland, OH 44195, USA; Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH 44195, USA.
Juan Young, Department of Psychiatry, Yale School of Medicine, New Haven, CT, USA.
Rakin Hoq, NeoMed/Summa Psychiatry Residency Program, Akron, OH, USA.
Kyle Resnick, NeoMed/Summa Psychiatry Residency Program, Akron, OH, USA.
Deena J. Tampi, Diamond Healthcare, Richmond, VA, USA
References
- 1. Gaebel W, Zielasek J. Focus on psychosis. Dialogues Clin Neurosci. 2015; 17: 9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Psychiatric Association. Desk reference to the diagnostic criteria from DSM–5. Washington, DC: American Psychiatric Publishing, 2013, p.xlviii, 395 p. [Google Scholar]
- 3. World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization, 1992, p.xii, 362 p. [Google Scholar]
- 4. Howard R, Rabins PV, Seeman MV, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: An international consensus. Am J Psychiatry 2000; 157: 172–178. [DOI] [PubMed] [Google Scholar]
- 5. Talaslahti T, Alanen HM, Hakko H, et al. Patients with very-late-onset schizophrenia-like psychosis have higher mortality rates than elderly patients with earlier onset schizophrenia. Int J Geriatr Psychiatry 2015; 30: 453–459. [DOI] [PubMed] [Google Scholar]
- 6. Gareri P, Segura-Garcia C, Manfredi VG, et al. Use of atypical antipsychotics in the elderly: a clinical review. Clin Interv Aging 2014; 9: 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colijn MA, Nitta BH, Grossberg GT. Psychosis in later life: a review and update. Harv Rev Psychiatry 2015; 23: 354–367. [DOI] [PubMed] [Google Scholar]
- 8. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). 5th ed. Arlington, VA: American Psychiatric Publishing, Inc. [Google Scholar]
- 9. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics 2000; 41: 519–522. [DOI] [PubMed] [Google Scholar]
- 10. Holroyd S, Laurie S. Correlates of psychotic symptoms among elderly outpatients. Int J Geriatr Psychiatry 1999; 14: 379–384. [PubMed] [Google Scholar]
- 11. Manepalli JN, Gebretsadik M, Hook J, et al. Differential diagnosis of the older patient with psychotic symptoms. Prim Psychiatry 2007; 14: 55–62. [Google Scholar]
- 12. Castle DJ, Murray RM. The epidemiology of late-onset schizophrenia. Schizophrenia Bull 1993; 19: 691–700. [DOI] [PubMed] [Google Scholar]
- 13. Copeland JRM, Dewey ME, Scott A, et al. Schizophrenia and delusional disorder in older age: community prevalence, incidence, comorbidity, and outcome. Schizophrenia Bull 1998; 24: 153–161. [DOI] [PubMed] [Google Scholar]
- 14. Diwan S, Cohen CI, Bankole AO, et al. Depression in older adults with schizophrenia spectrum disorders: prevalence and associated factors. Am J Geriatr Psychiatry 2007; 15: 991–998. [DOI] [PubMed] [Google Scholar]
- 15. Maglione JE, Vahia IV, Jeste DV. Schizophrenia spectrum and other psychotic disorders. In: The American Psychiatric Publishing Textbook of Geriatric Psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing, 2015, pp.309–332. [Google Scholar]
- 16. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophrenia Bull 1988; 14: 39. [DOI] [PubMed] [Google Scholar]
- 17. Stafford J, Howard R, Kirkbride JB. The incidence of very late-onset psychotic disorders: a systematic review and meta-analysis, 1960-2016. Psychol Med 2018; 48: 1775–1786. [DOI] [PubMed] [Google Scholar]
- 18. Stafford J, Howard R, Dalman C, et al. The incidence of nonaffective, nonorganic psychotic disorders in older people: a population-based cohort study of 3 million people in Sweden. Schizophrenia Bull 2019; 45: 1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ribe AR, Laursen TM, Charles M, et al. Long-term risk of dementia in persons with schizophrenia: a Danish population-based cohort study. JAMA Psychiatry 2015; 72: 1095–1101. [DOI] [PubMed] [Google Scholar]
- 20. Korner A, Lopez AG, Lauritzen L, et al. Late and very-late first-contact schizophrenia and the risk of dementia – a nationwide register based study. Int J Geriatr Psychiatry 2009; 24: 61–67. [DOI] [PubMed] [Google Scholar]
- 21. Cai L, Huang J. Schizophrenia and risk of dementia: a meta-analysis study. Neuropsychiatr Dis Treat 2018; 14: 2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palmer BW, McClure FS, Jeste DV. Schizophrenia in late life: findings challenge traditional concepts. Harv Rev Psychiatry 2001; 9: 51–58. [DOI] [PubMed] [Google Scholar]
- 23. Chen L, Selvendra A, Stewart A, et al. Risk factors in early and late onset schizophrenia. Compr Psychiatry 2018; 80: 155–162. [DOI] [PubMed] [Google Scholar]
- 24. Meesters PD, de Haan L, Comijs HC, et al. Schizophrenia spectrum disorders in later life: prevalence and distribution of age at onset and sex in a Dutch catchment area. Am J Geriatr Psychiatry 2012; 20: 18–28. [DOI] [PubMed] [Google Scholar]
- 25. Post F. Schizo-affective symptomatology in late life. Br J Psychiatry 1971; 118: 437–445. [DOI] [PubMed] [Google Scholar]
- 26. Baran XY, Young RC. Bipolar and depressive types of schizoaffective disorder in old age. Am J Geriatr Psychiatry 2006; 14: 382–383. [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez-Rodriguez A, Molina-Andreu O, Navarro V, et al. Delusional disorder: no gender differences in age at onset, suicidal ideation, or suicidal behavior. Braz J Psychiatry 2014; 36: 119–124. [DOI] [PubMed] [Google Scholar]
- 28. Maher B. Delusional thinking and cognitive disorder. Integr Physiol Behav Sci 2005; 40: 136–146. [DOI] [PubMed] [Google Scholar]
- 29. Owoeye O, Kingston T, Scully PJ, et al. Epidemiological and clinical characterization following a first psychotic episode in major depressive disorder: comparisons with schizophrenia and bipolar I disorder in the Cavan-Monaghan First Episode Psychosis Study (CAMFEPS). Schizophr Bull 2013; 39: 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gournellis R, Oulis P, Rizos E, et al. Clinical correlates of age of onset in psychotic depression. Arch Gerontol Geriatr 2011; 52: 94–98. [DOI] [PubMed] [Google Scholar]
- 31. Weissman M, Leaf P, Tischler G, et al. Affective disorders in five United States communities. Psychol Med 1988; 18: 141–153. [DOI] [PubMed] [Google Scholar]
- 32. Depp CA, Jeste DV. Bipolar disorder in older adults: a critical review. Bipolar Disord 2004; 6: 343–367. [DOI] [PubMed] [Google Scholar]
- 33. Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry 2005; 162: 2022–2030. [DOI] [PubMed] [Google Scholar]
- 34. Leroi I, Voulgari A, Breitner JC, et al. The epidemiology of psychosis in dementia. Am J Geriatr Psychiatry 2003; 11: 83–91. [PubMed] [Google Scholar]
- 35. Nagahama Y, Okina T, Suzuki N, et al. Classification of psychotic symptoms in dementia with Lewy bodies. Am J Geriatr Psychiatry 2007; 15: 961–967. [DOI] [PubMed] [Google Scholar]
- 36. Schneider LS, Dagerman KS. Psychosis of Alzheimer’s disease: clinical characteristics and history. J Psychiatr Res 2004; 38: 105–111. [DOI] [PubMed] [Google Scholar]
- 37. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017; 89: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keshavan MS, Kaneko Y. Secondary psychoses: an update. World Psychiatry 2013; 12: 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brunelle S, Cole MG, Elie M. Risk factors for the late-onset psychoses: a systematic review of cohort studies. Int J Geriatr Psychiatry 2012; 27: 240–252. [DOI] [PubMed] [Google Scholar]
- 40. Jeste DV, Symonds LL, Harris MJ, et al. Nondementia nonpraecox dementia praecox? Late-onset schizophrenia. Am J Geriatr Psychiatry 1997; 5: 302–317. [DOI] [PubMed] [Google Scholar]
- 41. Voisey J, Swagell CD, Hughes IP, et al. A novel DRD2 single-nucleotide polymorphism associated with schizophrenia predicts age of onset: HapMap tag-single-nucleotide polymorphism analysis. Genet Test Mol Bioma 2012; 16: 77–81. [DOI] [PubMed] [Google Scholar]
- 42. Sachdev P, Brodaty H, Rose N, et al. Schizophrenia with onset after age 50 years. 2: Neurological, neuropsychological and MRI investigation. Br J Psychiatry 1999; 175: 416–421. [DOI] [PubMed] [Google Scholar]
- 43. Corey-Bloom J, Jernigan T, Archibald S, et al. Quantitative magnetic resonance imaging of the brain in late-life schizophrenia. Am J Psychiatry 1995; 152: 447–449. [DOI] [PubMed] [Google Scholar]
- 44. Chen L, Chen X, Liu W, et al. White matter microstructural abnormalities in patients with late-onset schizophrenia identified by a voxel-based diffusion tensor imaging. Psychiatry Res 2013; 212: 201–207. [DOI] [PubMed] [Google Scholar]
- 45. Wake R, Miyaoka T, Araki T, et al. Regional cerebral blood flow in late-onset schizophrenia: a SPECT study using 99mTc-ECD. Eur Arch Psychiatry Clin Neurosci 2016; 266: 3–12. [DOI] [PubMed] [Google Scholar]
- 46. Howard RJ, Almeida O, Levy R, et al. Quantitative magnetic resonance imaging volumetry distinguishes delusional disorder from late-onset schizophrenia. Br J Psychiatry 1994; 165: 474–480. [DOI] [PubMed] [Google Scholar]
- 47. Wang E, Snyder DS. Handbook of the aging brain. San Diego: Academic Press, 1998. [Google Scholar]
- 48. Freudenreich O. Differential diagnosis of psychotic symptoms: medical ‘mimics’. Psychiatr Times 2010; 27: 56–61. [Google Scholar]
- 49. RE: Gaebel W: Status of Psychotic Disorders in ICD-11. Schizophr Bull. 2012 Sep;38(5):895–898. Schizophr Bull 2012; 38: 1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marsh CM. Psychiatric presentations of medical illness. Psychiatr Clin North Am 1997; 20: 181–204. [DOI] [PubMed] [Google Scholar]
- 51. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry 2009; 3: 10–18. [DOI] [PubMed] [Google Scholar]
- 52. Vahia IV, Lanouette NM, Jeste DV. Schizophrenia and paranoid disorders. In: Blazer DG, Steffens DC. (eds) Clinical Manual of Geriatric Psychiatry. Washington, DC: American Psychiatric Publishing, 2013. [Google Scholar]
- 53. Marsh L, Williams JR, Rocco M, et al. Psychiatric comorbidities in patients with Parkinson disease and psychosis. Neurology 2004; 63: 293–300. [DOI] [PubMed] [Google Scholar]
- 54. Khandanpour N, Hoggard N, Connolly DJ. The role of MRI and CT of the brain in first episodes of psychosis. Clin Radiol 2013; 68: 245–250. [DOI] [PubMed] [Google Scholar]
- 55. Goulet K, Deschamps B, Evoy F, et al. Use of brain imaging (computed tomography and magnetic resonance imaging) in first-episode psychosis: review and retrospective study. Can J Psychiatry 2009; 54: 493–501. [DOI] [PubMed] [Google Scholar]
- 56. Reinhardt MM, Cohen CI. Late-life psychosis: diagnosis and treatment. Curr Psychiatry Rep 2015; 17: 1. [DOI] [PubMed] [Google Scholar]
- 57. Javadpour A, Sehatpour M, Mani A, et al. Assessing diagnosis and symptoms profiles of late-life psychosis. GeroPsych 2013; 26: 205–209. [Google Scholar]
- 58. Targum SD. Treating psychotic symptoms in elderly patients. Prim Care Companion J Clin Psychiatry 2001; 3: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nerius M, Johnell K, Garcia-Ptacek S, et al. The impact of antipsychotic drugs on long-term care, nursing home admission, and death in dementia patients. J Gerontol A Biol Sci Med Sci 2018; 73: 1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chahine LM, Acar D, Chemali Z. The elderly safety imperative and antipsychotic usage. Harv Rev Psychiatry 2010; 18: 158–172. [DOI] [PubMed] [Google Scholar]
- 61. Jeste D, Rockwell E, Harris M, et al. Conventional vs. newer antipsychotics in elderly patients. Am J Geriatr Psychiatry 1999; 7: 70. [PubMed] [Google Scholar]
- 62. Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: managing safety concerns. Am J Psychiatry 2012; 169: 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry 2004; 161. [PubMed] [Google Scholar]
- 64. Benjamin James Sadock MD, Pedro Ruiz MD, Virginia Alcott Sadock MD. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2015. [Google Scholar]
- 65. Rothenberg KG, Boyd S, Sommerville R, et al. Clozapine in geriatric population-clinical indication and safety monitoring. Am J Geriatr Psychiatry 22: S73–S74. [Google Scholar]
- 66. Reeves S, Bertrand J, McLachlan E, et al. A population approach to guide amisulpride dose adjustments in older patients with Alzheimer’s disease. J Clin Psychiatry 2017; 78: e844–e851. [DOI] [PubMed] [Google Scholar]
- 67. OCEBM Levels of Evidence Working Group. The Oxford 2011 levels of evidence. Oxford Centre for Evidence-Based Medicine, 2011. [Google Scholar]
- 68. Howard R, Cort E, Bradley R, et al. Antipsychotic treatment of very late-onset schizophrenia-like psychosis (ATLAS): a randomised, controlled, double-blind trial. Lancet Psychiatry 2018; 5: 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tzimos A, Samokhvalov V, Kramer M, et al. Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension. Am J Geriatr Psychiatry 2008; 16: 31–43. [DOI] [PubMed] [Google Scholar]
- 70. Alexopoulos GS, Streim JE, Carpenter D. Commentary: expert consensus guidelines for using antipsychotic agents in older patients. J Clin Psychiatry 2004; 65: 100–102. [PubMed] [Google Scholar]
- 71. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev 2012; 2: CD004162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mukku SSR, Sivakumar PT, Varghese M. Clozapine use in geriatric patients: challenges. Asian J Psychiatr 2018; 33: 63–67. [DOI] [PubMed] [Google Scholar]
- 73. Reeves S, Eggleston K, Cort E, et al. Therapeutic D2/3 receptor occupancies and response with low amisulpride blood concentrations in very late-onset schizophrenia-like psychosis (VLOSLP). Int J Geriatr Psychiatry 2018; 33: 396–404. [DOI] [PubMed] [Google Scholar]
- 74. Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry 2015; 172: 731–742. [DOI] [PubMed] [Google Scholar]
- 75. Factor SA, Friedman JH, Lannon MC; Parkinson Study Group. Clozapine for the treatment of drug-induced psychosis in Parkinson’s disease: results of the 12 week open label extension in the PSYCLOPS trial. Mov Disord 2001; 16: 135–139. [DOI] [PubMed] [Google Scholar]
- 76. Merims D, Balas M, Peretz C, et al. Rater-blinded, prospective comparison: quetiapine versus clozapine for Parkinson’s disease psychosis. Clin Neuropharmacol 2006; 29: 331–337. [DOI] [PubMed] [Google Scholar]
- 77. Klein C, Gordon J, Pollak L, et al. Clozapine in Parkinson’s disease psychosis: 5-year follow-up review. Clin Neuropharmacol 2003; 26: 8–11. [DOI] [PubMed] [Google Scholar]
- 78. Goldman JG, Holden S. Treatment of psychosis and dementia in Parkinson’s disease. Curr Treat Options Neurol 2014; 16: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Desmarais P, Massoud F, Filion J, et al. Quetiapine for psychosis in Parkinson disease and neurodegenerative Parkinsonian disorders: a systematic review. J Geriatr Psych Neurol 2016; 29: 227-36. [DOI] [PubMed] [Google Scholar]
- 80. Cummings JL, Street J, Masterman D, et al. Efficacy of olanzapine in the treatment of psychosis in dementia with Lewy bodies. Dement Geriatr Cogn Disord 2002; 13: 67–73. [DOI] [PubMed] [Google Scholar]
- 81. McKeith I, Fairbairn A, Perry R, et al. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ 1992; 305: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gold G. Dementia with Lewy bodies: clinical diagnosis and therapeutic approach. Front Neurol Neurosci. 2009; 24: 107–113. [DOI] [PubMed] [Google Scholar]
- 83. Kianirad Y, Simuni T. Pimavanserin, a novel antipsychotic for management of Parkinson’s disease psychosis. Expert Rev Clin Pharmacol 2017; 10: 1161–1168. [DOI] [PubMed] [Google Scholar]
- 84. Markowitz JD, Narasimhan M. Delirium and antipsychotics: a systematic review of epidemiology and somatic treatment options. Psychiatry (Edgmont) 2008; 5: 29–36. [PMC free article] [PubMed] [Google Scholar]
- 85. Tampi RR, Tampi DJ, Balachandran S, et al. Antipsychotic use in dementia: a systematic review of benefits and risks from meta-analyses. Ther Adv Chronic Dis 2016; 7: 229–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rochon PA, Normand SL, Gomes T, et al. Antipsychotic therapy and short-term serious events in older adults with dementia. Arch Intern Med 2008; 168: 1090–1096. [DOI] [PubMed] [Google Scholar]
- 87. Neufeld KJ, Yue J, Robinson TN, et al. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64: 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mittal V, Kurup L, Williamson D, et al. Risk of cerebrovascular adverse events and death in elderly patients with dementia when treated with antipsychotic medications: a literature review of evidence. Am J Alzheimers Dis Other Demen 2011; 26: 10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jessop T, Harrison F, Cations M, et al. Halting Antipsychotic Use in Long-Term care (HALT): a single-arm longitudinal study aiming to reduce inappropriate antipsychotic use in long-term care residents with behavioral and psychological symptoms of dementia. Int Psychogeriatr 2017; 29: 1391–1403. [DOI] [PubMed] [Google Scholar]
- 90. Takeuchi H, Suzuki T, Remington G, et al. Effects of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia: an open-label, randomized, controlled, pilot study. Schizophrenia Bull 2013; 39: 993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Meyer JP, Swetter SK, Kellner CH. Electroconvulsive therapy in geriatric psychiatry: a selective review. Psychiatr Clin North Am 2018; 41: 79–93. [DOI] [PubMed] [Google Scholar]
- 92. Granholm E, McQuaid JR, McClure FS, et al. A randomized, controlled trial of cognitive behavioral social skills training for middle-aged and older outpatients with chronic schizophrenia. Am J Psychiatry 2005; 162: 520–529. [DOI] [PubMed] [Google Scholar]
- 93. Granholm E, McQuaid JR, McClure FS, et al. Randomized controlled trial of cognitive behavioral social skills training for older people with schizophrenia: 12-month follow-up. J Clin Psychiatry 2007; 68: 730–777. [DOI] [PubMed] [Google Scholar]
- 94. Patterson TL, Mausbach BT, McKibbin C, et al. Functional Adaptation Skills Training (FAST): a randomized trial of a psychosocial intervention for middle-aged and older patients with chronic psychotic disorders. Schizophr Res 2006; 86: 291–299. [DOI] [PubMed] [Google Scholar]
- 95. Bartels SJ, Pratt SI, Mueser KT, et al. Long-term outcomes of a randomized trial of integrated skills training and preventive healthcare for older adults with serious mental illness. Am J Geriatr Psychiatry 2014; 22: 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mueser KT, Pratt SI, Bartels SJ, et al. Randomized trial of social rehabilitation and integrated health care for older people with severe mental illness. J Consult Clin Psychol 2010; 78: 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cohen-Mansfield J, Libin A, Marx MS. Nonpharmacological treatment of agitation: a controlled trial of systematic individualized intervention. J Gerontol A Biol Sci Med Sci 2007; 62: 908–916. [DOI] [PubMed] [Google Scholar]
- 98. Twamley EW, Narvaez JM, Becker DR, et al. Supported Employment for Middle-Aged and Older People with Schizophrenia. Am J Psychiatr Rehabil 2008; 11: 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Twamley EW, Padin DS, Bayne KS, et al. Work rehabilitation for middle-aged and older people with schizophrenia: a comparison of three approaches. J Nerv Ment Dis 2005; 193: 596–601. [DOI] [PubMed] [Google Scholar]


