Abstract
Purpose
Primary vitreoretinal lymphoma [(P)VRL]) is a rare malignancy of the eye localized in the retina, vitreous or choroid. Here, we aim to determine the value of the combination of innovative diagnostic methods for accurate differentiation between (P)VRL and non‐(P)VRL in patients with suspect uveitis or vitritis.
Methods
Multicolour flow cytometric immunophenotyping of cells in the vitreous samples was performed using the EuroFlow small sample tube. Additionally, cytokines/chemokines and growth factors were measured in the vitreous specimens using a multiplex immunoassay. Data were evaluated in predefined clinical subgroups using omniviz unsupervised Pearson's correlation visualization and unsupervised heatmap analysis.
Results
A total of 53 patients were prospectively included in the period 2012–2015. In the (P)VRL subgroup (n = 10), nine cases showed aberrant surface membrane immunoglobulin (SmIg) light chain expression. In the non‐(P)VRL group (n = 43) clearly skewed SmIg light chain expression was observed in two multiple sclerosis‐related uveitis cases, but not in other uveitis types. Soluble mediator measurement revealed high interleukin (IL)‐10/IL‐6 ratios, and high IL‐1RA levels in 9/10 (P)VRL cases, but not in any non‐(P)VRL case. Further correlation and heatmap analysis revealed a minimal signature of cellular parameters (CD19+ B cells, aberrant SmIg light chain expression) and cytokine parameters (IL‐10/IL‐6 ratio >1, high IL‐10, high IL‐1 RA, high monocyte chemotactic protein‐1, high macrophage inflammatory protein‐1β) to reliably distinguish (P)VRL from non‐(P)VRL.
Conclusion
Here, we show the power of a combined cellular and proteomics strategy for detecting (P)VRL in vitreous specimens, especially in cases with minor cellular (P)VRL infiltrates.
Keywords: diagnostics, immunology, multiparameter flow cytometry, soluble mediators, uveitis, vitreoretinal lymphoma
Introduction
Vitreoretinal lymphoma (VRL) is a rare malignancy of the eye, localized in the retina, vitreous or choroid (Coupland et al. 2009; Chan & Sen 2013; Sagoo et al. 2014). It forms a subset of primary central nervous system lymphoma (PCNSL) and typically concerns the diffuse large B‐cell lymphoma type (DLBCL). It can present with only ocular findings, without CNS involvement, in which case the term primary vitreoretinal lymphoma (PVRL) is used. When found in combination with CNS involvement, the term VRL applies. Clinically, PVRL is a challenging masquerade syndrome, often presenting as an intermediate and/or posterior uveitis (Sen et al. 2009; Chan et al. 2011; Davis 2013). Due to this, a delay in diagnosis and therapeutic management is commonly encountered. Currently, the gold standard for the diagnosis of (P)VRL is the identification of malignant lymphoid cells in the vitreous, retina and/or optic nerve (Chan et al. 2011; Chan & Sen 2013). However, cytology results can be repeatedly inconclusive (Raparia et al. 2009; Chan & Sen 2013), which emphasizes the need to improve the diagnostic workup of (P)VRL.
In this respect, several characteristic findings of B‐cell lymphoma cells can be exploited to optimize diagnosing (P)VRL. One of these is the monoclonal character of the lymphoma cells, which can be assessed through molecular techniques as well as through flow cytometry. In an earlier study, Missotten et al. (2013) validated multicolour flow cytometric immunophenotyping as a strategy in the diagnosis of (P)VRL. Despite the relatively high sensitivity of the multicolour flow cytometry approach, a small percentage of the (P)VRL still remained undiagnosed with this approach. In this pilot study, we therefore aimed to determine the value of the combination of multiparameter flow cytometry with cytokine analysis as a diagnostic approach for the differentiation between (P)VRL and non‐(P)VRL in patients with suspect uveitis or vitritis.
Materials and Methods
Patients and inclusion criteria
A total of 53 patients were prospectively included in the period 2012–2015. All patients received full ophthalmologic investigation, including slit lamp examination and funduscopy. Patients typically presented with vitritis or with uveitis plus vitreous involvement and underwent pars plana vitrectomy for diagnostic and/or therapeutic reasons. There were no repeated vitrectomies for diagnostic reasons, nor were any of the eyes vitrectomized prior to the diagnostic procedure. Vitreous specimens were collected and analyzed via cytomorphology. In addition, screening for infectious causes of uveitis was performed on the specimens, which concerned microbiological culture, polymerase chain reaction and antibody analysis (including the Goldmann–Witmer coefficient), as well as screening for viruses (VZV, HSV, CMV, Rubella) and Toxoplasma gondii. All patients underwent a standard workup for uveitis, which included erythrocyte sedimentation rate, blood counts, serum angiotensin‐converting enzyme levels, serology for syphilis, as well as interferon gamma (IFNγ) release assay test (QuantiFERON–TB Gold In‐Tube test). Furthermore, radiologic chest imaging was performed and HLA‐B27 testing was performed in patients with anterior uveitis and panuveitis. Approval was obtained for this study from the Erasmus MC IRB (MEC 2012‐016/017), and the study was performed in accordance with the Declaration of Helsinki guidelines.
Flow cytometric immunophenotyping
Vitreous samples were freshly collected in syringes and immediately transported to the laboratory for further workup (within 1 hr). Samples were centrifuged and the supernatants were stored at −20°C until further use. Cell pellets were resuspended in 200 μl phosphate buffered saline with 0.5% bovine serum albumin. One third of this suspension was used for flow cytometric immunophenotyping using the EuroFlow 8‐colour small sample tube labelling, consisting of the following antibodies: CD20 (Pacific Blue; 2H7; Biolegend, San Diego, CA, USA); CD45 (Pacific Orange; HI30 Invitrogen, Waltham, MA, USA); CD8 (FITC, UCH‐T4; Cytognos) + surface membrane immunoglobulin lambda (SmIgλ; FITC, polyclonal; Cytognos, Salamanca, Spain); CD56 (PE, C5.9; Cytognos) + SmIg kappa (SmIgκ; PE, polyclonal; Cytognos); CD4 (PerCPCy5.5, SK3; Cytognos); CD19 (PECy7, J3‐119; Beckman Coulter, Atlanta, GA, USA); CD3 (APC, SK7; BD Biosciences) + CD14 (APC, MφP9; BD Biosciences); and CD38 (APC‐H7, HB7; BD Biosciences, San Jose, CA, USA) (Van Dongen et al. 2012). This allows identification of T cells (CD3+) and B cells (CD19+/CD20+), while in parallel expression of CD4 and CD8 as well as SmIgκ and SmIgλ can be evaluated within the T‐cell and B‐cell fraction, respectively. Based on the SmIgκ/SmIgλ ratio the monoclonal character of the B cells can be determined. The additional markers allow to distinguish monocytes, granulocytes (scatter characteristics) and NK cells, to be able to make a complete differential of leucocytes.
Measurement of cytokines/chemokines/growth factors
Supernatants of the vitreous samples were analyzed with a Bio‐plex Pro™ Human Cytokine, Chemokine, Growth Factor Assay (Bio‐Rad, Hercules, CA, USA) allowing simultaneous detection of the following cytokines, chemokines and growth factors: interleukin (IL) ‐1β, IL‐1RA, IL‐2, IL‐4, IL‐5, IL‐6, IL‐7, IL‐8/CXCL8, IL‐9, IL‐10, IL‐12 (p70), IL‐13, IL‐15, IL‐17, eotaxin, basic fibroblast growth factor, granulocyte colony‐stimulating factor (G‐CSF), granulocyte‐macrophage (GM) CSF, IFNγ, IFNγ‐induced protein‐10/CXCL10, monocyte chemotactic protein (MCP)‐1/CCL2, macrophage inflammatory protein (MIP)‐1α/CCL3, MIP‐1β/CCL4, platelet‐derived growth factor‐BB, regulated on activation and normal T cell expressed and secreted, tumour necrosis factor alpha, and vascular endothelial growth factor A (Bastiaans et al. 2014). The assay was performed according to the manufacturer's instructions.
Bioinformatic and statistical analysis
Flow cytometric data and cytokine/chemokine/growth factor data were evaluated via omniviz version 6.1.13.0 (Instem, Stone, UK) unsupervised Pearson's correlation visualization (Correlation View) and unsupervised heatmap analysis using HeatMapper. To this end percentages of the different cell types (B cells, T cells, NK cells, monocytes, neutrophils) were used with a minimum cut‐off of 0.1% and log2 geometric (GM) mean values were calculated. Additionally, log2 values of the SmIgκ/SmIgλ and CD4/CD8 ratios were included. For cytokine/chemokine/growth factor analysis, log2 GM values of the actual concentrations were used, while taking the lower limit of detection for every individual cytokine as a cut‐off for cases not showing expression. Statistical significance between (P)VRL and non‐(P)VRL groups was evaluated using the Mann–Whitney test.
Results
In this prospective pilot study, 53 patients were included. Mean age of the patients was 60 years, and 19/53 (34%) were males. In 20/53 patients the initial (P)VRL suspicion was high. Of these, six were previously diagnosed with histologically proven CNS lymphoma, while an additional two showed cerebral lesions suggestive of CNS lymphoma at the time of ocular surgery. Initial suspicion for (P)VRL was low in the other 33 patients. Two of these showed VZV‐induced retinal necrosis, one endogenous endophthalmitis (Candida albicans), one toxoplasma retinochoroiditis, and one longstanding retinal detachment. The remaining 28/33 patients with low suspicion of (P)VRL underwent therapeutic vitrectomy, and already had a presumptive diagnosis of either sarcoidosis or latent TB‐related uveitis, but were analyzed to rule out a diagnosis of (P)VRL. Based on the combination of clinical characteristics and imaging and laboratory findings, a definite diagnosis was made to divide the patients into different subgroups (Table S1 and S2). The (P)VRL group eventually consisted of 10 cases (Table 1, Table S3), whereas in the other 43 cases no compelling evidence could be obtained for (P)VRL from the combination of clinical, imaging and laboratory outcomes. This group was quite heterogeneous with different diagnoses (a.o. sarcoidosis and sarcoidosis suspect, n = 15; tuberculosis‐related uveitis, n = 6; multiple sclerosis (MS)‐related uveitis, n = 2; infectious uveitis, n = 2; other types of uveitis, n = 18), most subgroups being relatively small. Therefore, in the rest of the study, these 43 cases were all combined into a non‐(P)VRL group.
Table 1.
Detailed characterization of ten cases with primary vitreoretinal lymphoma diagnosis
| Vitreous specimen | Gender/age | % B cells | % SmIgK (within B) | % SmIgL (within B) | SmIgK/L ratio | IL‐6 | IL‐10 | IL‐10/IL‐6 ratio | IL‐1RA |
|---|---|---|---|---|---|---|---|---|---|
| 19 | M/58 | 43 | 0.1 | 100 | <0.1 | 41.54 | 369.7 | 8.9 | 257.38 |
| 20 | F/67 | 33 | 100 | 0.1 | >10 | 42.88 | 180.58 | 4.2 | 82.6 |
| 21 | M/45 | 89 | 98 | 2 | >10 | 299.26 | 2950.29 | 9.8 | 2005.85 |
| 22 | M/57 | 0.1 | 50 | 50 | 1,0 | 11.83 | 84.38 | 7.1 | 31.56 |
| 23 | F/85 | 0.1 | 0.1 | 100 | <0.1 | 4078.09 | 16486.91 | 4.0 | 6851.13 |
| 24 | M/71 | 2 | 0.1 | 100 | <0.1 | 104.70 | 838.66 | 8.0 | 496.66 |
| 25 | F/83 | 3 | ND | ND | ND | 760.21 | 2.27 | <0.1 | 6.53 |
| 26 | M/27 | 83 | 100 | 0.1 | >10 | 5.72 | 189.73 | 33.2 | 153.83 |
| 27 | F/75 | 78 | 100 | 0.1 | >10 | 36.19 | 1789.63 | 49.5 | 1309.58 |
| 28 | M/58 | 41 | ND | ND | ND | 68.96 | 502.31 | 7.3 | 251.91 |
IL = interleukin, SmIg = surface membrane immunoglobulin.
In 6/10 (P)VRL patients (cases 19–21 and 26–28) relatively high percentages of B cells (33–89%) were found (Table 1), and in five of these vitreous specimens the B‐cell populations displayed monotypic SmIgκ or SmIgλ expression translating into a skewed SmIgκ/SmIgλ ratio (Fig. 1). In one vitreous sample (case 28) neither SmIgκ nor SmIgλ expression was observed, which is also indicative of the presence of an abnormal cell population (Table 1). The other four (P)VRL (cases 22–25) only contained a small B‐cell infiltrate (<3%), yet two of these still showed clear SmIgλ restriction (cases 23, 24); from the other two vitreous specimens one showed abnormal lack of SmIgκ and SmIgλ expression on the B cells (case 25), while the other displayed a normal SmIgκ/SmIgλ ratio (case 22; Table 1). Thus, 9/10 (P)VRL cases showed signs of aberrant SmIg light chain expression (i.e. skewed ratio, n = 7 or SmIg‐, n = 2), while one case showed a normal SmIgκ/SmIgλ ratio. In the non‐(P)VRL group clear B‐cell infiltrates (9–28%) were seen in vitreous specimens of nine patients, which was accompanied by clearly skewed SmIg light chain expression in the 2 MS‐related uveitis cases, but not in other uveitis (Fig. 1). Hence, the sensitivity of the flow cytometric analysis was high (90%) for detecting aberrant B‐cell populations, but its specificity (95%) was somewhat lower than previously reported (100%) (Missotten et al. 2013). We then looked at the total cellular composition of the vitreous specimens and performed unsupervised cluster analysis (omniviz, correlation and heatmapper) taking into account the percentages of the various cell types (B cells, T cells, NK cells, monocytes, neutrophils) as well as SmIgκ/SmIgλ and CD4/CD8 ratios. However, this did not improve accurate identification of all (P)VRL cases; in fact, clustering was seen for the four (P)VRL that had a relatively large and aberrant B‐cell infiltrate, while the six (P)VRL with a mostly small B‐cell infiltrate did not cluster with these four (data not shown). Taken together, these data imply that (P)VRL can be diagnosed via flow cytometric immunophenotyping, especially when a clear monoclonal B‐cell infiltrate is present in the vitreous specimen, but that additional parameters are essential to reliably distinguish (P)VRL from non‐(P)VRL.
Figure 1.
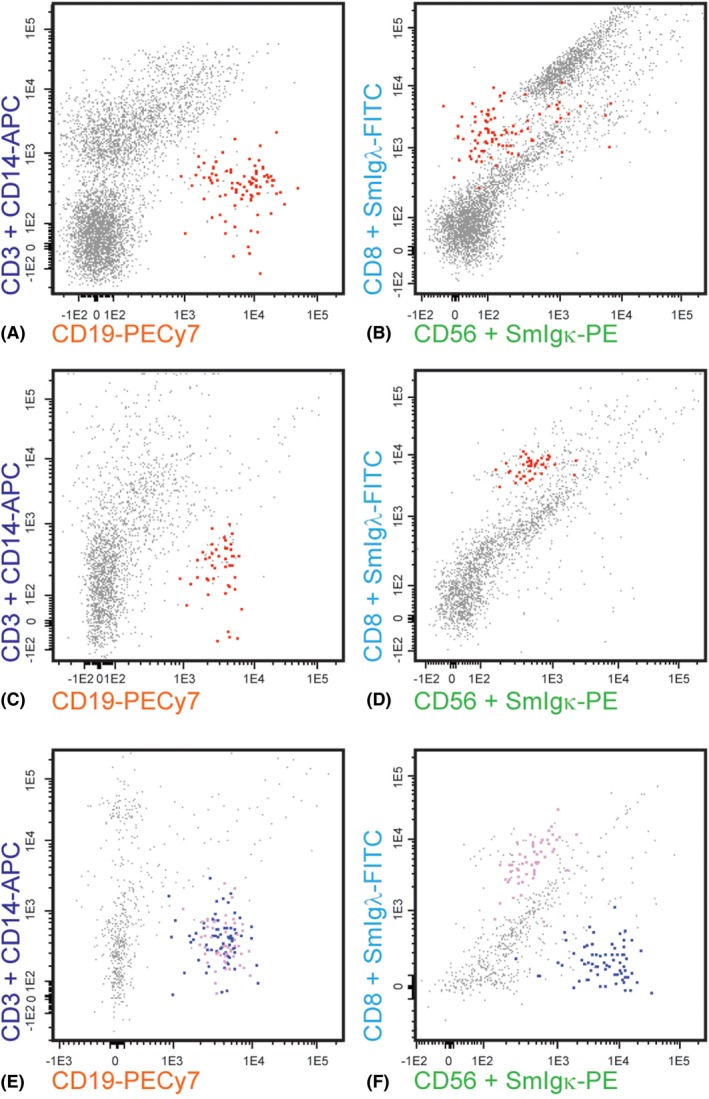
Flow cytometric analysis of B cells in vitreous specimens. (A, B) primary vitreoretinal lymphoma; all CD19+ B cells (marked in red) show monotypic surface membrane immunoglobulin lambda (SmIgλ) expression; (C, D) Multiple sclerosis‐related uveitis; all CD19+ B cells (marked in red) show monotypic SmIgλ expression; (E, F) Uveitis in the context of sarcoidosis; CD19+ B cells either show SmIgκ (blue) or SmIgλ (purple) expression.
We therefore performed multiplex cytokine/chemokine/growth factor analysis on 52 of the 53 available vitreous specimen supernatants. Taking note of published data on high IL‐10 levels and a high IL‐10/IL‐6 ratio in (P)VRL (Whitcup et al. 1997; Buggage et al. 1999; Cassoux et al. 2007; Costopoulos et al. 2016), we first focused on IL‐10 and IL‐6 levels in the cohort. Indeed, in 9/10 (P)VRL vitreous samples a high IL‐10 concentration was measured, combined with an IL‐10/IL‐6 ratio >1; in 1/10 (P)VRL the IL‐10 concentration was not increased and the ratio <1 (case 25; Table 1). Interestingly, in the non‐(P)VRL group only 1/42 vitreous specimens showed a high IL‐10 concentration, albeit that the IL‐10/IL‐6 ratio was still <1 due to a concomitant high IL‐6 concentration. Hence, sensitivity and specificity of IL‐10/IL‐6 measurements was 90% and 100%, respectively. Further detailed analysis of the cytokine data revealed that the IL‐1 receptor antagonist (IL‐1 RA) concentration was also high in 9/10 (P)VRL vitreous specimens, as compared to the non‐(P)VRL samples; the single exception was the abovementioned (P)VRL case 25 that showed a low IL‐10 concentration and an IL‐10/IL‐6 ratio <1 (Table 1). Subsequent unsupervised omniviz cluster analysis using concentrations of the various cytokines and chemokines in the vitreous samples displayed clear clustering of the nine (P)VRL, with the exception of the above discussed (P)VRL (low IL‐10, IL‐10/IL‐6 ratio <1 and low IL‐1 RA), and without clustering of the two MS‐related uveitis specimens (Fig. 2A).
Figure 2.
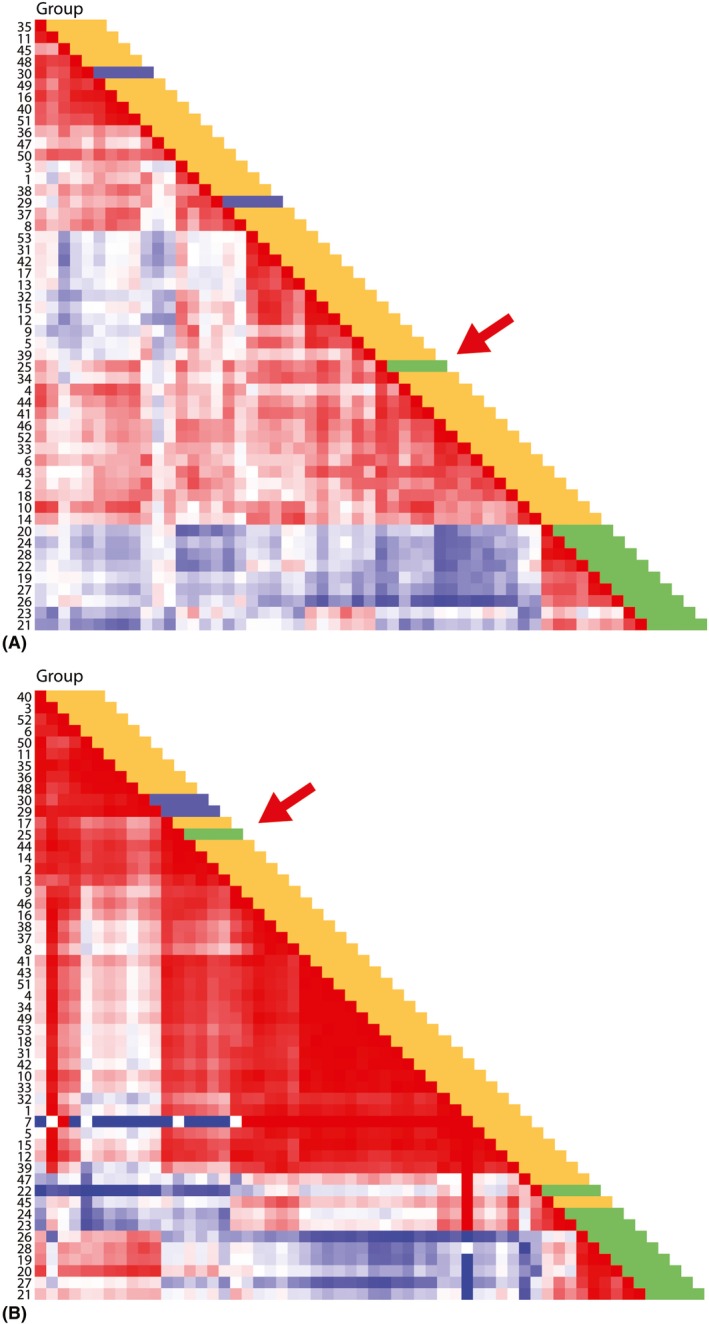
Omniviz‐based visualization of soluble mediators in vitreous specimens. (A) HeatMapper analysis of cytokine, chemokine, growth factor levels, including interleukin (IL)‐10/IL‐6 ratio, shows clustering of 9/10 primary vitreoretinal lymphoma [(P)VRL], while 1 (P)VRL (sample 25) does not cluster (red arrow). Colours represent different diagnosis: green: (P)VRL; blue: multiple sclerosis (MS)‐related uveitis; yellow: other uveitis. (B) Same analysis with top seven of cellular and soluble mediator parameters shows clustering of 9/10 (P)VRL, except for the same (P)VRL as in (A) (sample 25; red arrow). Notably, the (P)VRL with a small B‐cell infiltrate (samples 22, 23, 24) form a subcluster, with coclustering of one non‐(P)VRL case (sample 45). Colours represent different diagnosis: green: (P)VRL; blue: MS‐related uveitis; yellow: other uveitis.
Also after unsupervised clustering of the combined cellular and soluble mediator datasets, the same 9/10 (P)VRL clustered (data not shown). More detailed analysis revealed a top 7 of statistically significant markers to distinguish (P)VRL from non‐(P)VRL (Table 2). Using these seven parameters a new unsupervised analysis was performed that resulted in clustering of the same 9/10 (P)VRL specimens, still without the two MS‐related cases (Fig. 2B). Notably, the (P)VRL cluster consisted of two subclusters, mostly related to size of the B‐cell infiltrate (small versus large) with one non‐(P)VRL coclustering with the small infiltrate (P)VRL cases (Fig. 2B). Taken together, the data from this pilot study thus suggest that a signature of cellular parameters (CD19+ B cells, aberrant SmIgκ/SmIgλ ratio) and cytokine parameters (IL‐10/IL‐6 ratio >1, high IL‐10, high IL‐1 RA, high MCP‐1, high MIP‐1β) collectively allows to reliably distinguish (P)VRL from non‐(P)VRL.
Table 2.
Signature of seven statistically most significant markers to distinguish primary vitreoretinal lymphoma
| Parameter | Typical profile | Function |
|---|---|---|
| IL‐10 | Increased | Cytokine (anti‐inflammatory) |
| IL‐1RA | Increased | IL‐1 receptor antagonist |
| Ratio IL‐10/IL‐6 | >1 | Anti‐/pro‐inflammatory cytokines |
| Ratio SmIgK/SmIgL | Aberrant (skewed or SmIg‐) | Skewing B cells |
| Monocyte chemotactic protein‐1 | Increased | Chemokine |
| Macrophage inflammatory protein‐1β | Increased | Chemokine |
| CD19 | Increased | Pan B‐cell marker |
IL = interleukin, SmIg = surface membrane immunoglobulin.
Discussion
In a previous study, Missotten et al. (2013) showed fairly good sensitivity (82%) and specificity (100%) in patients with suspected (P)VRL when using six‐colour flow cytometric immunophenotyping. In our current study, we used 8 colours/fluorochromes to evaluate a total of 11 markers for both B cells and T cells, plus monocytes and NK cells. This new strategy gave more detailed information about the B cells and T cells, in addition to a better and more complete identification of all the cells present in the vitreous specimens. Furthermore, even though it did translate into an improved sensitivity (90%) to detect (P)VRL, its specificity was somewhat lower (95%).
Nevertheless, distinction between (P)VRL and non‐(P)VRL via multiparameter flow cytometric analysis is feasible with reasonable specificity and sensitivity. Notably, the sensitivity of flow cytometric immunophenotyping is strongly dependent on the size of the B‐cell infiltrate in the vitreous specimen, which can hamper proper detection of the malignant (P)VRL clone. Small numbers of malignant B cells in the vitreous may be explained on biological grounds (few (P)VRL cells being present in the vitreous sample), or by technical cause (fragility of cells in the vitreous material). The latter can, at least partly, be prevented by shortening transport and workup times and keeping the cells cooled on ice (De Jongste et al. 2014).
Cytokines and chemokines are important mediators of leucocyte recruitment, activation and differentiation, and are believed to reflect biologically relevant processes in the cells present in vitreous specimens. In line with this, IL‐10 that is implicated in growth and differentiation of malignant B cells, was previously found to be elevated in (P)VRL vitreous specimens with a sensitivity of 89% and specificity of 93% for the diagnosis of (P)VRL (Whitcup et al. 1997; Cassoux et al. 2007). Furthermore, an IL‐10/IL‐6 ratio of >1 was seen in vitreous material of (P)VRL patients, in contrast to a ratio <1 which was suggestive of uveitis, due to the fact that normal inflammatory cells secrete more IL‐6 (Wang et al. 2011). In our study, we found that besides the vitreous IL‐10 level and the IL‐10/IL‐6 ratio, also increased levels of IL1‐RA and the chemokines MCP‐1 and MIP‐1β contribute in differentiating (P)VRL from non‐(P)VRL uveitis. In a recent report, Kuiper et al. (2017) also found evidence for IL‐10 and MCP‐1 to distinguish (P)VRL from a.o. non‐infectious uveitis. The added value of cytokine analysis was most clearly seen in the two patients in our cohort who were clinically diagnosed as having uveitis in the context of MS. Based on flow cytometry only, these patients would have been (mis)classified as (P)VRL based on the monoclonal B‐cell population, but the cytokine profile (IL‐10, IL‐10/IL‐6 ratio, IL‐1RA) was clearly different from the (P)VRL cases. Similar false positive results were seen before in another flow cytometric study where the authors detected monotypic B‐cell populations in cerebrospinal fluid specimens of patients who were ultimately found to have MS (Vafaii & DiGiuseppe 2014), representing a possible diagnostic pitfall of flow cytometry.
In our cohort we encountered one peculiar case (case 25), which was originally classified as B‐cell lymphoma based on laboratory findings, but which showed a different cytokine profile (low IL‐10, low IL‐1 RA, IL‐10/IL‐6 ratio <1, plus few B cells without detectable SmIgκ and/or SmIgλ expression) compared to the other (P)VRL cases. Retrospective analysis of this single case that did not end up in the (P)VRL cluster in any of the clustering analyses, revealed that it was eventually clinically classified as lymphoma due to the presence of B‐cell type chronic lymphocytic leukaemia as detected in peripheral blood. Hence, the uveitis of this patient was not so much caused by (P)VRL, but the uveitis merely coexisted with the leukaemia. This implies that the results from the cytokine analyses would even be better than the current data suggests, if we do not consider this case as true (P)VRL.
The combination of flow cytometric and proteomics data appears the more optimal strategy to detect (P)VRL at diagnosis. Firstly, because the combination gives an optimal sensitivity and specificity, and secondly, because it couples detection of the malignant cells with analysis of the mediators that are produced by such cells. For the gold standard technique in diagnosing (P)VRL, that is cytology, a repeat vitrectomy is often required. One could argue if the gold standard is to be respected in the future, considering the promising results of current research on cell populations, soluble mediators and perhaps also genetic factors such as MYD88 mutations (Bonzheim et al. 2015; Raja et al. 2016; Cani et al. 2017) or miRNA profiles (Tuo et al. 2014; Kakkassery et al. 2017). Further validation of the combined cellular and cytokine signature is required in vitreous specimens of a new cohort of patients with chronic uveitis. In contrast to the combined role of cellular and soluble mediator evaluation for establishing the (P)VRL diagnosis, the here described cytokine profile by itself might be valuable for follow‐up analysis of (P)VRL patients upon therapy, both in vitreous samples and potentially also in anterior chamber fluid.
The here defined profile of cellular parameters (CD19 B cells, SmIgκ/SmIgλ ratio) and cytokines (MCP‐1, MIP1β, IL‐10, IL‐1RA, IL‐10/IL‐6 ratio) strongly suggest that (P)VRL are characterized by a monoclonal B‐cell infiltrate in a local environment characterized by increased anti‐inflammatory properties. The latter is supported by the high IL‐10 and IL‐1RA levels that could act to dampen the inflammatory reaction, thus contributing to survival of the (P)VRL cells in their intraocular niche. Similar observations on the importance of IL‐10 for cell survival and proliferation have been made in primary DLBCL and PCNSL (Nguyen‐Them et al. 2016; Cha et al. 2017).
Finally, the non‐(P)VRL group consists of different types of uveitis, which could not be divided in clear (sub)clusters based on the current combination of flow cytometric and proteomics data. It should be noted however that some of these diagnostic subgroups were rather small, which might hamper such classification in our current pilot study. In addition, it might well be that for proper (sub)clustering within the non‐(P)VRL group multiple and other parameters are needed. Novel high‐throughput multiparameter strategies should be evaluated to find such markers. Currently, we are exploring such novel strategies, while at the same time increasing cases in particular subgroups to increase the statistical power of the analyses.
In summary, here we show the power of a combined cellular and proteomics strategy for detecting (P)VRL in vitreous specimens. Implementation of specific cytokine measurements allows better distinction between (P)VRL and non‐(P)VRL, especially in cases with a minor cellular (P)VRL infiltrate, although there is always a small risk of overinterpretation of data when immunophenotyping evidence is lacking. Detailed analysis shows a signature of both cellular and cytokine parameters that provides good sensitivity and specificity for identifying (P)VRL.
Supporting information
Table S1. Cellular parameters.
Table S2. Soluble mediator parameters.
Table S3. Survival data lymphoma.
These authors contributed equally as first authors.
References
- Bastiaans J, van Meurs JC, Mulder VC et al. (2014): The role of thrombin in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 55: 4659–4666. [DOI] [PubMed] [Google Scholar]
- Bonzheim I, Giese S, Deuter C et al. (2015): High frequency of MYD88 mutations in vitreoretinal B‐cell lymphoma: a valuable tool to improve diagnostic yield of vitreous aspirates. Blood 126: 76–79. [DOI] [PubMed] [Google Scholar]
- Buggage RR, Whitcup SM, Nussenblatt RB & Chan CC (1999): Using interleukin 10 to interleukin 6 ratio to distinguish primary intraocular lymphoma and uveitis. Invest Ophthalmol Vis Sci 40: 2462–2463. [PubMed] [Google Scholar]
- Cani AK, Hovelson DH, Demirci H, Johnson MW, Tomlins SA & Rao RC (2017): Next generation sequencing of vitreoretinal lymphomas from small‐volume intraocular liquid biopsies: new routes to targeted therapies. Oncotarget 8: 7989–7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassoux N, Giron A, Bodaghi B et al. (2007): IL‐10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci 48: 3253–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha Z, Qian G, Zang Y et al. (2017): Circulating CXCR5+CD4+ T cells assist in the survival and growth of primary diffuse large B cell lymphoma cells through interleukin 10 pathway. Exp Cell Res 350: 154–160. [DOI] [PubMed] [Google Scholar]
- Chan CC & Sen HN (2013): Current concepts in diagnosing and managing primary vitreoretinal (intraocular) lymphoma. Discov Med 15: 93–100. [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Rubenstein JL, Coupland SE et al. (2011): Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist 16: 1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costopoulos M, Touitou V, Golmard JL et al. (2016): ISOLD: a new highly sensitive interleukin score for intraocular lymphoma diagnosis. Ophthalmology 123: 1626–1628. [DOI] [PubMed] [Google Scholar]
- Coupland SE, Chan CC & Smith J (2009): Pathophysiology of retinal lymphoma. Ocul Immunol Inflamm 17: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JL (2013): Intraocular lymphoma: a clinical perspective. Eye 27: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongste AH, Kraan J, van den Broek PD, Brooimans RA, Bromberg JE, van Montfort KA, Sillevis Smitt PA & Gratama JW (2014): Use of TransFix™ cerebrospinal fluid storage tubes prevents cellular loss and enhances flow cytometric detection of malignant hematological cells after 18 hours of storage. Cytometry B Clin Cytom 86: 272–279. [DOI] [PubMed] [Google Scholar]
- Kakkassery V, Schroers R, Coupland SE et al. (2017): Vitreous microRNA levels as diagnostic biomarkers for vitreoretinal lymphoma. Blood 129: 3130–3133. [DOI] [PubMed] [Google Scholar]
- Kuiper JJW, Beretta L, Nierkens S et al. (2017): An ocular protein triad can classify four complex retinal diseases. Sci Rep 7: 41595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missotten T, Tielemans D, Bromberg JE, van Hagen PM, van Lochem EG, van Dongen JJ, Baarsma GS & Langerak AW (2013): Multicolor flowcytometric immunophenotyping is a valuable tool for detection of intraocular lymphoma. Ophthalmology 120: 991–996. [DOI] [PubMed] [Google Scholar]
- Nguyen‐Them L, Costopoulos M, Tanguy ML et al. (2016): The CSF IL‐10 concentration is an effective diagnostic marker in immunocompetent primary CNS lymphoma and a potential prognostic biomarker in treatment‐responsive patients. Eur J Cancer 61: 69–76. [DOI] [PubMed] [Google Scholar]
- Raja H, Salomão DR, Viswanatha DS & Pulido JS (2016): Prevalence of MYD88 L265P mutation in histologically proven diffuse large B‐cell vitreoretinal lymphoma. Retina 36: 624–628. [DOI] [PubMed] [Google Scholar]
- Raparia K, Chan CC & Chévez‐Barrios P (2009): Intraocular lymphoma: diagnostic approach and immunophenotypic findings in vitrectomy specimens. Arch Pathol Lab Med 133: 1233–1237. [DOI] [PubMed] [Google Scholar]
- Sagoo MS, Mehta H, Swampillai AJ, Cohen VM, Amin SZ, Plowman PN & Lightman S (2014): Primary intraocular lymphoma. Surv Ophthalmol 59: 503–516. [DOI] [PubMed] [Google Scholar]
- Sen HN, Bodaghi B, Hoang PL & Nussenblatt R (2009): Primary intraocular lymphoma: diagnosis and differential diagnosis. Ocul Immunol Inflamm 17: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Shen D, Yang HH & Chan CC (2014): Distinct microRNA‐155 expression in the vitreous of patients with primary vitreoretinal lymphoma and uveitis. Am J Ophthalmol 157: 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafaii P & DiGiuseppe JA (2014): Detection of B‐cell populations with monotypic light chain expression in cerebrospinal fluid specimens from patients with multiple sclerosis by polychromatic flow cytometry. Cytometry B Clin Cytom 86: 106–110. [DOI] [PubMed] [Google Scholar]
- Van Dongen JJM, Lhermitte L, Böttcher S et al. (2012): EuroFlow antibody panels for standardized n‐dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 26: 1908–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shen D, Wang VM, Sen HN & Chan CC (2011): Molecular biomarkers for the diagnosis of primary vitreoretinal lymphoma. Int J Mol Sci 20: 5684–5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcup SM, Stark‐Vancs V, Wittes RE, Solomon D, Podgor MJ, Nussenblatt RB & Chan CC (1997): Association of interleukin‐10 in the vitreous and cerebrospinal fluid and primary central nervous system lymphoma. Arch Ophthalmol 115: 1157–1160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cellular parameters.
Table S2. Soluble mediator parameters.
Table S3. Survival data lymphoma.


