Short abstract
Background
Magnetic resonance spectroscopy quantitatively monitors biomarkers of neuron-myelin coupling (N-acetylaspartate (NAA)), and inflammation (total creatine (tCr), total choline (tCho), myo-inositol (mI)) in the brain.
Objective
This study aims to investigate how ocrelizumab and interferon beta-1a differentially affects imaging biomarkers of neuronal-myelin coupling and inflammation in patients with relapsing multiple sclerosis (MS).
Methods
Forty patients with relapsing MS randomized to either treatment were scanned at 3T at baseline and weeks 24, 48, and 96 follow-up. Twenty-four healthy controls were scanned at weeks 0, 48, and 96. NAA, tCr, tCho, mI, and NAA/tCr were measured in a single large supra-ventricular voxel.
Results
There was a time × treatment interaction in NAA/tCr (p = 0.04), primarily driven by opposing tCr trends between treatment groups after 48 weeks of treatment. Patients treated with ocrelizumab showed a possible decline in mI after week 48 week, and stable tCr and tCho levels. Conversely, the interferon beta-1a treated group showed possible increases in mI, tCr, and tCho over 96 weeks.
Conclusions
Results from this exploratory study suggest that over 2 years, ocrelizumab reduces gliosis compared with interferon beta-1a, demonstrated by declining ml, and stable tCr and tCho. Ocrelizumab may improve the physiologic milieu by decreasing neurotoxic factors that are generated by inflammatory processes.
Keywords: Multiple sclerosis, MRI, relapsing/remitting, magnetic resonance spectroscopy
Introduction
Magnetic resonance spectroscopy (MRS) can assess changes in brain metabolism associated with multiple sclerosis (MS) disease pathophysiology. Several MRS studies have previously reported decreased N-acetylaspartate levels (NAA) in MS compared with healthy controls,1,2 which recent literature suggests may reflect underlying mitochondrial dysfunction or insufficient myelin maintenance,3 rather than neuronal density, as previously attributed. In addition, MRS studies have repeatedly shown elevated and/or rising total creatine (tCr = creatine + phosphocreatine), total choline (tCho = choline containing compounds) and myo-inositol (mI) levels in MS, consistent with ongoing gliosis.2,4–6 MRS can also provide information about potential therapeutic mechanisms of action. Studies monitoring treatments for MS have posited that an increase in the NAA/tCr ratio demonstrates neuroprotection due to treatment.7–11
Ocrelizumab is a B-cell-depleting monoclonal antibody with greater efficacy on preventing clinical relapses, disability progression, and new focal inflammatory lesions on brain MRI in phase II & III trials compared with placebo and interferon beta-1a.12,13 The goals of this study were to use MRS to monitor patients with relapsing MS compared with healthy controls to investigate how ocrelizumab and interferon beta-1a differentially affect brain metabolites in relapsing MS, and to aid in the understanding of ocrelizumab’s mechanism of action.
Methods
Subjects
All patients recruited into the OPERA II (clinicaltrials.gov NCT01412333) double blind, double dummy, active control relapsing MS trial of ocrelizumab 600 mg IV every 24 weeks versus interferon beta-1a 44 μg subcutaneous three injections weekly (as detailed elsewhere13) at a single site (University of British Columbia, Vancouver, Canada) were invited to participate in an advanced MRI substudy. Forty patients were scanned with the MRS protocol at baseline and weeks 24, 48, and 96 follow-up. MRS data from 37 participants who completed at least two time points were included in the analysis (see Table 1 for details). Twenty-four healthy age and gender-matched controls were also scanned at baseline and weeks 48 and 96, and data from all healthy controls were included in the analysis. The study was approved by the local clinical research ethics board, and written, informed consent was obtained. Participant characteristics are summarized in Table 1.
Table 1.
Participant characteristics.
| Relapsing MS Patients |
Healthy Controls | ||
|---|---|---|---|
| Ocrelizumab Treated | Interferon Beta-1a Treated | ||
| Total Number of Subjects | 19 | 18 | 24 |
| Females : Males | 11 : 8 | 11 : 7 | 14 : 10 |
| Age in years median (range) | 37.0 (22.8–51.6) | 42.2 (18.1–55.5) | 35.2 (21.6–56.0) |
| Total Number of Subjects with MRS at Weeks 0/24/48/96 | 13/18/18/17 | 11/17/18/18 | 24/0/24/24 |
| EDSS at Baseline median (range) | 2.0 (0.75–4.5) | 2.5 (1.25–4) | |
| Disease Duration at Baseline in years median (range) | 2.9 (0.2–15.8) | 5.4 (0.4–19.3) | |
| Gadolinium MRI at Baseline Enhancing Lesions Yes : No | 3 : 16 | 3 : 15 | |
Magnetic resonance spectroscopy
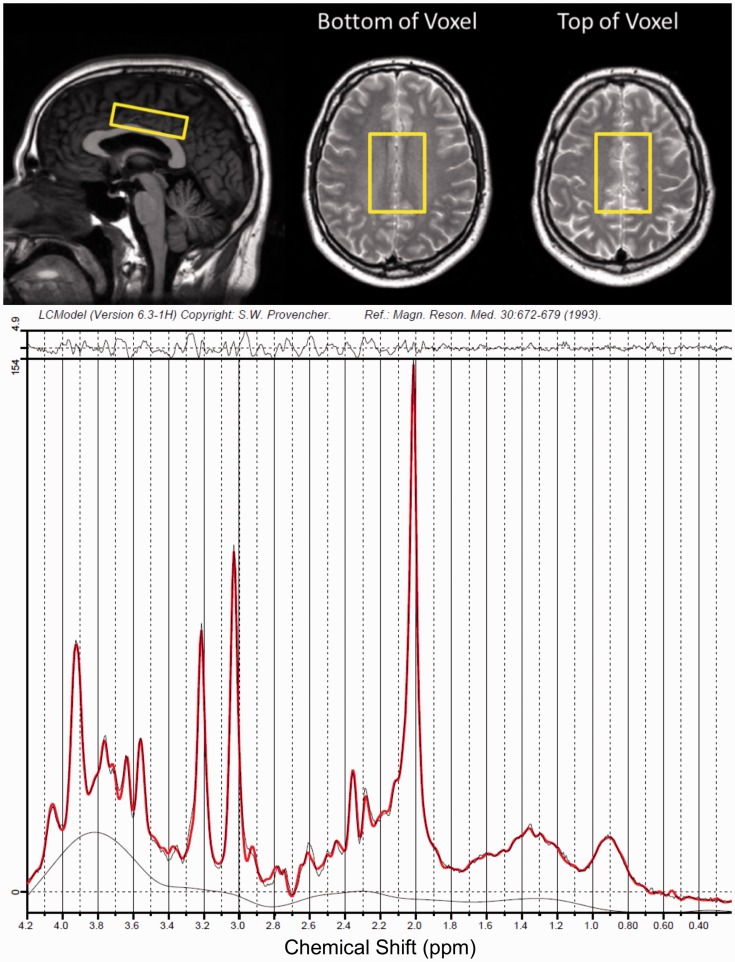
All participants were scanned with an 8-channel phased array head coil on a 3.0 T Philips Achieva MRI system (Best, The Netherlands). Proton density (TE/TR = 10/2000 ms, reconstructed voxel size 0.98 × 0.98 × 3.00 mm3) and T2 weighted images (TE/TR = 80/6100 ms, reconstructed voxel size 0.98 × 0.98 × 3.00 mm3) were acquired and used to prescribe a 6.5 × 4.5 × 1.8 cm3 primarily normal-appearing white matter voxel above the ventricles, as shown in Figure 1. Point RESolved Spectroscopy (PRESS) was used to acquire single-voxel spectra with TE/TR = 36/4000 ms, 8 phase cycle steps, 56 water-suppressed, and 8 non-water-suppressed acquisitions, which were saved as the final averaged spectrum. Automated pencil beam second-order shimming was performed on the same region as the voxel.
Figure 1.
Top: Localization of the MRS Voxel. Bottom: Example MR spectrum from a patient with MS.
Analysis was performed blinded to treatment allocation. Spectra were fit using LCModel version 6.3 with water scaling.14 MRS voxels were segmented into white matter, lesion, gray matter, and cerebrospinal fluid (CSF) using the proton density and T2 weighted images with an approach previously described elsewhere15 and resulting volume fractions are listed in Table 2. The tissue fractions were used to correct the water peak area for compartmentation and relaxation to produce absolute metabolite concentrations, as previously described.16 Individual metabolite fits were determined to be reliable if the absolute value of their error estimate was below 30% of the median metabolite concentration across all spectra.17 Outcome measures were the ratio of NAA/tCr, and the absolute concentrations of NAA (marker of neuron-myelin coupling),3,18 tCr (cellular energy metabolism),19 tCho (membrane building block),19 mI (glial cell marker),20,21 glutamate (excitatory neurotransmitter),19 and glutamine (involved in the glutamate uptake cycle).19
Table 2.
Mean voxel tissue composition for each group over time with 95% confidence intervals in brackets.
| Subject Group | Visit Week | Voxel Tissue Composition Fractions |
|||
|---|---|---|---|---|---|
| White Matter | Gray Matter | Cerebrospinal Fluid | Lesion | ||
| Relapsing MS: Orelizumab Treated | 0 | 68.4%(65.8 to 70.9%) | 21.4%(19.4 to 23.4%) | 8.8%(7.3 to 10.2%) | 1.5%(0.9 to 2.2%) |
| 24 | 68.2%(66.3 to 70.2%) | 20.7%(18.9 to 22.5%) | 10.0%(8.7 to 11.3%) | 1.9%(1.0 to 2.8%) | |
| 48 | 68.6%(66.4 to 70.8%) | 20.2%(18.2 to 22.3%) | 9.8%(8.1 to 11.5%) | 1.9%(1.2 to 2.7%) | |
| 96 | 67.4%(64.8 to 69.9%) | 21.0%(18.8 to 23.3%) | 9.7%(8.2 to 11.2%) | 1.8%(1.0 to 2.5%) | |
| Relapsing MS: Interferon Beta-1a Treated | 0 | 68.3%(64.7 to 71.8%) | 20.0%(16.9 to 23.1%) | 9.6%(7.4 to 11.8%) | 2.7%(0.6 to 4.8%) |
| 24 | 67.3%(64.1 to 70.5%) | 19.6%(17.3 to 21.8%) | 11.1%(9.1 to 13.1%) | 2.2%(1.0 to 3.4%) | |
| 48 | 66.6%(63.8 to 69.4%) | 19.2%(16.6 to 21.7%) | 11.9%(9.9 to 13.9%) | 2.4%(1.2 to 3.6%) | |
| 96 | 66.9%(63.9 to 69.9%) | 18.9%(16.9 to 20.8%) | 11.7%(9.5 to 13.9%) | 3.0%(1.4 to 4.5%) | |
| Healthy Controls | 0 | 69.8%(68.1 to 71.6%) | 22.3%(20.4 to 24.1%) | 8.4%(7.5 to 9.3%) | |
| 48 | 69.7%(68.0 to 71.5%) | 21.7%(19.9 to 23.5%) | 8.6%(7.5 to 9.6%) | ||
| 96 | 70.0%(67.8 to 72.2%) | 21.1%(19.2 to 23.0%) | 8.8%(7.7 to 10.0%) | ||
Statistics
Metabolite concentrations and the NAA/tCr ratio from the treatment groups were fit to a mixed effects model to account for repeated measures over time on each subject and to handle incomplete data from missing time points using the R Project for Statistical Computing.22 An interaction effect was included to allow the differences between treatment arms to vary by week. The visit week and treatment arms were kept as fixed effects while the subject was a random effect to account for individual variability. A separate mixed effects model was fit to the healthy controls because there were no data at week 24 for this group, with week as a fixed effect and subject as a random effect. An ANOVA was used to test for significant differences between healthy controls and treatment arms for measures of spectral quality and in the percent change over 96 weeks with the Tukey method to control for multiple comparisons. Raw uncorrected p-values are reported.
Results
This study obtained spectra with high signal to noise ratios (SNR) (median 47, range 33–58) and narrow linewidths (median 5.9 Hz, range 4.0–8.8 Hz), as calculated by LCModel based on the NAA peak. No spectra were rejected from the analysis. Across all visits, the SNR was not significantly different between patient groups, the mean SNR in the ocrelizumab cohort was 46.4 (95% confidence interval 45.4 to 47.4) and in the interferon beta-1a cohort was 46.9 (46.0 to 47.7). However, it was slightly higher in the healthy controls as compared with both patient groups (mean 48.9 (48.1 to 49.8), ANOVA p < 0.0001). The linewidths were not significantly different between any groups. The excellent spectral quality led to reliable fits for all metabolites listed above in each spectrum with the exception of glutamine, which was reliably fit in 103 out of 202 spectra.
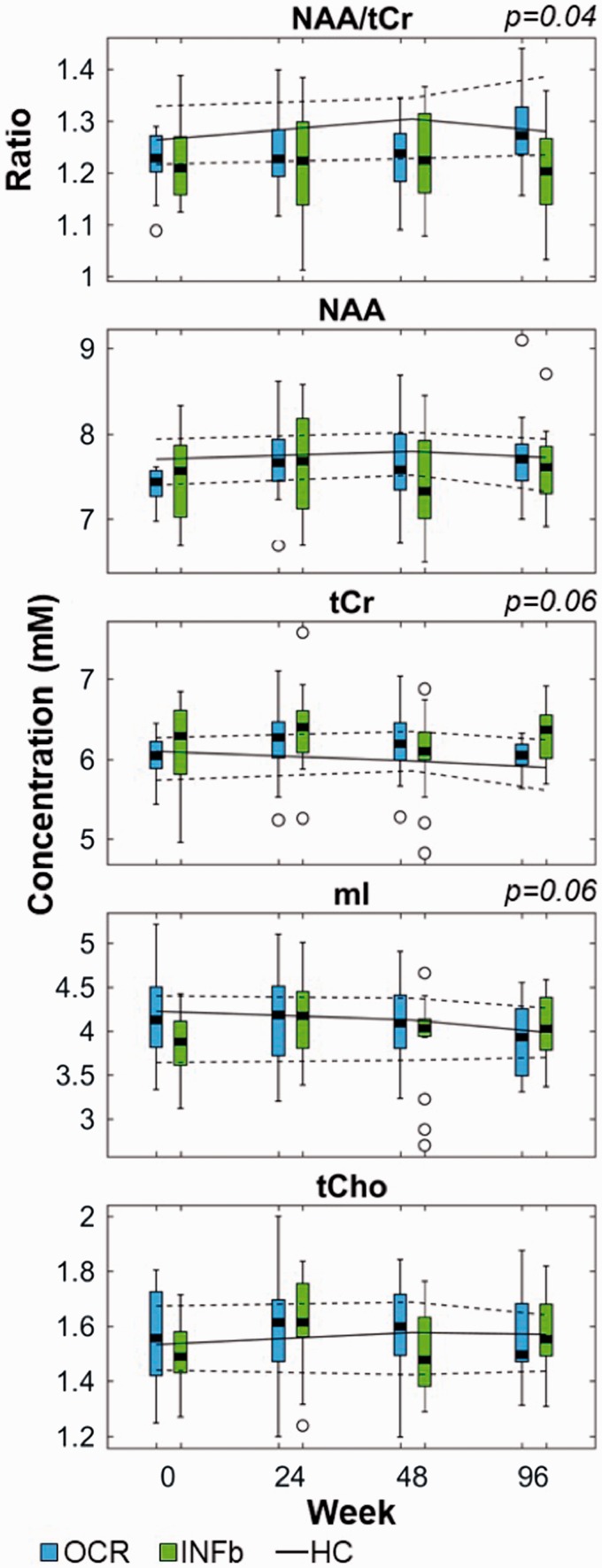
The NAA/tCr ratio and absolute concentrations at each time point for patients with relapsing MS in both treatment arms are shown in Figure 2. There was a time × treatment interaction for the ratio of NAA/tCr (p = 0.04). The absolute concentration of tCr also demonstrated a time × treatment interaction (p = 0.06), where patients receiving ocrelizumab exhibited stable levels over time while those receiving interferon beta-1a experienced an increase after 48 weeks of treatment. There was also a time × treatment interaction for mI (p = 0.06), arising from a decreasing trend in the ocrelizumab group and increasing trend in the interferon beta-1a group over 96 weeks.
Figure 2.
Metabolite concentrations over time in both treatment arms.
Boxplots of metabolite concentrations from patients treated with ocrelizumab (OCR) are shown in blue and those treated with interferon beta-1a (INFb) are shown in green. Data from matched healthy controls are demarcated by a solid line for the median with dashed lines for the 25th and 75th percentiles. p-values for the time × treatment interaction effects are listed above the plot when p ≤ 0.06.
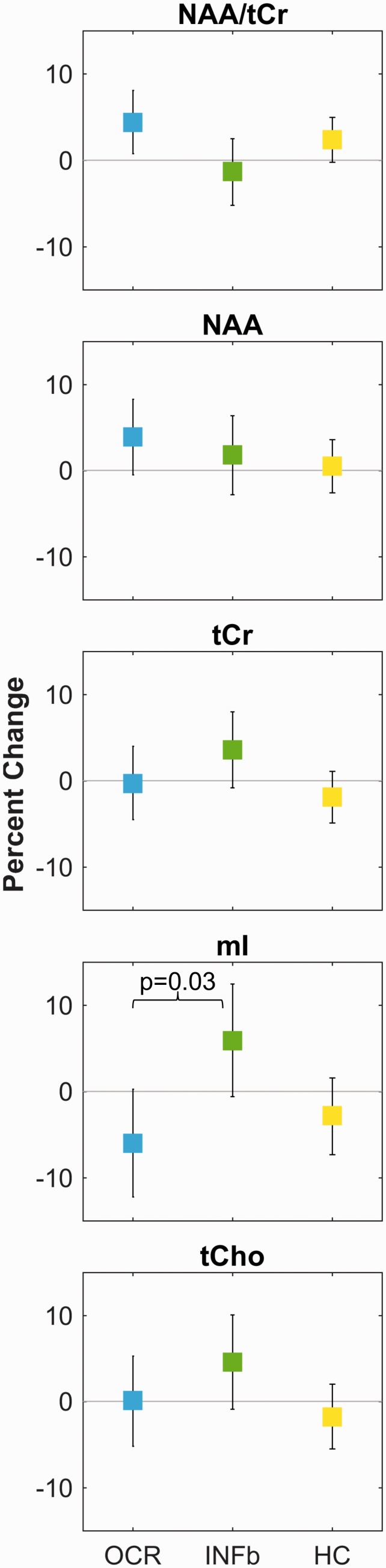
On average over 96 weeks, the NAA/tCr ratio was more likely to increase for ocrelizumab-treated patients (mean +4.4 (0.8 to 8.1) %) than for those treated with interferon beta-1a (–1.3 (–5.2 to 2.5) %), as shown in Figure 3 and Table 3. However, NAA alone tended to increase in both treatment arms, whereas tCr tended to increase in the interferon beta-1a group but remained constant in the ocrelizumab cohort. Myo-inositol was more likely to decrease in the ocrelizumab group than in the interferon beta-1a group (post-hoc contrast p = 0.03). Table 3 also demonstrates that the changes in metabolite concentrations over time were more similar between the ocrelizumab-treated patients and healthy control groups than the interferon beta-1a treated cohort for tCr, tCho, and mI.
Figure 3.
Percent change in mixed effects model means over 96 weeks.
Change in the mixed effects model means over 96 weeks shown with error bars representing the 95% confidence intervals. While the NAA/tCr ratio changes in opposite directions for the ocrelizumab and interferon beta-1a treated cohorts, these groups show the same direction of change in NAA over time. The opposing change in the NAA/tCr ratio arises from different directions of change in the tCr concentrations over time, thus the absolute concentrations are necessary for accurate interpretation of metabolic changes. The only difference between groups with p < 0.05 is the difference in the change in mI concentration over time between the two treatment arms.
Table 3.
Summary of percent change in metabolite concentrations from week 0 to 96.
| Metabolite | Relapsing MS Patients |
Healthy Controls | ANOVA Between Groups (p) | ||
|---|---|---|---|---|---|
| Ocrelizumab Treated | Interferon Beta-1a Treated | Time × Treatment Interaction (p) | |||
| NAA/tCr | 4.4 (0.8 to 8.1) % | –1.3 (–5.2 to 2.5) % | 0.04 | 2.4 (–0.2 to 5.0) % | 0.09 |
| NAA | 3.9 (–0.5 to 8.3) % | 1.8 (–2.8 to 6.4) % | 0.45 | 0.5 (–2.6 to 3.6) % | 0.44 |
| tCr | –0.3 (–4.5 to 4.0) % | 3.6 (–0.8 to 8.0) % | 0.06 | –1.9 (–4.9 to 1.1) % | 0.12 |
| mI | –6.0 (–12.2 to 0.3) % | 5.9 (–0.6 to 12.5) % | 0.06 | –2.8 (–7.3 to 1.6) % | 0.03 |
| tCho | 0.1 (–5.2 to 5.4) % | 4.6 (–0.9 to 10.1) % | 0.17 | –1.8 (–5.5 to 2.0) % | 0.17 |
Percent differences are expressed as mixed effects model means and 95% confidence intervals with associated p-values for the time × treatment interaction effect between the ocrelizumab and interferon beta-1a treated groups. Healthy control changes with time are given for comparison, as well as the p-value for the ANOVA between all three cohorts. p-values less than 0.05 are highlighted in bold.
Glutamate and glutamine did not exhibit any time × treatment interactions or differences between subject groups over 96 weeks (p > 0.23).
Discussion
This phase III clinical trial of ocrelizumab versus interferon beta-1a demonstrated a significant interaction of time × treatment for the ratio of NAA/tCr. Patients receiving ocrelizumab experienced a greater overall increase in NAA/tCr from baseline to week 96, while patients receiving interferon beta-1a were more likely to exhibit a decline over 96 weeks.
Investigating the absolute metabolite concentrations revealed that the NAA/tCr treatment interaction is primarily driven by the differing directions of tCr concentration changes between treatment groups. NAA levels were more likely to increase over time in both treatment groups and did not show a time × treatment interaction (p = 0.45). The tCr levels showed a time × treatment interaction trend over time (p = 0.06), with stable levels in the ocrelizumab-treated cohort and an overall possible increase in the interferon beta-1a group.
Changes in markers of neuron and myelin integrity
NAA is synthesized in neuronal mitochondria, released into the extracellular space, and is thought to be taken up by oligodendrocytes to provide acetate for lipid synthesis in myelin production.18 A case report of a 3-year-old child with no detectable NAA via in vivo MRS without extensive loss of neuroaxonal tissue23 suggests that NAA is not solely related to neuronal density. In addition, a recent histology finding of higher NAA concentrations in oligodendrocytes and myelin than in the axonal/neuronal cytosol or mitochondria of adult mice brains suggests that myelin synthesis is one of the primary roles of NAA in the brain.3 Hence, reduced NAA in MS brain may reflect a decline in neuron-myelin function,24 and treatment-related increases in NAA may be interpreted as improved neuron-myelin coupling.2,7–9,11,25 It should be noted that most previous studies are difficult to interpret since NAA is not often reported independently but as the confounding measure of NAA/tCr or the combination of NAA and N-acetylaspartylglutamate into a measure of total NAA (tNAA).2,7–9,11,25
In the present study, there was a trend that the absolute concentration of NAA may be more likely to increase in the ocrelizumab group compared with the interferon beta-1a group over 96 weeks; however, replication with a larger sample size is needed to confirm these observations. A previous longitudinal study of absolute metabolite concentrations that monitored 18 treated patients with relapsing–remitting MS (almost entirely treated with interferons or glatiramer acetate) found that NAA increased at a rate of 1.4% per year (raw uncorrected p = 0.04),2 which is in between the rates reported for both treatment groups in the present study. NAA was more likely to be constant over time in the healthy controls, consistent with trends observed in healthy cohorts of previous MS studies that reported absolute metabolite concentrations over a similar age range.2,25
Changes in markers of glial cell density
The total creatine and total choline signals in the brain arise from all cell types, including neurons and glia, whereas the myo-inositol signal has been shown to arise almost exclusively from glia.20,21 Thus, changes in mI levels can add cellular specificity to the interpretation of similar trends in tCr and tCho levels. Elevated levels of these three metabolites in the normal-appearing white matter of relapsing–remitting MS, or combined relapsing and progressive MS groups, have previously been suggested to indicate ongoing gliosis.2,4,5 Further evidence that these metabolites are markers of active gliosis was recently revealed by correlations between increased tCr and tCho levels in the normal-appearing white matter with increased intrathecal markers of inflammation in natalizumab-treated MS patients.6 In the present study, the interferon beta-1a cohort exhibited possible increases in concentrations of tCr, tCho, and mI between baseline and week 96, suggesting ongoing gliosis. These metabolite levels increased more rapidly than previously observed in a cohort of low Expanded Disability Status Scale (EDSS) relapsing–remitting MS patients primarily treated with glatiramer acetate or interferon beta.2 Conversely, the healthy control and ocrelizumab groups exhibited stable or possibly declining concentrations of these three metabolites, indicative of constant or decreasing glial cell density. Furthermore, mI was more likely to decline in the ocrelizumab-treated cohort than the interferon beta-1a treated group (p = 0.03). While stable tCr and tCho levels in treated relapsing–remitting MS patients have previously been reported,25 to our knowledge this is the first report of a declining trend in the glial cell marker mI over time in treated or untreated MS patients.
Study limitations
While there was a larger number of patients (40) enrolled in this study in comparison to similar MS clinical trial substudies, which involved 27 to 34 patients,6,7,11 the present substudy recruitment began after the initial study, and baseline spectra were not obtained from 6 out of 19 patients in the ocrelizumab cohort and 7 out of 18 patients in the interferon beta-1a cohort. To ensure that the lower number of baseline scans as compared with follow-up scans did not affect the overall results of this study, all analyses were also conducted separately on the subset of patients who had a baseline and at least one follow-up scan. Results from the subset of the subjects who had a baseline scan are listed in the supplemental data, and are not different from the full study results.
The excellent SNR of the spectra acquired in this study is due in part to the very large voxel size (6.5 × 4.5 × 1.8 cm3 = 53 mL) which was placed to encompass primarily white matter, with approximately 20% of the voxel being composed of gray matter (see Table 2 for voxel composition details). Thus, the metabolite concentration changes presented here reflect metabolic changes from a large, central, area of the brain above the ventricles, and it was not possible to capture regional changes in metabolite concentrations.
Conclusion
This in vivo MRS investigation demonstrated that patients treated with ocrelizumab were significantly more likely to experience declining gliosis, while patients treated with interferon beta-1a were more likely to exhibit increasing gliosis, based on MRS markers of inflammation measured in the normal-appearing white matter over 96 weeks. Ocrelizumab is an anti-CD20 B-cell depletion therapy, and is thought to reduce inflammation in MS by disrupting the role of CD20+ B-cells in antigen presentation and cytokine production.26 This targeted reduction in B-cell-mediated immune response is supported by the greater likelihood of declining glial cell density in patients treated with ocrelizumab reported here. In addition, the percent change in the marker of neuron-myelin function, NAA, over 96 weeks, appeared that it may be greater in the ocrelizumab group than in the cohort treated with interferon beta-1a. Furthermore, there were opposing changes in absolute tCr levels after week 48 between the treatment groups that would have led to the incorrect interpretation of NAA changes if only the NAA/tCr ratios were reported. This opposing change in tCr levels reemphasizes the importance of obtaining absolute concentrations to ensure maximal sensitivity to changes from the treatment, as well as correct interpretation of the biochemical mechanisms of action. Taken together, this study revealed unique, pathologically specific, insights into the potential mechanisms of action of two different treatments in patients with MS actually receiving these therapies, and demonstrates a practical approach to including MRS to unlock such insights in future clinical trials or clinical research.
Supplemental Material
Supplemental material, MSO879952 Supplemental Material1 for Magnetic resonance spectroscopy evidence for declining gliosis in MS patients treated with ocrelizumab versus interferon beta-1a by Erin L MacMillan, Julia J Schubert, Irene M Vavasour, Roger Tam, Alexander Rauscher, Carolyn Taylor, Rick White, Hideki Garren, David Clayton, Victoria Levesque, David KB Li, Shannon H Kolind and Anthony L Traboulsee in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental Material
Supplemental material, MSO879952 Supplemental Material2 for Magnetic resonance spectroscopy evidence for declining gliosis in MS patients treated with ocrelizumab versus interferon beta-1a by Erin L MacMillan, Julia J Schubert, Irene M Vavasour, Roger Tam, Alexander Rauscher, Carolyn Taylor, Rick White, Hideki Garren, David Clayton, Victoria Levesque, David KB Li, Shannon H Kolind and Anthony L Traboulsee in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Acknowledgements
We gratefully acknowledge support from UBC MRI Research Centre, patients with MS, and volunteers.
Contributor Information
Erin L MacMillan, Department of Radiology, University of British Columbia; Business Unit MR, Philips.
Julia J Schubert, Department of Medicine, University of British Columbia.
Irene M Vavasour, Department of Radiology, University of British Columbia; Department of Medicine, University of British Columbia.
Roger Tam, Department of Medicine, University of British Columbia; MS/MRI Research Group, University of British Columbia.
Alexander Rauscher, Department of Radiology, University of British Columbia; Department of Physics & Astronomy, University of British Columbia; Department of Pediatrics, University of British Columbia.
Rick White, Statistics, University of British Columbia.
Victoria Levesque, Genentech, Roche Pharmaceuticals.
David KB Li, Department of Radiology, University of British Columbia; MS/MRI Research Group, University of British Columbia.
Shannon H Kolind, Department of Radiology, University of British Columbia; Department of Medicine, University of British Columbia; MS/MRI Research Group, University of British Columbia; Department of Physics & Astronomy, University of British Columbia.
Anthony L Traboulsee, Department of Medicine, University of British Columbia; MS/MRI Research Group, University of British Columbia.
Conflicts of Interest
The author(s) declared the following potential conflicts ofinterest with respect to the research, authorship, and/orpublication of this article: Erin L. MacMillan received a postdoctoral fellowship from the MS Society of Canada and currently receives salary support from Philips Canada.
Roger Tam has received grant funding from the Natural Sciences and Engineering Research Council of Canada (NSERC), MS Society of Canada, and Mitacs, and research support as part of sponsored clinical studies from Novartis, Roche, and Sanofi Genzyme.
Alexander Rauscher has received speaking fees from Philips and research support from NSERC and the National Multiple Sclerosis Society.
Hideki Garren, David Clayton, and Victoria Levesque are employees of F. Hoffmann-La Roche.
David K.B. Li has received research funding from the MS Society of Canada. He is Emeritus Director of the UBC MS/MRI Research Group, which has been contracted to perform central analysis of MRI scans for therapeutic trials with Roche and Sanofi-Genzyme. The UBC MS/MRI Research Group has also received grant support for investigator-initiated studies from Genzyme, Novartis and Roche. He has been a consultant to Vertex Pharmaceuticals and Genzyme, served on the Scientific Advisory Board for Celgene and the PML-MS Steering Committee for Biogen He has given lectures, supported by non-restricted education grants from Academy of Health Care Learning, Consortium of MS Centers and Sanofi-Genzyme.
Shannon H. Kolind has received research support from Roche, Genzyme, the MS Society of Canada, NSERC, VCHRI, MSFHR and Milan & Maureen Ilich Foundation; consulting for Acorda, Genzyme.
Anthony L. Traboulsee has received research funding from Chugai, Roche, and Sanofi Genzyme; received honoraria or travel support from Consortium of MS Centers, MS Society of Canada, Biogen, Teva, Roche, Merck/EMD Serono, Sanofi Genzyme, Chugai.
Julia J. Schubert, Carolyn Taylor, Rick White, and Irene M. Vavasour have nothing to disclose.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by F. Hoffmann-La Roche Ltd and was supported in part by a postdoctoral fellowship from the MS Society of Canada.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.De Stefano N, Filippi M, Miller D, et al. Guidelines for using proton MR spectroscopy in multicenter clinical MS studies. Neurology 2007; 69: 1942–1952. [DOI] [PubMed] [Google Scholar]
- 2.Kirov II, Tal A, Babb JS, et al. Serial proton MR spectroscopy of gray and white matter in relapsing-remitting MS. Neurology 2013; 80: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordengen K, Heuser C, Rinholm JE, et al. Localisation of N-acetylaspartate in oligodendrocytes/myelin. Brain Struct Funct 2015; 220: 899–917. [DOI] [PubMed] [Google Scholar]
- 4.Hattingen E, Magerkurth J, Pilatus U, et al. Combined 1H and 31P spectroscopy provides new insights into the pathobiochemistry of brain damage in multiple sclerosis. NMR Biomed 2011; 24: 536–546. [DOI] [PubMed] [Google Scholar]
- 5.Vrenken H, Barkhof F, Uitdehaag BMJ, et al. MR spectroscopic evidence for glial increase but not for neuro-axonal damage in MS normal-appearing white matter. Magn Reson Med 2005; 53: 256–266. [DOI] [PubMed] [Google Scholar]
- 6.Mellergård J, Tisell A, Dahlqvist Leinhard O, et al. Association between change in normal appearing white matter metabolites and intrathecal inflammation in natalizumab-treated multiple sclerosis. PLoS One 2012; 7: e44739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold DL, Narayanan S, Antel S. Neuroprotection with glatiramer acetate: evidence from the PreCISe trial. J Neurol 2013; 260: 1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan O, Shen Y, Caon C, et al. Axonal metabolic recovery and potential neuroprotective effect of glatiramer acetate in relapsing-remitting multiple sclerosis. Mult Scler 2005; 11: 646–651. [DOI] [PubMed] [Google Scholar]
- 9.Narayanan S, De Stefano N, Francis GS, et al. Axonal metabolic recovery in multiple sclerosis patients treated with interferon beta-1b. J Neurol 2001; 248: 979–986. [DOI] [PubMed] [Google Scholar]
- 10.Sijens PE, Mostert JP, Irwan R, et al. Impact of fluoxetine on the human brain in multiple sclerosis as quantified by proton magnetic resonance spectroscopy and diffusion tensor imaging. Psychiatry Res Neuroimaging 2008; 164: 274–282. [DOI] [PubMed] [Google Scholar]
- 11.Filippi M, Rocca MA, Pagani E, et al. Placebo-controlled trial of oral laquinimod in multiple sclerosis: MRI evidence of an effect on brain tissue damage. J Neurol Neurosurg Psychiatry 2014; 85: 852–859. [DOI] [PubMed] [Google Scholar]
- 12.Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: A phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011; 378: 1779–1787. [DOI] [PubMed] [Google Scholar]
- 13.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376: 221–234. [DOI] [PubMed] [Google Scholar]
- 14.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30: 672–679. [DOI] [PubMed] [Google Scholar]
- 15.McAusland J, Tam R, Wong E, et al. Optimizing the use of radiologist seed points for improved multiple sclerosis lesion segmentation. IEEE Trans Biomed Eng 2010; 57: 2689–2698. [DOI] [PubMed] [Google Scholar]
- 16.MacMillan EL, Tam R, Zhao Y, et al. Progressive multiple sclerosis exhibits decreasing glutamate and glutamine over two years. Mult Scler J 2016; 22: 112–116. [DOI] [PubMed]
- 17.Kreis R. The trouble with quality filtering based on relative Cramér-Rao lower bounds. Magn Reson Med 2016; 75: 15–18. [DOI] [PubMed] [Google Scholar]
- 18.Francis JS, Strande L, Markov V, et al. Aspartoacylase supports oxidative energy metabolism during myelination. J Cereb Blood Flow Metab 2012; 32: 1725–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Graaf RA. In Vivo NMR Spectroscopy: Principles and Techniques. 3rd Edition Hoboken: John Wiley & Sons Ltd, 2019. Epub ahead of print 2019. DOI: 10.1002/9780470512968. [Google Scholar]
- 20.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 1993; 15: 289–298. [DOI] [PubMed] [Google Scholar]
- 21.Oz G, Alger JR, Barker PB, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 2014; 270: 658–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Team RC. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014. 2014; 3–36. [Google Scholar]
- 23.Martin E, Capone a, Schneider J, et al. Absence of N-acetylaspartate in the human brain: Impact on neurospectroscopy? Ann Neurol 2001; 49: 518–521. [PubMed] [Google Scholar]
- 24.Sajja BR, Wolinsky JS, Narayana PA. Proton magnetic resonance spectroscopy in multiple sclerosis. Neuroimaging Clin N Am 2009; 19: 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiberio M, Chard DT, Altmann DR, et al. Metabolite changes in early relapsing-remitting multiple sclerosis. A two year follow-up study. J Neurol 2006; 253: 224–230. [DOI] [PubMed] [Google Scholar]
- 26.Greenfield AL, Hauser SL. B-cell therapy for multiple sclerosis: Entering an era. Ann Neurol 2018; 83: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSO879952 Supplemental Material1 for Magnetic resonance spectroscopy evidence for declining gliosis in MS patients treated with ocrelizumab versus interferon beta-1a by Erin L MacMillan, Julia J Schubert, Irene M Vavasour, Roger Tam, Alexander Rauscher, Carolyn Taylor, Rick White, Hideki Garren, David Clayton, Victoria Levesque, David KB Li, Shannon H Kolind and Anthony L Traboulsee in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, MSO879952 Supplemental Material2 for Magnetic resonance spectroscopy evidence for declining gliosis in MS patients treated with ocrelizumab versus interferon beta-1a by Erin L MacMillan, Julia J Schubert, Irene M Vavasour, Roger Tam, Alexander Rauscher, Carolyn Taylor, Rick White, Hideki Garren, David Clayton, Victoria Levesque, David KB Li, Shannon H Kolind and Anthony L Traboulsee in Multiple Sclerosis Journal – Experimental, Translational and Clinical