Abstract
Background
Immune checkpoint inhibitors have improved clinical outcomes including survival in several malignancies but have also been associated with a range of immune-related adverse events (irAEs). Neurological irAEs are rare compared to the more typical skin, gastrointestinal, and endocrine toxicities, and are often underrecognized and challenging to diagnose. Here, we report a case of seronegative autoimmune autonomic ganglionopathy (AAG) induced by dual immune checkpoint inhibitor therapy (ICI) in a patient with metastatic melanoma.
Case presentation
A patient with metastatic melanoma was treated with ipilimumab and nivolumab. He developed a constellation of new symptoms including nausea, fatigue, and severe orthostatic hypotension refractory to fluid resuscitation. An infectious, cardiac, neurologic, and endocrine workup were unrevealing. Cardiovascular autonomic testing revealed poor sympathetic nervous system responses. He was diagnosed with seronegative AAG and significantly improved with immunomodulatory therapies including IVIG and steroids as well as varying doses of midodrine and fludrocortisone. He was able to restart nivolumab without recurrence of his symptoms. However, the AAG reoccurred when he was re-challenged with ipilimumab and nivolumab due to disease progression. While the AAG was manageable with steroids at that time, unfortunately his melanoma became resistant to ICI.
Conclusions
Immune checkpoint inhibitors can have a wide range of unusual, rare irAEs, including neurotoxicity such as AAG. Clinicians should maintain suspicion for this toxicity so that treatment can be rapidly provided to avoid disability.
Keywords: Immune checkpoint inhibitors, Ipilimumab, Nivolumab, Autoimmune, Autonomic ganglionopathy, Melanoma
Background
Monoclonal antibodies against the immune checkpoints cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) (ipilimumab) and programmed-death-1 (PD-1) (nivolumab, pembrolizumab) have the potential to induce long-term durable responses in patients with advanced melanoma [1–4]. Dual checkpoint inhibition with ipilimumab and nivolumab has led to 3-year overall survival rates of over 50%, but these improved clinical outcomes can be at the expense of immune-related toxicity. The rate of grade > 3 adverse events for patients treated with combination ipilimumab and nivolumab is greater than 50% [5]. While the most common toxicities impact the skin, gastrointestinal tract, and endocrine organs and are well-characterized, rare but serious neurological immune-related adverse events (irAE) have been described [6]. Neurotoxicity attributable to immune checkpoint inhibitors (ICI) is estimated to occur in up to 3% of patients [7, 8] and represents a heterogeneous constellation of syndromes including Guillan-Barre, peripheral neuropathies, myasthenia gravis, and encephalitis among others [9]. Here, we report a case of seronegative autoimmune autonomic ganglionopathy (AAG) induced by dual checkpoint inhibition in a patient with metastatic melanoma. To our knowledge, this is the first case of AAG attributed to ICI reported in the literature.
Case presentation
A 60-year-old man initially presented with rectal bleeding and discomfort. On physical exam, a rectal mass was initially identified as hemorrhoids. Hemorrhoidectomy was performed, and pathology showed an over 20 mm thick ulcerated mucosal melanoma extending to the margins with a high mitotic rate and the presence of lymphovascular invasion. Tumor profiling showed the malignancy to be BRAF wild-type and KIT mutated (D579 deletion). Upon referral to our institution, staging CT scans showed an enlarging anal mass, a right inguinal mass, and multiple pulmonary nodules consistent with metastatic disease. He underwent palliative trans-anal excision of the rectal mass and was urgently started on dual ICI with ipilimumab 3 mg/kg and nivolumab 1 mg/kg once every 3 weeks for a total of four planned doses. After the third cycle, he presented with a constellation of new symptoms including nausea, constipation, weight loss, fatigue, and hypotension (seated systolic BP as low as 70 mmHg systolic). ICI was held, and he was admitted for further work-up.
His blood pressure did not respond to an initial intravenous fluid challenge of 5 l of normal saline. There were no localizing signs of infection, leukocytosis, tachycardia, or fever, so both sepsis and cytokine release syndrome were felt to be unlikely. His examination was negative other than for orthostatic hypotension. His pupillary responses to light and accommodation, and motor and sensory examinations were normal. A cardiac workup with transthoracic echocardiogram showed preserved ejection fraction without diastolic dysfunction, no significant valvular disease, and no pericardial effusion. A cardiac MRI had no acute findings. An endocrinopathy was considered, however multiple morning cortisol levels were normal as were TSH and a comprehensive evaluation of pituitary function including LH, FSH, prolactin, and GH, thereby ruling out hypopituitarism. There was also no evidence of mineralocorticoid deficiency (normal aldosterone and renin). Other etiologies of autonomic neuropathy were investigated including a work-up for autoimmune (ANA, creatinine kinase), infectious (Lyme, syphilis, HIV), and neurologic (anti-cholinergic receptor antibodies, anti-GAD65 antibody) causes, nutritional deficiencies (B12), and paraneoplastic syndromes (Mayo Clinic paraneoplastic antibody panel), all of which were negative (Table 1). MRI of the brain was negative for intracranial metastases and had no abnormalities that could explain his symptoms. There was no family history of dysautonomia, synucleopathies, or other neurologic disorders.
Table 1.
Lab test results
| Lab | Result | Normal range |
|---|---|---|
| Aldosterone | 13 ng/dL | 3–16 ng/dL |
| Plasma renin activity | 0.51 ng/mL/h | 0.25–5.82 ng/mL/h |
| TSH | 3.500 uLU/mL | 0.300–4.200 uLU/mL |
| Free T4 | 1.12 ng/dL | 0.8–1.80 ng/dL |
| Total T3 | 69.9 ng/dL (slightly low likely due to impaired T4 to T3 conversion in illness) | 79–149 ng/dL |
| Prolactin | 20.1 ng/mL (mildly elevated thought to be from chronic prochlorperazine use) | 0–14.0 ng/mL |
| FSH | 7.1 mU/mL | 1–12 mU/mL |
| LH | 7.1 mU/mL | 1.6–9.6 mU/mL |
| AM cortisol | multiple unstimulated cortisol levels as high as 22.3 | 7–25 μg/dL |
| Testosterone total | 334 ng/dL | 250–1100 ng/dL |
| Free testosterone | 60.7 pg/mL | 35–155 pg/mL |
| Sex hormone binding globulin | 61 nmol/L | 22–77 nmol/L |
| ANA by IFA | < 1:80 | < 1:80 |
| B12 | 1187 pg/mL | 180–914 pg/mL |
| Lyme antibodies | 0.43 LI | < 0.90 LI |
| Treponema pallidum antibody | Nonreactive | nonreactive |
| Total CK | 61 U/L | 24–195 U/L |
| HIV | Negative | Negative |
| Anti-Neuronal Nuclear Ab, Type 1, 2, 3 | Negative | < 1:240 titer |
| Anti-Glial Nuclear Ab, Type 1 | Negative | < 1:240 titer |
| Purkinje Cell Cytoplasmic Ab Type 1, 2, Tr | Negative | < 1:240 titer |
| Amphiphysin Antibody | Negative | < 1:240 titer |
| CRMP-5, IgG | Negative | < 1:240 titer |
| Striational Antibody | Negative | < 1:120 titer |
| P/Q-Type Calcium Channel Ab | 0.00 | <=0.02 nmol/L |
| N-Type Calcium Channel Ab | 0.00 | <=0.03 nmol/L |
| ACh Receptor (Muscle) Binding Ab | 0.00 | <=0.02 nmol/L |
| AChr Ganglionic Neuronal Antibody | 0.00 | <=0.02 nmol/L |
| Neuronal (V-G) K+ Channel (CASPR2, LG11) Antibody | 0.01 | <=0.02 nmol/L |
| Anti GAD-65 Ab | 0.00 | <=0.02 nmol/L |
There was no evidence of volume depletion based on objective bioimpedance measures of total body water and extracellular water. On formal autonomic testing (Finapres NOVA, Finapres Medical Systems, Enschede, Netherlands), he had a low supine resting heart rate (51 bpm) and BP (91/50 mmHg). Slow deep breathing revealed blunted amplitude (4.5 bpm [normal > 7 bpm]) at a low heart rate range (45–52 bpm). Valsalva maneuver resulted in normal heart rate responses (Valsalva ratio 1.38–1.57 [normal > 1.29]) but a “flat top” blood pressure profile and absent phase 4 overshoot. This constellation of findings was indicative of significant sympathetic dysfunction and resultant parasympathetic predominance. His cold-pressor test resulted in only a modest rise in blood pressure (91/52 to 108/63 mmHg [normal: BP increase by > 20/10 mmHg]) and no change in heart rate, also indicative of poor sympathetic reserve. On orthostatic testing, systolic blood pressure dropped from supine average of 92 mmHg to 68 mmHg within 30 s of standing, and further down to 57 mmHg by the 50th second, at which time we terminated the test. His heart rate increased from 49 at baseline to 63 bpm at termination of orthostasis. Peripheral resistance averaged 860 dyn.s.cm− 5 at baseline and increased only minimally (to ~ 990 dyn.s.cm− 5) with standing. Concomitantly, stroke volume decreased from 83 ml supine to 54 ml at the end of standing (50 s), suggesting excessive venous pooling. His hypotension, chronotropic incompetence, suboptimal baroreflex-mediated responses, impaired increase in vascular resistance and significant venous pooling all indicated a loss of sympathetic tone, consistent with acute autonomic dysfunction due to an acute autonomic ganglionopathy which, in his case, was presumed to be autoimmune in nature given its development while on ICI.
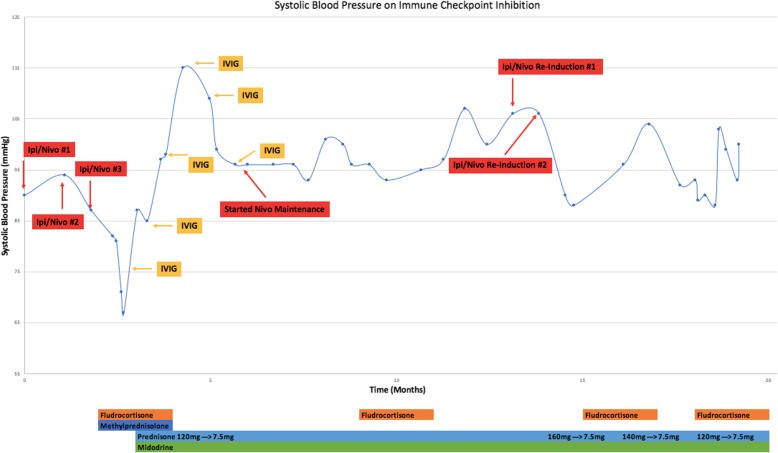
Treatment was initiated with pulse dose solumedrol 1 g IV for 6 days that was converted to oral prednisone upon hospital discharge and was slowly tapered over months. Although there was no serologic evidence of the presence of autoantibodies, seronegative cases of autoimmune autonomic ganglionopathy (AAG) have been shown to respond to intravenous immune globulin (IVIg) [10, 11]. Thus in addition to steroids, IVIG was administered initially as 0.4 g/kg daily for 5 days (total dose 2 g/kg), followed by 1 g/kg every 2 weeks as maintenance (Fig. 1). He was also maintained on midodrine (up to 20 mg three times daily), fludrocortisone (up to 0.3 mg per day in divided doses) and sodium chloride tablets (1 g three times daily). His blood pressure gradually improved to 100/60s mmHg and several months later fludrocortisone and salt were tapered off, midodrine dose was tapered down, IVIg was discontinued, and he was maintained on prednisone 7.5 mg daily, with systolic blood pressures ranging 100–120 s mmHg and minimal orthostatic changes on variable doses of midodrine as the sole treatment agent.
Fig. 1.
Laboratory testing at first presentation of symptoms
Restaging CT scans approximately 4 months after the last dose of combined ICI showed disease progression in the lung and anal region for which he underwent re-excision of the anal mass followed by palliative radiation. Nivolumab alone was restarted which was well tolerated and he did not have reoccurrence of AAG at this time. Six months later CT scans showed disease progression and he was re-induced with combination ICI, but with only 1 mg/kg of ipilimumab and 3 mg/kg of nivolumab. This led to a milder exacerbation of his orthostatic hypotension, which responded to 2 mg/kg of prednisone followed by a slow taper. After his symptoms improved, he was re-challenged with nivolumab alone but again became hypotensive and ICI was discontinued. He also developed autoimmune transaminitis that was rapidly responsive to oral prednisone. Other treatment options were offered, however he opted for hospice care and ultimately passed away from disease progression.
Discussion and conclusions
Dual ICI has improved clinical outcomes in advanced melanoma but often requires treatment delays and/or discontinuation due to irAEs [12]. While the most common irAEs of any grade are gastrointestinal (i.e. diarrhea 45%, colitis 13%, hepatitis 20%), skin (i.e. rash 30%, pruritus 35%), and endocrinopathies (hypothyroidism 17%, hypophysitis 7%), neurologic irAEs are far less common (~ 3%), are infrequently reported in ICI clinical trials, and are only more recently being recognized and reported. Polyneuropathies, Guillain-Barré syndrome, transverse myelitis [9], enteric neuropathy manifesting as constipation [13, 14], and myasthenia gravis with or without the presence of acetylcholine receptor antibodies have been described in case reports [15–18]. The rate of neurotoxicity attributable to ICI is rare, only 1–3%, however it is likely an underrecognized complication [19]. While the typical irAEs have a more identifiable symptomatology and time course in relation to ICI, neurotoxicity has variable manifestations making its diagnosis a challenge. Suspicious neurologic symptoms should prompt a comprehensive work-up because exclusion of alternative diagnoses and timely recognition is important to initiate the correct treatment. Patients should be managed in multidisciplinary fashion with input from neurologic specialists. Unfortunately, there are no clinically validated assays that can predict patients who are at risk for neurotoxicity or other irAEs, however this is currently an area of active research.
AAG can have a range of symptoms, many of which are quite debilitating [20] as was the case with our patient. Common symptoms may include sympathetic dysfunction manifesting as orthostatic hypotension, syncope, and anhidrosis, parasympathetic dysfunction such as dry eyes and dry mouth, and/or enteric dysfunction including constipation and gastroparesis [21]. Antibodies associated with AAG include most commonly the ganglionic nicotinic acetylcholine receptor antibodies [22], and cases have been described in paraneoplastic syndromes (several antibodies described, most commonly anti-neuronal antibody type 1 antibodies, also known as anti-Hu antibodies). However seronegative cases have also been reported [22] and it is thought that other auto-antibodies, potentially not yet discovered, are contributory in such cases [21, 23]. While the serologic testing in this patient was negative, the AAG was still attributed to an autoimmune mechanism given the clinical setting and in the absence of other apparent causes other than the melanoma itself and the ICI treatment.
AAG in non-ICI-induced settings is treated with steroids, IVIG, or plasma exchange, and there is some evidence that mycophenolate and rituximab may be used in patients who are refractory of these initial immunomodulatory agents [24]. This overlaps with the general principle of immunomodulatory agents recommended for treatment of ICI-induced irAEs and was successful in treating our patient [25, 26]. Case series have demonstrated that IVIg can be efficacious for AAG, as it was here, irrespective of whether antibodies against the acetylcholine receptor are detected or not [10, 11]. For example, of 6 AAG patients treated with immunosuppressive therapy in a case series reported by Iodice et al., patients with both seropositive (n = 3) and seronegative (n = 2) AAG responded to IVIg. Conversely, we acknowledge that in another case series, 4 seronegative AAG patients treated with IVIg achieved little to no clinical benefit, while high dose intravenous steroids seemed to induce the best responses in those cases [27]. Despite the limited data on treatment of AAG, IVIg is considered a first line therapy since it is an antibody-mediated process and was the reason it was selected as a treatment in this case, in addition to high dose intravenous steroids.
Careful thought has to be given to re-inducing patients with ICI who have had prior irAEs. In this case, our patient made a significant, albeit partial recovery of his AAG and the risk of his melanoma progression was felt to outweigh the risk of developing additional toxicity. Fortunately, when his dysautonmia recurred with reinduction of dual ICI, the symptoms were milder and were managed on an outpatient basis with a prednisone taper in addition to the adjunct use of midodrine and fludrocortisone.
Hypothetically, the autoimmunity causing AAG and other ICI-related toxicities is triggered by autoreactive T-cells. Blocking PD-1 and CTL4 can disrupt important self-tolerance balances, thereby causing a host of autoimmune pathologies. It has also been theorized that autoantibodies could be formed as a result of T-cell dependent activation of B-cells. For example, a case of ICI-associated Guillain-Barre syndrome describes a pan-dysautonomia that occurred after a single dose of ipilimumab – the patient had a tonically dilated pupil, gastrointestinal dysmotility, urinary retention, and orthostatic hypotension. Electromyography showed Guillan-Barre-like changes. The patient recovered with IVIG and droxidopa [28]. Other hypotheses for neurotoxicity from ICI have included inflammation of endoneurial microvessls and sub-perineural inflammation and edema [29]. In the case of AAG, auto-antibodies can be detected but are not mandatory for diagnosis in the correct clinical setting.
In summary, we report a case of AAG induced by dual ICI therapy, requiring high dose solumedrol and IVIG, which recurred with re-induction of ICI. While this is a rare neurotoxicity related to ICI use, clinicians should maintain high suspicion for this toxicity in a patient on ICI who presents with refractory hypotension, nausea, and other dysautonomic symptoms.
Acknowledgements
The authors would like to thank Richard Nowak MD, MS, Assistant Professor of Neurology, Dr. Kevan Herold MD, Professor of Immunobiology and Endocrinology, and Dr. Harriet Kluger MD, Professor of Medical Oncology for their input on this case.
Abbreviations
- AAG
Autoimmune autonomic ganglionopathy
- CTLA-4
Cytotoxic T-lymphocyte-associated antigen-4
- ICI
Immune checkpoint inhibitors
- irAE
Immune-related adverse events
- IVIG
Intravenous immune globulin
- PD-1
Programmed-death-1
Authors’ contributions
All authors were involved in care of the patient and in the writing of the manuscript. CAG, UW, AP, and SW contributed to collecting the data, writing the manuscript and revising the manuscript. All authors have read and approved the final manuscript.
Funding
S.W. has NIH support from K12 CA215110.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Patient is deceased.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Catherine A. Gao, Email: catherine.gao@yale.edu
Urs M. Weber, Email: urs.weber@yale.edu
Aldo J. Peixoto, Email: aldo.peixoto@yale.edu
Sarah A. Weiss, Email: sarah.weiss.sw842@yale.edu
References
- 1.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang XL, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank C, Petrella TM, Hamid O, Zhou HH, Ebbinghaus S, Ibrahim N, Robert C. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390:1853–1862. doi: 10.1016/s0140-6736(17)31601-x. [DOI] [PubMed] [Google Scholar]
- 5.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbe C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia CA, El-Ali A, Rath TJ, Contis LC, Gorantla V, Drappatz J, Davar D. Neurologic immune-related adverse events associated with adjuvant ipilimumab: report of two cases. J Immunother Cancer. 2018;6:83. doi: 10.1186/s40425-018-0393-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spain L, Walls G, Julve M, O'Meara K, Schmid T, Kalaitzaki E, Turajlic S, Gore M, Rees J, Larkin J. Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: a single centre experience and review of the literature. Ann Oncol. 2017;28:377–385. doi: 10.1093/annonc/mdw558. [DOI] [PubMed] [Google Scholar]
- 8.Kao JC, Liao B, Markovic SN, Klein CJ, Naddaf E, Staff NP, Liewluck T, Hammack JE, Sandroni P, Finnes H, Mauermann ML. Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol. 2017;74:1216–1222. doi: 10.1001/jamaneurol.2017.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hottinger AF. Neurologic complications of immune checkpoint inhibitors. Curr Opin Neurol. 2016;29:806–812. doi: 10.1097/wco.0000000000000391. [DOI] [PubMed] [Google Scholar]
- 10.Iodice V, Kimpinski K, Vernino S, Sandroni P, Fealey RD, Low PA. Efficacy of immunotherapy in seropositive and seronegative putative autoimmune autonomic ganglionopathy. Neurology. 2009;72:2002–2008. doi: 10.1212/WNL.0b013e3181a92b52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tijero B, Del Pino R, Perez-Concha T, Acera MA, Gabilondo I, Berganzo K, Graus F, Martinez-Alday JD, Barcena J, Gomez-Esteban JC. Seronegative and seropositive autoimmune autonomic ganglionopathy (AAG): same clinical picture, same response to immunotherapy. J Neuroimmunol. 2018;319:68–70. doi: 10.1016/j.jneuroim.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Weber JS. American Society of Clinical Oncology educational book American Society of Clinical Oncology Annual Meeting. 2012. Practical management of immune-related adverse events from immune checkpoint protein antibodies for the oncologist; pp. 174–177. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia S, Huber BR, Upton MP, Thompson JA. Inflammatory enteric neuropathy with severe constipation after ipilimumab treatment for melanoma: a case report. J Immunother. 2009;32:203–205. doi: 10.1097/CJI.0b013e318193a206. [DOI] [PubMed] [Google Scholar]
- 14.Gaudy-Marqueste C, Monestier S, Franques J, Cantais E, Richard MA, Grob JJ. A severe case of ipilimumab-induced guillain-barre syndrome revealed by an occlusive enteric neuropathy: a differential diagnosis for ipilimumab-induced colitis. J Immunother. 2013;36:77–78. doi: 10.1097/CJI.0b013e31827807dd. [DOI] [PubMed] [Google Scholar]
- 15.Makarious D, Horwood K, Coward JIG. Myasthenia gravis: an emerging toxicity of immune checkpoint inhibitors. Eur J Cancer. 2017;82:128–136. doi: 10.1016/j.ejca.2017.05.041. [DOI] [PubMed] [Google Scholar]
- 16.Mehta JJ, Maloney E, Srinivasan S, Seitz P, Cannon M. Myasthenia gravis induced by nivolumab: a case report. Cureus. 2017;9:e1702. doi: 10.7759/cureus.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirai T, Sano T, Kamijo F, Saito N, Miyake T, Kodaira M, Katoh N, Nishie K, Okuyama R, Uhara H. Acetylcholine receptor binding antibody-associated myasthenia gravis and rhabdomyolysis induced by nivolumab in a patient with melanoma. Jpn J Clin Oncol. 2016;46:86–88. doi: 10.1093/jjco/hyv158. [DOI] [PubMed] [Google Scholar]
- 18.Chen YH, Liu FC, Hsu CH, Chian CF. Nivolumab-induced myasthenia gravis in a patient with squamous cell lung carcinoma: case report. Medicine. 2017;96:e7350. doi: 10.1097/md.0000000000007350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin J, Chmielowski B, Lao CD, Hodi FS, Sharfman W, Weber J, Suijkerbuijk KPM, Azevedo S, Li H, Reshef D, Avila A, Reardon DA. Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist. 2017;22:709–718. doi: 10.1634/theoncologist.2016-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vernino S, Hopkins S, Wang Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton Neurosci. 2009;146:3–7. doi: 10.1016/j.autneu.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winston N, Vernino S. Recent advances in autoimmune autonomic ganglionopathy. Curr Opin Neurol. 2010;23:514–518. doi: 10.1097/WCO.0b013e32833d4c7f. [DOI] [PubMed] [Google Scholar]
- 22.Kimpinski K, Iodice V, Vernino S, Sandroni P, Low PA. Association of N-type calcium channel autoimmunity in patients with autoimmune autonomic ganglionopathy. Auton Neurosci. 2009;150:136–139. doi: 10.1016/j.autneu.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandroni P, Low PA. Other autonomic neuropathies associated with ganglionic antibody. Auton Neurosci. 2009;146:13–17. doi: 10.1016/j.autneu.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iodice V, Kimpinski K, Vernino S, Sandroni P, Low PA. Immunotherapy for autoimmune autonomic ganglionopathy. Auton Neurosci. 2009;146:22–25. doi: 10.1016/j.autneu.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Rudzki JD. Management of adverse events related to checkpoint inhibition therapy. Memo. 2018;11:132–137. doi: 10.1007/s12254-018-0416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv264–iv266. doi: 10.1093/annonc/mdy162. [DOI] [PubMed] [Google Scholar]
- 27.Golden EP, Bryarly MA, Vernino S. Seronegative autoimmune autonomic neuropathy: a distinct clinical entity. Clin Auton Res. 2018;28:115–123. doi: 10.1007/s10286-017-0493-8. [DOI] [PubMed] [Google Scholar]
- 28.Kelly Wu W, Broman KK, Brownie ER, Kauffmann RM. Ipilimumab-induced Guillain-Barre syndrome presenting as dysautonomia: an unusual presentation of a rare complication of immunotherapy. J Immunother. 2017;40:196–199. doi: 10.1097/cji.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 29.Manousakis G, Koch J, Sommerville RB, El-Dokla A, Harms MB, Al-Lozi MT, Schmidt RE, Pestronk A. Multifocal radiculoneuropathy during ipilimumab treatment of melanoma. Muscle Nerve. 2013;48:440–444. doi: 10.1002/mus.23830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.